Abstract
Noroviruses are the most common cause of acute viral gastroenteritis, accounting for >21 million cases annually in the U.S. alone. Norovirus infections constitute an important health problem for which there are no specific antiviral therapeutics or vaccines. In this study, a series of bisulfite adducts derived from representative transition state inhibitors (dipeptidyl aldehydes and α-ketoamides) was synthesized and shown to exhibit anti-norovirus activity in a cell-based replicon system. The ED50 of the most effective inhibitor was 60 nM. This study demonstrates for the first time the utilization of bisulfite adducts of transition state inhibitors in the inhibition of norovirus 3C-like protease in vitro and in a cell-based replicon system. The approach described herein can be extended to the synthesis of the bisulfite adducts of other classes of transition state inhibitors of serine and cysteine proteases, such as α-ketoheterocycles and α-ketoesters.
Keywords: norovirus 3CL protease, bisulfite salt adducts, transition state inhibitors
Noroviruses are a major cause of waterborne and foodborne acute gastroenteritis.1–4 Outbreaks of viral gastroenteritis are common because of the highly contagious nature of noroviruses. Noroviral gastroenteritis is the cause of significant morbidity and may lead to fatal infection in children, the elderly, and immuno-compromised individuals.5 There are currently no effective vaccines or antiviral agents for combating norovirus infection; consequently, there is an urgent need for the discovery of small molecule therapeutics for the management and treatment of norovirus infection.6–7 Recently-reported small molecule norovirus inhibitors include cyclic and acyclic sulfamide derivatives,8–10 piperazine derivatives,11 pyranobenzopyrones,12 nitazoxanide,13 and other chemotypes.14
The norovirus RNA genome encodes a polyprotein which is processed by a virus-encoded 3C-like cysteine protease (3CLpro) to generate mature non-structural proteins.15 Co- and post-translational processing of the polyprotein by norovirus 3CLpro is essential for virus replication (Figure 1); consequently, norovirus 3CLpro is an attractive target for the discovery of anti-norovirus small molecule therapeutics.
Figure 1.
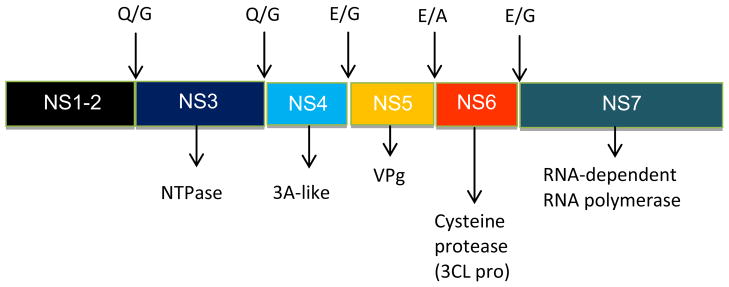
Proteolytic cleavage of the nonstructural polyprotein of norovirus (Norwalk virus) encoded in open reading frame 1 (ORF1). The indicated cleavage sites at Q/G, E/A or E/G (corresponding to the P1-P1′ scissile bond) are mediated by the virus-encoded 3CL cysteine protease.
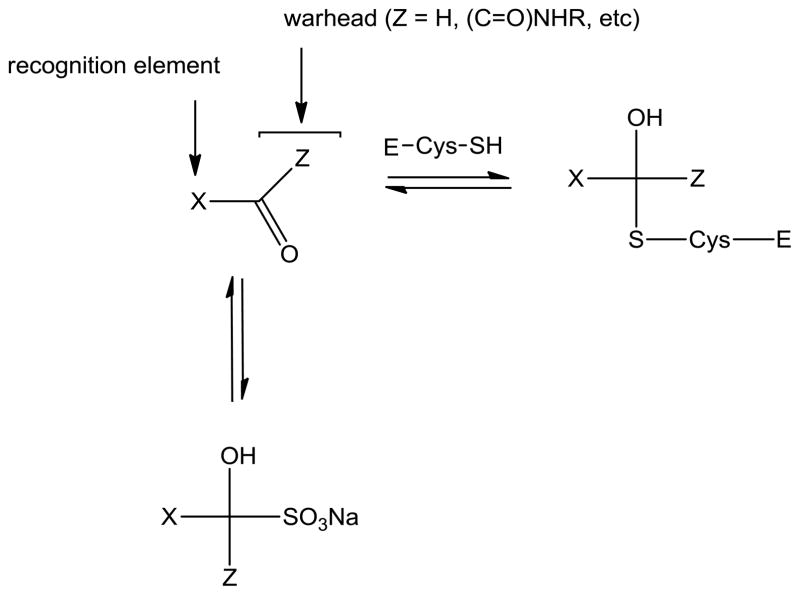
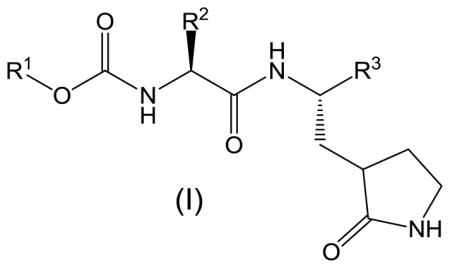
We have recently demonstrated that peptidyl aldehydes,16 α-ketoamides,17 and α-ketoheterocycles17 potently inhibit norovirus 3CLpro in vitro, as well as norovirus in a cell-based replicon system. In an attempt to identify suitably-functionalized dipeptidyl transition state inhibitors that possess potent pharmacological activity, as well as molecular properties that are important for oral bioavailability and favorable ADMET characteristics,18–24 we describe herein the synthesis of bisulfite adducts of transition state inhibitors (I) (Table 1), and their subsequent utilization in the inhibition of norovirus 3CLpro in vitro, as well as viral replication in a cell-based replicon system (Figure 2). To the best of our knowledge, this is the first report on the use of bisulfite adducts of transition state inhibitors to inhibit a cysteine protease25. We furthermore describe the results of preliminary structure-activity relationship studies related to the probing of the S2 subsite26 of norovirus 3CLpro, as well as the nature of the “cap” that projects toward the S3 subsite and beyond.
Table 1.
Inhibitory activity of compounds 6, 7a–j, 9, 10
| |||||
|---|---|---|---|---|---|
| Compounda | R1 | R2 | R3 | IC50 (μM) | ED50 (μM) |
| 6a | benzyl | Isobutyl | CHO | 0.6 | 0.2 |
| 7a | benzyl | Isobutyl | CH(OH) SO3Na | -b | 0.3 |
| 6b | benzyl | n-propyl | CHO | 6.1 | 2.3 |
| 7b | benzyl | n-propyl | CH(OH) SO3Na | -b | 1.5 |
| 6c | benzyl | n-butyl | CHO | 4.5 | 1.2 |
| 7c | benzyl | n-butyl | CH(OH) SO3 Na | -b | 1.3 |
| 6d | benzyl | cyclohexylmethyl | CHO | 0.5 | 0.05 |
| 7d | benzyl | cyclohexylmethyl | CH(OH) SO3 Na | -b | 0.06 |
| 6e | benzyl | benzyl | CHO | 5.1 | 1.8 |
| 7e | benzyl | benzyl | CH(OH) SO3Na | -b | 1.1 |
| 6f | p-fluorobenzyl | Isobutyl | CHO | 1.8 | 0.5 |
| 7f | p-fluorobenzyl | Isobutyl | CH(OH) SO3 Na | -b | 0.4 |
| 6g | m-fluorobenzyl | Isobutyl | CHO | 0.7 | 0.3 |
| 7g | m-fluorobenzyl | Isobutyl | CH(OH) SO3Na | -b | 0.2 |
| 6h | 2-phenylethyl | Isobutyl | CHO | 1.9 | 1.1 |
| 7h | 2-phenylethyl | Isobutyl | CH(OH) SO3Na | -b | 1.0 |
| 6j | 2-cyclohexylethyl | Isobutyl | CHO | 0.6 | 0.3 |
| 7j | 2-cyclohexylethyl | Isobutyl | CH(OH) SO3 Na | -b | 0.3 |
| 917 |
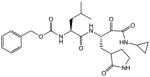
|
3.4 | 1.1 | ||
| 10 |
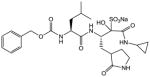
|
5.3 | 0.8 | ||
CC50: All compounds, except 6j and 7j, showed no toxicity up to 320 μM (CC50: > 320μM). The CC50 values for 6j and 7j were 210 μM, and 240 μM, respectively.
Not determined (see text).
Figure 2.
General representation of the interaction and interrelationships between 3CL protease (E-Cys-SH), transition state inhibitor, X(C=O)Z, and bisulfite adduct, XZ(OH)SO3Na.
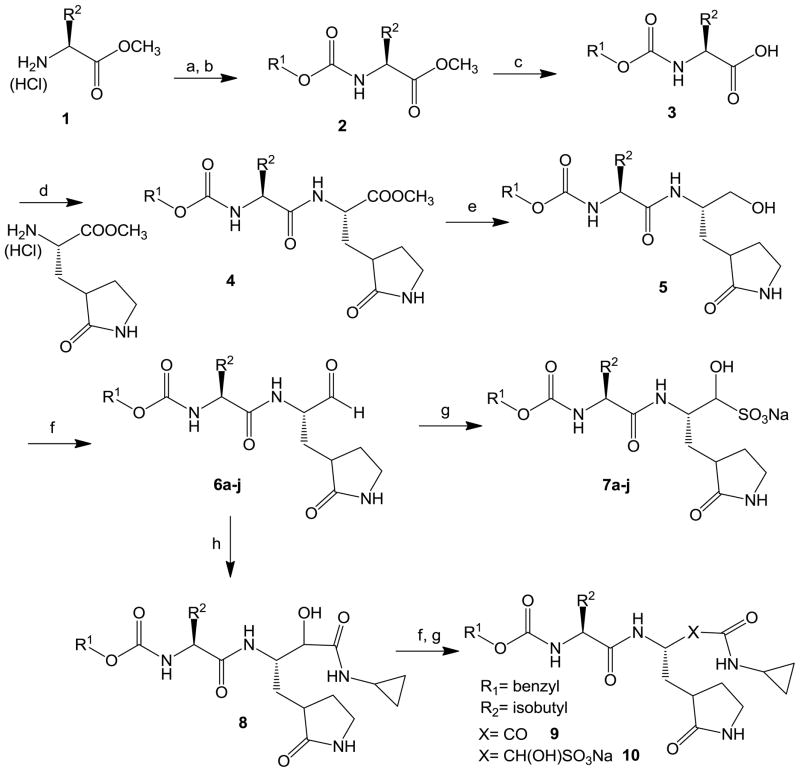
The synthesis of dipeptidyl inhibitors 6a–j, 7a–j, 9, and 10 is summarized in Scheme 1.
Scheme 1. Reagents.
(a) CCI30(C=0)CI/dioxane; (b)Triethylamine/R1OH; (c) Li0H/THF/H20; (cl) EDCI/HOBt/DIEA/DMF; (e) LiBH4/THF; (f) Dess-Martin periodinane/DCM; (g) NaHS03/EtOAc/EtOH/H20; (h) Cyclopropyl isonitrile/HOActhen K2CO3/CH3OH/H2O.
Reaction of an appropriate amino acid ester hydrochloride with trichloromethyl chloroformate yielded the corresponding isocyanate which was subsequently reacted with an appropriate alcohol in the presence of triethylamine to yield carbamate derivative 2. Hydrolysis with lithium hydroxide in aqueous THF followed by coupling with a glutamine surrogate27 yielded ester 4 which was further elaborated to yield aldehydes 6a–j via sequential reduction to the alcohol with lithium borohydride, followed by Dess-Martin oxidation.28 The reaction of aldehyde 6 (R1 = benzyl, R2 = isobutyl) with cyclopropyl isonitrile/HOAc followed by treatment with potassium carbonate in aqueous methanol yielded alcohol 8 which was then oxidized to the corresponding α-ketoamide 9 using Dess-Martin reagent. The generated aldehyde and α-ketoamide bisulfite adducts were readily obtained by stirring aldehydes 6a–j and α-ketoamide 9 with sodium bisulfite.29 The interaction of the generated compounds with norovirus 3CLpro was investigated in vitro as previously described.16–17 The activity of the compounds against norovirus was also investigated in a cell-based system30–34 and the combined results are listed in Table 1.
The rationale underlying the studies described herein rested on the following considerations: (a) bisulfite adducts of amino acid-derived isocyanates are readily-accessible, stable, water-soluble solids which function as latent isocyanates. These adducts have been shown to be highly effective, time-dependent, irreversible inhibitors of mammalian serine proteases, such as neutrophil elastase, cathepsin G, and proteinase 3;35 (b) bisulfite adducts of aldehydes, methyl or cyclic ketones, and α-ketoesters are readily-synthesized, stable solids having high aqueous solubility. Treatment of the addition products with acid or base yields the precursor carbonyl compounds;36 (c) we hypothesized that the bisulfite adducts of transition state (TS) inhibitors of proteases (serine and cysteine), such as peptidyl aldehydes, α-ketoamides, and others could potentially function as a latent form of the precursor TS inhibitor (Figure 2), generating the active form of the inhibitor in the gastrointestinal tract and blood plasma. In principle, the bisulfite adducts could also function as transition state mimics37 and, (d) the high aqueous solubility and pH-dependent equilibria between the precursor carbonyl compound and adduct were also envisaged to have a significant effect on potency and the ADMET and PK characteristics of the precursor TS inhibitors. It was envisioned that the bisulfite adducts might be suitable candidates for fulfilling such a role.
As shown in Table 1, the dipeptidyl aldehydes exhibited low to sub-micromolar inhibitory activity toward NV 3CLpro in vitro. The enzyme shows a strong preference for an R2 = isobutyl, which is in agreement with the known substrate specificity of the enzyme. The strong preference of NV 3CLpro for a P2 Leu is supported by substrate specificity studies using peptidyl p-nitroanilide substrates, as well as X-ray crystallographic studies.38 The results in Table 1 suggest that replacement of the isobutyl group by a cyclohexylmethyl group at R2 yields an inhibitor that is equipotent to 6a (Table 1, compounds 6a and 6d). However, in sharp contrast to compound 6a, the ED50 of compound 6d was found to be an order of magnitude lower than that of 6a, presumably because of its better cellular permeability. The nature of the “cap” (R1) which projects toward the S3 pocket and beyond was briefly explored by replacing the benzyl group with meta- and para-fluorobenzyl, 2-phenylethyl, and 2-cyclohexylethyl. The m-fluorobenzyl and 2-cyclohexylethyl groups were equipotent to the benzyl group, and about 2-fold better than other substitutions (Table 1, compounds 6a, 6g, 6j versus 6f and 6j).
The activity of the bisulfite adducts of the synthesized aldehydes was investigated in a cell-based system. The potency trends observed with the precursor aldehydes were generally reflected in the corresponding bisulfite salts, with bisulfite 7d (R2 = cyclohexylmethyl) being the most potent. In order to determine the nature of the active species, the behavior of aldehyde 6a and its corresponding bisulfite salt 7a was examined by mass spectroscopy. In separate experiments, compounds 6a and 7a were dissolved in dimethyl sulfoxide and diluted 1 to 1000 in either acetonitrile or water and examined by MS and tandem MS-MS. In acetonitrile the expected peaks for aldehyde 6a were 404.4 M + H+ (dominant peak) and 426.3 M+Na. The mass spectra of bisulfite salt 7a using negative mode detection, showed a dominant peak at 484.5 for (M-1)−, a loss of H+ from the sulfonic acid moiety. Aldehyde 6a in aqueous solution showed peaks corresponding to the aldehyde (404.6), the aldehyde + sodium (426.4) and hydrated aldehyde + sodium (444.2) in positive mode. In water, bisulfite adduct 7a displayed a dominant peak at 484.5 in negative mode and the relative intensities of this parent ion and other ions remained unchanged over 24 h (a time course study was carried out). In the case of 6a, the hydrated form was the dominant species after only five minutes exposure to water, while 7a remains unchanged as the bisulfite form after 24 h. The results indicate that the bisulfite adduct of 7a is stable in aqueous solution; however, in buffer solution, pH 7.4, compound 7a gradually dissociates into the corresponding aldehyde 6a within an hour, rapidly becoming hydrated. These observations are in agreement with the results of X-ray crystallographic studies showing that incubation of bisulfite adduct 7a with norovirus 3CLpro in buffer solution results in the formation of an enzyme-aldehyde complex, with the active site cysteine residue covalently bonded to the carbonyl carbon of aldehyde 6a.34 Lastly, the variable stability of the bisulfite values of the adducts in buffer solution has precluded accurate determination of the IC50 inhibitors.
In summary, the utilization of bisulfite adducts of transition state inhibitors of cysteine and serine proteases in the in vitro and cell-based inhibition of norovirus 3CL protease has been described for the first time.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Karst SM. Viruses. 2010;2:748. doi: 10.3390/v2030748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koo HL, Ajami N, Atmar RL, DuPont HL. Discov Med. 2010;10:61. [PMC free article] [PubMed] [Google Scholar]
- 3.Eckardt AJ, Baumgart DC. Rec Patents Anti-infect Drug Discov. 2011;6:54. doi: 10.2174/157489111794407877. [DOI] [PubMed] [Google Scholar]
- 4.Atmar RL. Food Environ Virol. 2010;2:117. doi: 10.1007/s12560-010-9038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan MA, Bass DM. Curr Opin Gastroenterol. 2010;26:26. doi: 10.1097/MOG.0b013e328333d7af. [DOI] [PubMed] [Google Scholar]
- 6.Tan M, Jiang X. Curr Opin Investig Drugs. 2008;9:146. [PubMed] [Google Scholar]
- 7.Glass RI, Parashar UD, Estes MK. New Engl J Med. 2009;361:1776. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dou D, Tiew KC, He G, Mandadapu SR, Aravapalli S, Alliston KR, Kim Y, Chang KO, Groutas WC. Bioorg Med Chem Lett. 2011;19:5975. doi: 10.1016/j.bmc.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dou D, Mandadapu SR, Alliston KR, Kim Y, Chang KO, Groutas WC. Eur J Med Chem. 2012;47:59. doi: 10.1016/j.ejmech.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dou D, Tiew KC, He G, Mandadapu SR, Gunnam MR, Alliston KR, Kim Y, Chang KO, Groutas WC. Bioorg Med Chem. 2012;20:2111. doi: 10.1016/j.bmc.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dou D, He G, Mandadapu SR, Aravapalli S, Kim Y, Chang KO, Groutas WC. Bioorg Med Chem Lett. 2012;22:377. doi: 10.1016/j.bmcl.2011.10.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pokheil L, Kim Y, Thi D, Nguyen T, Prior AM, Lu J, Chang KO, Hua DH. Bioorg Med Chem Lett. 2012;22:3480. doi: 10.1016/j.bmcl.2012.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddiq DM, Koo HL, Adachi JA, Viola GM. J Infect. 2011;63:394. doi: 10.1016/j.jinf.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dou D, Mandadapu SR, Alliston KR, Kim Y, Chang KO, Groutas WC. Bioorg Med Chem. 2011;19:5749. doi: 10.1016/j.bmc.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blakeney SJ, Cahill A, Reilly PA. Virology. 2003;308:216. doi: 10.1016/s0042-6822(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 16.Tiew K-C, He G, Aravapalli S, Mandadapu SR, Gunnam MR, Alliston K, Lushington GH, Kim Y, Chang K-O, Groutas WC. Bioorg Med Chem Lett. 2011;21:5315. doi: 10.1016/j.bmcl.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandadapu SR, Weerawarna PM, Gunnam MR, Alliston K, Lushington GH, Kim Y, Chang KO, Groutas WC. Bioorg Med Chem Lett. 2012;22:4820–4826. doi: 10.1016/j.bmcl.2012.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipinski CA. J Pharmacol Toxicol Meth. 2000;44:235. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 19.Veber DF. J Med Chem. 2002;45:2615. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 20.Ritchie TJ, Ertl P, Lewis R. Drug Discov Today. 2011;16:65. doi: 10.1016/j.drudis.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Gleeson MP. J Med Chem. 2008;51:817. doi: 10.1021/jm701122q. [DOI] [PubMed] [Google Scholar]
- 22.Johnson TW, Dress KR, Edwards M. Bioorg Med Chem Lett. 2009;19:5560. doi: 10.1016/j.bmcl.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 23.Perola E. J Med Chem. 2010;53:2986. doi: 10.1021/jm100118x. [DOI] [PubMed] [Google Scholar]
- 24.Edwards MP, Price DA. Ann Rep Med Chem. 2010;45:381. [Google Scholar]
- 25.Aldehyde bisulfite adducts have been previously used in the inhibition of glycollate oxidase (Corbett JR, Wright BJ. Phytochemistry. 1971;10:2015.) and thrombin (Ruterbories KJ, Shuman RT. US Patent 5,436,229. 1995 Jul 25;).
- 26.Schechter I, Berger A. Biochem Biophys Res Comm. 1967;27:157. doi: 10.1016/s0006-291x(67)80055-x. The residues on the N-terminus side of the peptide bond that is cleaved are designated P1-Pn and those on the C-terminus side are designated P1′-Pn′. The corresponding active site subsites are designated S1-Sn and S1′-Sn′. [DOI] [PubMed] [Google Scholar]
- 27.Dragovich PS, Prins TJ, Zhou R, Webber SE, Marakovits JT, Fuhrman SA, Patick AK, Matthews DA, Lee CA, Ford CE, Burke BJ, Rejto PA, Hendrickson TF, Tuntland T, Brown EL, Meador JW, Ferre RA, Harr JEV, Kosa MB, Wortland ST. J Med Chem. 1999;42:1213. doi: 10.1021/jm9805384. [DOI] [PubMed] [Google Scholar]
- 28.(a) Dess DB, Martin JC. J Org Chem. 1983;48:4155. [Google Scholar]; (b) Wells GJ, Tao MT, Josef KA, Bihovsky R. J Med Chem. 2001;44:3488–3503. doi: 10.1021/jm010178b. [DOI] [PubMed] [Google Scholar]
- 29.General synthesis of bisulfite salts. The procedure used was essentially that of Kjell DP, Slattery BJ, Semo MJ. J Org Chem. 1999;64:5722–5724. doi: 10.1021/jo990543v.. Briefly, aldehyde or α-ketoamide (1.24 mmol) was dissolved in ethyl acetate (2 mL) and a solution of sodium bisulfite (1.12 mmol) in ethanol (1 mL) and water (0.4 mL) was added. The mixture was heated to 40 °C with stirring. After the mixture was stirred for 2 h, it was allowed to cool to room temperature. The solution was filtered and the solid residue was washed with ethanol (5 mL). The filtrate was dried over anhydrous sodium sulfate, filtered, and concentrated, leaving an oily residue. Treatment with ether gave a solid (60–80% yield).
- 30.Chang KO, Sosnovtsev SV, Belliot G, King AD, Green KY. Virology. 2006;2:463. doi: 10.1016/j.virol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Chang KO, George DW. J Virol. 2007;22:12111. doi: 10.1128/JVI.00560-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang KO. J Virol. 2009;83:8587. doi: 10.1128/JVI.00005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim Y, Thapa M, Hua DH, Chang KO. Antiviral Res. 2011;89:165. doi: 10.1016/j.antiviral.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y, Lovell S, Tiew KC, Mandadapu SR, Alliston KR, Battaille KP, Groutas WC, Chang KO. J Virol. 2012;86:11754. doi: 10.1128/JVI.01348-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.(a) Groutas WC, Abrams WR, Theodorakis MC, Kasper AM, Rude SA, Badger RC, Ocain TD, Miller KE, Moi MK, Brubaker MJ, Davis KS, Zandler ME. J Med Chem. 1985;28:204. doi: 10.1021/jm00380a010. [DOI] [PubMed] [Google Scholar]; (b) Groutas WC, Brubaker MJ, Stanga MA, Castrisos JC, Huang TL, Crowley JP. Biochem Biophys Res Comm. 1985;128:90. doi: 10.1016/0006-291x(85)91648-1. [DOI] [PubMed] [Google Scholar]
- 36.Young PR, Jencks WP. J Am Chem Soc. 1978;100:1228. [Google Scholar]
- 37.Patel DV, Rielly-Gauvin K, Ryono DE. Tetrahedron Lett. 1990;31:5587. [Google Scholar]
- 38.Hussey RJ, Coates L, Gill RS, Erskine PT, Coker SF, Mitchell E, Cooper B, Wood S, Broadbridge R, Clarke IN, Lambden PR, Scholingin-Jordan MP. Biochemistry. 2011;50:240. doi: 10.1021/bi1008497. [DOI] [PMC free article] [PubMed] [Google Scholar]