Abstract
Aging is a complex process associated with physiological changes in numerous organ systems. In particular, aging of the immune system is characterized by progressive dysregulation of immune responses, resulting in increased susceptibility to infectious diseases, impaired vaccination efficacy and systemic low-grade chronic inflammation. Increasing evidence suggest that intracellular zinc homeostasis, regulated by zinc transporter expression, is critically involved in the signaling and activation of immune cells. We hypothesize that epigenetic alterations and nutritional deficits associated with aging may lead to zinc transporter dysregulation, resulting in decreases in cellular zinc levels and enhanced inflammation with age. The goal of this study was to examine the contribution of age-related zinc deficiency and zinc transporter dysregulation on the inflammatory response in immune cells. The effects of zinc deficiency and age on the induction of inflammatory responses were determined using an in vitro cell culture system and an aged mouse model. We showed that zinc deficiency, particularly the reduction in intracellular zinc in immune cells, was associated with increased inflammation with age. Furthermore, reduced Zip 6 expression enhanced proinflammatory response, and age-specific Zip 6 dysregulation correlated with an increase in Zip 6 promoter methylation. Furthermore, restoring zinc status via dietary supplementation reduced aged-associated inflammation. Our data suggested that age-related epigenetic dysregulation in zinc transporter expression may influence cellular zinc levels and contribute to increased susceptibility to inflammation with age.
Keywords: Aging, Epigenetics, Immunity, Inflammation, Zinc
1. Introduction
Aging of the immune system results in a progressive dysregulation of immune responses including immunosenescence, where there is a gradual decline in both cellular and humoral immune responses, and increased susceptibility to infectious diseases and compromised vaccination efficacy in the elderly [1]. At the same time, “inflammaging,” a low-grade systemic chronic inflammation characterized by constitutively elevated levels of proinflammatory cytokines in blood, is commonly observed in the elderly population [2,3]. Chronic inflammation has been implicated in the promotion of many age-related diseases including cancer, cardiovascular disease and autoimmune diseases. In addition, increases in inflammatory mediators in the blood are significant predictors of morbidity and mortality in aged individuals [4–6].
Zinc is an essential micronutrient required for many cellular processes, especially for the normal development and function of the immune system [7–9]. National surveys indicate that a significant portion of the aged population has inadequate zinc intake [10–13], and a decline in zinc status, as shown by plasma zinc concentrations, is observed with increasing age [14–17]. There are remarkable similarities between the hallmarks of zinc deficiency and age-related immunological dysfunction, both characterized by impaired immune responses and systemic chronic inflammation. Thus age-related zinc deficiency may play a significant role in age-associated dysregulation of immune function and may be a contributing factor in age-related inflammation and associated morbidities [18,19]. Importantly, zinc has anti-inflammatory properties and low zinc status is associated with increased susceptibility to infections and exaggerated inflammatory responses [20–23]. Recent studies indicate that intracellular zinc homeostasis is critically involved in the signaling events in immune cells, and the regulation of cellular zinc in these immune cells is mediated by changes in the expression of specific zinc transporters [24–26]. Zinc transporters comprises a family of multiple transmembrane spanning domain proteins that are encoded by two solute-linked carrier (SLC) gene families: SLC30 (ZnT) and SLC39 (Zip) [27,28]. ZnT and Zip family zinc transporters have opposing roles in regulating cellular zinc homeostasis; ZnT transporters reduce cytosolic zinc bioavailability by promoting zinc efflux and Zip transporters function by increasing cytosolic zinc. In the context of inflammation, stimulation of immune cells with inflammatory stimuli such as lipopolysaccharide (LPS) results in changes in cellular zinc that is mediated by alterations in zinc transporter expression [29]. Thus alterations and/or dysregulation of zinc transporter expression with age could potentially affect zinc homeostasis in immune cells and contribute to immune dysfunction and chronic inflammation [18,30].
The mechanisms contributing to age-related zinc loss and age-related inflammation are unclear. Accumulating evidence indicates that epigenetic dysregulation is a common feature of aging, characterized by global DNA hypomethylation and gene-specific promoter hypermethylation or hypomethylation, as well as alteration in histone modifications [31–34]. In the immune system, age-associated epigenetic modifications such as DNA methylation have been shown to affect immune cell activation and may also contribute to the decline of cellular zinc with age, as several zinc transporters have been shown to be susceptible to epigenetic regulation [35–38]. At the same time, nutrient deficits such as zinc deficiency may further modulate epigenetic regulation [39–41]. The goal of the current study was to examine the contribution of age-related zinc deficiency and zinc transporter dysregulation on the inflammatory response in immune cells using an in vitro cell culture system and an aged mouse model. We hypothesized that age-related decreases in cellular zinc levels, in part, are mediated by epigenetic alterations that result in zinc transporter dysregulation and contribute to enhanced inflammation with age. Moreover, enhancing zinc status in aged mice should mitigate age-related inflammation.
2. Materials and methods
2.1. Cell culture, in vitro zinc depletion and LPS treatments
Human monocytic cell line THP-1 was obtained from American Type Tissue Collection (Manassas, VA, USA). Cells were grown in RPMI 1640 culture medium with 10% fetal bovine serum (FBS) and maintained in humidified incubators with 5% CO2 at 37°C. Zinc-deficient (ZD) media were prepared, as previous published, using a chelation strategy in which zinc was removed from FBS by incubating with 10% Chelex 100 (wt/vol) (Sigma, St. Louis, MO, USA) overnight at 4°C with constant stirring [42]. THP-1 cells were cultured in zinc-adequate (ZA) medium (RPMI with 10% Chelex-treated FBS and 4 μM ZnCl2) or ZD medium (RPMI with 10% Chelex-treated FBS) for up to 14 days, and media were changed every 3 to 4 days. Mineral levels in tissue culture media and THP-1 cells were determined by inductively coupled plasma-optical emission spectroscopy (ICP-OES) and/or FluoZin-3 flow cytometry where appropriate. ZA or ZD THP-1 cells were treated with phorbol 12-myristate 13-acetate at 5 ng/ml for 48 h to induce the cells to differentiate into macrophages. Differentiated THP-1 macrophages were treated with 0, 10 or 100 ng/ml LPS (Sigma) for 6 h and harvested for zinc and gene expression analyses. Previous time-course studies indicated that LPS induced a rapid proinflammatory response (data not shown), and the 6-h time point was chosen to reflect optimal induction time for various genes of interest.
2.2. Animals, diets and study design
Female C57Bl/6 mice at various ages (2–26 months) were purchased from the aged rodent colonies at the National Institute on Aging (Bethesda, MD, USA). Mice were housed in a temperature- and humidity-controlled environment and were fed standard rodent diet where appropriate, or randomly assigned to a purified ZA diet containing 30 mg/kg zinc, or a zinc-supplemented (ZS) diet containing 300 mg/kg zinc that was previously shown to be well tolerated and were able to normalize plasma zinc levels in old mice to levels similar to that of young mice [43,44]. Purified ZA and ZS diets were purchased from Research Diets (New Brunswick, NJ, USA). Mice were fed ZA or ZS diets for 3 weeks. Food and water were provided ad libitum. Dietary intakes and body weights of all mice were monitored throughout the entire study. Mice were euthanized by CO2 asphyxiation at the termination of the experiments, and sera and tissues were collected. The animal protocol was approved by the Oregon State University Institutional Laboratory Animal Care and Use Committee. Tissues were processed immediately for ex vivo stimulation and/or differentiation or were preserved in RNALater (Life Technologies, Grand Island, NY, USA) for DNA and RNA isolation.
2.3. Ex vivo differentiation of bone marrow-derived dendritic cells and macrophages
Bone marrow (BM)-derived dendritic cells (BMDCs) and macrophages (BMM) were differentiated ex vivo according to published protocols [45,46]. BM cells were flushed and collected from the femurs and tibias of young and old mice. Red blood cells (RBCs) were removed using RBC lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM EDTA). BMs were adjusted to 1.5×106 cells/ml in RPMI media containing 10% FBS. BMDC and BMM were differentiated in media containing 20 ng/ml granulocyte/macrophage colony stimulating factor (GM-CSF) and 20 ng/ml macrophage colony stimulating factor (M-CSF), respectively (Peprotech, Rocky Hill, NJ, USA). Nonadherent cells were removed on days 2 and 4, and BMDC and BMM cells were harvested on day 7 for intracellular zinc analysis and siRNA transfections, respectively.
2.4. Inflammatory response assays
For ex vivo splenocyte stimulation, spleens collected from young and old mice were made into single-cell suspension, and RBCs were removed using RBC lysis buffer. To determine how age and zinc status affect inflammatory response on a per cell basis, an equal number of splenocytes from young or old mice were seeded at 5×106 cells per well in 24-well culture plates and treated with 0, 0.1 and 1 μg/ml LPS for 6 h and harvested for zinc and gene expression analyses. Serum interleukin (IL)-6 levels were detected using mouse IL6 Ready-SET-Go ELISA kit from eBioscience (San Diego, CA, USA).
2.5. Total and intracellular zinc measurements
Total zinc concentrations were determined using ICP-OES, as previously described, with minor modification [47]. Briefly, samples (plasma or cell pellets) were digested in 1 ml 70% ultrapure nitric acid and incubated overnight. Incubated samples were diluted with chelex-treated nanopure water to a final concentration of 7% nitric acid, centrifuged and analyzed using the Prodigy High Dispersion ICP-OES instrument (Teledyne Leeman Labs, Hudson, NH, USA) against known standards. Intracellular zinc levels were determined using FluoZin-3 acetoxymethyl ester (FluoZin-3), a cell permeable, zinc-specific fluorescent indicator that measures intracellular free zinc (Molecular Probes, Eugene, OR, USA), according to published methods [48]. Cells were labeled with 1 μM FluoZin-3 for 30 minutes at 37°C and washed once in phosphate-buffered saline. Intracellular zinc levels, as determined by FluoZin-3 mean fluorescence intensity, were analyzed by flow cytometry. Data were acquired using FACSCalibur (BD Biosciences, San Jose, CA, USA). Data analyses were performed using Summit software (DakoCytomation, Fort Collins, CO, USA).
2.6. Proinflammatory and zinc transporter gene expression
Total RNA from tissues and treated cells were isolated using TRIzol (Invitrogen). Total RNA was reverse transcribed into cDNA using SuperScript III First-Strand Synthesis SuperMix for quantitative real-time polymerase chain reaction (qRT-PCR) (Invitrogen). Real-time PCR was performed using the following PCR primers: human tumor necrosis factor α (TNF) (forward: 5′-CCCCAGGGACCTCTCTCTAATC-3′, reverse: 5′-GGTTTGCTACAACATGGGCTACA-3′), human IL1β (forward: 5′-CCTGTCCTGCGTGTTGAAAGA-3′, reverse: 5′-GGGAACTGGGCAGACTCAAA-3′), human GAPDH (forward: 5′-CGAGATCCCTC-CAAAATCAA-3′, reverse: 5′-TTCACACCCATGACGAACAT-3′), mouse TNFα (forward: 5′-CTGTAGCCCACGTCGTAGCA-3′, reverse: 5′-GTGTGGGTGAGGAGCACGTA-3′), mouse IL1β (forward: 5′-AAGATGAAGGGCTGCTTCCAA-3′, reverse: 5′-TGAAGGAAAAGAAGGTGCTCATG-3′), mouse Zip 6 (forward: 5′-AAGTGAGAAGAAGGCAGAAATCC-3′, reverse: 5′-GGAGAAGATGTAACAGAGCATCG-3′), mouse ZnT 1 (forward: 5′-TGGATGTACAAG-TAAATGGGAATCT-3′, reverse: 5′-GTCTTCAGTACAACCCTTCCAGTTA-3′), or mouse 18S ribosomal RNA (18S) (forward: 5′-CCGCAGCTAGGAATAATGGAAT-3′, reverse: 5′-CGAACCTCCGACTTTCGTTCT-3′). Real-time PCR reactions were performed using Fast SYBR Green Mastermix (Invitrogen) on 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Gene copies were determined using the standard curve method. A standard curvewas generated from serial dilutions of purified plasmid DNA that encoded for each gene of interest. Data represent the copy number of the gene of interest normalized to the copy number of GAPDH (for human) or 18S (for mouse) housekeeping genes.
2.7. Zip 6 gene silencing
BM macrophages were harvested and transfected with Zip 6-specific siRNA (Ambion Silencer siRNA S98750) using siPORT Amine and Ambion Silencer siRNA Transfection kit (Applied Biosystems). Control cells were transfected with scrambled siRNA (Ambion Silencer Select Negative Control No. 1). Transfected cells were seeded in 24-well tissue culture plates at 3×105 cells per well for 24 h and treated with 0, 10 and 100 ng/ml LPS for 6 h and harvested for gene expression analyses.
2.8. Genomic DNA isolation and DNA methylation analyses
Genomic DNA was isolated using DNeasy Blood and Tissue kit (Qiagen, Valencia, CA, USA). Global DNA methylation was measured using SuperSense Methylated DNA Quantification Kit (Epigentek, Brooklyn, NY, USA) and was reported as relative fluorescence units (RFUs) per 100 ng genomic DNA. Zip 6 promoter methylation was determined using Zip 6-specific EpiTect Methyl Profiler qPCR assay (Qiagen), which analyzed DNA methylation status of the CpG island within the Zip 6 promoter (Chr18 24761649–24762812).
2.9. Statistical analyses
Statistical analyses were performed using GraphPad Prism Version 5.02 (Graph-Pad, La Jolla, CA, USA). All data were reported as mean±S.E.M. P values were determined using unpaired t test, one-way analysis of variance (ANOVA) or two-way ANOVA, where appropriate. Bonferroni post hoc tests were performed to determine differences among the means when there was a significant main effect in one-way or two-way ANOVA. Statistical significance was defined as P≤.05.
3. Results
3.1. Proinflammatory response is associated with reduced intracellular zinc and is enhanced by zinc deficiency
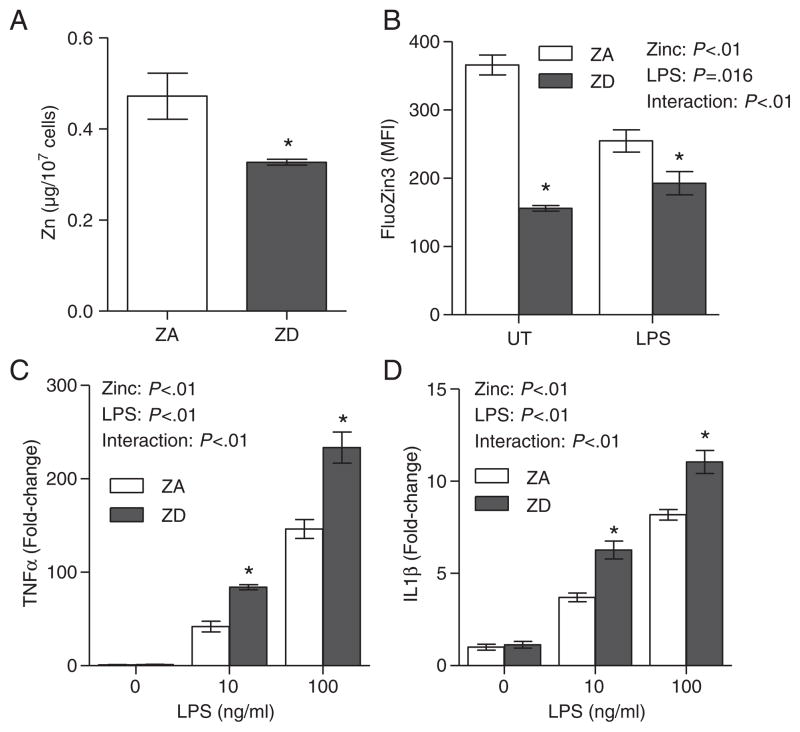
The effects of zinc deficiency on inflammation were determined in THP-1 cells in vitro. After 10 days of culture in ZD media, THP-1 cells had significantly lower total zinc compared to cells cultured in ZA media (Fig. 1A). In addition, stimulation of THP-1 cells with LPS (Toll-like receptor 4 agonist) significantly reduced intracellular zinc levels in ZA THP1 cells (Fig 1B). The intracellular zinc levels in ZD THP-1 cells remained significantly lower compared to ZA cells, both pre- and post-LPS stimulation (Fig. 1B). Zinc deficiency also enhanced the LPS-induced proinflammatory response. Stimulation of THP-1 cells with LPS elicited a well-characterized and dose-dependent proinflammatory response, as determined by the induction of two prototypical proinflammatory cytokines, TNFα and IL1β. Our data indicated that zinc deficiency significantly increased the expression of both TNFα (Fig. 1C) and IL1β (Fig. 1D) mRNA expression in ZD THP-1 cells compared to ZA THP-1 cells.
Fig 1.
Proinflammatory response was associated with reduced intracellular zinc and was enhanced by zinc deficiency. THP-1 cells (n=3 per treatment) were cultured in ZA or ZD media for 10 to 13 days. Total zinc (A) and intracellular zinc (B) were determined by ICP-OES and FluoZin-3 flow cytometry, respectively. Intracellular zinc and proinflammatory response were measured in ZA or ZD THP-1 cells that were left untreated (UT), or stimulated with 10 or 100 ng/ml LPS for 6 h. Expressions of TNFα (C) and IL1β mRNA (D) were determined by real-time PCR. Data represent mean±S.E.M. MFI, mean fluorescence intensity. *P<.05 vs. ZA. Results are representative of three independent experiments.
3.2. Age-related reduction in intracellular zinc in immune cells is associated with enhanced proinflammatory response
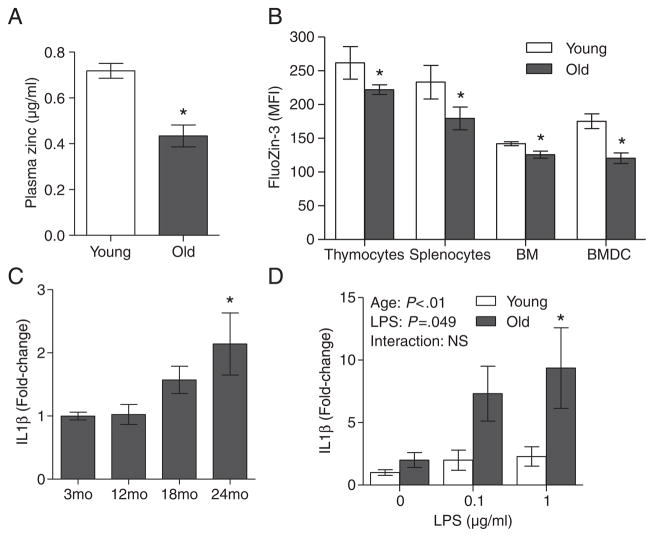
Aging was associated with a decline in plasma zinc concentrations (Fig. 2A). Despite a reduction in plasma zinc, total tissue zinc levels (including brain, liver, pancreas and spleen) measured by ICP-OES were not significantly different between young and old mice (data not shown). However, although total zinc levels did not differ in several tissues, FluoZin-3 analysis revealed that intracellular zinc levels were significantly lower in immune organs including thymocytes, splenocytes and BM cells of aged mice (Fig. 2B). Notably, BMDCs of aged mice had lower intracellular zinc compared to BMDC from young mice, despite ex vivo differentiation and culture in ZA media. Importantly, our data suggested that age-related decreases in intracellular zinc in immune cells were associated with increasing inflammation, as was demonstrated by a significant increase in baseline IL1β mRNA expression in the spleens in aged mice (24 months) compared to young mice (3 months) (Fig. 2C). There was also a significant age effect where aged splenocytes exhibited an increase in induced IL1β mRNA expression compared to young splenocytes, when stimulated with LPS ex vivo (Fig 2D).
Fig 2.
Age-related reduction in immune cell intracellular zinc levels was associated with enhanced proinflammatory responses. Plasma zinc (A) and intracellular zinc (B) in various immune cells including thymocytes, splenocytes, BM cells and BMDCs were determined in groups of young (2 months) and aged (26 months) mice (n=5 per age group) by ICP-OES and FluoZin-3 flow cytometry, respectively. (C) IL1β mRNA expression in the spleens of mice at various ages (n=10 per age group) were determined by real-time PCR. (D) Splenocytes from young (2 months) and aged (26 months) mice (n=4 per age group) were left untreated, or stimulated with 0.1 or 1 μg/ml LPS for 6 h. IL1β mRNA expression was determined by real-time PCR. Data represent mean normalized fold-change±S.E.M. vs. young mice (2 months; A, B and D) or 3-month-old mice (C). *P<.05 vs. young mice. NS, not significant.
3.3. Age-related increase in proinflammatory response is associated with dysregulation of Zip 6 zinc transporter expression
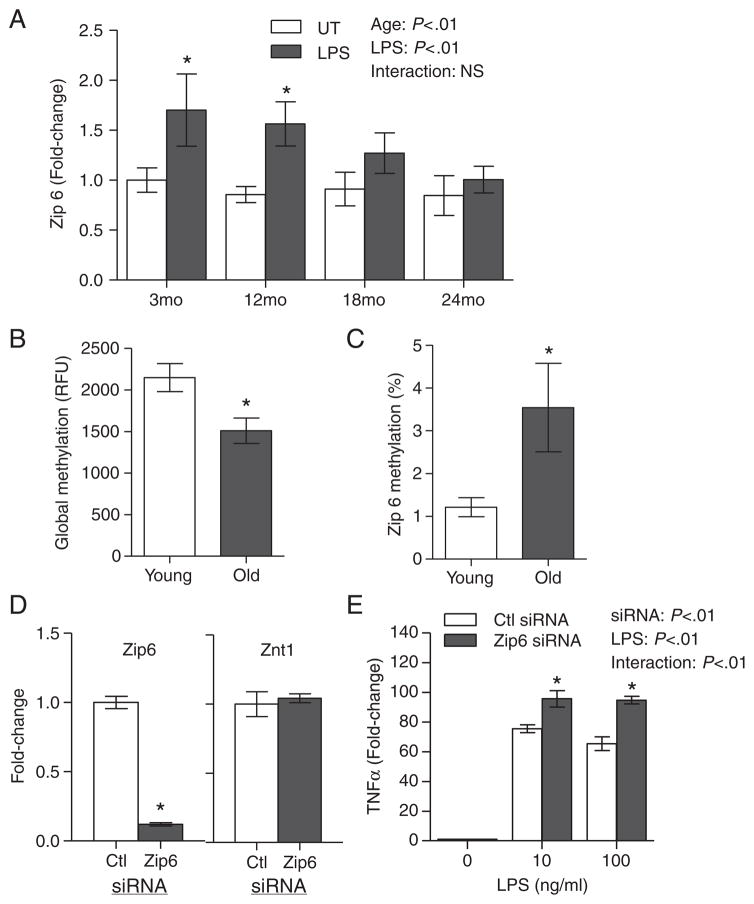
The regulation of cellular zinc homeostasis and immune activation is, in part, controlled by differential zinc transporter expression [25,29]. There were no significant age-related changes in the baseline expression of zinc transporters (Zip 1–6, ZnT 1–7) in the spleens between young and aged mice (data not shown). In contrast, ex vivo LPS stimulation induced a significant up-regulation of Zip 6 expression in the splenocytes of the younger mice (3 and 12 months). This induction was blunted in the splenocytes of older mice (18 and 24 months; Fig. 3A). While the gene expression of other zinc transporters has also been reported to alter during LPS treatment [29], only Zip 6 had age-associated alteration in gene expression in response to LPS treatment. We next examined whether Zip 6 dysregulation in aged mice was associated with age-related epigenetic alterations, particularly with respect to changes in DNA methylation. Global DNA methylation was significantly decreased with age (Fig. 3B). However, a significant age-specific increase in DNA methylation within the CpG island in the Zip6 promoter region was observed (Fig. 3C). To demonstrate that Zip 6 was involved in the induction of a proinflammatory response, Zip 6 expression was silenced in BMMs prior to the induction of a LPS-mediated proinflammatory response. Zip 6 expression in BMM transfected with Zip 6 siRNA was significantly reduced compared to control. Further, gene silencing was specific to Zip 6, as ZnT 1 expression was unaffected (Fig. 3D). Upon LPS stimulation, Zip 6-silenced BMM had significantly increased TNFα expression compared to control (Fig. 3E). Taken together, our data suggested that Zip 6 dysregulation was involved in enhanced inflammation during aging.
Fig 3.
Age-related increase in proinflammatory response was associated with dysregulation of Zip 6 zinc transporter mRNA expression. (A) Changes in Zip 6 expression were determined by real-time PCR in the splenocytes of mice at various ages (n=4 per age group) after stimulation with 1 μg/ml LPS for 6 h, or left untreated (UT). Data represent mean normalized fold-change±S.E.M. vs. UT control. (B) Global DNA methylation status in young (2 months) and aged (26 months) mice (n=11 per age group) was determined. Genomic DNA was isolated from the spleens from each group and analyzed for global DNA methylation changes. Data represent mean RFUs±S.E.M. per 100 ng DNA. *P<.05 vs. young. (C) Zip 6-specific promoter methylation in young (2 months) and aged (26 months) mice (n=15 per age group) was determined using Zip 6-specific EpiTect Methyl Profiler qPCR assay. Data represent percent DNA methylation compared to young mice. *P<.05 vs. young. (D) BMMs were transfected with Zip 6-specific siRNA or control siRNA (n=4 per transfection). Zip 6 and ZnT 1 gene expressions were determined by real-time PCR 24 h post-transfection. Data represent mean normalized fold-change±S.E.M. vs. ctl siRNA. *P<.05 vs. ctl siRNA. (E) siRNA-transfected BMMs were left untreated, or stimulated with 10 or 100 ng/ml LPS for 6 h, and TNFα expression was determined by real-time PCR (n=4). Data represent mean normalized fold-change±S.E.M. vs. ctl siRNA. *P<.05 vs. ctl siRNA. NS, not significant.
3.4. Dietary zinc supplementation decreases age-associated inflammation
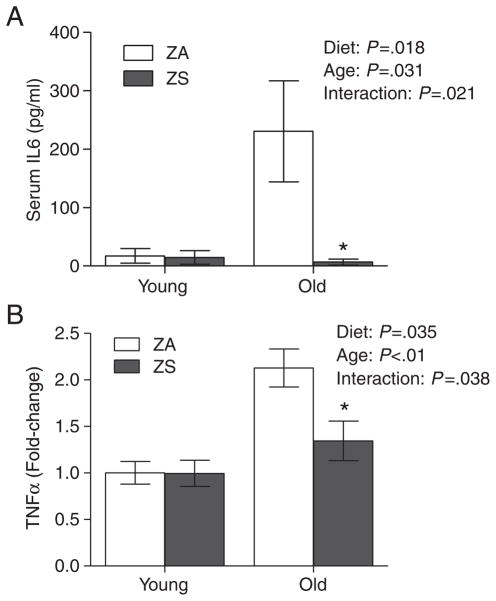
We had previously shown that dietary zinc supplementation can improve the zinc status in aged mice [44]. In this study, we found that dietary zinc supplementation for 3 weeks also mitigated aged-related increases in proinflammatory cytokine levels. Age-related increases in serum IL6 were significantly reduced in aged mice fed a ZS diet compared to aged mice fed a ZA diet (Fig. 4A). Similarly, age-related increases in TNFα expression were significantly reduced in the splenocytes of aged mice fed a ZS diet (Fig. 4B). Our data indicated that restoring zinc status in aged mice could overcome and decrease chronic inflammation in old age.
Fig 4.
Dietary zinc supplementation reduced age-associated inflammation. (A) Serum IL6 was determined in young (2 months) and aged (26 months) mice that were fed a ZA or ZS diet for 3 weeks (n=9 per group). Values were mean±S.E.M. (B) TNFα mRNA expression was determined by real-time PCR in the spleens of young and aged mice that were fed a ZA or ZS diet for 3 weeks (n=6 per group). Data represent mean normalized fold-change±S.E.M. vs. young. *P<.05 vs. ZA.
4. Discussion
Declining zinc status during aging may contribute to immune dysfunction and chronic inflammation, and many age-related health problems are conditions that are also associated with poor zinc status [30,49]. While zinc is known to act as an anti-inflammatory agent, however to date, the precise mechanisms linking zinc, age and inflammation are unclear. Our study is the first to report the potential role of age-related epigenetic control on zinc homeostasis. Specifically, these studies provide new evidence that suggests that methylation of specific zinc transporters with age may be one of the contributing factors resulting in zinc transporter dysregulation, and age-related zinc deficiency and inflammation. In this study, we demonstrated that zinc deficiency, particularly the reduction in intracellular zinc within immune cells, was associated with increased inflammation with increased age of the animal. We further showed that reduced Zip 6 mRNA expression enhanced proinflammatory responses, and age-specific Zip 6 dysregulation correlated with an increase in Zip 6 promoter methylation. Moreover, restoring zinc status via dietary supplementation reduced aged-associated inflammation. Our animal data suggested that age-related epigenetic dysregulation in zinc transporter expression may influence cellular zinc levels and contribute to increased susceptibility to inflammation with age.
Recent studies indicate that intracellular zinc homeostasis is critically involved in the signaling events in immune cells [24]. For example, T-cell receptor signaling and Th1 cell differentiation are controlled by activation-induced zinc influx during T-cell activation, and in DC, a reduction in intracellular zinc is required for DC maturation and antigen presentation [25,29,50]. In our in vitro study, LPS-stimulated proinflammatory response was accompanied by a significant reduction in intracellular zinc in THP-1 macrophages (Fig. 1). We further showed that reduced intracellular zinc in immune cells of aged mice was associated with increased inflammatory response (Fig. 2). Our data suggested that a decrease in cellular zinc was required for the induction of a proinflammatory response that was dysregulated with age and reaffirmed the importance of zinc homeostasis in controlling immune cell activation.
Zinc transporters play an important role in regulating cellular zinc homeostasis. Members of Zip and ZnT zinc transporter families exhibit tissue and cell-specific expression and possess differential responsiveness to dietary zinc, as well as to physiologic stimuli, including cytokines [27,51]. The expression of zinc transporters has been profiled in immune cell types, and their regulation plays an important role in specific immune cell activation. Intracellular zinc homeostasis in different leukocyte subsets is regulated by distinct patterns of zinc exporter expression [52,53]. Zinc transporter expression is important in controlling the activation and maturation of various immune cells [25,29]. In particular, Zip 6 and ZnT 1 have been shown to be key transporters that control free zinc levels in immune cells. Intracellular zinc levels and Zip 6 are involved in the maturation of DC, and a reduction in cellular zinc or Zip 6 expression leads to enhanced antigen presentation and DC-mediated immune responses [29]. We hypothesized that alteration and/or dysregulation of zinc transporter expression with age can potentially lead to age-associated decline in zinc status, aberrant immune activation and inflammation. We confirmed the role of Zip 6 in mediating the inflammatory response, where loss of Zip 6 mRNA expression by siRNA or with age resulted in an enhanced inflammatory response (Fig. 3).
Epigenetic alterations during aging are emerging as factors that can influence age-related processes, such as chronic inflammation. In the context of the immune system, epigenetic modifications including DNA methylation and histone modifications have been shown to control immune function [35]. For example, Agrawal et al. [36] recently demonstrated that epigenetic modifications in aged human DNA increase its immunogenicity, resulting in increased DC activation, and may be a potential mechanism leading to age-associated increases in autoimmune and proinflammatory responses. In addition to age-related epigenetic modifications, specific nutrients can also modulate epigenetic regulation and alter disease susceptibility [39,54,55]. In particular, there is strong evidence for a role of zinc in DNA methylation [56,57]. Zinc deficiency may cause methyl deficiency [58,59], similar to other methyl donors like folate, resulting in altered methylation patterns, abnormal gene expression and developmental defects. Interestingly, several zinc transporters have been reported to be susceptible to epigenetic regulation [37,38]. We hypothesized that age-related epigenetic modification of zinc transporters, particularly Zip 6, may contribute to Zip 6 dysregulation and the decline of zinc status with age. In our study, we determined changes in methylation status with age in immune cells and observed global DNA hypomethylation in the spleens of aged mice compared to young mice (Fig. 3). At the same time, DNA methylation status of CpG islands in the promoter region of Zip 6 was increased in the splenocytes of aged mice relative to young mice. Similar promoter hypermethylations were also observed in ZnT 1 and ZnT 5 (data not shown). Thus, age-related epigenetic alterations may represent one mechanism that contributes to age-related deficits in cellular zinc levels and enhanced inflammation. Preliminary results in zinc supplemented old mice with improved zinc status did not reveal changes in Zip 6 promoter methylation compared to old mice fed a ZA diet (data not shown). However, the methylation assay used in this report may not be sensitive enough to detect subtle changes in DNA methylation profile under dietary zinc supplementation conditions. Detailed characterization of the specific CpG residues within various zinc transporter promoter regions that are differentially methylated with age and zinc status is currently ongoing using methylation assays with improved sensitivity. Secondly, it is also possible that age-related epigenetic dysregulation of Zip6 is not reversible, but zinc supplementation simply overcomes losses in cellular zinc and exerts anti-inflammatory effects, despite continued dysregulation of methylation and zinc transporter expression. Taken together, our data suggested that age-related epigenetic alterations may contribute to deficits in cellular zinc levels in immune cells and enhance inflammation. Results from our animal studies will aid in providing rationale for future human studies that will help understand whether improving zinc status in the elderly will be helpful in correcting age-related immune defects and prevent chronic inflammation.
To date, the precise factors contributing to age-related zinc deficiency remain poorly defined. Results from the current study lend support to the hypothesis that age-related epigenetic modifications such as DNA methylation may be one of the contributing factors that lead to the dysregulation of zinc regulatory proteins such as zinc transporters. This may lead to impaired zinc utilization and age-associated decline in zinc status. The resulting zinc loss, particularly in immune cells, may subsequently contribute to immune dysfunction and enhanced inflammatory response. We further showed that improving zinc status in the aged mice via dietary zinc supplementation can overcome age-related chronic inflammation. Future studies will focus on further characterizing how age-related epigenetic modifications, either with age itself or in combination with age-related zinc deficiency, could impact key regulatory mechanisms that exacerbate intracellular zinc loss, immune dysregulation and chronic inflammation. Ultimately, these studies aid in the identification of factors that may influence zinc status and contribute to the promotion of inflammation-mediated disorders with age.
Footnotes
Funding support: Oregon Agricultural Experiment Station (OR00735), Environmental Health Science Center at Oregon State University (NIEHS P30 ES00210), Oregon State University General Research Fund, Linus Pauling Institute, and National Institute on Aging, NIH (R01 AG016322).
References
- 1.Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211:144–56. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Provinciali M, Barucca A, Cardelli M, Marchegiani F, Pierpaoli E. Inflammation, aging, and cancer vaccines. Biogerontology. 2010;11:615–26. doi: 10.1007/s10522-010-9280-9. [DOI] [PubMed] [Google Scholar]
- 4.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad A, Banerjee S, Wang Z, Kong D, Majumdar AP, Sarkar FH. Aging and inflammation: etiological culprits of cancer. Curr Aging Sci. 2009;2:174–86. doi: 10.2174/1874609810902030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–80. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 7.Fischer Walker C, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr. 2004;24:255–75. doi: 10.1146/annurev.nutr.23.011702.073054. [DOI] [PubMed] [Google Scholar]
- 8.Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277–98. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- 9.Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T. Zinc homeostasis and signaling in health and diseases: zinc signaling. J Biol Inorg Chem. 2011;16:1123–34. doi: 10.1007/s00775-011-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mares-Perlman JA, Subar AF, Block G, Greger JL, Luby MH. Zinc intake and sources in the US adult population: 1976–1980. J Am Coll Nutr. 1995;14:349–57. doi: 10.1080/07315724.1995.10718520. [DOI] [PubMed] [Google Scholar]
- 11.Ma J, Betts NM. Zinc and copper intakes and their major food sources for older adults in the 1994–96 Continuing Survey of Food Intakes by Individuals (CSFII) J Nutr. 2000;130:2838–43. doi: 10.1093/jn/130.11.2838. [DOI] [PubMed] [Google Scholar]
- 12.Ervin RB, Kennedy-Stephenson J. Mineral intakes of elderly adult supplement and non-supplement users in the third national health and nutrition examination survey. J Nutr. 2002;132:3422–7. doi: 10.1093/jn/132.11.3422. [DOI] [PubMed] [Google Scholar]
- 13.Prasad AS, Fitzgerald JT, Hess JW, Kaplan J, Pelen F, Dardenne M. Zinc deficiency in elderly patients. Nutrition. 1993;9:218–24. [PubMed] [Google Scholar]
- 14.Hotz C, Peerson JM, Brown KH. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976–1980) Am J Clin Nutr. 2003;78:756–64. doi: 10.1093/ajcn/78.4.756. [DOI] [PubMed] [Google Scholar]
- 15.Ravaglia G, Forti P, Maioli F, Nesi B, Pratelli L, Savarino L, et al. Blood micronutrient and thyroid hormone concentrations in the oldest-old. J Clin Endocrinol Metab. 2000;85:2260–5. doi: 10.1210/jcem.85.6.6627. [DOI] [PubMed] [Google Scholar]
- 16.Cakman I, Rohwer J, Schutz RM, Kirchner H, Rink L. Dysregulation between TH1 and TH2 T cell subpopulations in the elderly. Mech Ageing Dev. 1996;87:197–209. doi: 10.1016/0047-6374(96)01708-3. [DOI] [PubMed] [Google Scholar]
- 17.Prasad AS, Beck FW, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, et al. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85:837–44. doi: 10.1093/ajcn/85.3.837. [DOI] [PubMed] [Google Scholar]
- 18.Haase H, Rink L. The immune system and the impact of zinc during aging. Immunol Ageing. 2009;6:9. doi: 10.1186/1742-4933-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mocchegiani E, Costarelli L, Giacconi R, Piacenza F, Basso A, Malavolta M. Micronutrient (Zn, Cu, Fe)-gene interactions in ageing and inflammatory age-related diseases: implications for treatments. Ageing Res Rev. 2012;11:297–319. doi: 10.1016/j.arr.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Devirgiliis C, Zalewski PD, Perozzi G, Murgia C. Zinc fluxes and zinc transporter genes in chronic diseases. Mutat Res. 2007;622:84–93. doi: 10.1016/j.mrfmmm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Besecker BY, Exline MC, Hollyfield J, Phillips G, Disilvestro RA, Wewers MD, et al. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am J Clin Nutr. 2011;93:1356–64. doi: 10.3945/ajcn.110.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murr C, Pilz S, Grammer TB, Kleber ME, Bohm BO, Marz W, et al. Low serum zinc levels in patients undergoing coronary angiography correlate with immune activation and inflammation. J Trace Elem Med Biol. 2012;26:26–30. doi: 10.1016/j.jtemb.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Knoell DL, Julian MW, Bao S, Besecker B, Macre JE, Leikauf GD, et al. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit Care Med. 2009;37:1380–8. doi: 10.1097/CCM.0b013e31819cefe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakami M, Hirano T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008;99:1515–22. doi: 10.1111/j.1349-7006.2008.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu M, Lee WW, Tomar D, Pryshchep S, Czesnikiewicz-Guzik M, Lamar DL, et al. Regulation of T cell receptor signaling by activation-induced zinc influx. J Exp Med. 2011;208:775–85. doi: 10.1084/jem.20100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giacconi R, Malavolta M, Costarelli L, Busco F, Galeazzi R, Bernardini G, et al. Comparison of intracellular zinc signals in nonadherent lymphocytes from young-adult and elderly donors: role of zinc transporters (Zip family) and proinflammatory cytokines. J Nutr Biochem. 2011 doi: 10.1016/j.jnutbio.2011.07.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–72. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 28.Eide DJ. The SLC39 family of metal ion transporters. Pflugers Arch. 2004;447:796–800. doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, et al. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol. 2006;7:971–7. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- 30.Wong CP, Ho E. Zinc and its role in age-related inflammation and immune dysfunction. Mol Nutr Food Res. 2012;56:77–87. doi: 10.1002/mnfr.201100511. [DOI] [PubMed] [Google Scholar]
- 31.Kawakami K, Nakamura A, Ishigami A, Goto S, Takahashi R. Age-related difference of site-specific histone modifications in rat liver. Biogerontology. 2009;10:415–21. doi: 10.1007/s10522-008-9176-0. [DOI] [PubMed] [Google Scholar]
- 32.Grolleau-Julius A, Ray D, Yung RL. The role of epigenetics in aging and autoimmunity. Clin Rev Allergy Immunol. 2009;39:42–50. doi: 10.1007/s12016-009-8169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maegawa S, Hinkal G, Kim HS, Shen L, Zhang L, Zhang J, et al. Widespread and tissue specific age-related DNA methylation changes in mice. Genome Res. 2010;20:332–40. doi: 10.1101/gr.096826.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yung RL, Julius A. Epigenetics, aging, and autoimmunity. Autoimmunity. 2008;41:329–35. doi: 10.1080/08916930802024889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Elsen PJ, van Eggermond MC, Wierda RJ. Epigenetic control in immune function. Adv Exp Med Biol. 2011;711:36–49. doi: 10.1007/978-1-4419-8216-2_4. [DOI] [PubMed] [Google Scholar]
- 36.Agrawal A, Tay J, Yang GE, Agrawal S, Gupta S. Age-associated epigenetic modifications in human DNA increase its immunogenicity. Aging (Albany NY) 2010;2:93–100. doi: 10.18632/aging.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujishiro H, Okugaki S, Yasumitsu S, Enomoto S, Himeno S. Involvement of DNA hypermethylation in down-regulation of the zinc transporter ZIP8 in cadmium-resistant metallothionein-null cells. Toxicol Appl Pharmacol. 2009;241:195–201. doi: 10.1016/j.taap.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Coneyworth LJ, Mathers JC, Ford D. Does promoter methylation of the SLC30A5 (ZnT5) zinc transporter gene contribute to the ageing-related decline in zinc status? Proc Nutr Soc. 2009;68:142–7. doi: 10.1017/S0029665109001104. [DOI] [PubMed] [Google Scholar]
- 39.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12:949–57. [PubMed] [Google Scholar]
- 40.Wang J, Wu Z, Li D, Li N, Dindot SV, Satterfield MC, et al. Nutrition, epigenetics, and metabolic syndrome. Antioxid Redox Signal. 2012;17:282–301. doi: 10.1089/ars.2011.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park LK, Friso S, Choi SW. Nutritional influences on epigenetics and age-related disease. Proc Nutr Soc. 2011;71:75–83. doi: 10.1017/S0029665111003302. [DOI] [PubMed] [Google Scholar]
- 42.Ho E, Ames BN. Low intracellular zinc induces oxidative DNA damage, disrupts p53, NFkappa B, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc Natl Acad Sci U S A. 2002;99:16770–5. doi: 10.1073/pnas.222679399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho E, Quan N, Tsai YH, Lai W, Bray TM. Dietary zinc supplementation inhibits NFkappaB activation and protects against chemically induced diabetes in CD1 mice. Exp Biol Med (Maywood) 2001;226:103–11. doi: 10.1177/153537020122600207. [DOI] [PubMed] [Google Scholar]
- 44.Wong CP, Song Y, Elias VD, Magnusson KR, Ho E. Zinc supplementation increases zinc status and thymopoiesis in aged mice. J Nutr. 2009;139:1393–7. doi: 10.3945/jn.109.106021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inaba K, Swiggard WJ, Steinman RM, Romani N, Schuler G. Isolation of dendritic cells. In: Coligan, et al., editors. Current protocols in immunology. Unit 3.7. Chapter 3. New York: Greene Publishing Associates & Wiley-Interscience; 1998. pp. 3.7.1–3.7.15. [Google Scholar]
- 46.Weischenfeldt J, Porse B. Bone Marrow-Derived Macrophages (BMM): Isolation and Applications. CSH protocols. 2008;2008 doi: 10.1101/pdb.prot5080. pdb prot5080. [DOI] [PubMed] [Google Scholar]
- 47.Verbanac D, Milin C, Domitrovic R, Giacometti J, Pantovic R, Ciganj Z. Determination of standard zinc values in the intact tissues of mice by ICP spectrometry. Biol Trace Elem Res. 1997;57:91–6. doi: 10.1007/BF02803873. [DOI] [PubMed] [Google Scholar]
- 48.Kahmann L, Uciechowski P, Warmuth S, Plumakers B, Gressner AM, Malavolta M, et al. Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation Res. 2008;11:227–37. doi: 10.1089/rej.2007.0613. [DOI] [PubMed] [Google Scholar]
- 49.King JC, Keen CL. Zinc. In: Shils ME, Olson JA, Shike M, Ross AC, editors. Modern nutrition in health and disease. 9. Philadelphia: Lea & Febiger; 1999. pp. 223–39. [Google Scholar]
- 50.Bao B, Prasad AS, Beck FW, Bao GW, Singh T, Ali S, et al. Intracellular free zinc up-regulates IFN-gamma and T-bet essential for Th1 differentiation in Con-A stimulated HUT-78 cells. Biochem Biophys Res Commun. 2011;407:703–7. doi: 10.1016/j.bbrc.2011.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M. Overview of mammalian zinc transporters. Cell Mol Life Sci. 2004;61:49–68. doi: 10.1007/s00018-003-3148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aydemir TB, Blanchard RK, Cousins RJ. Zinc supplementation of young men alters metallothionein, zinc transporter, and cytokine gene expression in leukocyte populations. Proc Natl Acad Sci U S A. 2006;103:1699–704. doi: 10.1073/pnas.0510407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Overbeck S, Uciechowski P, Ackland ML, Ford D, Rink L. Intracellular zinc homeostasis in leukocyte subsets is regulated by different expression of zinc exporters ZnT-1 to ZnT-9. J Leukoc Biol. 2008;83:368–80. doi: 10.1189/jlb.0307148. [DOI] [PubMed] [Google Scholar]
- 54.Park LK, Friso S, Choi SW. The Proceedings of the Nutrition Society. 2011. Nutritional influences on epigenetics and age-related disease; pp. 1–9. [DOI] [PubMed] [Google Scholar]
- 55.Choi SW, Friso S. Epigenetics: a new bridge between nutrition and health. Adv Nutr Res. 2011;1:8–16. doi: 10.3945/an.110.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maret W, Sandstead HH. Possible roles of zinc nutriture in the fetal origins of disease. Exp Gerontol. 2008;43:378–81. doi: 10.1016/j.exger.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Keen CL, Hanna LA, Lanoue L, Uriu-Adams JY, Rucker RB, Clegg MS. Developmental consequences of trace mineral deficiencies in rodents: acute and long-term effects. J Nutr. 2003;133:1477S–80S. doi: 10.1093/jn/133.5.1477S. [DOI] [PubMed] [Google Scholar]
- 58.Wallwork JC, Duerre JA. Effect of zinc deficiency on methionine metabolism, methylation reactions and protein synthesis in isolated perfused rat liver. J Nutr. 1985;115:252–62. doi: 10.1093/jn/115.2.252. [DOI] [PubMed] [Google Scholar]
- 59.Sharif R, Thomas P, Zalewski P, Fenech M. The role of zinc in genomic stability. Mutat Res. 2011;733:111–21. doi: 10.1016/j.mrfmmm.2011.08.009. [DOI] [PubMed] [Google Scholar]