Abstract
Objective
Von Willebrand factor antigen (vWF-Ag) is a marker of pulmonary and systemic endothelial activation and injury. Adult studies indicate that patients with plasma vWF-Ag levels ≥450% of control early in the course of acute lung injury (ALI) have an increased risk of death. The objective of this study was to evaluate whether vWF-Ag is elevated in the early phase of ALI in children and whether the magnitude of the increase was predictive of two important outcomes: mortality or duration of mechanical ventilation.
Design
Two-center, prospective observational study.
Setting
Two pediatric intensive care units: one in an academic university setting and one in a major community children's hospital.
Patients
After appropriate consent, plasma was collected from 48 pediatric patients on day 1 of ALI, 45 patients on day 2 of ALI, and four intubated controls.
Interventions
None.
Measurements and Main Results
Mean Pao2/Fio2 at the onset of ALI was 140 ± 70, and mortality rate was 17%. vWF-Ag levels on day 1 of ALI were higher in patients compared with controls (287 ±183 vs. 87 ± 84% of control [mean ± sd], p < .05). Patients with vWF-Ag levels ≥450% of control on day 1 of ALI had a markedly greater risk of death (odds ratio, 7.0; confidence interval, 1.31, 37.30; p < .05). Multivariate analysis revealed that elevated vWF-Ag level and either presence of multiple organ system failure or Pediatric Risk of Mortality III score independently predict increased risk of death. vWF-Ag levels on day 2 of ALI were significantly higher in patients who required prolonged mechanical ventilation (316 ±173 vs. 191 ± 89% of control, p < .05).
Conclusions
Early injury to the systemic and pulmonary endothelium, as measured by plasma vWF-Ag levels, is associated with an increased risk of death and prolonged mechanical ventilation in pediatric patients with ALI. (Pediatr Crit Care Med 2007; 8:96–101)
Keywords: pediatric acute lung injury, von Willebrand factor antigen, mortality, mechanical ventilation, acute respiratory distress syndrome, biological markers
The process of acute lung injury (ALI) involves cell injury and activation in both the alveolar epithelium and lung endothelium (1, 2). Von Willebrand factor antigen (vWF-Ag), also known as factor VIII related antigen, is a large, multimeric glycoprotein that is produced predominantly by endothelial cells and, to a lesser extent, by platelets and megakaryocytes (3, 4). In the setting of endothelial cell activation or injury, vWF-Ag is released from preformed stores (Weibel-Palade bodies) into the circulation (5, 6). Since vWF-Ag is also a component of hyaline membranes (7), it is postulated that in ALI, this high molecular weight molecule enters the alveolar compartment as a result of increased permeability of the alveolar capillary barrier (8).
Several adult human studies have confirmed that plasma vWF-Ag is elevated in the plasma of patients with ALI/acute respiratory distress syndrome (ARDS) (8–17), with two smaller studies also suggesting that early elevations are prognostic of death (8, 10). Most recently, the early elevation of vWF-Ag, particularly ≥450% of control, was predictive of several important clinical outcomes, including mortality, duration of mechanical ventilation, and organ failure-free days, in 559 adults enrolled in the National Heart, Lung, and Blood Institute ARDS Network study of two tidal volume strategies (11).
In 1984, Mazzoni and colleagues (18) reported elevations of vWF-Ag in the plasma of 26 spontaneously breathing infants with severe respiratory syncytial virus bronchiolitis that subsequently decreased to control levels 1 month later in follow-up. No further studies have been done to date describing the potential significance of plasma vWF-Ag levels in the pediatric ALI/ARDS population.
Given that ALI represents not only injury to the alveolar epithelium but also injury to and activation of both the systemic and pulmonary endothelium, we hypothesized that plasma vWF-Ag levels would be elevated in pediatric patients with early ALI and would be associated with a greater risk of death and need for prolonged mechanical ventilation. To test these hypotheses, we measured vWF-Ag in the plasma in pediatric patients on days 1 and/or 2 of ALI and in intubated pediatric control patients.
Materials and Methods
As part of a larger observational study (19), all pediatric patients with ALI were prospectively identified from the pediatric intensive care unit (PICU) at Children's Hospital and Research Center Oakland from July 1996 through May 2000 and the PICU at the University of California San Francisco Medical Center from July 1996 through June 1998 and were included in a clinical database. Patients were included if they met the 1994 American-European Consensus Committee definitions of ALI or ARDS (bilateral infiltrates on chest radiograph, no evidence of left atrial hypertension, and Pao2/Fio2 <300 mm Hg for ALI and Pao2/Fio2 <200 for ARDS as measured on arterial blood gas) (2). Patients were excluded if they were <36 wks corrected gestational age or ≥19 yrs of age, if they had any evidence of intracardiac shunting on echocardiography, and if there was no arterial blood gas confirming a Pao2/Fio2 <300. A total of 320 patients were included in this database during this time period (19). As this was an observational study, medical therapies and modes of mechanical ventilation were not controlled and were instituted based solely on clinical need.
Of these 320 patients, a subset of 60 patients with ALI had plasma samples drawn or unused plasma retrieved from the laboratory at their respective institutions within the first 48 hrs of ALI. A total of 48 patients had samples measured on day 1 and 45 patients on day 2 of ALI. Of the 48, 33 patients had samples obtained from both days 1 and 2 of ALI. Patients were excluded if institutionally appropriate consent was unable to be obtained or if unused plasma was not available in the laboratory. This study was approved by the Children's Hospital and Research Center Oakland Institutional Review Board and the University of California San Francisco Committee on Human Research. At the University of California San Francisco Medical Center, consent was required for blood drawn additionally for research purposes. At Children's Hospital and Research Center Oakland, consent was required for both additional blood drawn for research purposes and unused blood that was retrieved from the clinical laboratory.
Four patients, ages 4–15 yrs old, without any known risk factors for the development of ALI and mechanically ventilated for >24 hrs were identified as control subjects. After appropriate consent was obtained, unused plasma samples from the first 24 hrs of mechanical ventilation were measured for vWF-Ag.
The primary outcome was all-cause PICU mortality. The secondary outcome was the duration of unassisted mechanical ventilation, defined in accordance with the ARDS Network protocol (20) as the number of days after the onset of ALI (per 28-day month) that the patient was alive and off mechanical ventilation. Because this outcome is not normally distributed, the duration of unassisted mechanical ventilation (ventilator-free days) was categorized into ≤14 and >14 days of mechanical ventilation per 28-day month as in our prior study (21). All patients who died while still mechanically ventilated during their PICU course were assigned a value of zero and included in the ≤14-day category; all patients not requiring mechanical ventilation were assigned a value of 28 and included in the >14-day category. Multiple organ system dysfunction was defined as the presence of two or more nonpulmonary organ system failures within the first 24 hrs after the onset of ALI. Definitions of each multiple organ system failure were the same as previously (19, 21). Risk of mortality was assessed using the unadjusted Pediatric Risk of Mortality (PRISM) III score that was calculated for the first 24 hrs after the onset of ALI (22). Clinical data were obtained by comprehensive chart review.
Levels of vWF-Ag were measured in plasma samples in duplicate by commercially available, enzyme-linked immunosorbent assays (Diagnostica Stago, Parsippany, NJ). Results are expressed as a percentage of a normal pooled plasma control reference supplied by the manufacturer that has been assayed against a secondary standard of the 4th International Standard of vWF-Ag (23).
Results were analyzed using the Stata6 statistical software package (24). Data were compared using parametric (Student's t-test) or nonparametric statistics (rank-sum) where appropriate. Univariable analysis was done using simple logistic and linear regression as well as chi-square analysis and Fisher's exact tests. We established pre hoc a cutoff value of vWF-Ag of ≥450% control as a potential predictor of poor clinical outcomes, as in our prior studies (8, 11, 25). Multivariable logistic regression analyses were completed using this cutoff value to test the association of categorized plasma vWF-Ag levels and the primary outcome variables of interest (mortality and need for prolonged mechanical ventilation) while controlling for presence of multiple organ system failure and for severity of illness as defined by unadjusted PRISM III score. Multi-variable models were confirmed using the Hosmer-Lemeshow goodness-of-fit test. Interactions were not evaluated due to the small sample size. A p value ≤.05 was considered statistically significant. Data are presented as mean ± sd of the mean unless otherwise specified.
Results
Patient characteristics are presented in Table 1. Overall, mortality was 17%. All ethnicities were represented (Caucasian 37%, African American 20%, Hispanic 23%, Asian 5%, other 15%). Pertinent past medical history for ALI patients included premature at birth (15%), genetic or neurologic abnormality (20%), oncologic process (10%), history of pulmonary disease (11%), other significant past medical history (27%), and no significant past medical history (17%). Associated clinical disorders included primary pneumonia (42%), aspiration pneumonia (13%), sepsis (12%), near drowning (5%), cardiac (7%), and other (22%). Approximately one half (53%) of patients had ALI due to an infectious cause and 60% had ALI due to a primary pulmonary process (primary pneumonia, aspiration, or near drowning).
Table 1.
Patient characteristics
| Characteristic | Value |
|---|---|
| Age, yrs, mean ± sd | 5.9 ± 6.0 |
| Age, yrs, median (95% CI) | 2.8 (1.44, 8.63) |
| Percent (n) male | 55 (33) |
| Pao2/Fio2 at onset of ALI, mean ± sd | 141 ± 72 |
| Pao2/Fio2 at onset of ALI, median (range) | 120 (35, 292) |
| Percent (n) presenting with ARDS (Pao2/Fio2 <200) | 75 (45) |
| Percent (n) of patients with ≥2 nonpulmonary organ system failures at onset of ALI | 38 (23) |
| Percent (n) presenting with neutropenia (ANC <1000) | 12 (7) |
| Percent (n) presenting with air leak (pneumothorax, pneumomediastinum, subcutaneous emphysema) | 3 (2) |
| Duration of unassisted mechanical ventilation, days, mean ± sd | 13 ± 10 |
| Duration of unassisted mechanical ventilation, days, median (range) | 16 (0, 28) |
| Unadjusted PRISM III score, mean ± sd | 10.5 ± 6.2 |
| Unadjusted PRISM III score, median (range) | 11 (0, 25) |
| Mortality, % (n) | 17 (10) |
CI, confidence interval; ALI, acute lung injury; ARDS, acute respiratory distress syndrome; ANC, absolute neutrophil count; PRISM, Pediatric Risk of Mortality.
Plasma von Willebrand Factor Antigen Levels and Outcome
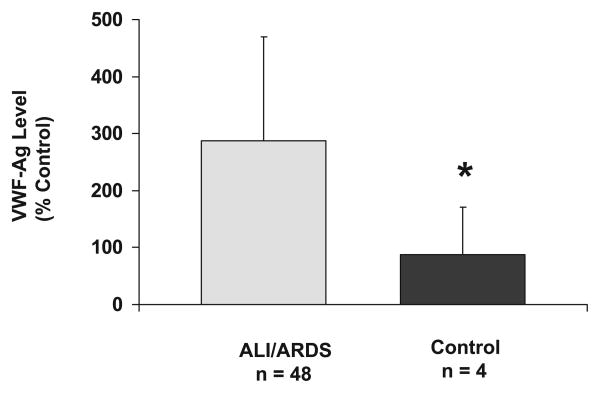
Mean and median plasma vWF-Ag levels were higher in the ALI cohort on day 1 of ALI (n = 48) than control (n = 4) subjects (mean 291 ± 183 vs. 87 ± 84% of control, respectively, Student's t-test, p = .03; the 95% confidence intervals for the median values are 186 and 323 for the first median value (229) and the 95% confidence interval for the second median value (59) are 23 and 207% of control, rank-sum, p = .01, Fig. 1).
Figure 1.
Plasma von Willebrand factor antigen (VWF-Ag) levels in acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) patients (n = 48) vs. intubated controls (n = 4). Mean plasma VWF-Ag levels were higher in ALI/ARDS patients compared with controls (*p = .03, Student's t-test). Data presented as mean ± sd.
Plasma vWF-Ag levels on day 1 of ALI were higher in nonsurvivors compared with survivors (384 ± 237, n = 9, vs. 264 ± 165, n = 39, percent of control), although not quite reaching statistical significance (Student's t-test, p = .08). There was no significant difference in plasma vWF-Ag levels on day 1 of ALI in patients requiring prolonged need for mechanical ventilation (≤14 ventilator-free days per 28-day month) compared with those with a lesser need for mechanical ventilation (290 ± 182, n = 23, vs. 284 ± 189, n = 25, percent of control, Student's t-test, p = .91).
Plasma vWF-Ag levels on day 2 of ALI were significantly elevated in patients with a prolonged need for mechanical ventilation (≤14 ventilator-free days per 28-day month) compared with those with a lesser need for mechanical ventilation (316 ± 173, n = 22, vs. 191 ± 89, n = 23, percent of control, Student's t-test, p=.004). There was no significant difference in vWF-Ag levels on day 2 of ALI in survivors vs. nonsurvivors (254 ± 154, n = 38, vs. 240 ± 130, n = 7, percent of control, Student's t-test, p = .82).
Approximately half (55%) of the 60 patients had vWF-Ag levels measured on both days 1 and 2 of ALI. Six (18%) of these patients died; all six had multiple organ system dysfunction (two or more nonpulmonary organ system failures) at onset of ALI. Five of these six patients (83%) had decreases in vWF-Ag levels from day 1 to day 2 of ALI compared with 52% (14 of 27) of survivors (chi-square, p = .16).
Thirteen (21%) of the 60 patients were not mechanically ventilated at the onset of ALI, and therefore tidal volume measurements were not available for those patients. There was no statistically significant difference in vWF levels on day 1 of ALI in those patients requiring mechanical ventilation on day 1 of ALI (n = 36) and those not requiring mechanical ventilation on day 1 of ALI (n = 12, p = .91, rank-sum). Furthermore, there was no association between plasma vWF-Ag levels on days 1 or 2 of ALI and diagnosis at ALI onset, gender, ethnicity, airway pressures (peak inspiratory pressure, positive end-expiratory pressure, mean airway pressure), exhaled tidal volume, Pao2/Fio2, pH, nonpulmonary organ system failure, or unadjusted PRISM III score. There was an expected (26), weak association between age and vWF-Ag levels on days 1 and 2 of ALI (Spearman ρ 0.32, p = .03, and Spearman ρ 0.33, p = .03, respectively) (Table 2).
Table 2.
Correlations between von Willebrand factor antigen levels and clinical variables of importance
| Variable | Spearman ρ Day 1 of ALI | p Value | Spearman ρ Day 2 of ALI | p Value |
|---|---|---|---|---|
| Gender | .14 | .33 | −.18 | .23 |
| Age | .32 | .03 | .32 | .03 |
| Diagnosis | .19 | .21 | .04 | .81 |
| Ethnicity | −.22 | .13 | .02 | .89 |
| PIP | .25a | .18 | .07b | .67 |
| PEEP | .35a | .06 | .24b | .13 |
| MAP | .32a | .08 | .14b | .39 |
| Exhaled tidal volume | .32a | .06 | −.25b | .13 |
| Pao2/Fio2 | −22a | .14 | −.06b | .72 |
| Presenting pH | .19 | .20 | −.14 | .35 |
| PRISM III | .12 | .41 | .16 | .28 |
| MOSF | .19 | .20 | .14 | .38 |
ALI, acute lung injury; PIP, peak inspiratory pressure; PEEP, positive end-expiratory pressure; MAP, mean arterial pressure; PRISM, Pediatric Risk of Mortality; MOSF, multiple organ system failure.
From onset of ALI;
after 24 hrs of ALI.
Categorized Plasma von Willebrand Factor Antigen Levels and Outcome
As described in the adult ALI literature (11, 25), a cutoff value for vWF-Ag associated with development of ARDS and poor clinical outcomes from ARDS of ≥450% of control has been validated since 1990. Fifty percent (four of eight) of pediatric patients with vWF-Ag levels ≥450% of control on day 1 of ALI died compared with 12.5% (five of 40) with levels <450% (exact test, p = .03, Fig. 2). Pediatric patients with plasma vWF-Ag levels ≥450% of control on day 1 of ALI had a greater odds of death (logistic regression, odds ratio 7, 95% confidence interval 1.3, 37.3, p = .02) than those with levels <450. There was no significant difference in need for prolonged mechanical ventilation in those patients with plasma vWF-Ag levels ≥450% or <450% of control (4 of 8 = 50% vs. 19 of 40 = 48%, exact test, p = 1.0).
Figure 2.
Categorized plasma von Willebrand factor antigen (VWF-Ag) levels on day 1 of acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) and mortality. Eight patients had plasma VWF-Ag levels ≥450% of control and 40 patients had plasma VWF-Ag levels <450% of control (*p = .03, Fisher's exact statistic).
For pediatric patients with vWF-Ag levels measured on day 2 of ALI, those with plasma vWF-Ag levels ≥450% of control on day 2 of ALI exhibited a prolonged need for mechanical ventilation compared with those with vWF-Ag levels <450 (5 of 5 = 100% vs. 17 of 40 = 42.5%, exact test, p = .02) (Fig. 3). Mortality was not significantly different between patients with plasma vWF-Ag levels ≥450% or <450% of control (exact test, 0 of 5 = 0% vs. 7 of 40 = 17.5%, p = .58).
Figure 3.
Categorized plasma von Willebrand factor antigen (VWF-Ag) levels on day 2 of acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) and need for prolonged mechanical ventilation (<15 ventilator-free days per 28-day month). Five patients had plasma VWF-Ag levels ≥450% of control and 40 patients had plasma VWF-Ag levels <450% of control (*p = .02, Fisher's exact statistic).
There was no association between categorized plasma vWF-Ag levels on days 1 or 2 of ALI and diagnosis at ALI onset, gender, ethnicity, airway pressures (peak inspiratory pressure, positive end-expiratory pressure, mean airway pressure), exhaled tidal volume, Pao2/Fio2, pH, nonpulmonary organ system failure, or unadjusted PRISM III score (data not shown). There was also no correlation between age and vWF-Ag levels ≥450% of control on either day 1 of ALI (Spearman ρ .25, p = .09) or day 2 of ALI (Spearman ρ .06, p = .68).
Multivariable Logistic Regression Analyses for Mortality
Due to the small sample size and relatively low mortality rate (17%) of this patient cohort, multiple variables could not be included into one model. Logistic regression analyses were completed to test the hypothesis that elevated vWF-Ag levels ≥450% of control are associated with mortality after adjusting for the presence of multiple, nonpulmonary organ system dysfunction at the onset of ALI and severity of illness, as measured by unadjusted PRISM III score. Both multiple organ system dysfunction and PRISM III score were also independent predictors of mortality when included as covariables in a model with vWF-Ag levels ≥450% control on day 1 of ALI (Tables 3 and 4). The addition of age, gender, or diagnosis at onset of ALI to these models did not significantly change the effect sizes.
Table 3.
Multivariable logistic regression analysis for plasma von Willebrand factor antigen (vWF-Ag) levels ≥450 on day 1 of acute lung injury, multiple organ system failure (OSF), and mortality
| Risk Factor | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| vWF-Ag ≥450 | 7.0 | 0.99, 49.3 | .05 |
| ≥2 OSF | 14.2 | 1.5, 138.5 | .02 |
Table 4.
Multivariable logistic regression analysis for plasma von Willebrand factor antigen (vWF-Ag) levels ≥450 on day 1 of acute lung injury, severity of illness score, and mortality
| Risk Factor | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| vWF-Ag ≥450 | 11.2 | 1.1, 115.0 | .04 |
| Pediatric Risk of Mortality III | 1.5 | 1.1, 1.9 | .01 |
Discussion
This study establishes that pediatric patients with ALI/ARDS have significant endothelial activation and injury resulting in early increases in plasma vWF-Ag. Elevations ≥450% of control on day 1 of ALI are independently predictive of mortality even when accounting for the presence of multiple organ system dysfunction. Similarly, elevations ≥450% of control on day 2 of ALI are independently predictive of the need for prolonged mechanical ventilation, also independent of the presence of multiple organ system dysfunction (data not shown). These increases are also independent of age and the diagnosis of sepsis.
Little is known about the similarities and differences in pathophysiology of acute lung injury and repair in children compared with animals and adults. Since 1982, Carvahlo et al. (16) and others (8, 10, 11, 25, 27, 28) have described increasing levels of vWF-Ag as correlating with severity of ALI in adults. Interestingly, the current pediatric study identified patients using the same criteria as that in the NHLBI study of 6 vs. 12 mL/kg tidal volume (20). Furthermore, vWF-Ag levels were measured in the same laboratory and using the same technique (vWF-Ag enzyme-linked immunosorbent assay, Diagnostica Stago) as that used for the adult study. Given that, it is interesting to note the modestly lower mean vWF-Ag level in our cohort compared with the National Heart, Lung, and Blood Institute adult cohort (287 ± 183% of control vs. 350 ± 265%, respectively) corresponding to the lower mortality in our cohort compared with the adult cohort (17% vs. 35%, respectively). These data suggest that the overall pediatric cohort meeting American-European Consensus Committee criteria for ALI may be healthier than their adult counterparts. However, when pediatric patients with ALI develop multiple organ system dysfunction and corresponding increases in plasma vWF-Ag, particularly ≥450% of control, there is a similar increase in the risk of death and need for prolonged mechanical ventilation, suggesting comparable injury to the systemic and pulmonary vascular endothelium in these more severely ill pediatric patients.
One limitation of this study is that vWF-Ag was measured predominantly in unused plasma samples retrieved from the central laboratory rather than samples collected specifically for this study at the bedside. This decision was made to allow for a) specimens to be collected as close to the onset of ALI as possible; b) patients without central venous or arterial access to be included in the study; and c) the likelihood of parental consent to be increased compared with studies requiring additional blood draws. Because vWF-Ag is a large molecular weight protein, it is unlikely that significant degradation occurred in the central laboratory, where the samples are stored at 4°C. One other limitation is that this was a study done only in two pediatric medical centers. Further study of biological markers would benefit from a larger, multiple-center approach. Last, patients were enrolled in this study before the publication by the Acute Respiratory Distress Syndrome Network (20) regarding the injurious nature of high tidal volume ventilation in adults with ALI. Therefore, elevations of vWF-Ag in this pediatric cohort may also reflect the injurious nature of high tidal volume ventilation. The average adjusted exhaled tidal volume in this cohort was 10.1 ± 3.5 mL/kg, higher than the 6 mL/kg that was associated with decreased mortality in the adult ARDS Network trial. Confirmation of these findings in the setting of mechanical ventilation using a lung-protective strategy is clearly indicated.
Although the results of this study are interesting, it is important to recognize that the use of more than one biological marker for prognosis may have even greater value than single biomarker studies alone. For example, the combination of an increased plasma vWF and an increased soluble intercellular adhesion molecule-1 (21) may have additive prognostic value in pediatric ALI. Future studies should test other candidate biological markers including inflammatory cytokines such as interleukin-6 and interleukin-8 (29) as well as markers of dysregulated coagulation (decreased protein C) (30–32) and impaired fibrinolysis (increased plasminogen-activation inhibitor-1 [9, 33, 34] and increased procollagen peptide III [35–37]). Furthermore, the combination of both biological markers and clinical predictors (29) may have greater prognostic value than either alone and may be useful for selecting patients for therapeutic trials.
Conclusions
Early elevation of plasma vWF-Ag has prognostic value for death and the need for prolonged mechanical ventilation and is independent of the presence of multiple organ system dysfunction. These results should be confirmed in larger, multiple-center pediatric observational studies as well as in future therapeutic trials of pediatric ALI. Several investigators to date have described intense activation and injury of the lung endothelium early in the course of ALI/ARDS in adults (8, 9, 11, 14, 28, 38, 39), prompting current interest in developing therapeutic strategies targeting the lung vasculature. These data support the inclusion of children with ALI/ARDS in future therapeutic trials that target the vascular endothelium in the treatment of ALI/ARDS.
Acknowledgments
Supported, in part, by grants RR01271, HL51856, HL70521, and RR15543 from the National Institutes of Health and by the Pediatric Clinical Research Center at Children's Hospital and Research Center Oakland, California. There are no additional financial disclosures for each author at this time.
Footnotes
The authors have not disclosed any potential conflicts of interest.
References
- 1.Ware L, Matthay M. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1346. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Bernard G, Artigas A, Brigham K, et al. The North American-European Consensus Conference on ARDS. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 3.Sporn L, Chavin S, Marder V, et al. Biosynthesis of von Willebrand protein by human megakaryocytes. J Clin Invest. 1985;76:1102–1106. doi: 10.1172/JCI112064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloom A, Giddings J, Wilks C. Factor VIII on the vascular intima: Possible importance in haemostasis and thrombosis. Nature. 1973;241:217–219. doi: 10.1038/newbio241217a0. [DOI] [PubMed] [Google Scholar]
- 5.Ribes J, Francis C, Wagner D. Fibrin induces release of von Willebrand factor from endothelial cells. J Clin Invest. 1987;79:117–123. doi: 10.1172/JCI112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton K, Sims P. Changes in Cytosolic Ca2+ associated with von Willebrand factor release in human endothelial cells exposed to histamine. J Clin Invest. 1987;79:600–608. doi: 10.1172/JCI112853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsubara O. Alveolar basement membrane breaks down in diffuse alveolar damage: An immunohistochemical study. Pathol Int. 1995;45:473–482. doi: 10.1111/j.1440-1827.1995.tb03488.x. [DOI] [PubMed] [Google Scholar]
- 8.Ware L, Conner E, Matthay M. von Willebrand factor antigen is an independent marker of poor outcome in patients with early acute lung injury. Crit Care Med. 2001;29:2325–2331. doi: 10.1097/00003246-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Moalli R, Doyle J, Tahhan H, et al. Fibrinolysis in critically ill patients. Am Rev Respir Dis. 1989;140:287–293. doi: 10.1164/ajrccm/140.2.287. [DOI] [PubMed] [Google Scholar]
- 10.Siemiatkowski A, Kloczko J, Galar M, et al. von Willebrand factor antigen as a prognostic marker in posttraumatic acute lung injury. Haemostasis. 2000;30:189–195. doi: 10.1159/000054134. [DOI] [PubMed] [Google Scholar]
- 11.Ware L, Eisner M, Thompson B, et al. Significance of von Willebrand Factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004;170:766–772. doi: 10.1164/rccm.200310-1434OC. [DOI] [PubMed] [Google Scholar]
- 12.Sabharwal A, Bajaj S, Ameri A, et al. Tissue factor pathway inhibitor and von Willebrand factor antigen in adult respiratory distress syndrome and in a primate model of sepsis. Am J Respir Crit Care Med. 1995;151:758–767. doi: 10.1164/ajrccm/151.3_Pt_1.758. [DOI] [PubMed] [Google Scholar]
- 13.Carvahlo A, Quinn D, DeMarinis S, et al. Platelet function in acute respiratory failure. Am J Hematol. 1987;25:377–388. doi: 10.1002/ajh.2830250404. [DOI] [PubMed] [Google Scholar]
- 14.Wenzel C, Kofler J, Locker G, et al. Endothelial cell activation and blood coagulation in critically ill patients with lung injury. Wiener Klinische Wochenschrift. 2002;114:853–858. [PubMed] [Google Scholar]
- 15.Modig J, Bagge L. Specific coagulation and fibrinolysis tests as biochemical markers in traumatic-induced adult respiratory distress syndrome. Resuscitation. 1986;13:87–93. doi: 10.1016/0300-9572(86)90012-2. [DOI] [PubMed] [Google Scholar]
- 16.Carvahlo A, Bellman S, Saullo V, et al. Altered factor VIII in acute respiratory failure. N Engl J Med. 1982;307:1113–1119. doi: 10.1056/NEJM198210283071803. [DOI] [PubMed] [Google Scholar]
- 17.Modig J, Borg T, Wegenius G, et al. The value of variables of disseminated intravascular coagulation in the diagnosis of adult respiratory distress syndrome. Acta Anaesthesiol Scand. 1983;27:369–375. doi: 10.1111/j.1399-6576.1983.tb01970.x. [DOI] [PubMed] [Google Scholar]
- 18.Mazzoni D, Fisher A, Practica G, et al. Factor VIII activity and factor VIII related antigen in infants with viral bronchiolitis. N Engl J Med. 1984;311:1257–1258. doi: 10.1056/NEJM198411083111915. [DOI] [PubMed] [Google Scholar]
- 19.Flori H, Glidden D, Rutherford G, et al. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–2001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 20.The Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 21.Flori H, Ware L, Glidden D, et al. Early elevation of plasma soluble intercellular adhesion molecule-1 in pediatric acute lung injury identifies patients at increased risk of death and prolonged mechanical ventilation. Ped Crit Care Med. 2003;4:315–321. doi: 10.1097/01.PCC.0000074583.27727.8E. [DOI] [PubMed] [Google Scholar]
- 22.Pollack M, Patel K, Ruttimann U. PRISM III: An updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Hubbard A, Rigsby P, Barrowcliffe T. Standardization of factor VIII and von Willebrand factor in plasma: Calibration of the 4th International Standard (97/586) Thromb Haemost. 2001;85:634–638. [PubMed] [Google Scholar]
- 24.Press S. Getting Started With Stata for Windows. College Station; TX, StataCorp: 2005. Vol. Release 9. [Google Scholar]
- 25.Rubin D, Wiener-Kronish J, Murray J, et al. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J Clin Invest. 1990;86:474–480. doi: 10.1172/JCI114733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conlan M, Folsom A, Finch A, et al. Associations of factor VIII and von Willebrand factor with age, race, sex and risk factors for atherosclerosis. Thromb Haemost. 1993;70:380–385. [PubMed] [Google Scholar]
- 27.Siemiatkowski A, Kloczko J, Wereszczynska-Siemiatkowska U, et al. Effect of severe multiple trauma complicated by acute lung injury on endothelial cell activity. Przeglad Lekarski. 2001;58:767–771. [PubMed] [Google Scholar]
- 28.Gando S, Kameue T, Matsuda T, et al. Systemic inflammation and disseminated intravascular coagulation in early stages of ALI and ARDS: Role of neutrophil and endothelial activation. Inflammation. 2004;28:237–244. doi: 10.1023/b:ifla.0000049049.81688.fe. [DOI] [PubMed] [Google Scholar]
- 29.Parsons P, Eisner M, Thompson B, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. [DOI] [PubMed] [Google Scholar]
- 30.Sheth S, Carvahlo A. Protein S and protein C alterations in acutely ill patients. Am J Hematol. 1991;36:14–19. doi: 10.1002/ajh.2830360104. [DOI] [PubMed] [Google Scholar]
- 31.Ware L, Fang X, Matthay M. Protein C and thrombomodulin in human acute lung injury. Am J Physiol. 2003;285:L514–L521. doi: 10.1152/ajplung.00442.2002. [DOI] [PubMed] [Google Scholar]
- 32.Matthay M, Ware L. Plasma protein C levels in patients with acute lung injury: Prognostic significance. Crit Care Med. 2004;32:S229–s232. doi: 10.1097/01.ccm.0000126121.56990.d3. [DOI] [PubMed] [Google Scholar]
- 33.Gando S, Kameue T, Nanzaki S, et al. Increased neutrophil elastase, persistent intravascular coagulation, and decreased fibrinolytic activity in patients with posttraumatic acute respiratory distress syndrome. J Trauma. 1997;42:1068–1072. doi: 10.1097/00005373-199706000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Idell S, Koenig K, Fair D, et al. Serial abnormalities of fibrin turnover in evolving adult respiratory distress syndrome. Am J Physiol. 1991;261:L240–L248. doi: 10.1152/ajplung.1991.261.4.L240. [DOI] [PubMed] [Google Scholar]
- 35.Chestnutt A, Matthay M, Tibayan F, et al. Early detection of type III procollagen peptide in acute lung injury. Am J Respir Crit Care Med. 1997;156:840–845. doi: 10.1164/ajrccm.156.3.9701124. [DOI] [PubMed] [Google Scholar]
- 36.Meduri G, Tolley E, Chinn A, et al. Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am J Respir Crit Care Med. 1998;158:1432–1441. doi: 10.1164/ajrccm.158.5.9801107. [DOI] [PubMed] [Google Scholar]
- 37.Pugin J, Verghese G, Widmer M, et al. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med. 1999;27:304–312. doi: 10.1097/00003246-199902000-00036. [DOI] [PubMed] [Google Scholar]
- 38.Idell S. Endothelium and disordered fibrin turnover in the injured lung: newly recognized pathways. Crit Care Med. 2002;30(5 Suppl):S274–S80. doi: 10.1097/00003246-200205001-00017. [DOI] [PubMed] [Google Scholar]
- 39.Zimmerman G, Albertine K, Carveth H, et al. Endothelial activation in ARDS. Chest. 1999;116:18S–24S. doi: 10.1378/chest.116.suppl_1.18s. [DOI] [PubMed] [Google Scholar]