Abstract
Background
While the Framingham Risk Score provides a reasonable estimation of risk in certain subgroups, the majority of MIs occur in individuals classified as low or moderate risk. Coronary Artery Calcium (CAC) testing provides an individualized measure of atherosclerotic burden that integrates an individual’s cumulative lifetime risk factor exposure that cannot be obtained from serum markers.
Methods and Results
We briefly summarize the existing evidence for the use of CAC scanning in primary prevention and performed a meta-analysis of the existing randomized controlled data investigating the impact of CAC screening on lifestyle modification, risk factors, and downstream testing. We identified four trials published between 2003 and 2011 with a total of 2,490 participants, >75% of whom came from the Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research (EISNER) trial. Three of the trials reported a non-significant increase in smoking cessation in the scan versus no-scan group with a pooled mean of 1.15 (95% CI 0.77 – 1.71). A significant reduction in SBP and LDL was noted in the EISNER trial, but the pooled estimates were 0.23mmHg (95% CI −2.25 – 2.71) and 0.23mg/dL (95% CI −5.96 – 6.42), respectively. Only the EISNER trial reported medication usage according to CAC score. They found a higher CAC score associated with an increased prescription of lipid lowering medications (p=<0.001) and a CAC=0 associated with fewer prescriptions for lipid lowering medications (p=0.02).
Conclusions
Our meta-analysis highlights the paucity of randomized evidence linking CAC scanning to improved intermediate and hard outcomes in primary prevention. Future trials are urgently needed to determine the impact of CAC screening on lifestyle modification, risk factor modification, and downstream testing.
Keywords: coronary artery disease, risk factors, primary prevention, imaging
Introduction
Over 600,000 myocardial infarctions (MI) occur yearly in the United States and more than half of patients whose MI presents as sudden cardiac death have no antecedent symptoms.1 Furthermore, men over 40 years of age have an almost 50% lifetime risk of developing coronary heart disease (CHD) and women of the same age have a risk of approximately one in three.2 The Framingham Risk Score (FRS) is the most commonly used CHD risk prediction model and is an integral component of cardiovascular screening and lipid-lowering guidelines.3 While the FRS provides a reasonable estimation of risk in certain subgroups, it was derived from a relatively homogenous population in an era when pharmacologic treatment options and usage were limited.4 Furthermore, chronologic age is the dominant risk factor in the FRS equation even though it is a poor surrogate for atherosclerotic burden and limits the personalization of risk estimates.5 As a result, the majority of MIs occur in individuals classified as low or moderate risk who therefore, do not meet the ATP III criteria for statin therapy.3, 6
To improve risk estimation, recent research has focused on identifying novel risk predictors. However, when they are added to existing risk prediction models there is little improvement in CHD risk stratification.7, 8 As a result, there is an increasing interest in selectively using atherosclerosis imaging to increase the accuracy of traditional risk prediction models in persons broadly classified as intermediate risk.9 Coronary artery calcium (CAC), measured using non-contrast cardiac computed tomography, is a relatively low cost and non-invasive imaging technique. CAC testing provides an individualized measure of atherosclerotic burden that integrates an individual’s cumulative lifetime risk factor exposure that cannot be obtained from serum markers.10, 11
The use of CAC screening in select patients is included as part of the most recent ACCF/AHA guidelines and adoption of CAC as part of a primary prevention strategy has garnered considerable interest.12–15 As such, this is an ideal time to reflect on the current state of literature supporting CAC. While the risk prediction data categorically demonstrate strong associations, most studies investigating CAC are of an observational cohort design and cannot definitively prove an independent impact of CAC screening on management decisions, patient behavior, and cardiovascular outcomes.
In this article, we briefly summarize the existing evidence for the use of CAC scanning in primary prevention and then shift to our primary aim of investigating the randomized evidence of the impact of CAC on lifestyle modification, risk factors, and downstream testing. We provide an updated meta-analysis and highlight the paucity of randomized data. Finally, we discuss the complex reasons for this literature gap and delineate questions that must be answered by future trials.
The Power of CAC
CAC provides a quantitative and reproducible measurement of the calcified portion of coronary plaque that is nearly pathognomonic for coronary atherosclerosis and strongly predictive of CHD.16–18 The Rotterdam Heart Study followed 1,795 asymptomatic individuals with a mean age of 71 years.19 Compared to individuals with little to no coronary calcium, individuals with extensive calcification, CAC >1,000 Agatston, units were more than 8 times as likely to develop incident myocardial infarction or CHD mortality. Taylor et al demonstrated analogous results in a younger patient population with a mean age of 43 years in which there was an almost 12-fold increased risk for sudden cardiac death, MI, or unstable angina in those subjects with any coronary calcium compared to none.20
The ability of CAC score to predict CHD risk has also been observed between genders, ethnic groups, and among those with only a small amount of calcification. The Multi-Ethnic Study of Atherosclerosis (MESA) cohort measured CAC in a diverse sample of about 6,800 asymptomatic men and women with no prior history of a CHD event. Individuals with even mild calcification, CAC 1–100, had a significantly increased risk of CHD compared to those with no calcification, hazard ratio (HR) of 3.61 (95% CI 1.9–6.7).21 Amongst individuals with normal LDL cholesterol in MESA, the presence of any CAC was still strongly associated with an increased risk of CHD (HR 6.65, 95% CI 2.99–14.78).22 Conversely, an elevated high sensitivity C-reactive protein, the common competitor of CAC for added risk prediction, did not predict CHD events in MESA (HR 0.98, 95% CI 0.62–1.57).
The Power of Zero
A CAC scan with zero coronary calcification stands alone as the most powerful negative risk factor for CHD and re-stratifies a significant proportion of individuals from an intermediate to a low risk group where conservative treatment strategies can be used.23, 24 A recent meta-analysis incorporated results from 13 studies of 71,595 asymptomatic subjects with a mean follow-up of more than 4 years.25 Those with a CAC of 0 had a relative risk (RR) of 0.15 (95% CI 0.11 – 0.21) for developing a cardiovascular event compared to individuals with any CAC.
In a different cohort, Blaha et al reported on 44,052 asymptomatic patients in which a CAC of 0 was associated with < 1% 10-year risk of all-cause mortality, indicating an excellent long term prognosis.26 Even among patients with multiple conventional risk factors, a CAC of 0 is associated with a low absolute number of events.27 The strong negative risk predictive value of a CAC of 0 has also been demonstrated across a diverse range of patient populations including women, diabetics, and the elderly.22–25
The Power of Addition
CAC is an established independent risk factor for CHD, but ultimately the clinical utility of any new CHD risk marker lies in its ability to improve upon the current risk prediction models. The incremental value of CAC was investigated in MESA using a model adding CAC to the Framingham risk factors, demonstrating an increase in the C-statistic from 0.77 to 0.82 (p <0.001).21 This change represents an improvement from a C-statistic with an acceptable discriminative value (0.7–0.8) to an excellent discriminative value (0.8–0.9).28
In the same study this combined model was examined within different ethnic groups and the addition of CAC to the Framingham risk factors increased the C-statistic by as much as 0.11. By comparison, other individual novel risk factors have shown little incremental value when integrated into the FRS. For example, Melander et al incorporated several novel serum biomarkers into a conventional risk model and found no improvement with a C-statistic of 0.009 (p-value 0.08).7
To further investigate CAC’s additional discriminative power, Polonsky et al investigated the Net Reclassification Index (NRI) of CAC in MESA.23 NRI determines the frequency of appropriate versus inappropriate reclassification (defined as whether or not an event occurs) from one risk group to another. When CAC was added to the MESA-recalibrated FRS there was a NRI of 0.25 (95% CI 0.16–0.34) for the entire study group and an NRI of 0.55 (95%CI 0.41–0.69) for the intermediate risk group. Therefore, among intermediate risk participants 55% were reclassified as either high risk (16%) or low risk (39%). Incorporating CAC into the FRS resulted in an additional 23% of patients who went on to develop CHD being reclassified from intermediate to high risk and an additional 13% who did not develop CHD during follow up to be reclassified as low risk. These findings demonstrate the significant additional discriminative value of CAC and underscore the clinically utility of this information.
The impact of CAC screening on lifestyle modification, risk factors, and downstream testing: a meta-analysis
While requiring proof that a test improves outcomes is nearly unprecedented in cardiovascular medicine, this level of evidence may be required for routine use of CAC given the tremendous public health implications of worldwide CHD screening. At this time there are few studies that have investigated how the results from a CAC scan may impact clinical outcomes and they are predominantly observational.
For example, Wong et al reported on a group of 703 asymptomatic individuals who were primarily self-referred for CAC scanning.29 Those with any CAC were more likely to start aspirin RR 1.86 (p <0.01) or a cholesterol lowering medication RR 3.54 (p = 0.01), compared to those without CAC. A similar relationship was observed in a group of 1,640 men with a mean age of 43 years. Those men with any CAC were more than three times as likely to use either aspirin (p<0.001) or a statin (p<0.001) and almost seven times as likely to use both aspirin and a statin (p<0.001) in comparison to men without CAC.30 Orakzai et al followed 980 individuals for a mean of three years and found that those with a CAC score ≥400 were more likely to increase their amount of exercise OR 2.03 (95% CI 1.26–3.27) and modify their diet OR 2.66 (95% CI 1.63–4.32) compared to individuals without CAC.31
Although these results are suggestive of true clinical reclassification, there are important limitations. These studies did not have a control group without CAC testing; therefore, no comparison can be made to individuals who received risk assessment in the usual fashion. There was no long term follow-up for clinical events. Additionally, the subjects in these groups were either self-selected or referred by their primary care physician to undergo CAC scanning and may be more highly motivated than the general population.
Hackam et al investigated the downstream effect of imaging on behavior, but this meta-analysis included trials with a variety of cardiac imaging techniques.32 Since then, results from EISNER, the largest randomized controlled trial yet to investigate the impact of CAC screening on CHD were published.33 We performed an update to the meta-analysis reported by Hackam et al by conducting a thorough up-to-date literature review, incorporating the EISNER trial results, and only including trials using CAC.
We identified four trials with a total of 2,490 participants, >75% of whom came from the EISNER trial (table 1).33–36 The trials were published between 2003 and 2011 with follow-up time ranging from one to four years. Three of the trials reported a change in cardiovascular risk factors or FRS as their primary outcome.33, 34, 36 Obuchowski et al performed total body computed tomography in addition to CAC and the results were not stratified between the two imaging modalities.35 The number of participants in each trial varied from 50 to 1,934. The trial conducted by Lederman et al was conducted exclusively among women and the other three trials consisted of at least 50% male participants.36
Table 1.
Characteristics of the four included trials investigating the impact coronary artery calcium scanning on cardiovascular risk factors and downstream testing
| Author, year | Imaging Modality | Primary Outcome | Number of Participants |
Age, mean (yrs) |
Male (%) | Follow-up (yrs) |
Imaging Abnormality (%)* |
|---|---|---|---|---|---|---|---|
| Lederman et al, 200736 | DHCT1 | CVD Risk Factors | 56 | 65 | 0 | 1 | n/a |
| O’Malley et al, 200334 | EBT2 | 10yr FRS5 event rate | 450 | 42 | 79 | 1 | 15 |
| Obuchowski et al, 200735 | Total Body CT / MDCT3 | Incident Clinical Disease | 50 | 55 | 50 | 2 | 64 |
| Rozanski et al, 201133 | EBT2/MCT4 | CAD Risk Factors | 1,934 | 59 | 53 | 4 | 52 |
– Double Helical Computed Tomography
– Electron Beam Tomography
– Multidetector Computed Tomograph
– Multislice Computed Tomography
– Framingham Risk Score
Defined as any abnormality.
All four trials collected data on smoking habits with three of the trials reporting a non-significant increase in smoking cessation in the scan versus no-scan group, the pooled mean was 1.15 (95% CI 0.77 – 1.71). Three trials reported results for BMI and two for glycated hemoglobin with non-significant pooled estimates of −0.05kg/m2 (95% CI−0.16 – 0.06) and 0.04% (95% CI −0.06 – 0.15), respectively for the scan versus no-scan groups.
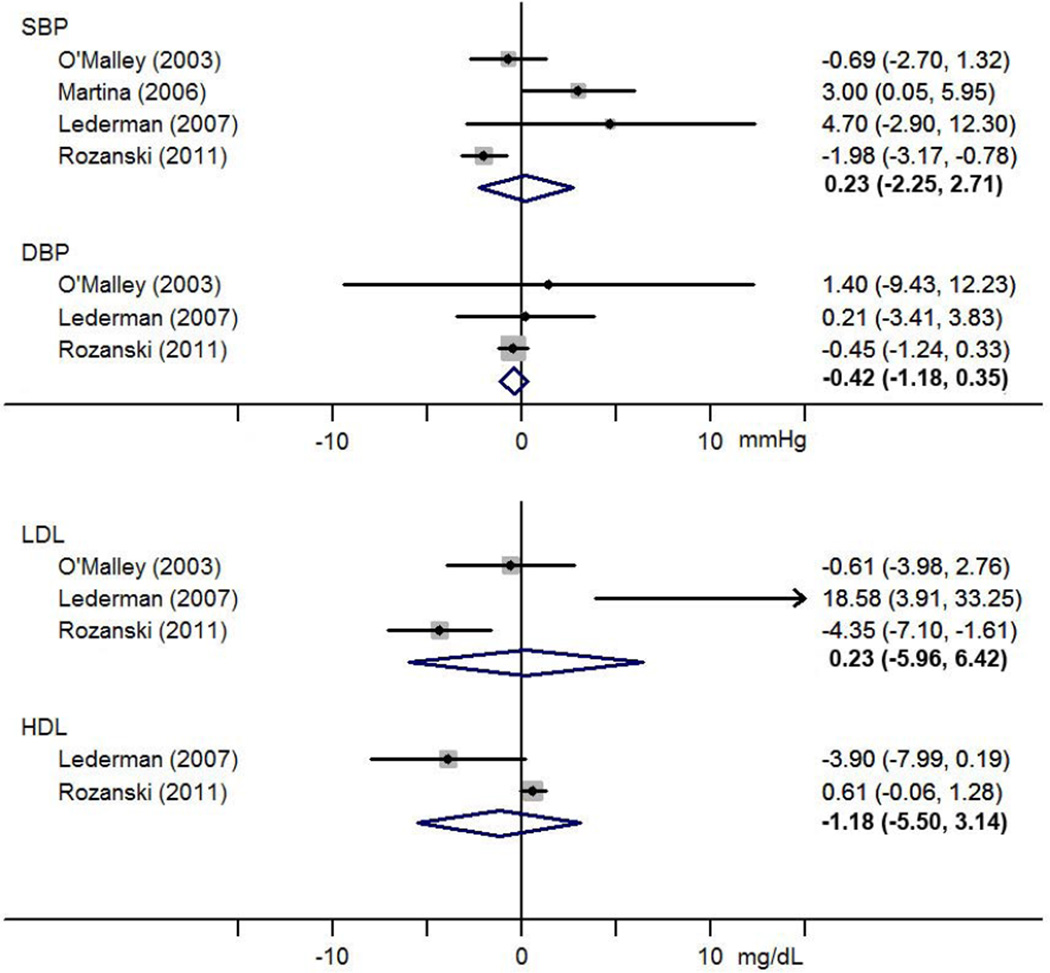
Change in blood pressure and cholesterol for the scan versus no-scan group was reported in three trials, with the exception of HDL which was only available for two of the trials (figure 1). The EISNER trial reported a significant reduction in systolic blood pressure (SBP), although the pooled estimate was −0.23mmHg (95% CI −2.25 – 2.71) for SBP, −0.42mmHg (95% CI −1.18 – 0.35) for diastolic blood pressure, and −1.18mg/dL (−5.50 – 3.14) for HDL. A significant reduction in LDL was noted in the EISNER trial, but the pooled estimate was 0.23mg/dL (95% CI −5.96 – 6.42).
Figure 1.
Forest plot of the effect of coronary artery calcium screening on the mean change in blood pressure and cholesterol level
Only the EISNER trial reported medication usage according to CAC score.33 Rozanski et al reported that a higher CAC score was associated with a significant increase in the prescription of lipid lowering medications (p<0.001). In contrast, those with CAC=0 were prescribed fewer lipid lowering medications than the non-scan group (p=0.02) and had a 25% reduction in their medication costs (p=0.005).
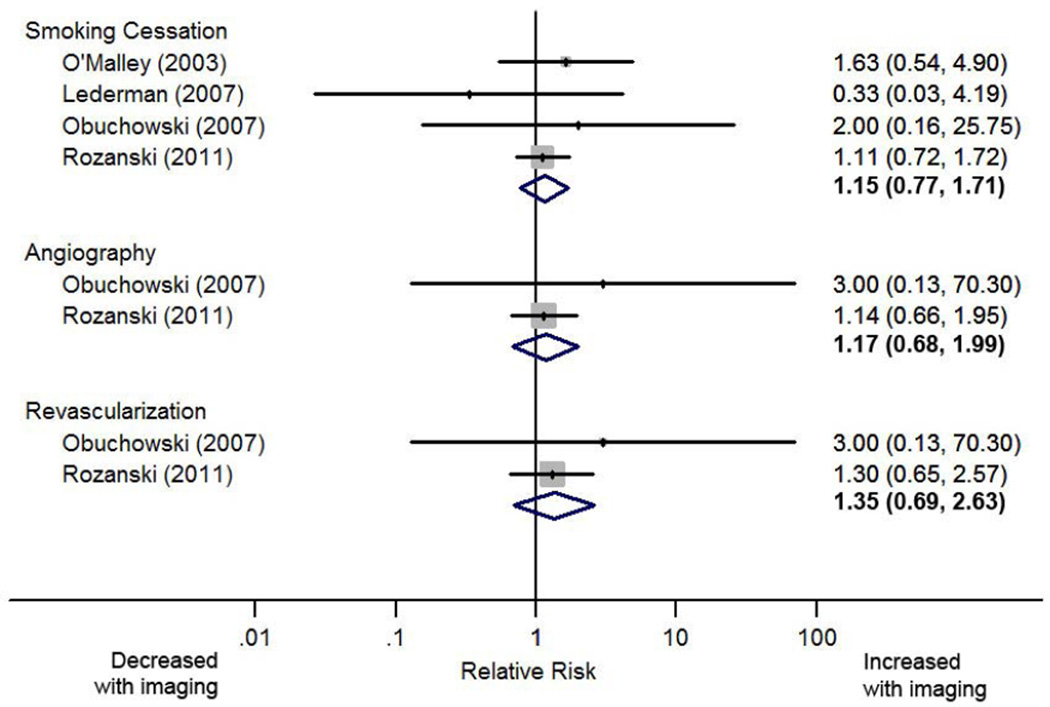
Two studies investigated downstream testing with both reporting a non-significant increase in patients undergoing catheterization and angiography in the CAC scan group, pooled estimates were RR 1.17 (95% CI 0.68 – 1.99) for angiography and RR 1.35 (95% CI 0.69 – 2.63) for revascularization (figure2). In the EISNER trial more than 60% of patients with CAC ≥400 had some form of downstream stress testing versus approximately a quarter of those with CAC=0 (p <0.001).33 Those with CAC=0 had overall medical costs that were approximately 70% less than those incurred by individuals with a CAC ≥400 (p <0.001).
Figure 2.
Forest plot of the effect of coronary artery calcium screening on the relative risk of smoking cessation and downstream testing
Both the no-scan and scan group had a low absolute number of invasive cardiovascular procedures and there was no significant difference in medical costs between these groups with a median of $3,649 spent for the no-scan group and $4,053 for the scan group (p=0.09). Only the EISNER trial reported data on cardiovascular outcomes. Two participants in the no-scan group were diagnosed with MI versus ten patients in the scan group (p=0.36). There were three cardiac deaths, one in the no-scan group and two in the scan group.
Discussion
Current primary prevention guidelines rely on the FRS to discriminate among low, intermediate, and high risk individuals.3 Regrettably, a significant percentage of individuals classified as low risk still go on to develop CHD.6 An important reason for misclassification in conventional risk models is the use of age as a surrogate marker for atherosclerotic burden, which overlooks the considerable variation among individuals with similar levels of traditional risk factors. CAC is a unique risk predictor, because it provides an individualized measure of atherosclerotic burden, integrating risk exposure over a lifetime, which conventional risk models are unable to assess.11
The most recent ACCF/AHA guidelines give a class IIa recommendation for the use of CAC as a reasonable procedure to aid in the risk assessment of patients with an intermediate 10-year cardiovascular risk.9 However, guidelines from other agencies vary considerably, mostly as a result of the limited evidence from randomized controlled trials.14, 37, 38 This leaves the decision of whether or not to include CAC as part of a risk stratification protocol to the individual clinician’s judgment. CAC scanning does also have some small risks compared to serum markers. A CAC scan exposes an individual to approximately 1mSv of radiation, which is comparable to a lumbar spine roentgenogram and much less than the 9–12mSv dose from a typical nuclear myocardial perfusion scan.39–41 Incidental extra-cardiac findings may also be revealed by CAC scanning. The majority of these findings are pulmonary nodules of uncertain clinical significance, which are more likely to be found in older individuals and those with a history of smoking.42 However, only about 10% of these incidental findings necessitate further testing and observation.43
Our meta-analysis highlights the paucity of randomized evidence linking CAC scanning to improved intermediate and hard outcomes in primary prevention. We found only four randomized controlled trials that have investigated the relationship between CAC and CHD risk factors, downstream resource utilization, or hard outcomes. Of these, two trials had sample sizes of approximately 50 participants while the EISNER trial accounts for >75% of the total participants studied to date.33 In contrast, observational CAC studies have enrolled up to 44,000 participants.44–46
Our meta-analysis of course has a number of limitations, the most significant being the small number of available trials and modest total number of participants. As a result there is inadequate power to draw meaningful conclusions, even after pooling the results. In addition, due to the relatively short follow-up, it was unfeasible for the majority of trials to investigate fatal and non-fatal MI in a primary prevention population where the absolute event rate is expected to be low. The trial conducted by O’Malley et al consisted of relatively young and healthy volunteers largely classified as low risk by the FRS who were not in the risk group most likely to benefit from CAC screening. However, these limitations underscore our primary message: there remains a remarkable paucity of randomized data.
Defining the optimal use of CAC was identified in 2009 as a top 100 priority by the Institute of Medicine and future trials are urgently needed to determine the impact of CAC screening on lifestyle modification, risk factor modification, and downstream testing.47 These trials will need to be randomized with a control group in which treatment is based on conventional risk stratification methods compared to an intervention group in which conventional risk assessment and treatment is augmented with CAC.
However, a number of challenges must be addressed before these trials can be implemented. Such trials will require long-term follow up of individuals who have a low to intermediate 10-year risk of developing CHD. Using this lower risk population will necessitate very large sample sizes and enormous expense (likely supported by the NIH/NHLBI) in order to be powered for hard CHD outcomes. Additionally, CAC screening is an increasingly low cost and low risk strategy. Therefore, some may consider it improper to withhold CAC screening for patients who would qualify as per the AHA’s IIa recommendation.48 Others will argue that generic statins and aspirin cannot be withheld from patients deemed low risk by FRS, but with a heavy burden of CAC. It must also be considered that many physicians already aggressively prescribe statin therapy outside of existing guidelines to intermediate risk patients even without the use of CAC, which may significantly diminish the potential risk reduction of CAC screening.
In order to receive a class I recommendation, a screening exam must demonstrate significant benefit with minimal or no harm. At present, randomized controlled trial evidence of a reduction in cardiovascular risk factors or hard outcomes likely represents the evidence threshold necessary to determine if a class I recommendation is justified for any CHD screening tool. It is expected that the upcoming ATP IV guidelines will lower the threshold for instituting statin therapy.48 In light of this expected change, CAC trials that focus on individuals with a 5–10% 10-year risk will likely provide the most clinically meaningful results. However, over the typical 5-year follow-up of a trial only a small proportion of these individuals will experience a hard CHD outcome and therefore even larger sample sizes will be required to investigate these outcomes.
This is an interesting crossroads in the field of cardiology, because there appears to be a "raising of the bar" regarding the prerequisite level of benefit prior to accepting strategies for CHD risk screening. It is interesting that no such randomized evidence for improvement in CVD risk factors or outcomes currently exists for the FRS, SCORE, QRISK, any individual test such as serum cholesterol or hsCRP, or global risk assessment in general.
It will be many years before the results of CAC trials are available, which in the interim provides little guidance to determine which intermediate risk patients should receive CAC scanning for further risk stratification and treatment decisions. Given the significant challenges that must be overcome to conduct such a trial, it is uncertain whether or not it would even be funded. In fact, a proposal submitted by Greenland et al to perform a prospective randomized CAC trial has to date not received funding. Therefore, the use of non-randomized data may take on a greater importance. One approach to bridge the literature gap is through the use of propensity matched analyses as have been used to asses downstream outcomes in coronary computed tomographic angiography.49 These studies provide a higher level of evidence than observational cohorts and are inexpensive compared to randomized controlled trials. Furthermore, if performed at high volume imaging centers they could readily accrue large numbers of participants. Rigorous randomized assessment of CAC, FRS, or any other method of risk assessment such as use of hsCRP is long overdue, and it is important to clarify the conclusive "level of benefit" that is required before CAC or any tool is used as part of a routine and universal risk prediction strategy.
Acknowledgments
Disclosures:
Dr. Berman has research grants from Siemens and GE/Amersham. He is on the speaker’s bureau and has research grants for Astelles and Lantheus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics-2011 update: A report from the american heart association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353:89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- 3.Third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 4.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 5.Berry JD, Lloyd-Jones DM, Garside DB, Greenland P. Framingham risk score and prediction of coronary heart disease death in young men. American heart journal. 2007;154:80–86. doi: 10.1016/j.ahj.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee GK, Lee LC, Liu CW, Lim SL, Shi LM, Ong HY, Lim YT, Yeo TC. Framingham risk score inadequately predicts cardiac risk in young patients presenting with a first myocardial infarction. Annals of the Academy of Medicine, Singapore. 2010;39:163–167. [PubMed] [Google Scholar]
- 7.Melander O, Newton-Cheh C, Almgren P, Hedblad B, Berglund G, Engstrom G, Persson M, Smith JG, Magnusson M, Christensson A, Struck J, Morgenthaler NG, Bergmann A, Pencina MJ, Wang TJ. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA : the journal of the American Medical Association. 2009;302:49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wannamethee SG, Welsh P, Lowe GD, Gudnason V, Di Angelantonio E, Lennon L, Rumley A, Whincup PH, Sattar N. N-terminal pro-brain natriuretic peptide is a more useful predictor of cardiovascular disease risk than c-reactive protein in older men with and without pre-existing cardiovascular disease. Journal of the American College of Cardiology. 2011;58:56–64. doi: 10.1016/j.jacc.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 9.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK. 2010 accf/aha guideline for assessment of cardiovascular risk in asymptomatic adults: Executive summary: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2010;122:2748–2764. doi: 10.1161/CIR.0b013e3182051bab. [DOI] [PubMed] [Google Scholar]
- 10.Roger Blumenthal JF, Wong Nathan. Preventative cardiology: Companion to braunwald's heart disease. Philadelphia, PA: Elsevier; 2011. [Google Scholar]
- 11.McClelland RL, Nasir K, Budoff M, Blumenthal RS, Kronmal RA. Arterial age as a function of coronary artery calcium (from the multi-ethnic study of atherosclerosis [mesa]) The American journal of cardiology. 2009;103:59–63. doi: 10.1016/j.amjcard.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenland P, Polonsky TS. Time for a policy change for coronary artery calcium testing in asymptomatic people? Journal of the American College of Cardiology. 2011;58:1702–1704. doi: 10.1016/j.jacc.2011.06.048. [DOI] [PubMed] [Google Scholar]
- 13.Lauer MS. Screening asymptomatic subjects for subclinical atherosclerosis: Not so obvious. Journal of the American College of Cardiology. 2010;56:106–108. doi: 10.1016/j.jacc.2010.01.059. [DOI] [PubMed] [Google Scholar]
- 14.Using nontraditional risk factors in coronary heart disease risk assessment: U.S. Preventive services task force recommendation statement. Annals of internal medicine. 2009;151:474–482. doi: 10.7326/0003-4819-151-7-200910060-00008. [DOI] [PubMed] [Google Scholar]
- 15.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS. Accf/aha 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: A report of the american college of cardiology foundation clinical expert consensus task force (accf/aha writing committee to update the 2000 expert consensus document on electron beam computed tomography) developed in collaboration with the society of atherosclerosis imaging and prevention and the society of cardiovascular computed tomography. Journal of the American College of Cardiology. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Detrano RC, Anderson M, Nelson J, Wong ND, Carr JJ, McNitt-Gray M, Bild DE. Coronary calcium measurements: Effect of ct scanner type and calcium measure on rescan reproducibility--mesa study. Radiology. 2005;236:477–484. doi: 10.1148/radiol.2362040513. [DOI] [PubMed] [Google Scholar]
- 17.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, american heart association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 18.Sabour S, Rutten A, van der Schouw YT, Atsma F, Grobbee DE, Mali WP, Bartelink ME, Bots ML, Prokop M. Inter-scan reproducibility of coronary calcium measurement using multi detector-row computed tomography (mdct) European journal of epidemiology. 2007;22:235–243. doi: 10.1007/s10654-007-9123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vliegenthart R, Oudkerk M, Hofman A, Oei HH, van Dijck W, van Rooij FJ, Witteman JC. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572–577. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- 20.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O'Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: Mean three-year outcomes in the prospective army coronary calcium (pacc) project. Journal of the American College of Cardiology. 2005;46:807–814. doi: 10.1016/j.jacc.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 21.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. The New England journal of medicine. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 22.Blaha MJ, Budoff MJ, DeFilippis AP, Blankstein R, Rivera JJ, Agatston A, O'Leary DH, Lima J, Blumenthal RS, Nasir K. Associations between c-reactive protein, coronary artery calcium, and cardiovascular events: Implications for the jupiter population from mesa, a population-based cohort study. Lancet. 2011;378:684–692. doi: 10.1016/S0140-6736(11)60784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA : the journal of the American Medical Association. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaha MJ, Blumenthal RS, Budoff MJ, Nasir K. Understanding the utility of zero coronary calcium as a prognostic test: A bayesian approach. Circulation. Cardiovascular quality and outcomes. 2011;4:253–256. doi: 10.1161/CIRCOUTCOMES.110.958496. [DOI] [PubMed] [Google Scholar]
- 25.Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffmann U, Cury RC, Abbara S, Brady TJ, Budoff MJ, Blumenthal RS, Nasir K. Diagnostic and prognostic value of absence of coronary artery calcification. JACC. Cardiovascular imaging. 2009;2:675–688. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 26.Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, Callister T, Raggi P, Blumenthal RS, Nasir K. Absence of coronary artery calcification and all-cause mortality. JACC. Cardiovascular imaging. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Michael G, Silverman MJB, Budoff Matthew J, Blankenstein Ron, Sibley Christopher T, Blumenthal Roger S, Nasir Khurram. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: The multi-ethnic study of atherosclerosis (mesa) American Heart Association Scientific Sessions 2011. 2011 doi: 10.1093/eurheartj/eht508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosmer D. Applied logistic regression. New York, NY: John Wiley and Sons; 2000. [Google Scholar]
- 29.Wong ND, Detrano RC, Diamond G, Rezayat C, Mahmoudi R, Chong EC, Tang W, Puentes G, Kang X, Abrahamson D. Does coronary artery screening by electron beam computed tomography motivate potentially beneficial lifestyle behaviors? The American journal of cardiology. 1996;78:1220–1223. doi: 10.1016/s0002-9149(96)00599-1. [DOI] [PubMed] [Google Scholar]
- 30.Taylor AJ, Bindeman J, Feuerstein I, Le T, Bauer K, Byrd C, Wu H, O'Malley PG. Community-based provision of statin and aspirin after the detection of coronary artery calcium within a community-based screening cohort. Journal of the American College of Cardiology. 2008;51:1337–1341. doi: 10.1016/j.jacc.2007.11.069. [DOI] [PubMed] [Google Scholar]
- 31.Orakzai RH, Nasir K, Orakzai SH, Kalia N, Gopal A, Musunuru K, Blumenthal RS, Budoff MJ. Effect of patient visualization of coronary calcium by electron beam computed tomography on changes in beneficial lifestyle behaviors. The American journal of cardiology. 2008;101:999–1002. doi: 10.1016/j.amjcard.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 32.Hackam DG, Shojania KG, Spence JD, Alter DA, Beanlands RS, Dresser GK, Goela A, Davies AH, Badano LP, Poldermans D, Boersma E, Njike VY. Influence of noninvasive cardiovascular imaging in primary prevention: Systematic review and meta-analysis of randomized trials. Archives of internal medicine. 2011;171:977–982. doi: 10.1001/archinternmed.2011.69. [DOI] [PubMed] [Google Scholar]
- 33.Rozanski A, Gransar H, Shaw LJ, Kim J, Miranda-Peats L, Wong ND, Rana JS, Orakzai R, Hayes SW, Friedman JD, Thomson LE, Polk D, Min J, Budoff MJ, Berman DS. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the eisner (early identification of subclinical atherosclerosis by noninvasive imaging research) prospective randomized trial. Journal of the American College of Cardiology. 2011;57:1622–1632. doi: 10.1016/j.jacc.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Malley PG, Feuerstein IM, Taylor AJ. Impact of electron beam tomography, with or without case management, on motivation, behavioral change, and cardiovascular risk profile: A randomized controlled trial. JAMA : the journal of the American Medical Association. 2003;289:2215–2223. doi: 10.1001/jama.289.17.2215. [DOI] [PubMed] [Google Scholar]
- 35.Obuchowski NA, Holden D, Modic MT, Cheah G, Fu AZ, Brant-Zawadzki M, Seballos R, Mohammed TL. Total-body screening: Preliminary results of a pilot randomized controlled trial. Journal of the American College of Radiology : JACR. 2007;4:604–611. doi: 10.1016/j.jacr.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Lederman J, Ballard J, Njike VY, Margolies L, Katz DL. Information given to postmenopausal women on coronary computed tomography may influence cardiac risk reduction efforts. Journal of clinical epidemiology. 2007;60:389–396. doi: 10.1016/j.jclinepi.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Nishimura R, Ohman EM, Page RL, Stevenson WG, Tarkington LG, Yancy CW. 2010 accf/aha guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Journal of the American College of Cardiology. 2010;56:e50–e103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 38.De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, Ebrahim S, Faergeman O, Graham I, Mancia G, Cats VM, Orth-Gomer K, Perk J, Pyorala K, Rodicio JL, Sans S, Sansoy V, Sechtem U, Silber S, Thomsen T, Wood D. European guidelines on cardiovascular disease prevention in clinical practice: Third joint task force of european and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of eight societies and by invited experts) European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2003;10:S1–S10. doi: 10.1097/01.hjr.0000087913.96265.e2. [DOI] [PubMed] [Google Scholar]
- 39.Simpson AK, Whang PG, Jonisch A, Haims A, Grauer JN. The radiation exposure associated with cervical and lumbar spine radiographs. Journal of spinal disorders & techniques. 2008;21:409–412. doi: 10.1097/BSD.0b013e3181568656. [DOI] [PubMed] [Google Scholar]
- 40.Halpern EJ, Fischman D, Savage MP, Koka AR, DeCaro M, Levin DC. Decision analytic model for evaluation of suspected coronary disease with stress testing and coronary ct angiography. Academic radiology. 2010;17:577–586. doi: 10.1016/j.acra.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 41.Budoff MJ, McClelland RL, Chung H, Wong ND, Carr JJ, McNitt-Gray M, Blumenthal RS, Detrano RC. Reproducibility of coronary artery calcified plaque with cardiac 64-mdct: The multi-ethnic study of atherosclerosis. AJR. American journal of roentgenology. 2009;192:613–617. doi: 10.2214/AJR.08.1242. [DOI] [PubMed] [Google Scholar]
- 42.Lee CI, Tsai EB, Sigal BM, Plevritis SK, Garber AM, Rubin GD. Incidental extracardiac findings at coronary ct: Clinical and economic impact. AJR. American journal of roentgenology. 2010;194:1531–1538. doi: 10.2214/AJR.09.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horton KM, Post WS, Blumenthal RS, Fishman EK. Prevalence of significant noncardiac findings on electron-beam computed tomography coronary artery calcium screening examinations. Circulation. 2002;106:532–534. doi: 10.1161/01.cir.0000027136.56615.de. [DOI] [PubMed] [Google Scholar]
- 44.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–833. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 45.LaMonte MJ, FitzGerald SJ, Church TS, Barlow CE, Radford NB, Levine BD, Pippin JJ, Gibbons LW, Blair SN, Nichaman MZ. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. American journal of epidemiology. 2005;162:421–429. doi: 10.1093/aje/kwi228. [DOI] [PubMed] [Google Scholar]
- 46.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS. Long-term prognosis associated with coronary calcification: Observations from a registry of 25,253 patients. Journal of the American College of Cardiology. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 47.Insitute of medicine. Initial national priorities for comparative effectiveness research. 2009 [Google Scholar]
- 48.Blumenthal RS, Hasan RK. Actually, it is more of a guideline than a rule. Journal of the American College of Cardiology. 2011;57:1601–1603. doi: 10.1016/j.jacc.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 49.McEvoy JW, Blaha MJ, Nasir K, Yoon YE, Choi EK, Cho IS, Chun EJ, Choi SI, Rivera JJ, Blumenthal RS, Chang HJ. Impact of coronary computed tomographic angiography results on patient and physician behavior in a low-risk population. Archives of internal medicine. 2011;171:1260–1268. doi: 10.1001/archinternmed.2011.204. [DOI] [PubMed] [Google Scholar]