Abstract
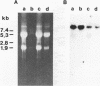
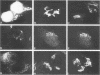
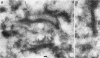
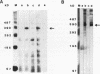
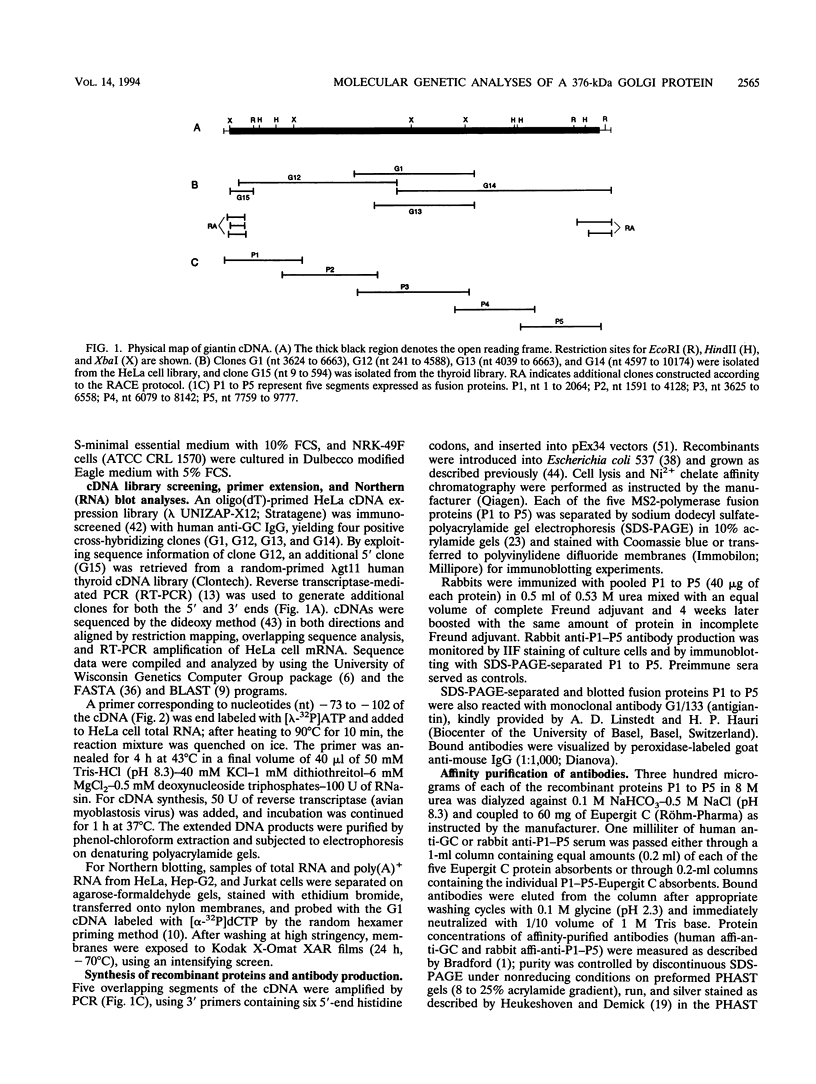
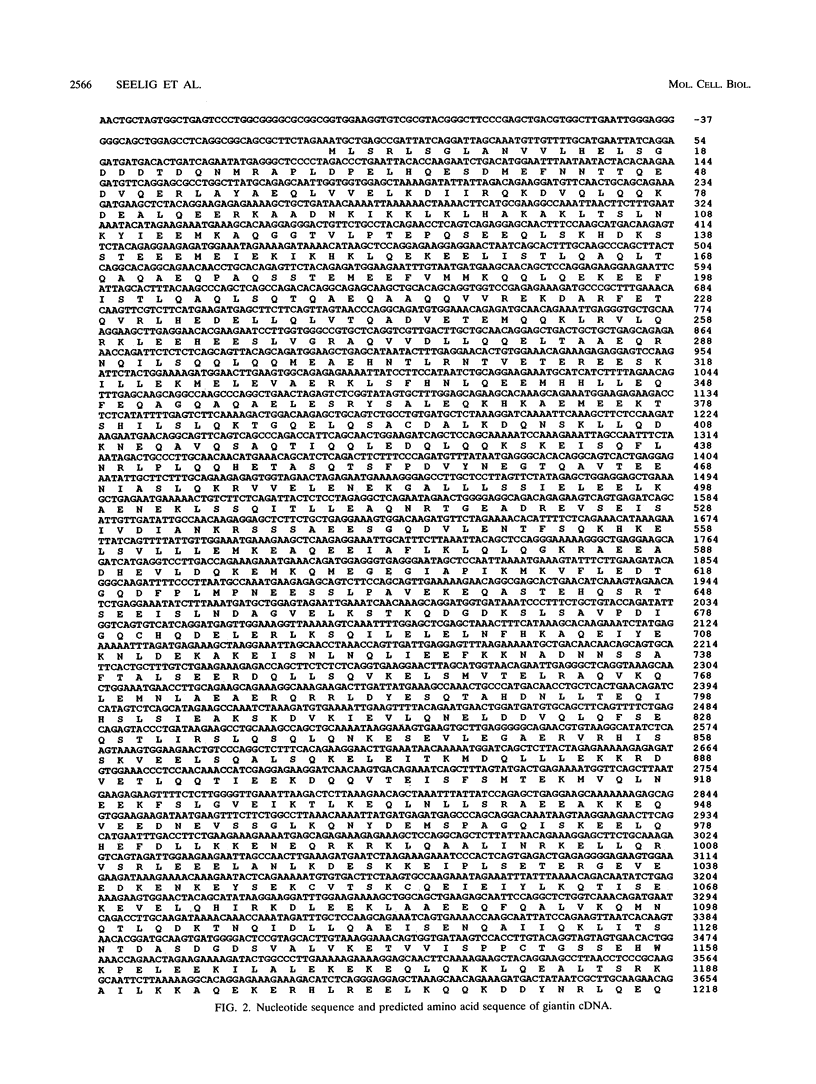
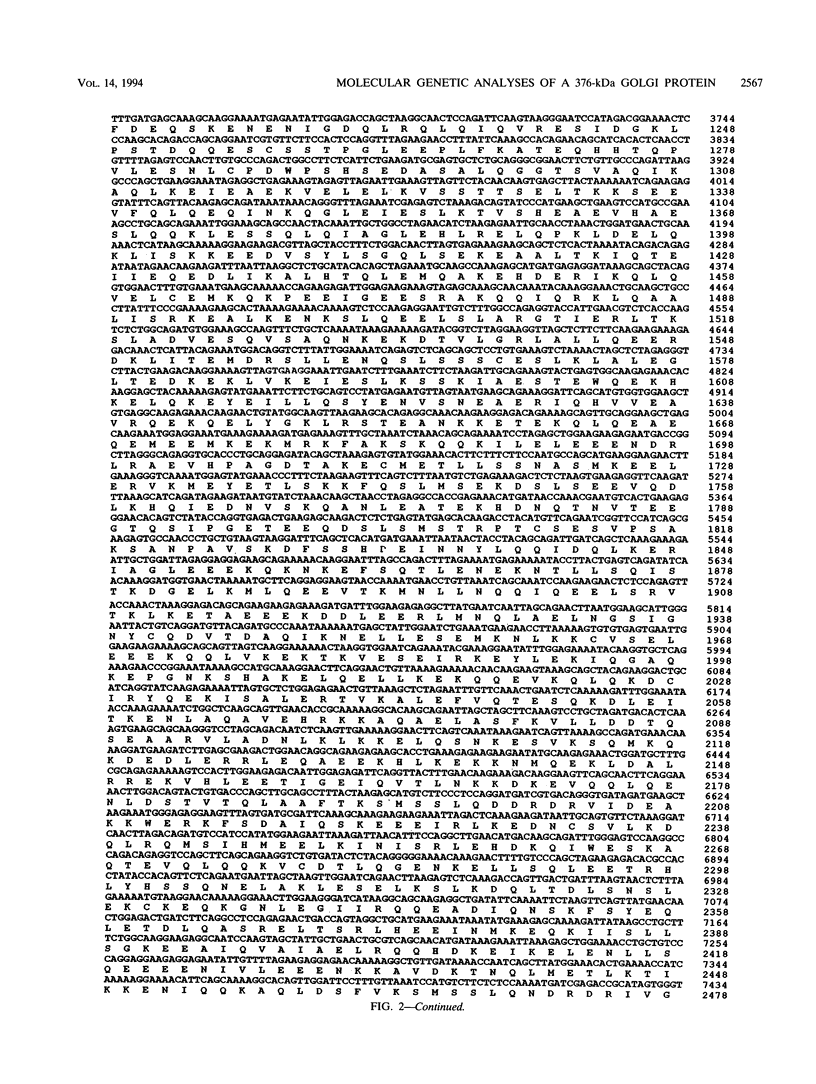
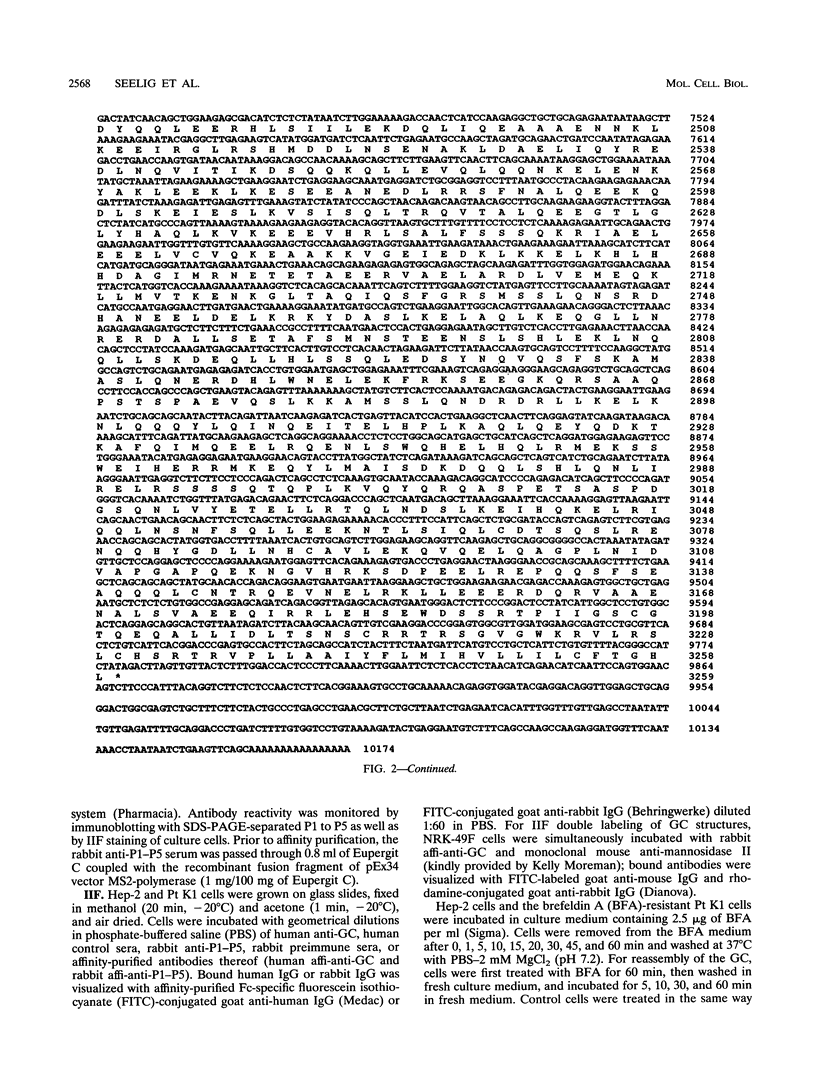
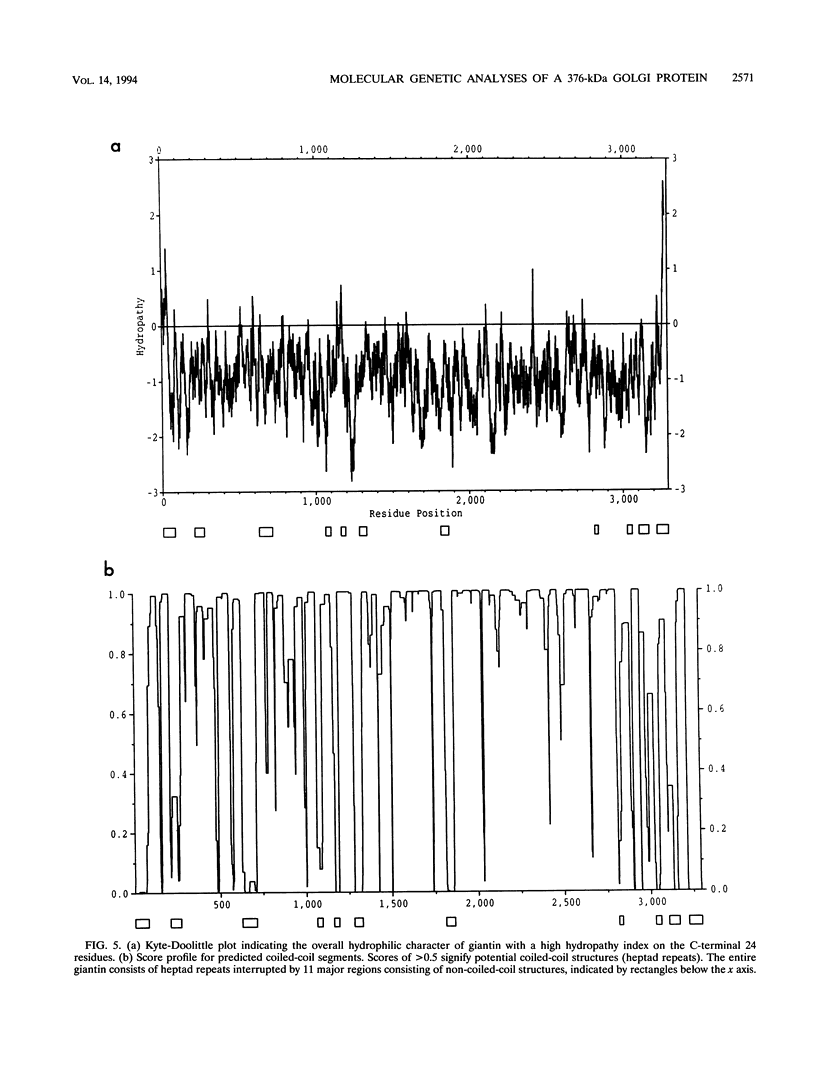
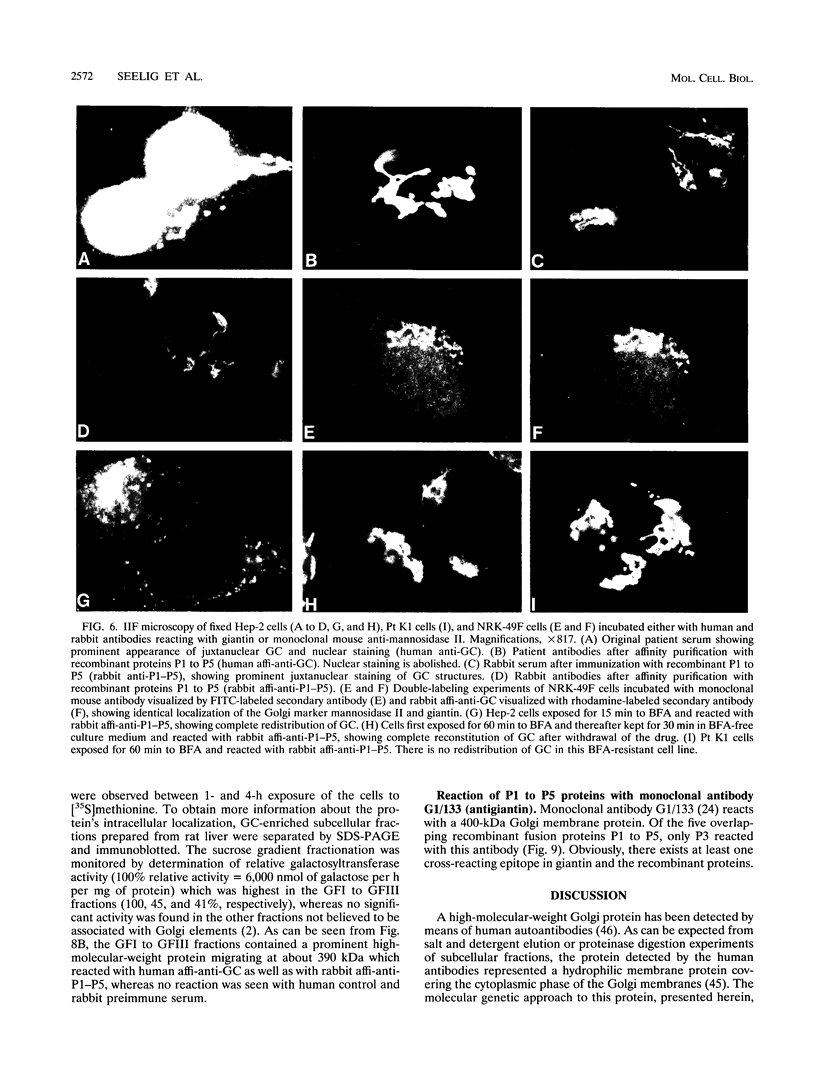
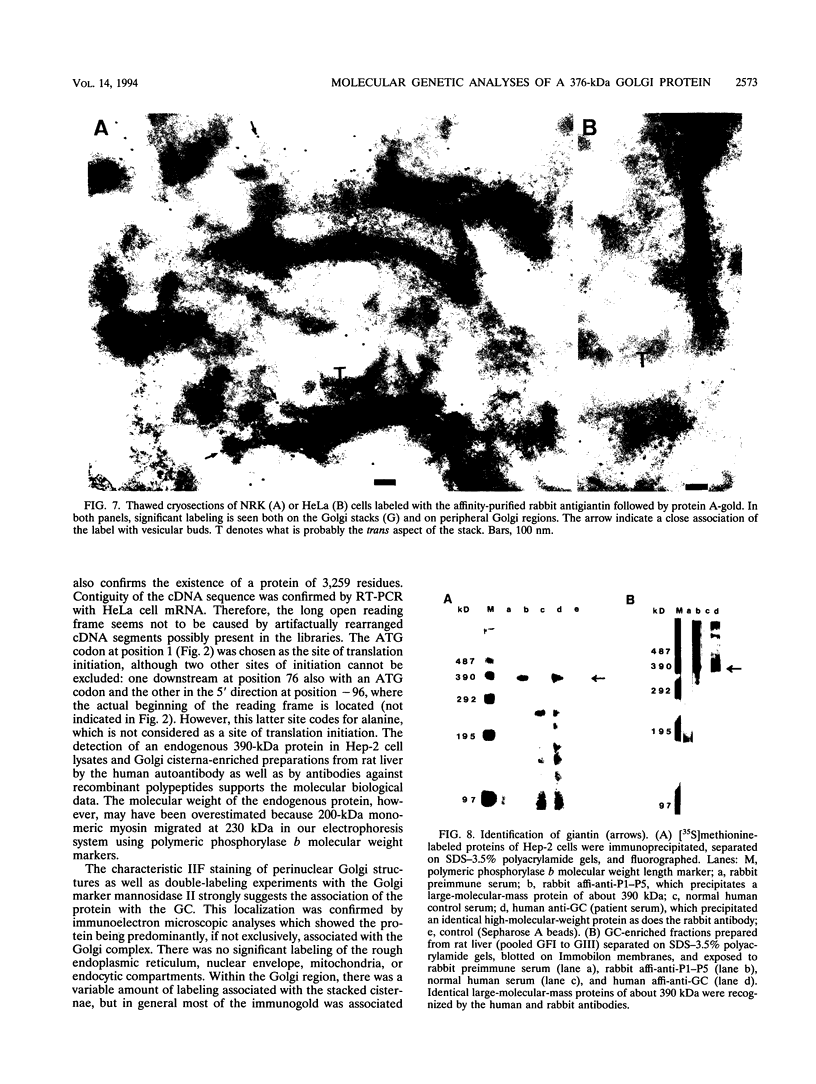
Molecular genetic analyses of a 376-kDa Golgi complex (GC) membrane protein (giantin) are described. The immunoglobulin G fraction of a human serum containing antibodies against GC antigens as revealed by indirect immunofluorescence microscopy with Hep-2 cells was used to screen a HeLa cDNA expression library, yielding four overlapping cross-hybridizing clones. Additional cDNA clones were retrieved from a lambda gt11 human thyroid cDNA library or generated by reverse transcriptase-mediated PCR from HeLa cell mRNA. Alignment of the clones resulted in a consensus cDNA of 10,300 bp encoding a protein of 376 kDa. The corresponding mRNA with a size of about 10 kb was detected by Northern (RNA) blotting of HeLa, Hep-G2, and Jurkat cell RNA. Sequence analyses of the protein revealed an extraordinarily high content of heptad repeats with the probability of forming coiled coils similar to the proteins of the myosin family. Five overlapping recombinant proteins covering the entire sequence were synthesized and used for antibody production in rabbits and for affinity purification of human and rabbit antibodies. Indirect immunofluorescence experiments also done with brefeldin A-treated Hep-2 and Pt K1 cells revealed an identical GC staining of both the affinity-purified human and rabbit antibodies. Double labeling experiments with antibodies against the GC marker mannosidase II as well as immunoelectron microscopic studies confirmed the localization of the protein within the GC. A corresponding endogenous large-molecular-mass protein of about 390 kDa was found in [35S]methionine-labeled Hep-2 cell lysates as well as in GC-enriched subcellular fractions from rat liver. The protein as well as the recently described proteins golgin-95 and golgin-160 (M. J. Fritzler, J. C. Hamel, R. L. Ochs, and E. K. L. Chan, J. Exp. Med. 178:49-62, 1993) may belong to a new group of Golgi proteins with a high content of heptad repeats which may exert functions in scaffold formation or vesicle transport. As far as can be concluded from immunological and personally communicated partial cDNA sequence data, the protein seems to be identical with a 400-kDa Golgi protein (giantin) recently described (A. D. Linstedt and H. P. Hauri, Mol. Biol. Cell 4:679-693, 1993). Therefore, we agreed to adopt the name giantin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bretz R., Bretz H., Palade G. E. Distribution of terminal glycosyltransferases in hepatic Golgi fractions. J Cell Biol. 1980 Jan;84(1):87–101. doi: 10.1083/jcb.84.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz R., Stäubli W. Detergent influence on rat-liver galactosyltransferase activities towards different acceptors. Eur J Biochem. 1977 Jul 1;77(1):181–192. doi: 10.1111/j.1432-1033.1977.tb11656.x. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. Trimeric G proteins in Golgi transport. Trends Biochem Sci. 1992 Mar;17(3):87–88. [PubMed] [Google Scholar]
- Burgoyne R. D. Trimeric G proteins in Golgi transport. Trends Biochem Sci. 1992 Mar;17(3):87–88. [PubMed] [Google Scholar]
- Clary D. O., Griff I. C., Rothman J. E. SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell. 1990 May 18;61(4):709–721. doi: 10.1016/0092-8674(90)90482-t. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J. G., Lippincott-Schwartz J., Bloom G. S., Kreis T. E., Klausner R. D. Dissociation of a 110-kD peripheral membrane protein from the Golgi apparatus is an early event in brefeldin A action. J Cell Biol. 1990 Dec;111(6 Pt 1):2295–2306. doi: 10.1083/jcb.111.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duden R., Griffiths G., Frank R., Argos P., Kreis T. E. Beta-COP, a 110 kd protein associated with non-clathrin-coated vesicles and the Golgi complex, shows homology to beta-adaptin. Cell. 1991 Feb 8;64(3):649–665. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fritzler M. J., Hamel J. C., Ochs R. L., Chan E. K. Molecular characterization of two human autoantigens: unique cDNAs encoding 95- and 160-kD proteins of a putative family in the Golgi complex. J Exp Med. 1993 Jul 1;178(1):49–62. doi: 10.1084/jem.178.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. J., Parry D. A., Steinert P. M., Virata M. L., Wagner R. M., Angst B. D., Nilles L. A. Structure of the human desmoplakins. Implications for function in the desmosomal plaque. J Biol Chem. 1990 Feb 15;265(5):2603–2612. [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Helms J. B., Rothman J. E. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature. 1992 Nov 26;360(6402):352–354. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- Heukeshoven J., Dernick R. Improved silver staining procedure for fast staining in PhastSystem Development Unit. I. Staining of sodium dodecyl sulfate gels. Electrophoresis. 1988 Jan;9(1):28–32. doi: 10.1002/elps.1150090106. [DOI] [PubMed] [Google Scholar]
- Kahn R. A., Gilman A. G. Purification of a protein cofactor required for ADP-ribosylation of the stimulatory regulatory component of adenylate cyclase by cholera toxin. J Biol Chem. 1984 May 25;259(10):6228–6234. [PubMed] [Google Scholar]
- Kooy J., Toh B. H., Pettitt J. M., Erlich R., Gleeson P. A. Human autoantibodies as reagents to conserved Golgi components. Characterization of a peripheral, 230-kDa compartment-specific Golgi protein. J Biol Chem. 1992 Oct 5;267(28):20255–20263. [PubMed] [Google Scholar]
- Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991 Oct 25;266(30):19867–19870. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linstedt A. D., Hauri H. P. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol Biol Cell. 1993 Jul;4(7):679–693. doi: 10.1091/mbc.4.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig T., Griffiths G., Hoflack B. Distribution of newly synthesized lysosomal enzymes in the endocytic pathway of normal rat kidney cells. J Cell Biol. 1991 Dec;115(6):1561–1572. doi: 10.1083/jcb.115.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A., Van Dyke M., Stock J. Predicting coiled coils from protein sequences. Science. 1991 May 24;252(5009):1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Malhotra V., Orci L., Glick B. S., Block M. R., Rothman J. E. Role of an N-ethylmaleimide-sensitive transport component in promoting fusion of transport vesicles with cisternae of the Golgi stack. Cell. 1988 Jul 15;54(2):221–227. doi: 10.1016/0092-8674(88)90554-5. [DOI] [PubMed] [Google Scholar]
- Mellman I., Simons K. The Golgi complex: in vitro veritas? Cell. 1992 Mar 6;68(5):829–840. doi: 10.1016/0092-8674(92)90027-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misumi Y., Misumi Y., Miki K., Takatsuki A., Tamura G., Ikehara Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem. 1986 Aug 25;261(24):11398–11403. [PubMed] [Google Scholar]
- Nakajima H., Hirata A., Ogawa Y., Yonehara T., Yoda K., Yamasaki M. A cytoskeleton-related gene, uso1, is required for intracellular protein transport in Saccharomyces cerevisiae. J Cell Biol. 1991 Apr;113(2):245–260. doi: 10.1083/jcb.113.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narula N., McMorrow I., Plopper G., Doherty J., Matlin K. S., Burke B., Stow J. L. Identification of a 200-kD, brefeldin-sensitive protein on Golgi membranes. J Cell Biol. 1992 Apr;117(1):27–38. doi: 10.1083/jcb.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Palmer D. J., Ravazzola M., Perrelet A., Amherdt M., Rothman J. E. Budding from Golgi membranes requires the coatomer complex of non-clathrin coat proteins. Nature. 1993 Apr 15;362(6421):648–652. doi: 10.1038/362648a0. [DOI] [PubMed] [Google Scholar]
- Orci L., Tagaya M., Amherdt M., Perrelet A., Donaldson J. G., Lippincott-Schwartz J., Klausner R. D., Rothman J. E. Brefeldin A, a drug that blocks secretion, prevents the assembly of non-clathrin-coated buds on Golgi cisternae. Cell. 1991 Mar 22;64(6):1183–1195. doi: 10.1016/0092-8674(91)90273-2. [DOI] [PubMed] [Google Scholar]
- Pavelka M., Ellinger A. Early and late transformations occurring at organelles of the Golgi area under the influence of brefeldin A: an ultrastructural and lectin cytochemical study. J Histochem Cytochem. 1993 Jul;41(7):1031–1042. doi: 10.1177/41.7.8515046. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo P. A., Yang Y. C., Rulka C., Kahn R. A. Activation of ADP-ribosylation factor by Golgi membranes. Evidence for a brefeldin A- and protease-sensitive activating factor on Golgi membranes. J Biol Chem. 1993 May 5;268(13):9555–9563. [PubMed] [Google Scholar]
- Remaut E., Tsao H., Fiers W. Improved plasmid vectors with a thermoinducible expression and temperature-regulated runaway replication. Gene. 1983 Apr;22(1):103–113. doi: 10.1016/0378-1119(83)90069-0. [DOI] [PubMed] [Google Scholar]
- Robinson M. S., Kreis T. E. Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cells: effects of brefeldin A and G protein activators. Cell. 1992 Apr 3;69(1):129–138. doi: 10.1016/0092-8674(92)90124-u. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987 May 22;49(4):527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig H. P., Ehrfeld H., Schroeter H., Heim C., Renz M. A recombinant 70K protein ELISA. Screening for antibodies against U1snRNP proteins in human sera. J Immunol Methods. 1991 Sep 20;143(1):11–24. doi: 10.1016/0022-1759(91)90267-j. [DOI] [PubMed] [Google Scholar]
- Serafini T., Orci L., Amherdt M., Brunner M., Kahn R. A., Rothman J. E. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 1991 Oct 18;67(2):239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- Serafini T., Stenbeck G., Brecht A., Lottspeich F., Orci L., Rothman J. E., Wieland F. T. A coat subunit of Golgi-derived non-clathrin-coated vesicles with homology to the clathrin-coated vesicle coat protein beta-adaptin. Nature. 1991 Jan 17;349(6306):215–220. doi: 10.1038/349215a0. [DOI] [PubMed] [Google Scholar]
- Shohet R. V., Conti M. A., Kawamoto S., Preston Y. A., Brill D. A., Adelstein R. S. Cloning of the cDNA encoding the myosin heavy chain of a vertebrate cellular myosin. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7726–7730. doi: 10.1073/pnas.86.20.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel K., Beck E., Strohmaier K., Schaller H. Characterization of foot-and-mouth disease virus gene products with antisera against bacterially synthesized fusion proteins. J Virol. 1986 Mar;57(3):983–991. doi: 10.1128/jvi.57.3.983-991.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Waters M. G., Serafini T., Rothman J. E. 'Coatomer': a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature. 1991 Jan 17;349(6306):248–251. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weidman P. J., Melançon P., Block M. R., Rothman J. E. Binding of an N-ethylmaleimide-sensitive fusion protein to Golgi membranes requires both a soluble protein(s) and an integral membrane receptor. J Cell Biol. 1989 May;108(5):1589–1596. doi: 10.1083/jcb.108.5.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Hamada Y., Katsuragawa Y., Imamura M., Mikawa T., Masaki T. Complete primary structure of vertebrate smooth muscle myosin heavy chain deduced from its complementary DNA sequence. Implications on topography and function of myosin. J Mol Biol. 1987 Nov 20;198(2):143–157. doi: 10.1016/0022-2836(87)90302-0. [DOI] [PubMed] [Google Scholar]