Abstract
Exposure to androgens during prenatal development shapes both physiological and behavioral developmental trajectories. Notably, in rhesus macaques, prenatal androgen exposure has been shown to increase rough-and-tumble play, a prominent behavioral feature in males during the juvenile period in primates. While macaques are an Old World, polygamous species with marked sexually dimorphic behavior, New World callitrichine primates (marmosets and tamarins) live in cooperative breeding groups and are considered to be socially monogamous and exhibit minimal sexual dimorphism in social play, which suggests that androgen may affect this species in different ways compared to macaques. In addition, we previously described considerable variation in maternal androgen production during gestation in marmosets. Here we tested the association between this variation and variation in offspring rough-and-tumble play patterns in both males and females. We measured testosterone and androstenedione levels in urine samples collected from pregnant marmoset mothers and then observed their offspring's play behavior as juveniles (5–10 months of age). In contrast to findings in rhesus macaques, hierarchical regression analyses showed that higher gestational testosterone levels, primarily in the second semester, were associated with decreased rough-and-tumble play in juveniles, and this relationship appears to be driven more so by males than females. We found no reliable associations between gestational androstenedione and juvenile play behavior. Our findings provide evidence to suggest that normative variation in levels of maternal androgen during gestation may influence developmental behavioral trajectories in marmosets in a way that contradicts previous findings in Old World primates.
Keywords: Rough-and-tumble play, Prenatal programming, Androstenedione, Testosterone, Intrauterine environment, Maternal androgens, Organizational effects
Introduction
Exposure to androgenic hormones during sensitive periods of development can substantially alter a number of developmental trajectories. This so-called organizational effect of androgens (Phoenix et al., 1959) can account for masculinization/defeminization of genitalia and other secondary sexual characteristics as well as shaping behavioral predispositions. Of the behaviors influenced by early exposure to androgens, social rough-and-tumble play, or play fighting, during the juvenile period is of particular importance due to its proposed role in shaping outcomes related to foraging/hunting, cognition, learning, reproduction, and sociality in mammals (reviewed in Power, 2000). In rats, juvenile males show higher rates of rough-and-tumble play than their female counterparts and androgen exposure during early postnatal life increases rates of rough-and-tumble play in females (reviewed in Thor and Holloway, 1985). Furthermore, in rats it appears that exposure to androgens during both the pre- and early post-natal periods is required for sexually dimorphic play and other sex-typical behaviors to fully develop (Casto et al., 2003). Evidence for prenatal androgen effects on juvenile play behavior in humans is provided by studies showing that girls with congenital adrenal hyperplasia (CAH)—a genetic disorder that causes enzyme deficiencies resulting in excessive androgen production beginning in utero. Girls with CAH tend to prefer male-typical toys during early childhood and adolescence, though no striking differences in rough-and-tumble play behavior have been found between girls with CAH and their normal peers (Berenbaum and Hines, 1992; Berenbaum et al., 2000; Hines, 2003; Hines and Kaufman, 1994; Meyer-Bahlburg et al., 2008; Nordenstrom et al., 2002).
In non-human primates, research on the effects of manipulation of maternal gestational androgens has focused on the rhesus macaque (Macaca mulatta). Macaques are a polygynous species that exhibits both physical and behavioral sexual dimorphism. For example, juvenile males initiate more rough-and-tumble play bouts than juvenile females (reviewed in Wallen and Hassett, 2009). Exogenous testosterone (T) propionate given during gestation masculinizes and/or defeminizes both morphological and behavioral development of fetuses in this species (Goy et al., 1988; Thornton et al., 2009), while early postnatal manipulations (without prenatal manipulations) of T have produced no striking effects on later juvenile or adult behavior in macaques (Thornton et al., 2009; Wallen, 2005; Wallen and Hassett, 2009). It may instead be that, similar to rats, both pre- and postnatal androgens are necessary to develop a fully masculinized/defeminized behavioral repertoire in macaques as Gibber and Goy (1985) found that prenatally androgenized females showed little behavioral dimorphism from neonatally castrated males as juveniles. Taken together, these results support a masculinizing/defeminizing organizational role of prenatal androgens in the behavioral development of the rhesus macaque similar to that seen in rodents. Thus, exposure to prenatal androgen – particularly during late gestation – can influence sexually dimorphic behavior throughout the primate lifespan.
Callitrichine primates (marmosets and tamarins), on the other hand, exhibit few sexually dimorphic traits (Ford, 1994; Plavcan, 2001). These New World primates typically give birth to fraternal twins and live in socially monogamous, extended family groups with fathers and older siblings providing a significant role in caring for the young (reviewed in Tardif et al., 1993). These features (reduced sexual dimorphism and a cooperative breeding social structure) might portend patterns of gender-based differences in rough-and-tumble play that would deviate from those observed in other polygynous primate species. For example, in squirrel monkeys (Biben, 1998) and rhesus macaques (reviewed in Wallen, 2005), the rate, intensity, and duration of rough-and-tumble play are all higher in males than females. However, the sex differences in rough-and-tumble play in socially monogamous primates are not completely understood. Sex differences in play patterns appear to be subtler, if at all present. For example, in common marmosets (Stevenson and Poole, 1982) and cotton-top tamarins (Cleveland and Snowdon, 1984), there appears to be little, if any, sexual dimorphism in the overall rate of play. Chau et al. (2008), however, did find that while the rate of play did not differ between captive male and female coppery titi monkeys – a socially monogamous, new world primate that also lives in extended family groups – there are sex differences in play partner preferences: juvenile females engage in more rough-and-tumble play with their fathers than do juvenile males. Also, while juvenile marmosets engage in rough-and-tumble play with all members of their extended families, the co-twin is generally the preferred play partner (Stevenson and Poole, 1982). One report suggests that males initiate more rough-and-tumble play bouts with female co-twins whereas male–male co-twin pairs initiate bouts equally (Abbott and Hearn, 1978a). In addition, administration of T early in postnatal life appears to increase rough-and-tumble twin play initiations in female marmosets (Abbott & Hearn, 1978a, 1978b; Abbott, 1984). Thus, in socially monogamous new world primate species, early exposure to androgens may differentially influence play patterns, which could include the rate of rough-tumble-play and play partner preference. However, to our knowledge, no reports have investigated how prenatal exposure to androgen might influence rough-and-tumble play in a socially monogamous species such as the marmoset.
Our laboratory recently reported that urinary testosterone (T) levels rise during the first trimester of pregnancy reaching peak levels during the second trimester and decline in the third trimester in the white-faced marmoset (Callithrix geoffroyi; French et al., 2010; Smith et al., 2010). These results parallel previous reports of gestational T and concentrations measured in plasma in the common marmoset (Chambers and Hearn, 1979). Interestingly, we also observed considerable variation in maternal urinary androgen levels across individual females and across different pregnancies of the same female. This variation in androgen levels was not related to fetal sex or number of male fetuses (French et al., 2010). However, it was associated with variations in the offspring's somatic growth: high maternal androgen levels during early gestation were associated with lower growth rates early in postnatal life followed by a subsequent period of catch-up growth during the juvenile period (Smith et al., 2010). The goal of the current study was to characterize the relationship, if any, between these variations in gestational androgens and juvenile rough-and-tumble play.
Androstenedione (A4) is a steroid precursor to both T and estrogens and has been associated with in utero effects on development in several mammals including the hyena (Licht et al., 1992; Yalcinkaya et al., 1993) and cavy (Kraus et al., 2008). Chambers and Hearn (1979) report high concentrations of A4 during gestation in the common marmoset. Findings from perinatal administration of A4 in male rats (Gilroy and Ward, 1978; Goldfoot et al., 1969) and female mice (Edwards, 1971) suggest that this androgen is involved in masculinization but not the defeminization of adult sexual behavior in rodents; i.e., intromission and ejaculation behaviors in males may be retained simultaneously with lordosis following neonatal A4 treatment.
Given the variation in gestational T observed in our previous studies (French et al., 2010; Smith et al., 2010), the present study sought to evaluate variation in rough-and-tumble play in juvenile white-faced marmosets and maternal concentrations of urinary androgens. To the extent that the behavioral development of marmosets is sensitive to natural levels of androgen exposure in utero, we predicted that higher levels of maternal androgen within normative variation during late gestation of these hormones would be associated with higher rates of rough-and-tumble play in juvenile offspring.
Materials and methods
Subjects
Androgen levels were measured in urine from 6 white-faced marmoset (C. geoffroyi) mothers across 18 pregnancies, ranging from 1 to 6 pregnancies per female and resulting in 29 viable offspring (16 males, 13 females). Mothers ranged in age at conception from 2.2 to 7.7 years old. Animals were socially housed in groups of 3 to 9 animals at the University of Nebraska at Omaha Callitrichid Research Center in wire-mesh enclosures no smaller than 1 m×2 m×2 m with no less than 1 m3 per animal. Cages were furnished with branches, a nest box, and various enrichment devices. Animals had access to water ad libitum and were fed Zupreem® Marmoset chow and fiber blocks each day, supplemented with varying combinations of meal worms, crickets, various fruits, yogurt and eggs. Adequate steps were taken to ensure minimal pain and discomfort and all procedures complied with and were approved by the University of Nebraska Medical Center/University of Nebraska at Omaha Institutional Animal Care and Use Committee (IACUC).
Urine collection
We collected one to two urine samples per week from each mother using noninvasive techniques described by (French et al., 1996). Briefly, subjects were trained to urinate into handheld pans for a preferred food item in their home cage. We collected 0.05–1.0 ml of first void urine samples from each animal between 0600 and 0830 h after the animals first woke. Samples were transferred to plastic vials, centrifuged at 7000 rpm for 2 min to remove sediments, and then the supernatant portion of each sample was transferred to a clean vial and stored at −20 °C until assayed.
Enzyme immunoassays (EIAs)
To determine dates of conception and gestation periods, urine samples were evaluated for concentrations of the progesterone metabolite, pregnanediol-3-glucuronide (PdG) using an enzyme immunoassay (EIA). Conception dates were determined retrospectively by estimating the date at which urinary PdG concentrations reached a nadir that was immediately followed by a sharp and sustained rise in urinary PdG concentrations which terminated in pregnancy (French et al., 1996). Three different raters scored their estimates of conception dates independently and inter-rater reliability was over 98% (within 2 days). Levels of urinary creatinine (Cr), a muscle metabolite excreted at near constant rates, were assayed and used to correct urinary PdG concentrations for variations in solute concentration and fluid intake. Cr levels were determined using a modified Jaffe-endpoint assay (Tietz, 1976).
Concentrations of maternal testosterone were determined by assaying urine samples using a T enzyme immunoassay that has previously been characterized and validated for marmosets (Fite et al., 2005; Nunes et al., 2000). Briefly, urine samples (10 μl) were extracted in 5 ml diethyl ether after enzyme hydrolysis with β-glucuronidase (Sigma Chemical, St. Louis MO). The ether was evaporated in a warm water bath under a gentle stream of air, and samples were reconstituted in 1.0 ml phosphate buffered saline. Sample concentrations were corrected for procedural loss of androgen, which was calculated by the recovery of radiolabelled testosterone. Assay sensitivity was 7.8 pg T/well. All standards, quality controls, and samples were assayed in duplicate. The testosterone antibody (provided by C.J. Munro, UC Davis; R156/7) used in the assay system cross-reacts with dihydrotestosterone (DHT) at 57.37% (other steroid cross-reactivity rates were<2%). Therefore, these data are expressed as μg/mg T-DHT. Inter-assay coefficients of variation averaged 18.2% and 7.9% for the high and low concentration quality control pools, respectively. Intra-assay coefficients of variation for the same pools averaged 9.9% and 12.7% for the high and low concentration pools, respectively. In order to control for variable fluid intake and urinary output, all androgen concentrations were corrected by urinary creatinine concentrations, as described above. Portions of these urinary T-DHT data were published previously by Smith et al. (2010).
A4 Radioimmunoassays (RIAs)
The same samples that were assayed for T-DHT were assayed for A4. A 10 μl aliquot of each sample was extracted using 5.0 ml diethyl ether after enzyme hydrolysis with β-glucuronidase. The ether was evaporated in a warm-water bath and samples were reconstituted in 1.0 ml of phosphate buffered saline (PBS). This solution underwent a second extraction by adding 6.0 ml of an ethyl acetate:hexane (3:2) solution to the reconstituted samples which were mixed thoroughly and then the phases were allowed to separate at room temperature for 60 min. We then transferred 5.0 ml of the ethyl acetate:hexane portion (upper phase) to a new test tube. This solution was then evaporated in a warm-water bath and samples were reconstituted in 2.5 ml of steroid diluent (MP Biomedicals). Samples were then diluted 1:7 (sample:steroid diluent) and 500 μl of solution was taken to assay.
We used an androstenedione radioimmunoassay (RIA) kit (MP Biomedicals) to measure urinary A4 levels in marmoset mothers. Serial dilutions of a quality control pool produced a displacement curve that was parallel to the standard curve. The assay's sensitivity was 0.10 ng/ml. A total of eight assays were conducted. Inter- and intra-assay coefficients of variations, calculated from a pooled urine sample analyzed on each assay, were 10.89% and 6.77%, respectively. Cross-reactivity of the antibody with steroids was as follows: A4: 100%; Dehydroepiandrosterone (DHEA) sulfate: 4.40%; DHEA: 3.50%; Estrone: 1.79%; Testosterone: 0.64%; all others: < 0.10%. To adjust for fluid intake and variations in urine solute concentrations, A4 concentrations were divided by the same Cr concentrations (mg/ml) used to correct other hormone concentrations as described above.
Behavioral observations
Behavioral observations (20 min each) were conducted two to five times per week for all offspring from 5 to 10 months of age. This time period coincides with the juvenile period in marmosets in which a well-developed and highly variable repertoire of play behaviors is exhibited (Yamamoto, 1993). Moreover, male marmoset androgen levels do not reach adult levels until approximately 16–18 months of age (Birnie, et al., 2011) and females generally do not experience their first ovulation cycle until after the first year of life, if at all, in their natal group (reviewed in French, 1997); thus, play data were collected from pre-pubertal animals. A trained observer sat approximately 1 m from the home cage with a laptop computer and recorded behaviors with either Noldus the Observer 5.0 or Noldus the Observer XT 8.0 (Noldus Technologies, Houston, TX). All occurrences of behaviors for play-bout frequencies were scored between all family members. For each bout, three components of rough-and-tumble play were scored: initiate play, receive play, and overall play. Both successful and unsuccessful (i.e., attempted) play initiations were combined to give a composite initiate play score. The animal that was engaged in rough-and-tumble play with the play initiator was given a receive play score. Each animal's number of successful play initiations (but not attempted play initiations) and the number of play receptions were summed for each observation to give an overall play score. A new play bout was scored only if the subjects engaged in a play bout had not been playing with each other during the previous five second interval. Table 1 displays a full ethogram of play behavior scored.
Table 1.
Ethogram of play behaviors.
| Behavior | Description |
|---|---|
| Rough-and tumble play | Any single display or combination of wrestling, biting, hitting, kicking, scratching, or pinning another animal in a non-agonistic context |
| Complete play initiation | Approach another animal and begin rough-and-tumble play |
| Attempted play initiation | Approach another animal and attempt to initiate rough-and-tumble play bout in the absence of a receive play by the target animal; includes chasing |
| Initiate play | Sum of complete play initiations and attempted play initiation |
| Receive play | Engage in play with another animal that has initiated a play bout |
| Overall play | Summation of complete play initiations and receive play behavior (does not include attempted play initiations) |
Hormone data analysis
All statistical analyses were conducted using PASW 17.0.2. We derived mean A4 and T-DHT levels for each semester of each pregnancy. Pearson's correlations were calculated to analyze the relationship between maternal A4 and T-DHT levels and variables of maternal age, parity, family size, litter size, the number of males in the litter, and the male ratio of the litter. In the event that any of these variables were significantly related to gestational A4 or T-DHT, that variable was then controlled for in appropriate subsequent analyses. Next, to examine the relationship between A4 and T-DHT excretion patterns, a Pearson's correlation analysis was conducted for all samples.
Gestational androgens were divided into semesters by finding the midpoint between the estimated conception (described above) and offspring's date of birth for each pregnancy. Mean first and second semester A4 and T-DHT levels were then compared using paired sample t-tests. Mean semester length±SE was 74.11±.52 days.
Play behavior data analysis
Two male offspring twins were dropped from analyses of play behavior due to the death of their father during their juvenile period; therefore, a total of 27 marmoset offspring (14 males, 13 females) from 5 mothers and 17 pregnancies were used in analyses involving juvenile play behavior. To test for differences across time in play initiations, play receptions, and overall play, separate two-way repeated measures factorial analyses of covariance (ANCOVA) were conducted across the juvenile period (5–10 months of age) with sex of the offspring as the between subjects variable and the number of play partners as a covariate.
Of the 17 pregnancies used in play behavior analysis, there were 10 sets of twins and 7 singletons. Of the sets of twins, one set was a male–male pair, seven were male–female and two were female–female pairs. Therefore, due to a large skew in litter sex ratios, meaningful analyses comparing male–male, female–female, and male–female could not be conducted. All post-hoc analyses of significant effects were conducted using least-squared difference (LSD) comparisons.
Maternal gestational androgens and juvenile offspring play data analysis
We first separated data by sex of the offspring and conducted separate partial correlations between first or second semester A4 or T-DHT and play behaviors controlling for family size. Partial correlations were also conducted between gestational androgens play behaviors with male siblings controlling for the number of male siblings present.
Data for males and females were then combined and hierarchical regressions were conducted to examine the relationship between maternal gestational androgen levels and the play behavior of her juvenile offspring. Separate analyses with the same dependent play variables were conducted for A4 and T-DHT. Since behavioral phenotypes are affected more so by the degree of exposure late in gestation compared to early gestation in macaques (Goy et al., 1988), we also separately regressed play behaviors on first and second semester hormones to test if this difference exists in marmosets as well. Controlling for the number of play partners present, we used a hierarchical model comparison approach to predict various play behaviors testing for main effects of offspring sex (contrast coded: female=−1, male=1), main effects of either first or second semester gestational A4 or T-DHT (centered by mean deviation), and the interaction of offspring sex and gestational A4 or T-DHT. Dependent play variables were initiate play, receive play, overall play (sum of initiations and receptions), initiate play with twin, receive play from twin, overall play with twin, initiate play with siblings (twins and older/younger siblings), receive play from siblings, overall play with siblings, initiate play with mother, receive play from mother, overall play with mother, initiate play with father, receive play from father, and overall play with father. See supplemental online material (SOM) for complete regression tables.
Another set of hierarchical regressions was conducted to examine the relationship between maternal gestational androgen levels and the play behavior of juvenile offspring with male siblings. The same hierarchical approach described above was used with the exception of controlling for the number of males in the group instead of number of play partners in the group. Dependent play variables were overall play, play initiations, and play receptions with male siblings.
We examined the data for the presence of outliers in both hormone and behavioral data and removed outliers from analyses that were ±2 standard deviations from the mean. We report the cases in which outliers were removed in the results below. All analyses were two-tailed and the criterion for statistical significance was set to p≤.05.
Results
Gestational A4 and T-DHT
Fig. 1 presents urinary A4 and T-DHT concentrations for individual pregnancies for each 10-day block during gestation and for two 10-day blocks postpartum. The considerable variation in extent and temporal pattern of changes in A4 and T-DHT are evident in this figure. While some marmosets exhibited comparable patterns across multiple pregnancies (e.g., female 5), in other cases the pattern for the same marmoset varied across pregnancies (e.g. female 3).
Fig. 1.
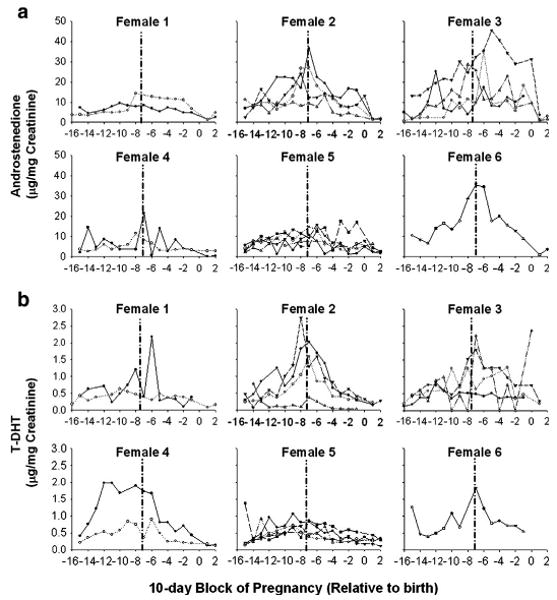
Urinary concentrations of A4 (panel a) and T-DHT (panel b) for each pregnancy relative to birth, grouped by female. Hormones were averaged in 10-day blocks. The first point represents estimated date of conception. Day “0” represents date of birth. Dashed vertical lines represent semester divisions.
When A4 and T-DHT data were averaged by semester, we found no significant correlations between A4 level litter size, the number of males in the litter, and the male ratio of the litter (data not shown). However, first semester T-DHT levels were significantly and negatively correlated with maternal age [r=−.59, p<.01], parity [r=−.54, p<.05], and family size at conception [r=−.57, p<.05]. No significant correlations were found between variables of maternal or group variables and second semester T-DHT levels (data not shown).
First semester gestational A4 levels were significantly lower than second semester A4 levels [t(17)= −2.56, p<.05]. No significant differences were found between first and second semester T-DHT [t(17)= −.41, p=.68].
Play behavior across the juvenile period
Monthly rates of play frequencies (the sum of initiations and play receptions), and play initiations did not differ significantly across the juvenile period [F(5, 120)=.27, p=.92; F(5, 120)=.17, p=.97; F(5, 120)=.52, p=.76] nor was there a significant interaction of age with sex [F(5, 120)=.22, p=.95]. Since play patterns did not differ as a function of time across the juvenile period, one composite mean for each play behavior was computed for each animal across this time period, and these values were used in all subsequent analyses.
Play behavior with various group members as a function of offspring sex and gestational androgens
When separating analyses by the sex of the offspring, partial correlations controlling for family size (or the number of males siblings present) show that males generally exhibit a negative relationship between gestational androgen exposure and juvenile play behavior (see SOM Table 1), while there were no significant correlations between gestational androgens and juvenile play in females.
For males, second semester gestational A4 was significantly and negatively correlated with overall rates of receiving play from other group members (r(11)= −.61, p<.05), receiving play from siblings (r(9)= −.61, p<.05), initiating play with male siblings (r(7)= −.81, p<.01), and overall rates of play with male siblings (r(7)= −.90, p<.01). Second semester A4 was positively correlated with play initiations with mothers (r(11)=.60, p<.05) and play initiations with fathers (r(9)=.65, p<.05). Only one significant relationship was found between first semester A4 and play behavior: first semester A4 levels were positively correlated with play initiations with fathers (r(9)=.80, p<.01; see SOM Table 1 for complete results).
First semester T-DHT was significantly and negatively correlated with receiving play from twins (r= −.72, p<.05) and initiating play with male siblings (r= −.68, p<.05) for male offspring. First semester T-DHT tended to be negatively correlated with receiving play from other group members for males (r(11)=.54, p=.057).
Second semester T-DHT was significantly and negatively correlated for male offspring with receiving play from other group members (r(11)= −.77, p<.01), receiving play from the twin (r(6)= −.74, p<.05), overall play with siblings (r(9)= −.63, p<.05), play initiations with male siblings (r(7)= −.89, p<.05), and overall play with male siblings (r(7)= −.79, p<.05). Second semester T-DHT tended to be negatively correlated with receiving play from siblings for males (r(9)=.59, p=.057).
Again, no significant relationships were found between gestational hormones during any semester and juvenile play behavior for females (see SOM Table 2).
While the partial correlation approach employed above provides information specific for each sex, the following hierarchical modeling approach we used allowed us to directly test the main effects of sex on play behavior, main effects of gestational androgen on play behavior, and sex by gestational hormone interactions while controlling for family size.
Main effects of sex on play behavior
Compared to female offspring, male offspring initiated more play bouts with other group members overall [FΔ(1, 22)=10.91, p<.01; outliers removed], with co-twins [FΔ(1, 17)=4.51, p<.01], and with all siblings overall [FΔ(1, 20)=9.35, p<.01; outliers removed]. No other main effects of sex were found (see SOM for full results).
Gestational androgens and juvenile play behavior
Models predicting play behaviors from gestational T-DHT controlling for family size and the sex of the offspring were then analyzed. These analyses indicated that higher levels of T-DHT during pregnancy are associated with decreased rough-and-tumble play during the juvenile period. Higher levels of first semester T-DHT were associated with lower rates of receiving play from all group members [R2=.48, b=−2.33, FΔ(1, 23)=4.60, p<.05], but not overall play [R2=.29, b= −.37, FΔ(1, 21)=.08, p=.79; outliers removed] or play initiations [R2=.38, b=.54, FΔ(1, 21)=.40, p=.53; outliers removed; see Figs. 2 a–c]. Higher levels of first semester T-DHT were also associated with lower rates of initiating play with male siblings [R2=.39, b= −1.16, FΔ(1, 18)=6.17, p<.05] and males exposed to higher levels of first semester T-DHT tended to engage in less play overall with their co-twin compared to females [interaction: R2=.27, b= −1.18, FΔ(1, 13)=3.58, p=.08; data not shown]. No other significant relationships were found between first semester T-DHT and juvenile play behavior.
Fig. 2.
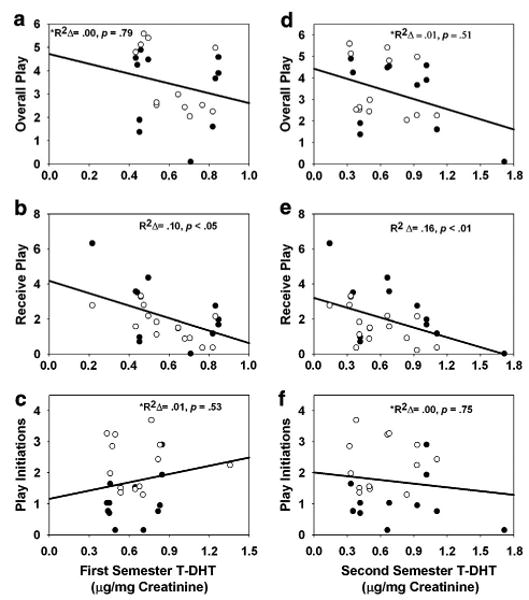
Rates of overall play, play receptions, and play initiations with all members of the family group as a function of first semester gestational T-DHT (a-c) and second semester T-DHT (d-f). Play behaviors (on vertical axes) are average frequencies per 20-min focal observation period. Open circles represent males; closed circles represent females. *Indicates instance in which an outlier was removed.
Higher levels of second semester T-DHT were associated with lower rates of receiving play from all group members [R2=.53, b= −1.94, FΔ(1, 23)=7.68, p <.05], but not overall play [R2 = .30, b = −.61, FΔ(1, 21) =.45, p=.51; outliers removed] or play initiations [R2=.37, b=.18, FΔ(1, 21)=.10, p=.75; outliers removed; see Figs. 2 d–f].
Higher second semester T-DHT levels were associated with lower rates of receiving play from siblings [R2=.54, b= −2.24, FΔ(1, 21)=4.72, p<.05; Fig. 3d] and overall play with siblings [R2=.45, b= −4.62, FΔ(1, 21)=6.33, p<.05; Fig. 3e], but not play initiations with siblings [R2=.33, b=.34, FΔ(1, 19)=.35, p=.56; outliers removed; data not shown]. However, higher levels of T-DHT during the second semester were associated with lower rates of play initiation with male siblings when controlling for the number of male siblings present [R2=.54, b= −2.06, FΔ(1, 19)=19.45, p<.001; outliers removed; Fig. 3f]. Also, males exposed to higher levels of second semester T-DHT received significantly fewer play receptions from their co-twin than females [interaction: R2=.46, b= −1.02, FΔ(1, 14)=6.62, p<.05; outliers removed; data not shown].
Fig. 3.
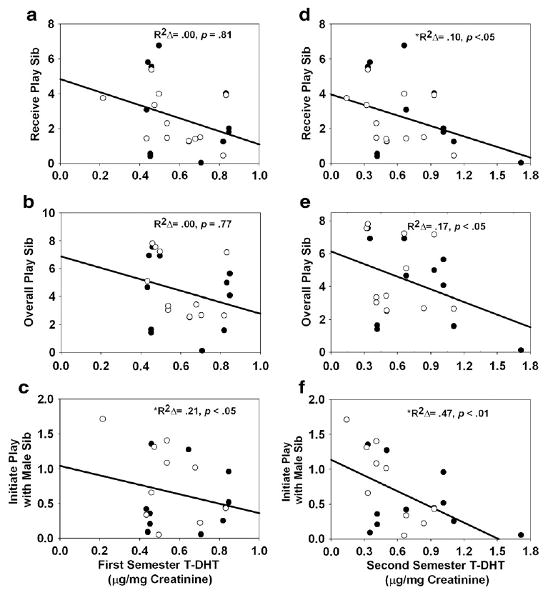
Play behaviors with all siblings and male siblings as a function of T-DHT during the first (a-c) and second (d-f) semesters of gestation. Play behaviors (on vertical axes) are average frequencies per 20-min focal observation period. Open circles represent males; closed circles represent females. *Indicates instance in which an outlier was removed.
Regression analyses predicting juvenile rough-and-tumble play from either first or second semester gestational A4 levels controlling for the number of play partners in the group yielded no significant main effects of sex or gestational A4, nor were there any significant sex by gestational A4 interactions. See SOM Tables 3–6 for complete regression results.
Discussion
In the present study, we observed considerable variation in patterns of T-DHT and A4 concentrations across individuals and across different pregnancies within the same female. This variation constitutes a possible mechanism for inducing variability in offspring characteristics. In contrast to findings in rhesus macaques (reviewed in Wallen and Hassett, 2009), we found that maternal androgen levels during pregnancy were negatively correlated with some aspects of rough-and-tumble play in juvenile offspring. Specifically, juveniles born to mothers with higher gestational levels of T-DHT, but not A4, received fewer play initiations from other group members overall, received fewer play initiations from siblings, engaged in less overall play with siblings, and initiated fewer play bouts with male siblings. However, previous studies demonstrate that early postnatal injections of androgens increase rates of rough-and-tumble play and sexual behavior in female callitrichine primates (Abbott, 1984; Abbott and Hearn, 1978b; Dixson, 1993a, 1993b, 1993c; Epple et al., 1987, 1990). Thus, our results appear to highlight a difference between androgen functions during two distinct life periods, prenatal and early postnatal, in marmosets. Furthermore, more significant relationships were found during the second semester of gestation than the first, suggesting that in marmosets, like macaques (reviewed in Wallen, 2005) exposure to androgen later in gestation is associated with changes in later-life behavior while earlier gestational exposure has fewer effects.
This negative relationship between gestational exposure to androgen and juvenile play behavior also appears to be driven more so by marmoset males than females as we found significant associations between gestational androgens and juvenile play in males, but not in females, when analyses were separated by offspring sex. This parallels the results from a human study that found that boys with CAH – who are exposed to higher levels of androgen during pregnancy – show reduced rough-and-tumble play during early childhood (Hines and Kaufman, 1994). This negative relationship in humans appears to generalize to other psychological traits as well, since boys with CAH are less dominant, report less physical aggression, and exhibit more tender mindedness than boys without CAH (Mathews et al., 2009). Moreover, compared to unaffected controls, boys with CAH show reduced ability in mental rotation tasks (Hines et al., 2003). The authors of these studies have suggested that one possible explanation for such findings is that elevated exposure to non-testicular T during pregnancy can invoke negative feedback mechanisms on the production of testicular T in male fetuses. Therefore, males that are initially exposed to higher levels of androgen might adjust testicular androgen production to achieve normal or even sub-normal levels, ultimately leading to a demasculinizing effect in CAH males (Mathews et al., 2009). However, in clinical populations of human boys with CAH, there remains the possibility that socialization factors relating to their illness may cause a reduction in more masculine-like traits. Hines and Kaufman (1994) found a negative correlation between the amount of time boys with CAH spent in the hospital and rough-and-tumble play, providing some evidence for this interpretation. The current study, however, provided an animal model which examined healthy animals from intact families and found that increased levels of normative androgens during gestation were associated with reduced rough-and-tumble play and that the relationship appears to be driven more so by males. Therefore, to the extent that our findings in marmosets can be generalized, our results support the hypothesis that exposure to higher levels of gestational androgen can induce negative feedback in testicular androgen production in male fetuses.
While experimental manipulations have provided a wealth of information about the masculinization/defeminization effects of androgen during pregnancy on both somatic and behavioral development, the role of natural variation in androgen production during gestation remains to be fully characterized. Our findings contribute to a growing body of evidence suggesting that natural variation in hormone exposure in utero can affect developmental trajectories of offspring (Dloniak et al., 2006; Licht et al., 1992; Smith et al., 2010; current study). We previously proposed that androgen production during pregnancy may be one mechanism by which female marmosets influence the morphological phenotype (as measured by somatic growth) of their offspring and that such a relationship may represent a tradeoff in terms of immediate versus long-term resource allocation and investment in offspring (Smith et al., 2010). Since, in our earlier report, higher gestational androgen levels were inversely associated with growth rates early in life, we argued that marmoset mothers might modify androgen production rates during gestation in order to influence the physical phenotype of her offspring based on environmental conditions. Results from the present study might also be explained within this framework. Rough-and-tumble play performs an integral role in developing a number of skills that influence fitness (reviewed in Power, 2000). The ability to influence such an important developmental characteristic in offspring based on environmental conditions might therefore provide a fitness advantage. We propose that maternal androgens during pregnancy may influence this class of behaviors to increase reproductive fitness by optimizing the behavioral profile in accordance with the environment. On the other hand, play behavior is energetically expensive and can leave animals vulnerable to predation. Influencing play behavior tendencies by manipulating prenatal androgens could be one route by which marmoset mothers signal environmental cues to offspring in an attempt to balance these costs and benefits.
This type of environmental signaling from mother to fetus via endocrine pathways has been documented in other species. For example, female zebra finches (Taeniopygia guttata) fed a high quality diet allocate more T to their egg clutches compared to females who are fed a low quality diet (Rutstein et al., 2004) and female collard flycatchers (Ficedula albicollis) allocate more T to their clutches after mating with a younger male compared to after mating with an older male (Michl et al., 2004). In rats, prenatal stress decreases the late gestational surge in T in male fetuses (Ward and Weisz, 1980) and feminizes juvenile male play behavior (Ward and Stehm, 1991). From our results, however, precisely which environmental cues marmoset mothers may be relaying to her offspring is unclear. Second semester T-DHT levels were not associated with maternal or group characteristics that we measured and all monkeys were fed the same diet. Future studies are warranted to examine if social dynamics or other group member characteristics might account for variations in maternal androgen production during gestation.
It is also interesting to note that the neural circuits underlying different categories of behavior often have sensitive periods that can span development (Thornton et al., 2009), and more complex behaviors, presumably under the control of multiple neural circuits, are likely to have multiple sensitive periods (Knudsen, 2004). Thus, since the different neural circuits involved in complex behaviors – such as social play – are developing at different time points and at different rates, we cannot assume that the same manipulations during different developmental time points will result in similar behavioral outcomes. It is therefore possible that, in callitrichine primates, androgens affect behavioral development differentially depending on the timing of exposure (i.e., pre- vs. postnatal). Admittedly speculative, this argument would obviously benefit with the support of evidence from experimental manipulations of maternal androgens during pregnancy in marmosets.
Our results also provide evidence of the source of androgen production. Neither T-DHT nor A4 concentrations were associated with litter size, family size, or male composition of the litter (sex ratio and number of males in the litter). The lack of association of maternal hormones with both the sex ratios of the litter and the number of males in the litter suggest that these hormones are not of fetal origin, but rather of maternal or placental origin (French et al., 2010), or that one or both are synthesized from hormones produced by the placenta and/or metabolized through placental enzymes (Husen et al., 2003). However, while our data provides evidence to suggest that urinary androgens measured during gestation are maternal in origin, the possibility remains that male fetuses are producing their own androgens, and we therefore cannot know precisely how much androgen they are exposed to as fetuses with our methods.
We also observed minimal sexual dimorphism in rough-and-tumble play in juvenile marmosets. While juvenile males did initiate more play bouts than their female counterparts, this was the only sexually dimorphic feature we found. Similar to our findings, Chau et al. (2008) report that titi monkeys do not exhibit sexually dimorphic rates of overall play as juveniles. However, play partner choice is sexually dimorphic in that female titi monkeys direct more play behaviors towards their fathers than to juvenile males. By contrast, polygamous species typically show marked sexual dimorphism in the rates of rough-and-tumble play in that males exhibit higher rates of play than females, and treatment with prenatal androgens increases rates of play in juvenile females (reviewed in Wallen, 2005; Wallen and Hassett, 2009). However, we found no marked sexually dimorphic play patterns in marmosets, and while the relationships that were found appeared to be driven more by male offspring, there were few significant sex by gestational androgen interactions on juvenile play behavior. This suggests that, unlike species that exhibit a higher degree of sexual dimorphism, male and female marmosets may not exhibit highly marked differences in their sensitivity to androgens, at least in terms of physical growth (Smith et al., 2010) and behavior (current study). Since marmosets typically give birth to fraternal twins, raising the possibility of mixed-sex pairs, it may be that fetal males exhibit some degree of testicular quiescence or at least suppressed testicular activity compared to other primate species in order to prevent virilization of a female co-twin.
Maternal concentrations of A4 were correlated with offspring play behavior only for a very few specific measures, which suggests that exposure to A4 during gestation has minimal effects on postnatal behavior. Given the similar patterns of A4 and T concentrations, and the fact that A4 is a metabolic precursor to T, this difference in T and A4 association with play behavior is somewhat surprising. In addition, exogenous A4 has been reported to alter developmental trajectories in ways paralleling those of other androgens in rats (Edwards, 1971; Whalen and Luttge, 1971). However, A4 is an inactive steroid that is also a precursor to estradiol. Varying metabolic fates of A4 may be one variable that modulates and/or alters any effects related to variation in concentrations of A4. Future investigations might attempt to examine this relationship as the frequency of sampling in the current study did not provide sufficient resolution by which to analyze an androgen ratio (e.g. A4:T) that could provide some measure of metabolic conversion rates.
It is possible that exposure to androgen in utero alters stimulus properties of an animal, which in turn influences the way in which other group members interact with them. If this is the case, it is not clear if this relationship is due to alterations in physical or behavioral characteristics. In the current study, higher T-DHT levels were associated with fewer play receptions from other group members. It is possible that this is due to behavioral characteristics of the juvenile. For example, if a juvenile marmoset initiates more play bouts with other group members, there are fewer chances for other group members to initiate play bouts with that juvenile. However, our data suggests that this is not the case. Instead, as a function of prenatal exposure to androgens, play behavior directed towards juvenile marmosets appears to be independent of the play behaviors of the juveniles measured in the current study as we found no relationship between the number of play initiations by young marmosets and late gestational androgen levels when interactions with all group members and all siblings were analyzed. Moreover, when analyzing play interactions with male siblings, juvenile marmosets initiated significantly more play bouts with male siblings as a function of decreased late gestational androgen, but no relationship was found with the number of play bouts juveniles received from male siblings. Thus, it is possible that prenatal androgens might alter stimulus properties other than play behavior patterns of juvenile marmosets that make the animal a less attractive play partner.
In rhesus macaques, genital and behavioral masculinization due to prenatal androgen exposure appear to occur independently of one another (Goy et al., 1988); androgens masculinize genital morphology early in pregnancy, but not during late pregnancy and vice versa for behavioral masculinization. However, macaques are a polygynous species with relatively marked physical and behavioral sexual dimorphism. Marmosets, on the other hand, exhibit far fewer sexually dimorphic traits, which portends a different sensitivity to sex steroids compared to macaques. However, little is known about the effects of prenatal androgens on the genital morphology of marmoset monkeys. While postnatal androgen administration masculinizes genitalia and causes increased rates of rough-and-tumble play with the co-twin in female marmosets (Abbott and Hearn, 1978a, b; Dixson, 1993a; Dixson and Lunn, 1987), it is unclear if changes in behavior are due to androgen-dependent neural reorganization or the changes in genital morphology which might cause other group members to interact with a masculinized female differently, which in turn could alter a female's behavior towards other group members. In addition, there is evidence to suggest that in monogamous mammals, androgens can operate in a manner opposite of what would be considered virilization. For instance, in the male California mouse (Peromyscus californicus) T promotes paternal behavior whereas castration reduces it (Trainor and Marler, 2001). This relationship appears to be mediated via aromatase conversion of T to estradiol (Trainor and Marler, 2002). The authors argue that this may be one mechanism by which monogamous mammals maintain paternal behavior in the presence of elevated circulating T. While little is known regarding aromatase activity in marmosets, we might also draw similar conclusions about the mechanisms that translate the effects of prenatal androgens into postnatal behavior in the monogamous marmoset. We found that maternal prenatal androgens are associated with decreased early-life growth (Smith et al., 2010) and decreased rough-and-tumble play during the juvenile period (current study), contradicting to the virilizing effects of prenatal androgens seen in polygamous species. Therefore, marmosets, like California mice, may employ mechanisms by which increased androgen levels can facilitate both physical and behavioral characteristics that favor a monogamous social structure.
Supplementary Material
Acknowledgments
We thank Heather Jensen, Danny Revers and Liz Gunkelman for their excellence in animal care and husbandry. We would also like to thank the UNO Neuroscience and Behavior Journal Club and the journal's reviewers for providing helpful comments on previous versions of this manuscript. The research program from which these data are derived was initiated by Jeffrey E. Fite, and was supported by funds from the National Institutes of Health (HD 42882) and the National Science Foundation (IBN 00-91030) awarded to JAF. All procedures were approved by the University of Nebraska at Omaha/Medical center Institutional Animal Care and Use Committee and adhered to all local, state, and national laws regulating research on nonhuman primates.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.yhbeh.2012.05.014.
References
- Abbott DH. Differentiation of sexual behaviour in female marmoset monkeys: effects of neonatal testosterone or a male co-twin. Prog Brain Res. 1984:349–358. doi: 10.1016/S0079-6123(08)64446-5. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Hearn JP. Physical, hormonal and behavioural aspects of sexual development in the marmoset monkey, Callithrix jacchus. J Reprod Fertil. 1978a;53:155–166. doi: 10.1530/jrf.0.0530155. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Hearn JP. The effects of neonatal exposure to testosterone on the development of behaviour in female marmoset monkeys. Ciba Foundation Symposium. 1978b:299–327. doi: 10.1002/9780470720448.ch14. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Hines M. Early androgens are related to childhood sex-typed toy preferences. Psychol Sci. 1992;3:203–206. [Google Scholar]
- Berenbaum SA, Duck SC, Bryk K. Behavioral effects of prenatal versus postnatal androgen excess in children with 21-hydroxylase-deficient congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2000;85:727–733. doi: 10.1210/jcem.85.2.6397. [DOI] [PubMed] [Google Scholar]
- Biben M. Squirrel monkey playfighting: making the case for a cognitive training function for play. In: Bekoff M, Byers JA, editors. Animal Play: Evolutionary, Comparative, and Ecological Perspectives. Cambridge University Press; New York: 1998. pp. 167–182. [Google Scholar]
- Birnie AK, Smith AS, Nali C, French JA. Social and developmental influences on urinary androgen levels in young male white-faced marmosets (Callithrix geoffroyi) Am J Primatol. 2011;73:378–385. doi: 10.1002/ajp.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casto JM, Ward OB, Bartke A. Play, copulation, anatomy, and testosterone in gonadally intact male rats prenatally exposed to flutamide. Phys Behav. 2003;79:633–641. doi: 10.1016/s0031-9384(03)00120-3. [DOI] [PubMed] [Google Scholar]
- Chambers PL, Hearn JP. Peripheral plasma levels of progesterone, oestradiol-17β, oestrone, testosterone, androstenedione and chorionic gonadotrophin during pregnancy in the marmoset monkey, Callithrix jacchus. J Reprod Fertil. 1979;56:23–32. doi: 10.1530/jrf.0.0560023. [DOI] [PubMed] [Google Scholar]
- Chau MJ, Stone AI, Mendoza SP, Bales KL. Is play behavior sexually dimorphic in monogamous species? Ethology. 2008;114:989–998. [Google Scholar]
- Cleveland J, Snowdon CT. Social development during the first twenty weeks in the cotton-top tamarin (Saguinus oedipus) Ann Behav. 1984;32:432–444. [Google Scholar]
- Dixson AF. Effects of testosterone propionate upon the sexual and aggressive behavior of adult male marmosets (Callithrix jacchus) castrated as neonates. Horm Behav. 1993a;27:216–230. doi: 10.1006/hbeh.1993.1016. [DOI] [PubMed] [Google Scholar]
- Dixson AF. Observations on effects of neonatal castration upon sexual and aggressive behavior in the male common marmoset (Callithrix jacchus) Am J Primatol. 1993b;31:1–10. doi: 10.1002/ajp.1350310102. [DOI] [PubMed] [Google Scholar]
- Dixson AF. Sexual and aggressive behaviour of adult male marmosets (Callithrix jacchus) castrated neonatally, prepubertally, or in adulthood. Phys Behav. 1993c;54:301–307. doi: 10.1016/0031-9384(93)90115-v. [DOI] [PubMed] [Google Scholar]
- Dixson AF, Lunn SF. Post-partum changes in hormones and sexual behaviour in captive groups of marmosets (Callithrix jacchus) Phys Behav. 1987;41:577–583. doi: 10.1016/0031-9384(87)90314-3. [DOI] [PubMed] [Google Scholar]
- Dloniak SM, French JA, Holekamp KE. Rank-related maternal effects of androgens on behaviour in wild spotted hyaenas. Nature. 2006;440:1190–1193. doi: 10.1038/nature04540. [DOI] [PubMed] [Google Scholar]
- Edwards DA. Neonatal administration of androstenedione, testosterone or testosterone propionate: effects on ovulation, sexual receptivity and aggressive behavior in female mice. Phys Behav. 1971;6:223–228. doi: 10.1016/0031-9384(71)90030-8. [DOI] [PubMed] [Google Scholar]
- Epple G, Alveario MC, Andre ES. Sexual and social behavior of adult saddle-back tamarins (Saguinus fuscicollis), castrated as neonates. Am J Primatol. 1987;13:37–49. doi: 10.1002/ajp.1350130106. [DOI] [PubMed] [Google Scholar]
- Epple G, Alveario MC, Belcher AM. Copulatory behavior of adult tamarins (Saguinus fuscicollis) castrated as neonates or juveniles: effect of testosterone treatment. Horm Behav. 1990;24:470–483. doi: 10.1016/0018-506x(90)90036-w. [DOI] [PubMed] [Google Scholar]
- Fite JE, French JA, Patera KJ, Hopkins EC, Rukstalis M, Ross CN. Elevated urinary testosterone excretion and decreased maternal caregiving effort in marmosets when conception occurs during the period of infant dependence. Horm Behav. 2005;47:39–48. doi: 10.1016/j.yhbeh.2004.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford SM. Evolution of sexual dimorphism in body weight in platyrrhines. Am J Primatol. 1994;34:221–244. doi: 10.1002/ajp.1350340211. [DOI] [PubMed] [Google Scholar]
- French JA. Proximate regulation of singular breeding in Callitrichid primates. In: Solomon NG, French JA, editors. Cooperative breeding in mammals. Cambridge Press University; New York: 1997. pp. 34–75. Cooperative Breeding in Mammals. [Google Scholar]
- French JA, Brewer KJ, Schaffner CM, Schalley J, Hightower-Merritt D, Smith TE, Bell SM. Urinary steroid and gonadotropin excretion across the reproductive cycle in female Wied's black tufted-ear marmosets (Callithrix kuhli) Am J Primatol. 1996;40:231–245. doi: 10.1002/(SICI)1098-2345(1996)40:3<231::AID-AJP2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- French JA, Smith AS, Birnie AK. Maternal gestational androgen levels in female marmosets (Callithrix geoffroyi) vary across trimesters but do not vary with the sex ratio of litters. Gen Comp Endocrinol. 2010;165:309–314. doi: 10.1016/j.ygcen.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibber JR, Goy RW. Infant-directed behavior in young rhesus monkeys: sex differences and effects of prenatal androgens. Am J Primatol. 1985;8:225–237. doi: 10.1002/ajp.1350080305. [DOI] [PubMed] [Google Scholar]
- Gilroy AF, Ward IL. Effects of perinatal androstenedione on sexual behavior differentiation in male rats. Behav Biol. 1978;23:243–248. doi: 10.1016/s0091-6773(78)91894-1. [DOI] [PubMed] [Google Scholar]
- Goldfoot DA, Feder HH, Goy RW. Development of bisexuality in the male rat treated neonatally with androstenedione. J Comp Physiol Psychol. 1969;67:41–45. doi: 10.1037/h0026653. [DOI] [PubMed] [Google Scholar]
- Goy RW, Bercovitch FB, McBrair MC. Behavioral masculinization is independent of genital masculinization in prenatally androgenized female rhesus macaques. Horm Behav. 1988;22:552–571. doi: 10.1016/0018-506x(88)90058-x. [DOI] [PubMed] [Google Scholar]
- Hines M. Sex steroids and human behavior: prenatal androgen exposure and sex-typical play behavior in children. Ann N Y Acad Sci. 2003;1007:272–282. doi: 10.1196/annals.1286.026. [DOI] [PubMed] [Google Scholar]
- Hines M, Kaufman FR. Androgen and the development of human sex-typical behavior: rough-and-tumble play and sex of preferred playmates in children with congenital adrenal hyperplasia (CAH) Child Dev. 1994;65:1042–1053. [PubMed] [Google Scholar]
- Hines M, Fane BA, Pasterski VL, Mathews GA, Conway GS, Brook C. Spatial abilities following prenatal androgen abnormality: targeting and mental rotations in individuals with congenital adrenal hyperplasia. Psychoneuroendocrinology. 2003;28:1010–1026. doi: 10.1016/s0306-4530(02)00121-x. [DOI] [PubMed] [Google Scholar]
- Husen B, Adamski J, Brüns A, Deluca D, Fuhrmann K, Möller G, Schwabe I, Einspanier A. Characterization of 17β-hydroxysteroid dehydrogenase type 7 in reproductive tissues of the marmoset monkey. Biol Reprod. 2003;68:2092–2099. doi: 10.1095/biolreprod.102.012476. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Kraus C, Pfannkuche KA, Trillmich F, Groothuis TGG. High maternal androstenedione levels during pregnancy in a small precocial mammal with female genital masculinisation. Max Planck Inst Demog Res. 2008 Working Papers WP-2008-017. [Google Scholar]
- Licht P, Frank LG, Pavgi S, Yalcinkaya TM, Siiteri PK, Glickman SE. Hormonal correlates of ‘masculinization’ in female spotted hyaenas (Crocuta crocuta) 2 Maternal and fetal steroids. J Reprod Fertil. 1992;95:463–474. doi: 10.1530/jrf.0.0950463. [DOI] [PubMed] [Google Scholar]
- Mathews GA, Fane BA, Conway GS, Brook CGD, Hines M. Personality and congenital adrenal hyperplasia: possible effects of prenatal androgen exposure. Horm Behav. 2009;55:285–291. doi: 10.1016/j.yhbeh.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bahlburg H, Dolezal C, Baker S, New M. Archives of Sexual Behavior. Springer; Netherlands: 2008. Sexual orientation in women with classical or non-classical congenital adrenal hyperplasia as a function of degree of prenatal androgen excess; pp. 85–99. [DOI] [PubMed] [Google Scholar]
- Michl G, Török J, Péczely P, Garamszegi LZ, Schwabl H. Female collared flycatchers adjust yolk testosterone to male age, but not to attractiveness. Behav Ecol. 2004;16:383–388. [Google Scholar]
- Nordenstrom A, Servin A, Bohlin G, Larsson A, Wedell A. Sex-typed toy play behavior correlates with the degree of prenatal androgen exposure assessed by CYP21 genotype in girls with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2002;87:5119–5124. doi: 10.1210/jc.2001-011531. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, French JA. Variation in steroid hormones associated with infant care behaviour and experience in male marmosets (Callithrix kuhlii) Ann Behav. 2000;60:857–865. doi: 10.1006/anbe.2000.1524. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Plavcan JM. Sexual dimorphism in primate evolution. Am J Phys Anthropol. 2001;116:25–53. doi: 10.1002/ajpa.10011.abs. [DOI] [PubMed] [Google Scholar]
- Power T. Play and Exploration in Children and Animals. Lawrence Erlbaum Associations; London: 2000. [Google Scholar]
- Rutstein AN, Gilbert L, Slater PJB, Graves JA. Sex-specific patterns of yolk androgen allocation depend on maternal diet in the zebra finch. Behav Ecol. 2004;16:62–69. [Google Scholar]
- Smith AS, Birnie AK, French JA. Maternal androgen levels during pregnancy are associated with early-life growth in Geoffroy's marmosets, Callithrix geoffroyi. Gen Comp Endocrinol. 2010;166:307–313. doi: 10.1016/j.ygcen.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson MF, Poole TB. Playful interactions in family groups of the common marmoset (Callithrix jacchus jacchus) Ann Behav. 1982;30:886–900. [Google Scholar]
- Tardif SD, Harrison ML, Simek MA. Communal infant care in marmosets and tamarins: relation to energetics, ecology, and social organization. In: Rylands AB, editor. Marmosets and Tamarins: Systematics, Behaviour, and Ecology. Oxford Press; Oxford: 1993. pp. 220–234. [Google Scholar]
- Thor DH, Holloway WR., Jr Social play in juvenile rats: a decade of methodological and experimental research. Neurosci Biobehav Rev. 1985;8:455–464. doi: 10.1016/0149-7634(84)90004-6. [DOI] [PubMed] [Google Scholar]
- Thornton J, Zehr JL, Loose MD. Effects of prenatal androgens on rhesus monkeys: a model system to explore the organizational hypothesis in primates. Horm Behav. 2009;55:633–644. doi: 10.1016/j.yhbeh.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz NW. Fundamental of Clinical Chemistry. W. B. Saunders; Philadelphia: 1976. [Google Scholar]
- Trainor BC, Marler CA. Testosterone, paternal behavior, and aggression in the monogamous California mouse (Peromyscus californicus) Horm Behav. 2001;40:32–42. doi: 10.1006/hbeh.2001.1652. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc R Soc B Biol Sci. 2002;269:823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front Neuroendocrinol. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Wallen K, Hassett JM. Sexual differentiation of behaviour in monkeys: role of prenatal hormones. J Neuroendocrinol. 2009;21:421–426. doi: 10.1111/j.1365-2826.2009.01832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward IL, Stehm KE. Prenatal stress feminizes juvenile play patterns in male rats. Phys Behav. 1991;50:601–605. doi: 10.1016/0031-9384(91)90552-y. [DOI] [PubMed] [Google Scholar]
- Ward I, Weisz J. Maternal stress alters plasma testosterone in fetal males. Science. 1980;207:328–329. doi: 10.1126/science.7188648. [DOI] [PubMed] [Google Scholar]
- Whalen RE, Luttge WG. Testosterone, androstenedione and dihydrotestosterone: effects on mating behavior of male rats. Horm Behav. 1971;2:117–125. doi: 10.1016/0018-506x(74)90035-x. [DOI] [PubMed] [Google Scholar]
- Yalcinkaya T, Siiteri P, Vigne J, Licht P, Pavgi S, Frank L, Glickman S. A mechanism for virilization of female spotted hyenas in utero. Science. 1993;260:1929–1931. doi: 10.1126/science.8391165. [DOI] [PubMed] [Google Scholar]
- Yamamoto ME. From dependence to sexual maturity: the behavioral ontogeny of Callitrichidae. In: Rylands AB, editor. Marmosets and Tamarins: Systematics, Behaviour, and Ecology. Oxford University Press; New York: 1993. pp. 235–254. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


