Abstract
Transport of GluA1-containing AMPA glutamate receptors to synapses in the nucleus accumbens, a process that involves phosphorylation of key serine residues by CaMKII, is associated with the reinstatement of cocaine-seeking behavior. A growing body of evidence indicates that the dorsal striatum contributes to aspects of cocaine addiction. However, the potential role of CaMKII-mediated phosphorylation of GluA1 subunits in the dorsolateral (DL) striatum during cocaine reinstatement has not been examined. In this study, rats were trained to self-administer cocaine and were partnered with saline-yoked rats that received injections of saline. Following extinction, each pair of rats received either a systemic priming injection of cocaine (10 mg/kg, i.p.) or saline. As expected, cocaine-experienced rats displayed robust reinstatement of cocaine seeking in response to a challenge injection, whereas yoked saline controls did not. The DL striatum was dissected immediately following the reinstatement test session. Results from Western blotting experiments showed increased pGluA1-ser831 and pCaMKII-thr286 in the DL striatum of saline-yoked rats given an acute injection of cocaine. This effect was absent in cocaine-experienced rats that received a saline injection, and no changes were observed following a priming injection of cocaine in cocaine-experienced rats. These results indicate that acute exposure to cocaine in drug naïve rats increased CaMKII-mediated phosphorylation of GluA1-containing AMPA receptors in the DL striatum, an effect that was not observed during cocaine priming-induced reinstatement of drug seeking. It is possible; therefore, that increased phosphorylation of CaMKII and GluA1 following acute cocaine is a compensatory mechanism in the DL striatum.
Keywords: psychostimulant, addiction, relapse, striatum, CaMKII, glutamate
Introduction
It is well documented that changes in glutamatergic transmission in subregions of the striatum play a role in the reinstatement of cocaine seeking, an animal model of relapse [25]. Although the majority of this work has focused on the nucleus accumbens [1, 11, 27], recent evidence indicates that pharmacological inhibition of the dorsolateral (DL) striatum attenuates cocaine seeking in rats following short-access (1- or 2-hour daily) self-administration [23, 29]. In terms of glutamatergic transmission, infusion ofα -amino-3-hydroxy-5-methyl-4-isoxazolepropionic (AMPA) glutamate receptor antagonists into the DL striatum blocked cue-controlled reinstatement and attenuated cocaine self-administration when injected into the dorsomedial (DM) striatum [11, 22, 28, 31]. Though there is an established role for the DL striatum in cue-induced reinstatement, there is little to no data on DL striatum involvement in cocaine priming-induced reinstatement of drug seeking.
AMPA glutamate receptors are ionotropic, excitatory ion channels comprised of four subunits (GluA1-GluA4) that are involved in several forms of neuronal plasticity [6]. The GluA1 subunit in particular has been associated with synaptic plasticity in animals exposed to cocaine [2, 5]. Activity-mediated autophosphorylation of calcium/calmodulin-dependent protein kinase II (CaMKII) at threonine 286 promotes phosphorylation of GluA1 at serine 831, resulting in enhanced GluA1 subunit trafficking to the plasma membrane and associated increases in synaptic strength [6, 18]. Indeed, there is evidence that reinstatement of cocaine seeking in rats involves CaMKII phosphorylation of the GluA1 subunit in the nucleus accumbens, and pharmacological inhibition of CaMKII in the accumbens prevents cocaine seeking [1, 19].
A single cocaine injection promotes glutamate release in the DL striatum of cocaine-naïve rats [3, 21]. Acute or repeated experimenter-delivered cocaine increases levels of phosphorylated GluA1 AMPA receptor subunits and GluA1 surface expression in the DL striatum [12, 16]. Here, we extend this work by examining expression of phospho-CaMKII and –GluA1 in the DL striatum during cocaine priming-induced reinstatement of drug seeking.
Materials and Methods
Animals and Housing
Male Sprague-Dawley rats (Rattus novergicus) weighing 250–300 g were obtained from Taconic Laboratories (Germantown, NY, USA). Animals were individually housed with food and water available ad libitum. A 12h light/dark cycle was used and all experiments were performed during the light cycle. All experiments used Med-Associates (East Fairfield, VT, USA) operant chambers equipped with response levers, house light, and pumps for injecting drugs intravenously. Operant chambers were enclosed within ventilated, sound attenuating chambers. All experimental procedures were consistent with the ethical guidelines of the U.S. National Institutes of Health and were approved by the University of Pennsylvania School of Medicine Institutional Animal Care and Use Committee.
Drugs
Cocaine hydrochloride was obtained from the National Institute on Drug Abuse (Rockville, MD, USA) and dissolved in bacteriostatic 0.9% saline.
Surgery
Prior to surgery, the rats were anesthetized with 80 mg/kg ketamine (i.p.; Sigma-Aldrich, St. Louis, MO, USA) and 12 mg/kg xylazine (i.p.; Sigma-Aldrich, St. Louis, MO, USA). An indwelling silastic catheter (CamCaths, UK) was placed into the right jugular vein and sutured in place. The catheter was routed to a mesh backmount platform and implanted subcutaneously dorsal to the shoulder blades. Catheters were flushed daily with 0.3 ml of an antibiotic (Timentin, 0.93 mg/ml, Henry Schein, Melville, NY, USA) dissolved in heparinized saline. The catheters were sealed with plastic obturators when not in use.
Cocaine Self-Administration and Extinction
Following a 7-day recovery period from surgery, 50% of the rats were randomly selected to self-administer cocaine. The remaining 50% were paired to a cocaine self-administering animal as yoked-saline controls that only receive infusions of saline. Cocaine self-administering rats were placed in operant chambers and allowed to lever press for intravenous cocaine infusions (0.25 mg/59 µl saline over 5 s) over a 2-hour time period daily for 16 days. Yoked-saline controls received the same number and temporal pattern of infusions as the paired cocaine self-administering rat. Rats were initially trained using a fixed ratio (FR) 1 schedule of reinforcement, with each daily self-administration session initiated by a priming injection of cocaine (0.25 mg, i.v.) to fill the catheter (limited to 30 injections per 120 min). Once animals achieved stable responding, as defined by at least 20 infusions over the 2 h session for 2 consecutive days, they were transitioned to an FR5 schedule of reinforcement (limited to 30 injections per 120 min). For both schedules of reinforcement, a 20s time-out period followed each cocaine infusion, during which time active, drug-paired lever responses were tabulated but had no scheduled consequence. Each operant chamber was also equipped with an inactive lever. Responses made on the inactive lever, which had no scheduled consequence, were also recorded during all training sessions.
Following cocaine self-administration, drug-taking behavior in cocaine self-administering rats was extinguished by replacing cocaine with 0.9% bacteriostatic saline. Extinction continued until responding on the drug-paired lever was <15% of the response rate on the last day of cocaine self-administration using the FR5 schedule of reinforcement. Typically, it took rats 5 days to meet this criterion.
Cocaine Priming-Induced Reinstatement of Drug Seeking
Following extinction, animals entered the reinstatement phase of the experiment. During reinstatement, satisfaction of the response requirements for FR5 resulted in saline rather than cocaine infusions. The FR5 schedule was used for the reinstatement test session. Pairs of cocaine self-administering/yoked-saline rats were randomly assigned to receive saline or cocaine (10 mg/kg, i.p.) injections immediately prior to the start of the reinstatement test session. Rats were placed into the operant chambers immediately following injection of saline or cocaine. Responding was recorded for 30 minutes, after which the pairs of rats were removed from the operant chambers and immediately decapitated. Whole brains were extracted and flash-frozen in isopentane on dry ice, then stored at −80° C. Brains were sliced on a cryostat (300 µm sections) and striatal subregions (+1.7 mm to −0.08 mm A/P; −3.8 mm to −6.0mm D/V; DM: ±1.2 mm – 2.8 mm M/L and DL: ±2.8 mm – 4.4 mm M/L) dissected by tissue punch (2.0 mm Harris Uni-core stainless steel punchers, Ted Pella, Inc.). Tissue samples were stored at −80° C until processing for Western blot as described below.
Western Blot
Whole-cell tissue was processed for Western blot as described previously (Anderson et al., 2008). For all samples, protein concentration was quantified using a Pierce BCA Protein Assay Kit (Thermo Scientific). Equal amounts of protein (10 µg for whole-cell) were loaded and separated in 10% Tris-Glycine gels (Invitrogen) and transferred to nitrocellulose membranes using the i-Blot dry transfer system (Invitrogen). Membranes were blocked with either 5% nonfat dry milk in TBST or 5% BSA in TBST, according to antibody instructions. Membranes were incubated overnight at 4° C with selective antibodies to: GluA1 (1:1000, Abcam), phosphorylated (p) GluA1-S831 (1:500, Millipore), CaMKII (1:1000, Millipore), pCaMKII-T286 (1:1000, Cell Signaling), and GAPDH (1:2000, Cell Signaling). Membranes were then incubated with fluorescent secondary antibodies (1:5000, IR-dye 680 or IR-dye 800), before being imaged on an Odyssey fluorescent scanner (Licor Biosciences). To ensure equal loading, GAPDH expression was used as a loading control.
Behavioral Experiment Data Analyses
Drug-paired and inactive lever responding was analyzed using a two-way ANOVA. The between subjects factors were pretreatment (cocaine self-administration or yoked-saline) and drug challenge injection (10 mg/kg cocaine or saline). Bonferroni post-tests were used to establish significant difference (p<0.05).
Western Blot Data Analyses
Quantification was performed by normalizing the intensity of all bands with protein-specific antibodies to the GAPDH intensity, followed by normalizing that value to saline-control (yoked-saline, i.p. saline) values. The immunoblot analyses were performed using a two-way ANOVA. The between-subjects factors were pretreatment (cocaine self-administration or yoked-saline) and drug challenge injection (10 mg/kg cocaine i.p. or saline). Bonferroni post-tests were used to establish significant difference (p<0.05).
Results
A priming injection of cocaine, but not saline, elicits robust reinstatement of drug-seeking behavior in animals with cocaine self-administration experience
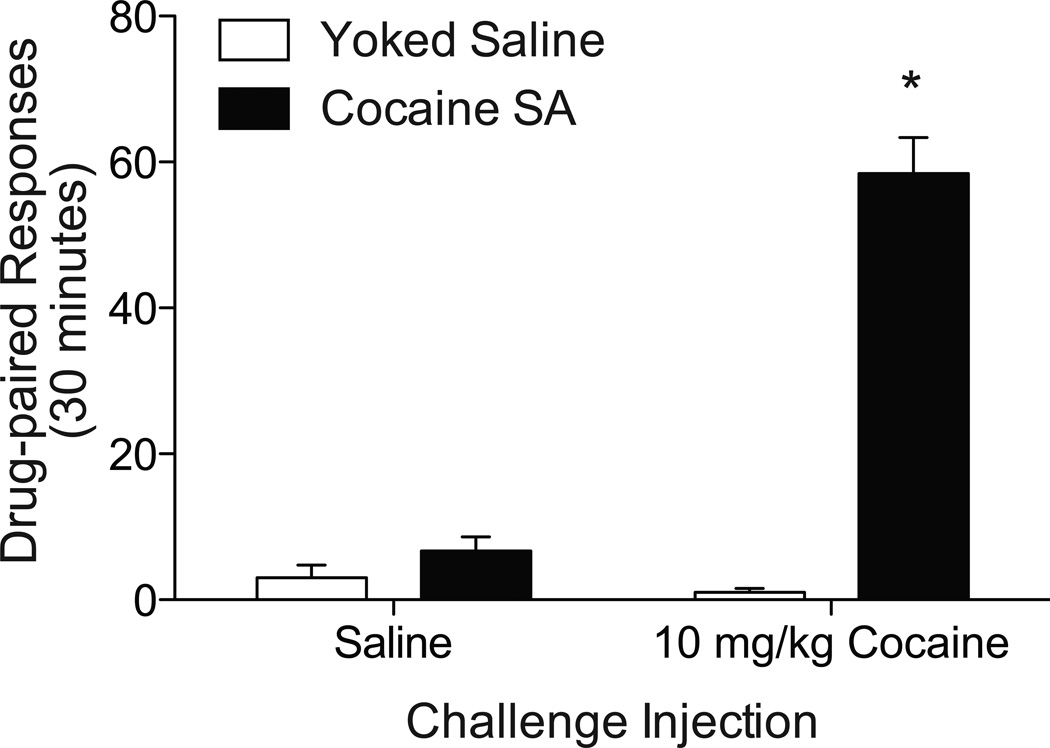
Total drug-paired lever responding (mean +/− SEM) for the reinstatement test session are plotted in Fig 1. Responding was analyzed using a two-way ANOVA. Analyses revealed a significant main effect of pretreatment (F1,24=119.39, p<0.0001), a significant main effect of challenge injection (F1,24=92.14, p<0.0001), as well as a significant pretreatment × challenge interaction (F1,24=78.93, p<0.0001). Post-hoc tests revealed a significant difference of drug-paired lever responding due to cocaine challenge injection (Bonferroni p<0.001). There was no significant effect of pretreatment (F1,24=0.03, p=0.8543) or challenge (F1,24=3.68, p=0.0671) on inactive lever responding (data not shown).
Figure 1.
An acute, priming injection of cocaine elicits robust reinstatement of drug-seeking behavior in rats with cocaine self-administration experience. Total drug-paired lever responding for the 30-minute reinstatement test session is plotted for animals that received saline or cocaine challenge injection. There was a significant increase of drug-paired lever responding for cocaine-experienced rats that received cocaine compared to animals that received a saline injection. N = 7/group, “SA” = self-administration (The asterisk represents a significant difference from yoked-saline controls. 2-way ANOVA: Bonferroni p<0.001)
Exposure to acute cocaine in drug naïve animals increases expression of pGluA1-ser831 and pCaMKII-thr286 in the DL Striatum
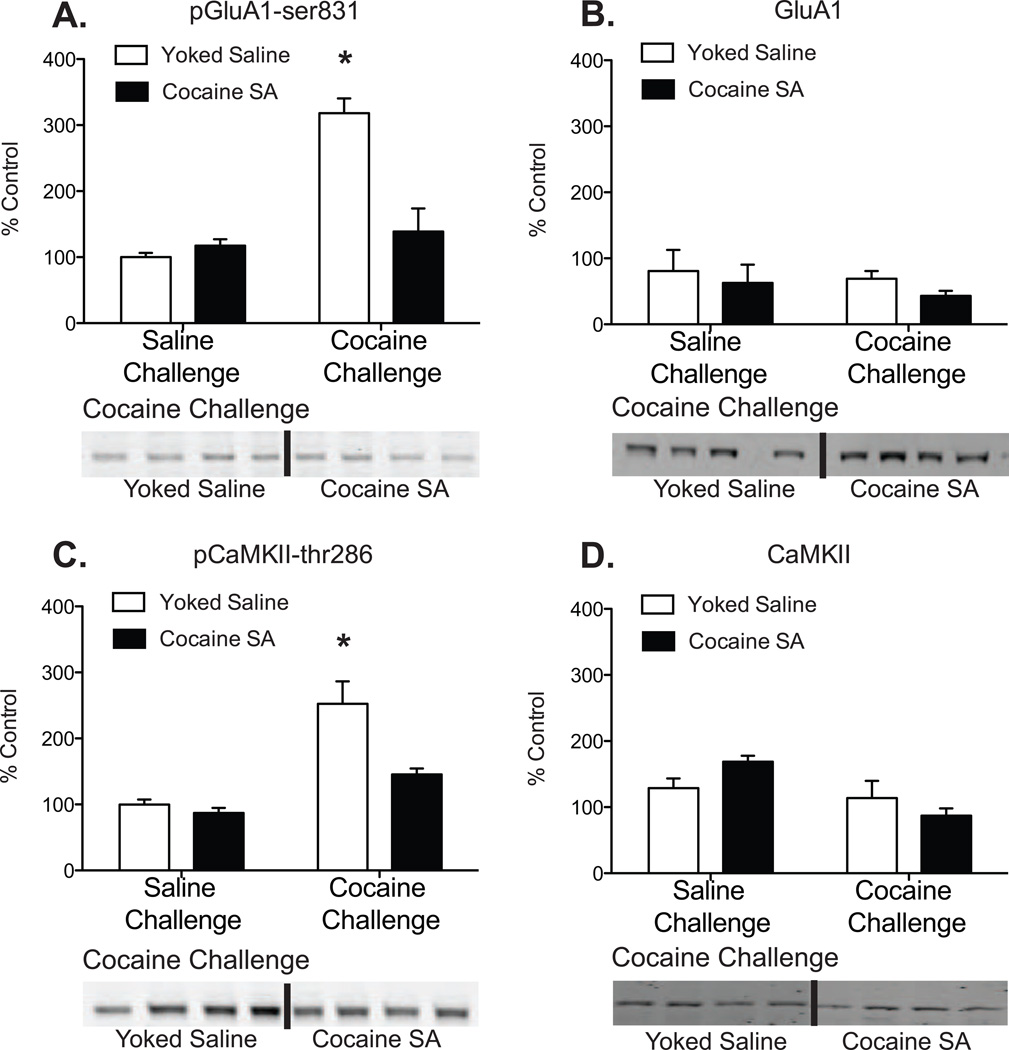
The average fluorescent intensity for pGluA1-ser831 in the DL striatum was expressed as percent change from control and is plotted in Fig 2A. Percentages were analyzed by two-way ANOVA, which revealed a significant main effect of pretreatment (cocaine self-administration vs. yoked-saline, F1,12=14.03, p<0.005), a significant main effect of challenge (10 mg/kg cocaine or saline, F1,12=30.68, p<0.0001), as well as a significant pretreatment × challenge interaction (F1,12=20.75, p<0.0007). Post hoc tests revealed a significant difference in pGluA1-ser831 expression between yoked-saline and cocaine self-administering animals after cocaine challenge injection (Bonferroni p<0.001). There was no significant effect of pretreatment or challenge on the native GluA1 protein (Fig 2B).
Figure 2.
Acute cocaine exposure increases expression of pGluA1-ser831 and pCaMKII-thr286 in the DL striatum and these effects are reversed after cocaine self-administration. Following an acute cocaine injection, yoked-saline controls show a significant increase in pGluA1-ser831 (A) and pCaMKII-thr286 (B) protein expression in the DL striatum. No significant changes in pGluA1-ser831 (A) and pCaMKII-thr286 (B) protein expression were observed in cocaine-experienced animals when compared to yoked-saline controls that received saline injections. There was no significant effect of training or challenge injection on native GluA1 (C) or native CaMKII (D) protein expression for any treatment. N = 4–7/group, “SA” = self-adminstration (Asterisks represent significant differences from yoked-saline controls that received a saline challenge injection. 2-way ANOVA: pGluA1 Bonferroni p<0.001; pCaMKII Bonferroni p<0.01)
The average fluorescent intensity for pCaMKII-thr286 in the DL striatum was expressed as percent change from control and plotted in Fig 2C. Percentages were analyzed by two-way ANOVA, which revealed a significant main effect of pretreatment (F1,11=15.73, p<0.0022), a significant main effect of challenge (F1,11=48.62, p<0.0001), as well as a significant pretreatment × challenge interaction (F1,11=9.78, p<0.0096). Post hoc tests revealed a significant difference in pCaMKII-thr286 expression between yoked-saline and cocaine self-administering animals after cocaine challenge injection (Bonferroni p<0.01). There was no significant effect of pretreatment or challenge on native CaMKII protein (Fig 2D).
Acute cocaine decreases expression of pGluA1-ser831, but not pCaMKII-thr286, in the DM striatum regardless of cocaine exposure
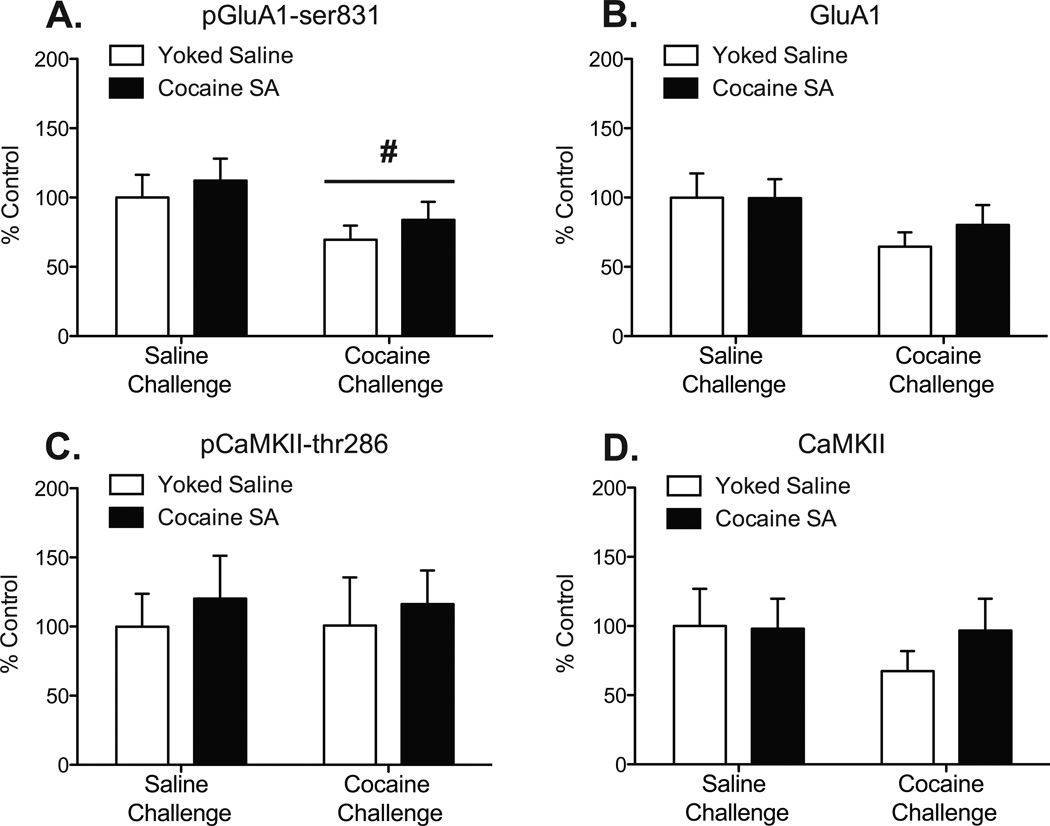
The average fluorescent intensity for pGluA1-ser831 in the DM striatum was expressed as percent change from control and plotted in Fig 3A. Percentages were analyzed with a two-way ANOVA, which revealed no significant effect of pretreatment (p=0.3542) and no pretreatment × challenge interaction (p=0.9403), but a significant main effect of challenge (F1,24=4.33, p<0.05). There was no significant effect of pretreatment or challenge on the average fluorescent intensity of native GluA1 protein in the DM striatum (Fig 3B).
Figure 3.
An acute, priming cocaine injection decreases pGluA1-ser831 in the DM striatum regardless of cocaine experience. (A) pGluA1-ser831 protein expression was decreased in both cocaine-experienced rats and yoked-saline controls in the DM striatum after a cocaine challenge injection. There is no change in native GluA1 (B), pCaMKII-286 (C), or native CaMKII (D) protein expression in the DM striatum, regardless of cocaine experience. N = 4–7/group, “SA” = self-administration (The # represents a significant effect of challenge injection, p<0.05. There was no significant effect of pretreatment and no significant interaction.)
The average fluorescent intensity for pCaMKII-thr286 in the DM striatum was expressed as percent change from control and is plotted in Fig 3C. Percentages were analyzed with a two-way ANOVA, which revealed no significant effect of pretreatment (p=0.1615) or challenge (p=0.2906). There was no significant effect of pretreatment or challenge on the average fluorescent intensity of native CaMKII protein in the DM striatum (Fig 3D).
Discussion
The present findings indicate that an acute injection of cocaine increased expression of pCaMKII-thr286 and pGluA1-ser831 in the DL, but not the DM, striatum of drug-naïve rats. Since the phosphorylation of CaMKII at threonine 286 is associated with increased phosphorylation of GluA1 at serine 831 and subsequent trafficking of these subunits [4], these results suggest that an acute cocaine injection may increase the surface expression of AMPA receptors in the DL striatum. However, following cocaine self-administration and extinction, a cocaine challenge injection had no effect on DL striatal pCaMKII-thr286 or pGluA1-ser831 expression. Taken together, these results indicated that tolerance to cocaine-induced increases in the phosphorylation of DL striatal CaMKII and GluA1 develops following cocaine self-administration.
Our findings are consistent with previous work that showed increased pCaMKII-thr286 and pGluA1-ser831 in the DL striatum following acute cocaine [10, 15]. A single exposure to cocaine also increases immediate early gene expression in the dorsal striatum [14, 17, 24]. Additionally, a single microinjection of cocaine into the dorsal striatum enhances locomotor activity [9]. Taken together, these results indicate that cocaine-induced hyperlocomotion is associated with increased neuronal activation in the DL striatum and increased phosphorylation of CaMKII and GluA1. Future studies are required to determine the functional significance of increased phosphorylation of CaMKII and GluA1 following acute cocaine exposure.
There also is evidence of altered glutamatergic transmission in the DL striatum following prolonged cocaine administration [26]. Animals identified as vulnerable to cocaine relapse following cocaine self-administration showed down-regulation of genes for GluA1 and activity-regulated cytoskeletal protein, both of which are involved in synaptic plasticity and AMPA receptor trafficking [7]. While we did not investigate alternate phosphorylation sites of GluA1 subunits in this study, there is also evidence of decreased expression of phospho-GluA1 ser845, a known target of protein kinase A, in the dorsal striatum in animals with chronic cocaine self-administration experience [10, 20]. Functionally, pharmacological inactivation of the DL striatum attenuates cocaine seeking in rats with a history of short- or long-access to cocaine self-administration [23]. Moreover, an intra-striatal infusion of an AMPA/kainate receptor antagonist attenuates cue-controlled cocaine seeking [23, 30]. Furthermore, overexpression of a dominant negative peptide that prevents trafficking of GluA1 to the membrane surface in the DL striatum attenuated cocaine-induced behavioral sensitization in juvenile mice [15]. It is important to note, however, that unlike adult mice, the juveniles exhibited increased GluA1 surface expression following repeated cocaine, suggesting that age at time of cocaine exposure influences DL striatal glutamatergic plasticity. Transgenic mice that express constitutively active CaMKII, where threonine 286 is replaced with aspartic acid, in the dorsal striatum exhibit impairments in goal-directed behaviors, with impairments in both cue-and reward-primed reinstatement [32]. As a whole, these results indicate that the changes in AMPA receptor transmission in adult rodents that contribute to cocaine sensitization and reinstatement are not likely due to increases in CaMKII or GluA1 phosphorylation in the DL striatum. Our data showed changes the phosphorylation state of CaMKII and GluA1 in DL striatum of drug naïve rats following acute cocaine only, offering novel biochemical support for such conclusions.
The present findings suggest differential involvement of the glutamatergic system in the DL striatum relative to the ventral striatum, particularly after cocaine self-administration and the reinstatement of cocaine seeking. It is well known that CaMKII plays a key role in phosphorylating GluA1 subunits at serine 831 and promotes AMPA receptor trafficking to the membrane surface [4]. In fact, plasticity of the glutamatergic system in the ventral striatum, including increased phosphorylation of CaMKII and GluA1 as well as increased GluA1 surface expression occurs in a variety of paradigms involving chronic cocaine exposure [5, 8, 13]. More specifically, during cocaine priming-induced reinstatement, there is increased phosphorylation of CaMKII and GluA1 AMPA receptor subunits, as well as increased trafficking of GluA1 to the plasma membrane in the accumbens shell [1]. Inhibiting CaMKII or preventing the trafficking of GluA1 subunits in the shell is sufficient to block reinstatement of cocaine seeking [1]. These findings coupled with the data presented here suggest that phosphorylation of CaMKII and GluA1 in the ventral, but not DL, striatum is required for reinstatement of cocaine seeking. Thus, changes in phospho-CaMKII and –GluA1 in the DL striatum likely reflect a compensatory mechanism in response to acute cocaine.
Conclusions
Our results indicate that acute cocaine increases expression of pCaMKII-thr286 and pGluA1-ser831 in the DL, but not DM, striatum and that these effects are not associated with the reinstatement of cocaine seeking. Therefore, changes in phospho-CaMKII and –GluA1 most likely reflect a compensatory adaptation in the DL striatum during early stages of drug use. It is possible that acute cocaine promotes transient biochemical modifications in the DL striatum that reverse with additional input from areas like the ventral striatum and/or prefrontal cortex as cocaine experience is extended [10].
Highlights.
We modeled cocaine addiction and relapse via rodent drug self-administration and reinstatement.
We measured changes in protein expression in subregions of the dorsal striatum with western blot.
Acute cocaine increased phosphorylation of CaMKII and GluA1 in the dorsolateral striatum.
Dorsolateral striatum phospho-CaMKII and –GluA1 is unchanged in cocaine-experienced rats.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Samantha L. White, Email: samwhite@mail.med.upenn.edu.
Heath D. Schmidt, Email: hschmidt@mail.med.upenn.edu.
Fair M. Vassoler, Email: fair.vassoler@tufts.edu.
R. Christopher Pierce, Email: rcpierce@mail.med.upenn.edu.
References
- 1.Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- 2.Bachtell RK, Choi KH, Simmons DL, Falcon E, Monteggia LM, Neve RL, Self DW. Role of GluR1 expression in nucleus accumbens neurons in cocaine sensitization and cocaine-seeking behavior. Eur J Neurosci. 2008;27:2229–2240. doi: 10.1111/j.1460-9568.2008.06199.x. [DOI] [PubMed] [Google Scholar]
- 3.Barrot M, Abrous DN, Marinelli M, Rouge-Pont F, Le Moal M, Piazza PV. Influence of glucocorticoids on dopaminergic transmission in the rat dorsolateral striatum. Eur J Neurosci. 2001;13:812–818. doi: 10.1046/j.1460-9568.2001.01434.x. [DOI] [PubMed] [Google Scholar]
- 4.Boehm J, Malinow R. AMPA receptor phosphorylation during synaptic plasticity. Biochem Soc Trans. 2005;33:1354–1356. doi: 10.1042/BST0331354. [DOI] [PubMed] [Google Scholar]
- 5.Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 7.Brown AL, Flynn JR, Smith DW, Dayas CV. Down-regulated striatal gene expression for synaptic plasticity-associated proteins in addiction and relapse vulnerable animals. Int J Neuropsychopharmacol. 2011;14:1099–1110. doi: 10.1017/S1461145710001367. [DOI] [PubMed] [Google Scholar]
- 8.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. J Neurosci. 1990;10:303–310. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards S, Graham DL, Bachtell RK, Self DW. Region-specific tolerance to cocaine-regulated cAMP-dependent protein phosphorylation following chronic self-administration. Eur J Neurosci. 2007;25:2201–2213. doi: 10.1111/j.1460-9568.2007.05473.x. [DOI] [PubMed] [Google Scholar]
- 11.Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha JH, Pierce RC. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28:11061–11070. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME. The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacology. 2010;35:818–833. doi: 10.1038/npp.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galinanes GL, Heng LJ, Tseng KY, Wolf ME. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca(2)(+)-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011;61:1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim M, Au E, Neve R, Yoon BJ. AMPA receptor trafficking in the dorsal striatum is critical for behavioral sensitization to cocaine in juvenile mice. Biochem Biophys Res Commun. 2009;379:65–69. doi: 10.1016/j.bbrc.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Kim SM, Ahn SM, Go BS, Wang JQ, Choe ES. Alterations in AMPA receptor phosphorylation in the rat striatum following acute and repeated cocaine administration. Neuroscience. 2009;163:618–626. doi: 10.1016/j.neuroscience.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 17.Larson EB, Akkentli F, Edwards S, Graham DL, Simmons DL, Alibhai IN, Nestler EJ, Self DW. Striatal regulation of DeltaFosB, FosB, and cFos during cocaine self-administration and withdrawal. J Neurochem. 2010;115:112–122. doi: 10.1111/j.1471-4159.2010.06907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 19.Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- 20.Mattson BJ, Bossert JM, Simmons DE, Nozaki N, Nagarkar D, Kreuter JD, Hope BT. Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. J Neurochem. 2005;95:1481–1494. doi: 10.1111/j.1471-4159.2005.03500.x. [DOI] [PubMed] [Google Scholar]
- 21.McKee BL, Meshul CK. Time-dependent changes in extracellular glutamate in the rat dorsolateral striatum following a single cocaine injection. Neuroscience. 2005;133:605–613. doi: 10.1016/j.neuroscience.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Murray JE, Belin D, Everitt BJ. Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology. 2012;37:2456–2466. doi: 10.1038/npp.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pacchioni AM, Gabriele A, See RE. Dorsal striatum mediation of cocaine-seeking after withdrawal from short or long daily access cocaine self-administration in rats. Behav Brain Res. 2011;218:296–300. doi: 10.1016/j.bbr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philibin SD, Hernandez A, Self DW, Bibb JA. Striatal signal transduction and drug addiction. Front Neuroanat. 2011;5:60. doi: 10.3389/fnana.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierce RC, Vanderschuren LJ. Kicking the habit: the neural basis of ingrained behaviors in cocaine addiction. Neurosci Biobehav Rev. 2010;35:212–219. doi: 10.1016/j.neubiorev.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission. Ann N Y Acad Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneck N, Vezina P. Enhanced dorsolateral striatal activity in drug use: The role of outcome in stimulus-response associations. Behav Brain Res. 2012;235:136–142. doi: 10.1016/j.bbr.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- 30.Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 32.Wiltgen BJ, Law M, Ostlund S, Mayford M, Balleine BW. The influence of Pavlovian cues on instrumental performance is mediated by CaMKII activity in the striatum. Eur J Neurosci. 2007;25:2491–2497. doi: 10.1111/j.1460-9568.2007.05487.x. [DOI] [PubMed] [Google Scholar]