Abstract
Blebbistatin is a recently discovered myosin II inhibitor. It is rapidly becoming a compound of choice to reduce motion artifacts during cardiac optical mapping, as well as to study cell motility and cell invasion. Although blebbistatin has a number of advantages over other electromechanical uncouplers, many of its properties have yet to be addressed. Here we describe several methodological issues associated with the use of blebbistatin, including its spectral properties, reversibility, and its effect on tissue metabolic state. We show that if precautions are not taken, perfusion with blebbistatin may result in blebbistatin precipitate that accumulates in the vasculature. Although such precipitate is fluorescent, it is not detectable within wavelength bands that are typically used for transmembrane voltage fluorescence imaging (i.e., emission wavelengths >600 nm). Therefore, blockage of the microcirculation by blebbistatin may cause data misinterpretation in studies that use voltage-sensitive dyes. Blebbistatin may also impact imaging of green fluorophores due to the spectral shift it causes in endogenous tissue fluorescence. 3D excitation–emission matrices of blebbistatin in precipitate form and in various solutions (DMSO, water, and 1 % aqueous albumin) revealed significant changes in the fluorescence of this molecule in different environments. Finally, we examined the reversibility of blebbistatin’s uncoupling effect on cardiac contraction. Our findings provide important new information about the properties of this myosin II inhibitor, which will aid in the proper design and interpretation of studies that use this compound.
Introduction
Blebbistatin (1-phenyl-1,2,3,4-tetrahydro-4-hydroxypyrrolo[2,3-β]-7-methylquinolin-4-one) is a newly discovered reversible inhibitor of myosin II and other myosin isoforms [9, 23, 32, 33]. The name derives from its ability to inhibit cell membrane blebbing [33]. Blebbistatin acts by lowering myosin II affinity to actin. It has a growing number of applications in cancer, developmental biology, and the field of cell motility [1, 10, 25, 26, 34]. Blebbistatin is used in optical mapping of cardiac preparations to minimize motion artifacts caused by heart contraction. It has been shown to have minimal effect on the heart’s electrical activity, including ECG parameters, atrial and ventricular effective refractory periods, and atrial and ventricular activation patterns [14, 24].
Blebbistatin has been successfully used for cardiac optical mapping in a number of species including zebrafish [16], mouse [8], rat, rabbit [14], dog [20], horse [15], and humans [12, 13]. Blebbistatin’s toxicity and side effects on ion channels are less pronounced compared to other electromechanical uncouplers, such as cytochalasin D [29] and 2,3-butanedione monoxime [14, 22, 24]. Its main known limitations include photosensitivity and phototoxicity [19, 30]. We have successfully used blebbistatin in a number of our recent studies [2, 18, 28, 31]. These studies also revealed a need to more closely examine the spectral properties of blebbistatin with physiologically relevant experimental conditions. Here we present our observations and discuss the formation of blebbistatin precipitate, the effects of blebbistatin washout, and other properties of this compound that may impact experimental design and data interpretation.
Methods
Animal protocols
Adult Sprague Dawley rats (300–600 g) were heparinized and anesthetized using standard procedures [2, 18, 28]. Excised hearts were retrograde perfused via Langendorff at constant pressure (60 mmHg) with oxygenated, HEPES-buffered Tyrode solution. Experiments were conducted at room temperature unless noted otherwise. All anesthesia and euthanasia procedures were in full compliance with the Institutional Animal Care and Use Committee-approved protocols.
Dual fluorescence imaging
The fluorescence imaging system consisted of two CCD cameras (Andor IXON DV860s) fitted to a dual-port adaptor (Andor CSU Adapter Dual Cam) containing a 550-nm dichroic mirror. The two cameras were aligned to image the same field of view. The first camera acquired fluorescence in the green emission range (475/50 nm) while illuminating the epicardial surface with UV light (365±5 nm 250 mW UV LEDs, Mightex Systems). The other camera acquired fluorescence in the red emission range using a long-pass 680-nm filter while illuminating the epicardial surface with green light (LumiLEDs, 530/35 nm). Images from the first camera corresponded to fluorescence of endogenous NADH and/or precipitated blebbistatin. Images from the second camera were typically used to examine transmembrane action potentials using voltage-sensitive dyes.
Blebbistatin solutions
A stock solution of blebbistatin was prepared by adding 1 mL of DMSO to 5 mg of (−)-blebbistatin (Sigma B0560). Aliquots of blebbistatin in DMSO (100 μL) were then stored at −20 °C. To immobilize the hearts, a 100-μL aliquot was mixed into 70 mL of pre-warmed oxygenated Tyrode solution, which was then added to a recirculating perfusion system for a final solution volume of 170 mL. The final concentration of blebbistatin in the recirculating perfusate was 10 μM, unless stated otherwise.
Confocal imaging
An LSM510 Zeiss confocal imaging system mounted on an inverted Axiovert 200M microscope was used to acquire images of blebbistatin precipitate. A 458-nm argon laser was used for excitation, and emitted light was long pass filtered at 475 nm. Fluorescence intensity values were compared using identical settings for all samples. The Lambda mode of an LSM710 Zeiss confocal imaging system mounted on an upright Axio Examiner microscope was used to collect fluorescent emission spectra from the epicardial surface of the heart. For these experiments, the excised heart was perfused with Tyrode solution in Langendorff mode, and epicardial fluorescence was acquired before and after a bolus administration of 10 μM blebbistatin.
Spectra acquisition
In vitro fluorescence was measured using a FluoroMax-3 spectrofluorometer (HORIBA Jobin Yvon) and quartz cuvettes. Blebbistatin solutions were kept in the dark between measurements unless noted otherwise. To form blebbistatin precipitate on a glass coverslip, 10 μl of stock blebbistatin solution in DMSO was placed next to a water drop to create a boundary and allowed to dry. The coverslip was then placed inside the spectrofluorometer for spectra acquisition across specified wavelength ranges. To acquire 3D excitation/emission matrices of soluble blebbistatin, 1 μM blebbistatin was dissolved in DMSO, water, or 1 % bovine serum albumin solution.
TTC staining
Triphenyltetrazolium chloride (TTC) vital staining is a standard procedure for assessing acute necrosis. It relies on the ability of dehydrogenase enzymes and NADH to react with tetrazolium salts to form a formazan pigment. Immediately after the imaging protocol, the tissue was retrograde perfused with Tyrode solution containing 1.0 % TTC. Afterward, the heart was submerged in the TTC solution for an additional 8 min. Metabolically active tissue appeared crimson and necrotic tissue appeared white.
Statistical analysis of data
Mean values were compared by Student’s t test. Data were considered significantly different if p≤0.05. Images shown in Figs. 1a and b, 3b, 4b, 6, and 7 are representative of at least three independent experiments using different hearts each time. The data shown in Fig. 9 were derived from ten experiments (five hearts in each group). Representative snapshots are shown. The emission/ excitation matrices similar to the one shown in Fig. 4 have been acquired at least four times each using freshly prepared blebbistatin solutions.
Fig 1.
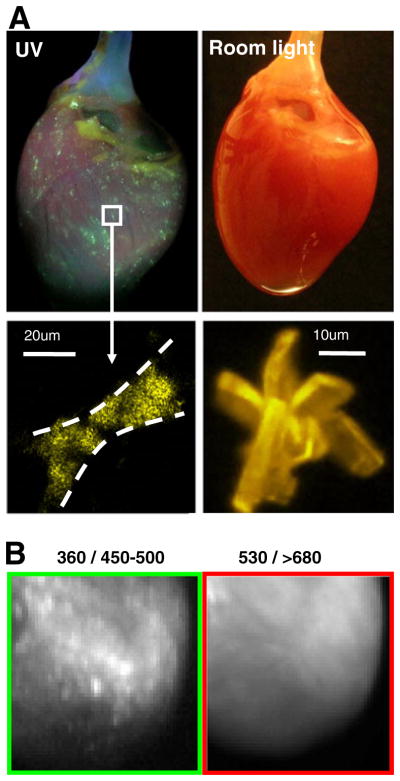
Blebbistatin precipitate within epicardial tissue. a Top left: A rat heart perfused with 10 μM blebbistatin under UV light illumination. Top right: The same heart under room light illumination. Bottom left: A close-up view of blebbistatin precipitate within epicardial tissue. Bottom right: An example of a large blebbistatin crystal. b Left: Epicardial surface of a heart perfused with 10 μM blebbistatin as it appears using 360 nm excitation and 450–530 nm emission settings. Right: The same surface when imaged at longer wavelengths (530 nm excitation and >680 nm emission)
Fig 3.
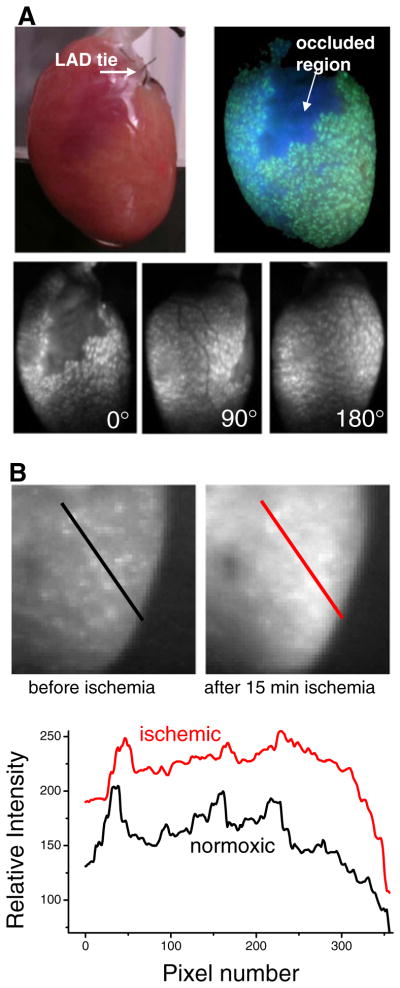
Blebbistatin precipitation during ischemia studies. a An extreme case of blebbistatin precipitation within epicardial tissue. After ligating a small branch of a coronary artery, the heart was Langendorff perfused (recirculated) with perfusate containing blebbistatin (10 μM). The occluded area remained clear of blebbistatin precipitate while the rest of the coronary vasculature was quickly filled with blebbistatin crystals. Top left: A photo of the heart under room light. Top right: A photo of the heart under UV light (360 nm). Bottom row: images of the heart using excitation and emission wavelengths that are used for imaging NADH fluorescence. Images are shown for three rotations of the heart to illustrate that the precipitate was observed over the entire epicardial surface. b Heart perfused with poorly dissolved blebbistatin before and after global ischemia. Top: the anterior left ventricle is shown. After 15 min of global ischemia, epicardial NADH fluorescence increases to a level that obscures the fluorescence of blebbistatin precipitate. Bottom: Fluorescence signals along the diagonal lines in the images above (black before ischemia, red after ischemia)
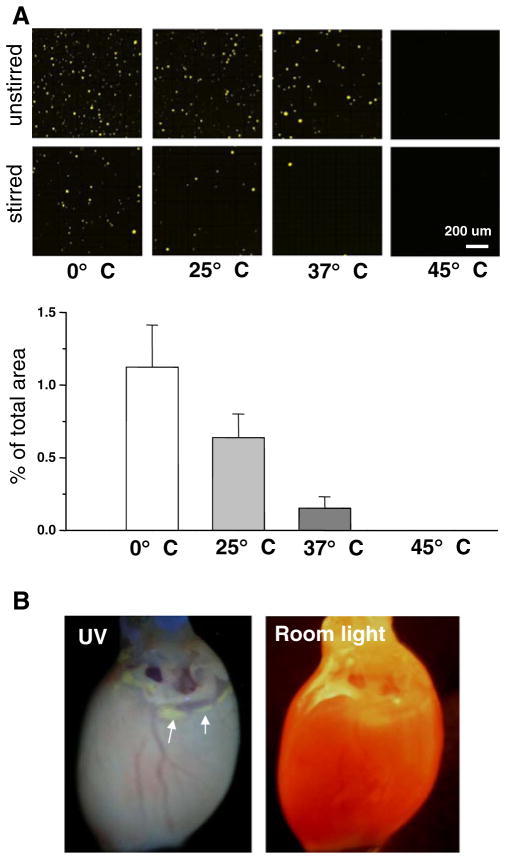
Fig 4.
Blebbistatin in solution. a Top: images showing blebbistatin precipitate that forms upon diluting a stock solution of blebbistatin in DMSO with aqueous media. Bottom: the amount of blebbistatin precipitate drops (measured as precipitate fluorescence) in stirred samples as temperature increases. The graph below compares the percentage of total area occupied by fluorescent speckles between the samples. b A heart after several hours of perfusion with a properly dissolved solution of blebbistatin (10 μM). Left: photo of the heart under UV light. Arrows point to the slow accumulation of blebbistatin in the adipose tissue, which becomes evident when the heart is illuminated with UV light. Right: photo of the heart under room light
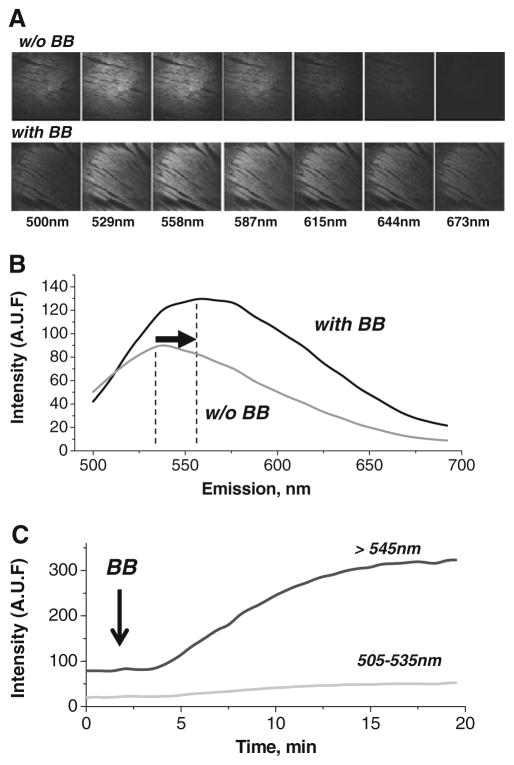
Fig 6.
Blebbistatin results in a shift of the epicardial fluorescence spectrum. Images of the epicardial surface acquired at specific emission wavelengths while illuminating with 488 nm light. Emission wavelengths (±5 nm) are indicated below each column. The top row shows images for blebbistatin-free perfusate. The bottom row shows images after perfusion with 10 μM blebbistatin. a The fluorescence profile of the epicardial surface before and after perfusion with 10 μM blebbistatin (derived from images similar to those shown in panel a). The profiles show the shift in epicardial peak fluorescence, as well as the overall increase in emission intensity. b The time course of elevations in epicardial fluorescence caused by perfusion with blebbistatin. Fluorescence signals for two emission wavelength ranges are shown
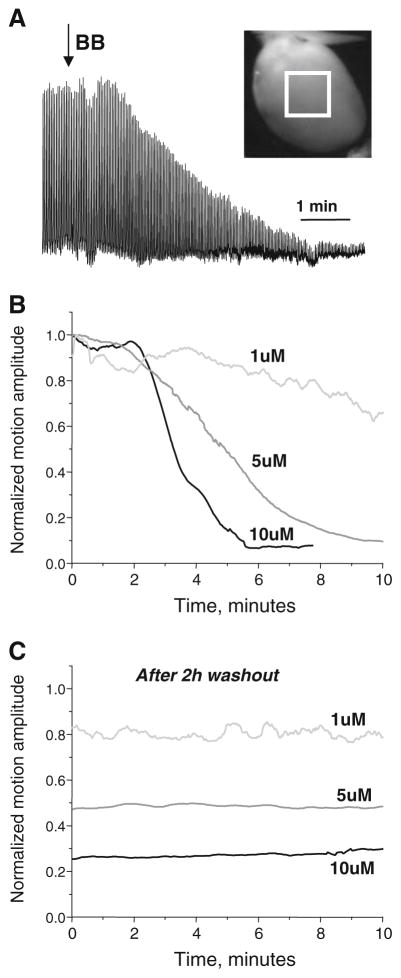
Fig 7.
Blebbistatin-induced reduction of contraction is not reversed by washout. a Contractile motion amplitude is plotted for a heart after administering blebbistatin. Hearts contracting at sinus rhythm were illuminated with dim room light and imaged at 2 fps. A 4×4-mm region of interest was placed at the center of the epicardium (white square) within the field of view. The motion associated with each heart contraction resulted in a shift of the epicardial surface, changing the amount of reflected light within the specified region of interest. b The three representative signals that illustrate a concentration and time-dependent drop in cardiac motion amplitude upon perfusion with 1, 5, and 10 μM of blebbistatin. Contractile motion amplitudes were computed as the standard deviation of the average frame-by-frame signal for the region of interest and then normalized to the initial pre-blebbistatin values. c The blebbistatin-induced reduction of contractile motion was not reversible upon washout. After 2 h of blebbistatin-free perfusion, contractile motion was only partially restored. The washout traces shown in Fig. 7c are continuation of recordings shown in Fig. 7b
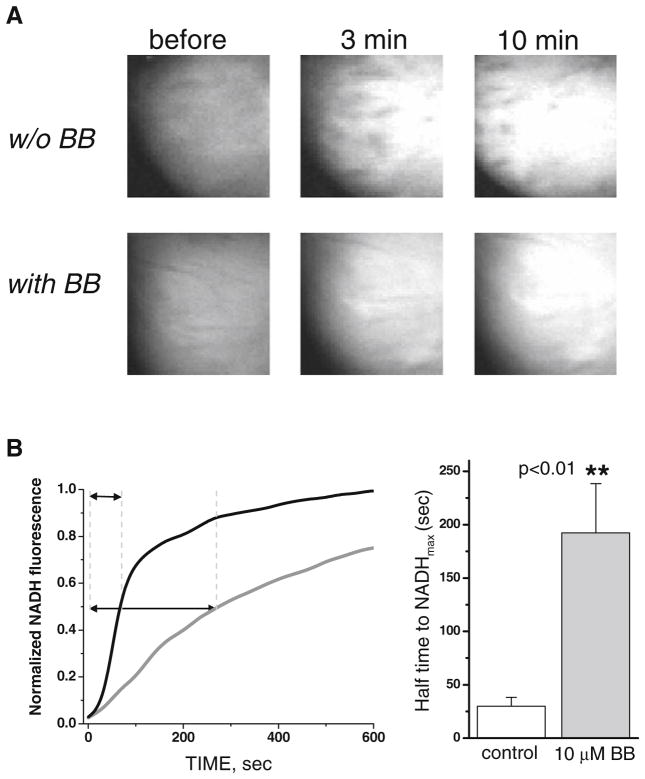
Fig 9.
Oxygen consumption for hearts perfused with blebbistatin is drastically reduced. a Images of NADH fluorescence before (left) and after (middle and right) global ischemia. NADH fluorescence was lower for hearts perfused with blebbistatin. b The time course of NADH fluorescence after global ischemia for contracting hearts (black line) and hearts perfused with blebbistatin (gray line). Increases in NADH fluorescence were drastically reduced in hearts perfused with blebbistatin. Bottom, right: The bar graph shows the time to reach half of the maximum NADH fluorescence during global ischemia in hearts perfused with and without blebbistatin. The data were derived using five hearts for each group, p<0.01
Results
Blebbistatin precipitate in perfused hearts
In some studies where we used blebbistatin as an electromechanical uncoupler, we observed the formation of blebbistatin precipitate within epicardial tissue (Fig. 1a). These hearts were otherwise normal in appearance when observed under room light conditions. The blebbistatin precipitate was noticeable only when the heart was illuminated with UV light. The method of dissolving the blebbistatin was an important factor in the formation of precipitate, as described below. If our experiments had been performed using standard potentiometric probes, such as RH237, Di-4 ANNEPS, or Di-8ANNEPS, this effect would have gone unnoticed. This is illustrated in Fig. 1b, which shows a heart perfused with blebbistatin precipitate-containing solution as it appears on two different channels. The left panel is an image of the epicardium at 360/450–500 nm excitation/emission settings and the bright blebbistatin speckles are clearly seen. The right panel is the same field of view at the 530/>680 nm excitation/emission settings (these are typically used to image hearts stained with potentiometric dye RH237 or other red dyes). Blebbistatin precipitate is not discernible using these settings.
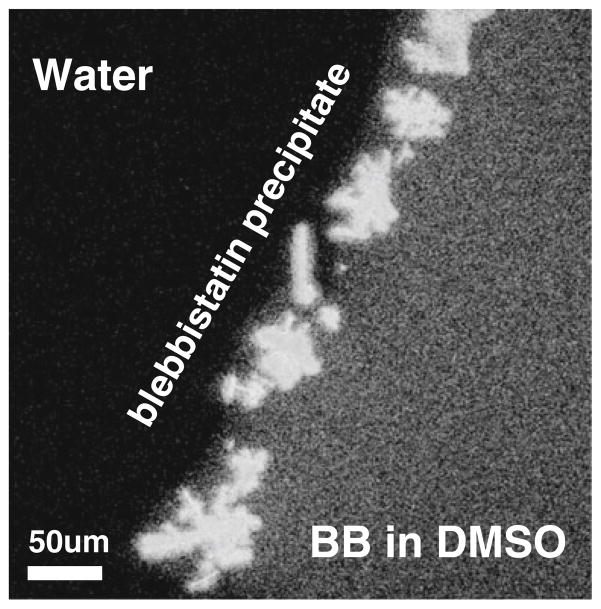
Blebbistatin precipitated when a concentrated DMSO stock solution of blebbistatin came in contact with aqueous media (Fig. 2). Once precipitated, blebbistatin would not readily dissolve into solution, even upon sonication (the sonication protocol included 6 cycles of 10-s bursts at 6 W using a Sonic Dismembrator, Model 100) or warming it up (more below). Addition of Pluronic® F-127 at the final working concentration of 0.1 % did not prevent the formation of the blebbistatin precipitate (data not shown).
Fig 2.
Blebbistatin precipitate. Blebbistatin precipitate is formed when a concentrated solution of blebbistatin in DMSO comes in contact with water. A drop of 10 mM blebbistatin in DMSO was placed on a glass coverslip next to a water drop. A boundary with multiple crystals formed upon contact of the two solutions
Figure 3a shows an extreme example of blebbistatin precipitate within the epicardial tissue. In this particular experiment, the branch of a coronary artery was ligated, and the heart was Langendorff perfused. Blebbistatin was then added to the recirculating perfusate. Over time the blebbistatin precipitate aggregated in the vasculature and was visible throughout the epicardial tissue except the occluded region (arrow).
Blebbistatin precipitate can be masked by the presence of other dyes or an increase in endogenous fluorophores such as mitochondrial NADH. The latter accumulates when oxygen availability is limited and is an indirect indicator of tissue hypoxia [6]. Since NADH and blebbistatin precipitate fluoresce within an overlapping wavelength band (more below), either global or local ischemia may obscure the appearance of blebbistatin precipitate. This effect is shown in Fig. 3b.
Blebbistatin solubility
We systematically evaluated the impact of temperature and stirring on the formation of blebbistatin precipitation. A 10-mM stock solution of blebbistatin in DMSO was diluted 1,000-fold with Tyrode solution on ice, at room temperature, at physiological temperature, and in a warm solution (0, 25, 37, and 45 °C, respectively). The amount of blebbistatin precipitate was then compared between the samples (Fig. 4a, n=3 for each condition). At 0 °C, blebbistatin precipitated even if the stock was added into a vigorously agitated solution. Warming the solution minimized blebbistatin precipitation in a temperature-dependent manner. Notably, once formed at room temperatures, blebbistatin precipitate did not go readily into solution upon heating it to 45 °C.
Our final protocol for optical mapping studies consisted of mixing a DMSO-based stock solution of blebbistatin into vigorously agitated media pre-warmed to 42–45 °C. Once dissolved, blebbistatin did not precipitate when cooled to room temperature. Perfusion with properly dissolved blebbistatin did not result in accumulation of precipitate within epicardial tissue. Due to slow blebbistatin accumulation in adipose tissue, slight elevation of fluorescence was observed after >1 h of perfusion in fatty tissue regions (arrows, Fig. 4b; compare to Fig. 1a).
Spectral properties of blebbistatin
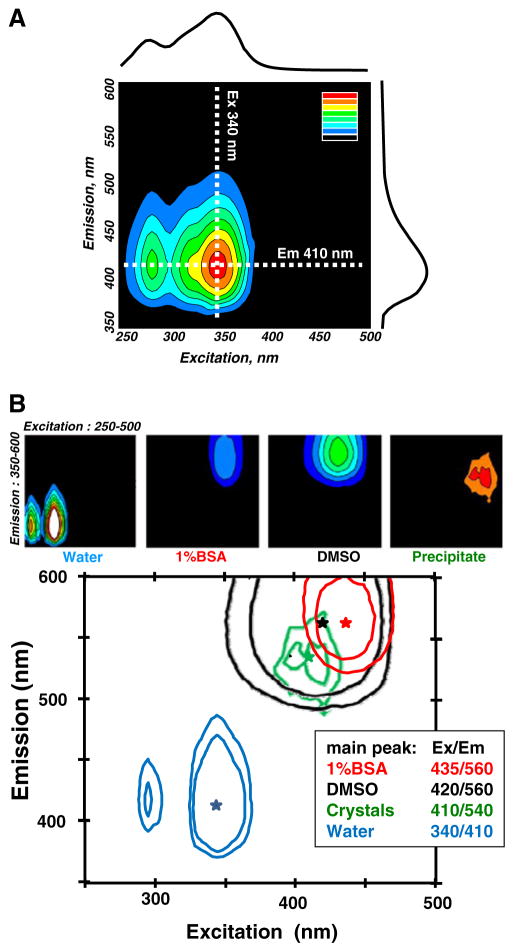
Aqueous solutions of fully dissolved non-illuminated blebbistatin (1–10 μM) exhibit negligible fluorescence in the spectral range typically used for biological fluorescence imaging (i.e., excitation above 400 nm and emission above 500 nm). This is illustrated in Fig. 5a, which shows the 3D emission/excitation spectrum of 10 μM blebbistatin in water. The excitation spectrum for 410 nm emission (i.e., values along the dotted horizontal line on the 3D map in Fig. 5a) is plotted above the 3D spectrum. The emission spectrum for 340 nm excitation (i.e., values along the dotted vertical line in Fig. 5a) is plotted to the right of the 3D spectrum.
Fig 5.
3D excitation/emission spectra of blebbistatin solutions. a 3D excitation/emission matrices of an aqueous blebbistatin solution. The emission profile for 340 nm excitation is shown on the right. It corresponds to the intensity values along the vertical dotted line. The excitation profile corresponding to 410 nm emission is shown at the top. It corresponds to the intensity values along the horizontal dotted line. b Top: 3D excitation/emission matrices of blebbistatin dissolved in water, aqueous 1 % bovine serum albumin solution, and DMSO. Background 3D excitation/emission values for each solvent without blebbistatin were subtracted from each spectrum. The map on the right was acquired from dry blebbistatin precipitate on a glass coverslip. Bottom: A combination of contours from 3D excitation/emission matrices with fluorescence peaks overlaid for different solutions
As seen in Fig. 5a, aqueous blebbistatin solutions exhibit negligible fluorescence levels for excitation and emission ranges of above 400 and 500 nm, respectively. Yet, when the heart is perfused with such a solution, it becomes fluorescent in these ranges (Figs. 1 and 3). To address this issue, we acquired 3D excitation/emission matrices for blebbistatin dissolved in different solutions, including water, DMSO, and an aqueous solution of 1 % bovine serum albumin (BSA). Due to the known photosensitivity of blebbistatin, caution was taken not to preexpose the samples to light. 3D excitation/emission matrices of each solvent (without blebbistatin) served as a corresponding blank. Blebbistatin dissolved in water had a two-peak excitation profile that consisted of a major peak at ~340 nm and a minor peak at ~280 nm (peak emission at ~410 nm). The same concentration of blebbistatin in DMSO had a different fluorescence profile with a peak at ~420/560 nm. Inclusion of protein (1 % BSA) shifted the spectra in a similar fashion as DMSO. The spectrum for the solution of 1 % BSA and blebbistatin was similar to that of DMSO and blebbistatin. A slightly different peak was observed for blebbistatin precipitate dried on a glass coverslip (410/540 nm excitation/emission peak). Presence of 10 mM phosphate buffer did not alter the spectral profile of blebbistatin as compared to water. Changes in pH of the phosphate buffer within a physiological range (6.0–8.0 pH) did not significantly shift emission profiles of aqueous blebbistatin solutions (data not shown).
The ~560-nm emission peak was confirmed in situ using the lambda mode of a LSM710 confocal imaging system to measure the fluorescence spectra from the epicardial surface of blebbistatin-perfused hearts (Fig. 6a, b). The relative fluorescence intensity of the epicardium for seven emission wavelengths, before and after heart perfusion with 10 μM blebbistatin, is shown. Excitation with a laser line at 488 nm revealed a red shift in epicardial fluorescence. This shift matched the 560-nm peak observed in the in vitro spectrofluorometer measurements of blebbistatin in 1 % BSA solution (Fig. 5b).
Lastly, the time course of epicardial fluorescence after adding blebbistatin to the perfusate is shown in Fig. 6c. Fluorescence steadily increases when observed on both 500–530- and >545-nm channels, with a more pronounced increase at >545 nm. These findings are consistent with the 560-nm emission peak of blebbistatin when it comes in contact with tissue proteins.
Reversibility and effects of epicardial UV illumination of blebbistatin-loaded hearts
Panels a and b of Figure 7 illustrate time- and concentration-dependent decrease in the amplitude of the cardiac contractions upon addition of blebbistatin to the perfusate. Washout of blebbistatin (for up to 2 h) did not restore cardiac contractions to the original levels, even when only 1 μM blebbistatin was used (Fig. 7c). Importantly, without blebbistatin, the heart motion amplitude remained stable over a 2.5-h period, declining no more than 5–10 % (data not shown).
Since blebbistatin is known to be inactivated by UV light, UV illumination has been used to restore contraction in cardiomyocytes exposed to blebbistatin [19, 30]. However, UV illumination, at least at intensities sufficient to record epicardial NADH levels, did not restore motion in our whole heart studies [18, 28]. This is most likely because UV light does not penetrate below the top 200–300 μm of the epicardial surface. Therefore, most of the media within the heart muscle remains unexposed to UV light, and the illuminated volume is miniscule when compared to the total volume of the recirculating solution.
Contractile motion is reduced in hearts exposed to blebbistatin due to its direct effect as a myosin II inhibitor. However, one can suggest that a continuous UV light illumination of hearts loaded with blebbistatin damages the tissue and therefore reduces motion due to cell death. Indeed, UV illumination of cells or cardiac trabeculae loaded with blebbistatin has been shown to promote cell death via phototoxic by-products and/or free radicals formed during illumination [11, 19, 30]. To distinguish between these two possibilities, we illuminated the epicardial surface using a UV wavelength and intensity we typically use to image epicardial NADH [18, 28]. A mask was placed over the heart to shield half of the epicardial surface from UV illumination. After 100 min of continuous UV illumination, optical action potential amplitudes from the shielded and exposed areas were then compared (Fig. 8). UV illumination of hearts loaded with blebbistatin did not significantly alter the amplitude of optical action potentials. This suggests that the illuminated layer of epicardial cardiomyocytes that contribute to the optical action potential remains viable throughout the experiments. Therefore, although one cannot exclude long-term detrimental effects of UV illumination, the data suggest that the immediate viability of these preparations was not affected. The absence of sizeable cell necrosis at the UV-illuminated side was further confirmed by TTC staining. The latter showed no discernible difference between the two sides.
Fig 8.
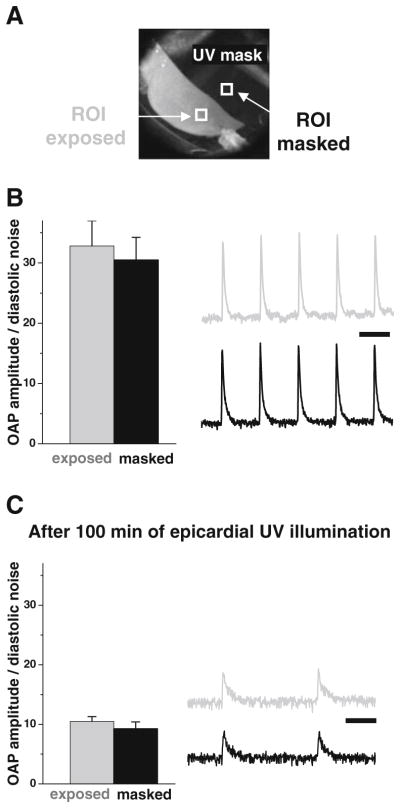
Continuous illumination with UV light does not acutely damage the epicardial tissue of hearts perfused with blebbistatin. a Hearts were perfused with blebbistatin then stained with the potentiometric dye RH237. Optical action potentials (OAP) were acquired from the epicardial surface. Half of the heart was covered by a mask, and the epicardial surface was continuously illuminated with UV light. b Before UV illumination, the ratio of the amplitude of optical action potentials to diastolic noise was identical across the epicardial surface. Representative signals from the two ROIs in Fig. 8a are shown. Scale bar—200 ms. c After 100 min of continuous UV illumination, the mask was removed and RH237 signals were acquired. The OAP amplitudes decreased due to gradual washout of RH237, and the intrinsic heart rate slowed (scale bar—200 ms). However, the OAP amplitude-to-diastolic noise ratio remained identical between UV-illuminated and non-illuminated regions (six regions of interest were used to compare the two sides). Note: the lack of visible motion artifacts in RH237 signals points to the fact that contractions did not return after prolonged UV illumination
Effect of blebbistatin on metabolism
Many groups, including ours, study effects of ischemia on electrical wave propagation in the heart and use blebbistatin to avoid motion artifacts in optical mapping studies. Blebbistatin significantly impacts how quickly the heart becomes ischemic. This is shown in Fig. 9 where NADH serves as an endogenous index of ischemia [4, 27]. In spontaneously beating healthy hearts, shutting down coronary flow was accompanied by a rapid increase in epicardial NADH fluorescence with a ~35-s half time. In contrast, when 10 μM blebbistatin was added to the perfusate, it took much longer for the heart to become ischemic, and the half time increased to nearly 3 min. These results suggest that blebbistatin decreases the rate of energy consumption by as much as ~5-fold.
Discussion
Optical mapping of the heart allows one to visualize propagation of electrical activity with high spatial resolution. Despite wide use of optical mapping, a number of technical challenges continue to limit the utility of this approach. The primary challenge is heart contraction and the associated movement that distorts optical recordings. Various techniques have been used to limit motion artifact, including mechanical restraint, changes in ionic composition of perfusate, and/or aggressive post-acquisition processing. Yet, the most commonly used approach is to immobilize the heart using electromechanical uncouplers. Unfortunately, many uncoupling agents, such as 2,3-butanedione monoxime and cytochalasin D, have been shown to detrimentally affect calcium cycling, ion channel kinetics, and action potential morphology [3, 5, 7, 24].
Blebbistatin is a cell-permeant molecule that functionally blocks myosin II in an actin-detached state [21]. It preferentially binds to the ATPase intermediate with ADP and phosphate at the active site without interfering with ATP-mediated actomyosin dissociation [23]. Blebbistatin has a minimal effect on ion channels, calcium dynamics, and major electrophysiological parameters [14, 24]. Several studies report blebbistatin precipitates upon mixing [25, 26, 34], yet no systematic studies of this effect and/or fluorescent properties of blebbistatin have been reported.
We have used blebbistatin in many of our studies [2, 18, 28, 31], and similar to others [16, 17, 24], we found blebbistatin to significantly improve the fidelity of potentiometric recordings. Our imaging setup includes a second camera and a UV light source, and with this, we have observed blebbistatin precipitation within some of our heart preparations. The awareness of blebbistatin precipitation is important for two main reasons. First, unnoticed blebbistatin precipitation can impair coronary perfusion. Blebbistatin crystals may interrupt normal vascular flow (crystals can be larger than 20 μm) and create regions of underperfused tissue. Notably, occasional arrhythmic events were reported when high blebbistatin concentrations (100 μM) were used in atrial preparations [17]. Secondly, in studies that use GFP or other green/yellow dyes, blebbistatin precipitate could be mistaken for labeled cells. Preventing blebbistatin precipitation is straightforward, i.e., one has to dissolve blebbistatin in a pre-warmed and vigorously stirred media (note: although one can filter the precipitate, the effective concentration of the uncoupler may be reduced).
It is important to emphasize that even if blebbistatin is completely dissolved, it may cause complications for visualization of weak green fluorophores, including grafted stem cells or cells genetically modified to express GFP-based calcium sensors [31]. Although an aqueous solution of blebbistatin is not fluorescent within the 500–600-nm emission range, when blebbistatin forms a precipitate or is placed in a lipophilic environment, its fluorescence within this emission range increases (Fig. 4). Therefore, when blebbistatin binds to albumin or other proteins, it becomes more fluorescent in the same range as green fluorescent dyes. This effect can potentially obscure, or even block, weak signals. Blebbistatin fluorescence may also be disguised as an apparent increase in the level of diastolic calcium. This happens when non-ratiometric calcium probes such as Fluo-3, Fluo-4, or Fluo 5F are used [14]. Subsequent use of ratiometric probes has shown that calcium transient amplitude is unaffected by blebbistatin [11].
We emphasize that blebbistatin significantly alters the metabolic state of cardiac tissue. Blebbistatin reduces, approximately 5-fold, the rate at which reduced NADH accumulates within ischemic tissue (Fig. 9). This is intuitively obvious, because diminished contraction is associated with a reduced demand for oxygen. Interestingly, when we paced blebbistatin-loaded motionless hearts, we observed frequency-dependent NADH accumulation (data not shown), pointing to involvement of other energy-consuming processes. The latter might include primary and secondary active transport systems engaged in ionic fluxes across the plasma membrane and intracellular compartments (i.e., Na/K pump, SERCA, and others). We conclude that, when conducting excised heart studies using blebbistatin, the following should be considered: (a) hearts perfused with blebbistatin require more time to become ischemic and (b) pacing rate can significantly impact the rate of endogenous NADH accumulation, even if these hearts are not contracting.
Several papers have reported that blue light can be used to reverse the uncoupling effects of blebbistatin in vitro. These studies included individual actin and myosin filaments, cells, and trabeculae [11, 19, 30]. The current consensus is that photoconversion destroys the active form of blebbistatin and that free radicals produced in the process of photoconversion can damage the surrounding cells [19, 30]. It was suggested that using blue light to photo-inactivate blebbistatin could be used in whole heart studies [14]. However, in our experiments, neither intermittent nor continuous epicardial UV illumination restored contractions in hearts perfused with blebbistatin (Fig. 8). Moreover, consistent with earlier observations [14], blebbistatin washout did not fully restore heart contractile motion (Fig. 7). Therefore, we conclude that blebbistatin cannot be considered a reversible inhibitor in the context of the whole heart studies.
Conclusion
A systematic study by Fedorov et al. [14] introduced blebbistatin to the optical mapping community. Indeed, blebbistatin is less toxic, is a more specific inhibitor of contraction, and is easier to use when compared to other electromechanical uncouplers. In this report, we present nontrivial fluorescent properties of blebbistatin that are particularly important when blebbistatin is used in conjunction with other fluorophores, particularly those excited by blue light. We have also described blebbistatin’s effect on the rate of NADH accumulation during ischemia. Finally, we have shown that continuous epicardial illumination does not restore contraction of rat hearts perfused with blebbistatin. Our findings will help to establish proper experimental conditions, avoid data misinterpretation, and promote the use of blebbistatin as an electromechanical uncoupling agent.
Acknowledgments
We gratefully acknowledge helpful discussions with Drs. Vadim Fedorov and Alexey Glukhov and thank Dr. Anastas Popratiloff for his guidance with epicardial spectrum measurements. This study was supported by the National Institutes of Health (HL095828, NCRR S10RR025565, F32ES019057).
Contributor Information
Luther M. Swift, Department of Pharmacology and Physiology, The George Washington University Medical Center, 2300 Eye Street, Washington, DC 20037, USA
Huda Asfour, Department of Electrical and Computer Engineering, The George Washington University, 801 22nd Street NW, Washington, DC 20052, USA.
Nikki G. Posnack, Department of Pharmacology and Physiology, The George Washington University Medical Center, 2300 Eye Street, Washington, DC 20037, USA
Ara Arutunyan, Department of Electrical and Computer Engineering, The George Washington University, 801 22nd Street NW, Washington, DC 20052, USA.
Matthew W. Kay, Department of Pharmacology and Physiology, The George Washington University Medical Center, 2300 Eye Street, Washington, DC 20037, USA. Department of Electrical and Computer Engineering, The George Washington University, 801 22nd Street NW, Washington, DC 20052, USA
Narine Sarvazyan, Email: phynas@gmail.com, Department of Pharmacology and Physiology, The George Washington University Medical Center, 2300 Eye Street, Washington, DC 20037, USA. Pharmacology and Physiology Department, The George Washington University, 2300 Eye Street, Washington, DC 20037, USA.
References
- 1.Allingham JS, Smith R, Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol. 2005;12(4):378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- 2.Asfour H, Swift LM, Sarvazyan N, Doroslovacki M, Kay MW. Signal decomposition of transmembrane voltage-sensitive dye fluorescence using a multiresolution wavelet analysis. IEEE Trans Biomed Eng. 2011;58(7):2083–2093. doi: 10.1109/TBME.2011.2143713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker LC, Wolk R, Choi BR, Watkins S, Plan P, Shah A, Salama G. Effects of mechanical uncouplers, diacetyl monoxime, and cytochalasin-D on the electrophysiology of perfused mouse hearts. Am J Physiol Heart Circ Physiol. 2004;287(4):H1771–H1779. doi: 10.1152/ajpheart.00234.2004. [DOI] [PubMed] [Google Scholar]
- 4.Barlow CH, Rorvik DA, Kelly JJ. Imaging epicardial oxygen. Ann Biomed Eng. 1998;26(1):76–85. doi: 10.1114/1.51. [DOI] [PubMed] [Google Scholar]
- 5.Biermann M, Rubart M, Moreno A, Wu J, Josiah-Durant A, Zipes DP. Differential effects of cytochalasin D and 2,3 butane-dione monoxime on isometric twitch force and transmembrane action potential in isolated ventricular muscle: implications for optical measurements of cardiac repolarization. J Cardiovasc Electrophysiol. 1998;9(12):1348–1357. doi: 10.1111/j.1540-8167.1998.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 6.Chance B, Cohen P, Jobsis F, Schoener B. Intracellular oxidation-reduction states in vivo. Science. 1962;137(3529):499–508. doi: 10.1126/science.137.3529.499. [DOI] [PubMed] [Google Scholar]
- 7.Coulombe A, Lefevre IA, Deroubaix E, Thuringer D, Coraboeuf E. Effect of 2,3-butanedione 2-monoxime on slow inward and transient outward currents in rat ventricular myocytes. J Mol Cell Cardiol. 1990;22(8):921–932. doi: 10.1016/0022-2828(90)90123-j. [DOI] [PubMed] [Google Scholar]
- 8.Dou Y, Arlock P, Arner A. Blebbistatin specifically inhibits actin-myosin interaction in mouse cardiac muscle. Am J Physiol Cell Physiol. 2007;293(3):C1148–C1153. doi: 10.1152/ajpcell.00551.2006. [DOI] [PubMed] [Google Scholar]
- 9.Duxbury MS, Ashley SW, Whang EE. Inhibition of pancreatic adenocarcinoma cellular invasiveness by blebbistatin: a novel myosin II inhibitor. Biochem Biophys Res Commun. 2004;313(4):992–997. doi: 10.1016/j.bbrc.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Eddinger TJ, Meer DP, Miner AS, Meehl J, Rovner AS, Ratz PH. Potent inhibition of arterial smooth muscle tonic contractions by the selective myosin II inhibitor, blebbistatin. J Pharmacol Exp Ther. 2007;320(2):865–870. doi: 10.1124/jpet.106.109363. [DOI] [PubMed] [Google Scholar]
- 11.Farman GP, Tachampa K, Mateja R, Cazorla O, Lacampagne A, de Tombe PP. Blebbistatin: use as inhibitor of muscle contraction. Pflugers Arch. 2008;455(6):995–1005. doi: 10.1007/s00424-007-0375-3. [DOI] [PubMed] [Google Scholar]
- 12.Fedorov VV, Glukhov AV, Ambrosi CM, Kostecki G, Chang R, Janks D, Schuessler RB, Moazami N, Nichols CG, Efimov IR. Effects of KATP channel openers diazoxide and pinacidil in coronary-perfused atria and ventricles from failing and non-failing human hearts. J Mol Cell Cardiol. 2011;51(2):215–225. doi: 10.1016/j.yjmcc.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fedorov VV, Glukhov AV, Chang R, Kostecki G, Aferol H, Hucker WJ, Wuskell JP, Loew LM, Schuessler RB, Moazami N, Efimov IR. Optical mapping of the isolated coronary-perfused human sinus node. J Am Coll Cardiol. 2010;56:1386–1394. doi: 10.1016/j.jacc.2010.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, Efimov IR. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Hear Rhythm. 2007;4(5):619–626. doi: 10.1016/j.hrthm.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 15.Fenton FH, Cherry EM, Kornreich BG. Termination of equine atrial fibrillation by quinidine: an optical mapping study. J Vet Cardiol. 2008;10(2):87–103. doi: 10.1016/j.jvc.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Jou CJ, Spitzer KW, Tristani-Firouzi M. Blebbistatin effectively uncouples the excitation-contraction process in zebrafish embryonic heart. Cell Physiol Biochem. 2010;25(4–5):419–424. doi: 10.1159/000303046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanlop N, Sakai T. Optical mapping study of blebbistatin-induced chaotic electrical activities in isolated rat atrium preparations. J Physiol Sci. 2010;60(2):109–117. doi: 10.1007/s12576-009-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kay M, Swift L, Martell B, Arutunyan A, Sarvazyan N. Locations of ectopic beats coincide with spatial gradients of NADH in a regional model of low-flow reperfusion. Am J Physiol Heart Circ Physiol. 2008;294(5):H2400–H2405. doi: 10.1152/ajpheart.01158.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolega J. Phototoxicity and photoinactivation of blebbistatin in UV and visible light. Biochem Biophys Res Commun. 2004;320 (3):1020–1025. doi: 10.1016/j.bbrc.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 20.Kong W, Ideker RE, Fast VG. Transmural optical measurements of Vm dynamics during long-duration ventricular fibrillation in canine hearts. Hear Rhythm. 2009;6(6):796–802. doi: 10.1016/j.hrthm.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004;279(34):35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 22.Li D, Nattel S. Pharmacological elimination of motion artifacts during optical imaging of cardiac tissues: is blebbistatin the answer? Hear Rhythm. 2007;4(5):627–628. doi: 10.1016/j.hrthm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Limouze J, Straight AF, Mitchison T, Sellers JR. Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell Motil. 2004;25(4–5):337–341. doi: 10.1007/s10974-004-6060-7. [DOI] [PubMed] [Google Scholar]
- 24.Lou Q, Li W, Efimov IR. The role of dynamic instability and wavelength in arrhythmia maintenance as revealed by panoramic imaging with blebbistatin vs. 2,3-butanedione monoxime. Am J Physiol Heart Circ Physiol. 2012;302(1):H262–H269. doi: 10.1152/ajpheart.00711.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loudon RP, Silver LD, Yee HF, Jr, Gallo G. RhoA-kinase and myosin II are required for the maintenance of growth cone polarity and guidance by nerve growth factor. J Neurobiol. 2006;66 (8):847–867. doi: 10.1002/neu.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matson S, Markoulaki S, Ducibella T. Antagonists of myosin light chain kinase and of myosin II inhibit specific events of egg activation in fertilized mouse eggs. Biol Reprod. 2006;74(1):169–176. doi: 10.1095/biolreprod.105.046409. [DOI] [PubMed] [Google Scholar]
- 27.Mayevsky A, Chance B. Oxidation-reduction states of NADH in vivo: from animals to clinical use. Mitochondrion. 2007;7 (5):330–339. doi: 10.1016/j.mito.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Mercader M, Swift LM, Sood S, Asfour H, Kay MW, Sarvazyan N. Use of endogenous NADH fluorescence for real-time in situ visualization of epicardial radiofrequency ablation lesions and gaps. Am J Physiol Heart Circ Physiol. 2012;302:H2131–H2138. doi: 10.1152/ajpheart.01141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nygren A, Baczko I, Giles WR. Measurements of electrophysiological effects of components of acute ischemia in Langendorff-perfused rat hearts using voltage-sensitive dye mapping. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S113–S123. doi: 10.1111/j.1540-8167.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 30.Sakamoto T, Limouze J, Combs CA, Straight AF, Sellers JR. Blebbistatin, a myosin II inhibitor, is photoinactivated by blue light. Biochemistry. 2005;44(2):584–588. doi: 10.1021/bi0483357. [DOI] [PubMed] [Google Scholar]
- 31.Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA. Human ESC-derived cardiomyocytes electrically integrate and suppress arrhythmias in a guinea pig infarct model. Nature. 2012 doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart M, Franks-Skiba K, Cooke R. Myosin regulatory light chain phosphorylation inhibits shortening velocities of skeletal muscle fibers in the presence of the myosin inhibitor blebbistatin. J Muscle Res Cell Motil. 2009;30(1–2):17–27. doi: 10.1007/s10974-008-9162-9. [DOI] [PubMed] [Google Scholar]
- 33.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299 (5613):1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe M, Yumoto M, Tanaka H, Wang HH, Katayama T, Yoshiyama S, Black J, Thatcher SE, Kohama K. Blebbistatin, a myosin II inhibitor, suppresses contraction and disrupts contractile filaments organization of skinned taenia cecum from guinea pig. Am J Physiol Cell Physiol. 2010;298(5):C1118–C1126. doi: 10.1152/ajpcell.00269.2009. [DOI] [PubMed] [Google Scholar]