Abstract
Chronic degenerative diseases are increasing with the aging U.S. population. One consequence of this phenomenon is the need for long-term osteoporosis therapies. Parathyroid hormone (PTH), the only FDA-approved treatment that adds bone to the aged skeleton, loses its potency within two years of initial treatment but the mechanism regulating its limited “anabolic window” is unknown. We have discovered that disabling the nucleocytoplasmic shuttling transcription factor nuclear matrix protein 4/cas interacting zinc finger protein (Nmp4/CIZ) in mice extends the PTH bone-forming capacity. Nmp4 was discovered during our search for nuclear matrix transcription factors that couple this hormone’s impact on osteoblast cytoskeletal and nuclear organization with its anabolic capacity. CIZ was independently discovered as a protein that associates with the focal adhesion-associated mechanosensor p130Cas. The Nmp4/CIZ-knockout (KO) skeletal phenotype exhibits a modestly enhanced bone mineral density but manifests an exaggerated response to both PTH and to BMP2 and is resistant to disuse-induced bone loss. The cellular basis of the global Nmp4/CIZ-KO skeletal phenotype remains to be elucidated but may involve an expansion of the bone marrow osteoprogenitor population along with modestly enhanced osteoblast and osteoclast activities supporting anabolic bone turnover. As a shuttling Cys2His2 zinc finger protein, Nmp4/CIZ acts as a repressive transcription factor perhaps associated with epigenetic remodeling complexes, but the functional significance of its interaction with p130Cas is not known. Despite numerous remaining questions, Nmp4/CIZ provides insights into how the anabolic window is regulated, and itself may provide an adjuvant therapy target for the treatment of osteoporosis by extending PTH anabolic efficacy.
Keywords: BMP2, osteoblasts, osteoclast, osteoporosis, osteoprogenitors, p130Cas
I. THE PARATHYROID HORMONE ANABOLIC WINDOW
As the U.S. population ages1 and life expectancy continues to increase,2 more people experience chronic degenerative diseases3 as observed with the burgeoning osteoporosis epidemic.4,5 This necessitates improvements in strategies for long-term osteoporosis therapy.
Bisphosphonates, which are antiresorptive drugs, are the most prescribed medication for the management of osteoporosis and are highly effective at reducing the risk of osteoporotic fractures,6 but recent concerns have emerged that their suppression of bone turnover or remodeling may actually weaken the skeleton after years of treatment.7–9 Additionally, bisphosphonates are only marginally effective in subjects that present with significant loss of bone mineral density.10
Ideally, the optimum strategy for long-term osteoporosis therapy is to increase skeletal turnover or remodeling with a positive bone balance (anabolic remodeling), thus improving biomechanical properties while adding more bone.11,12 Bone remodeling occurs in the basic multicellular unit (BMU) and is the process by which osteoblasts synthesize new bone within pits that have been excavated by osteoclasts.12 This bone resorption and formation process is temporally and spatially linked, occurs throughout the life of the skeleton, contributes to maintaining the biomechanical integrity of the bone, and maintains calcium and phosphorous exchange between the skeleton and extracellular fluid. Bone modeling differs from remodeling in that bone formation and resorption are uncoupled in both time and space; this process mediates changes in bone size and shape during development.12
Parathyroid hormone (PTH) is the sole FDA-approved anabolic therapy for osteoporosis and indeed enhances anabolic modeling and remodeling; however, its bone-forming activity is intrinsically limited. Specifically, treatment with PTH induces a rapid increase in bone-formation markers, followed by increases in bone-resorption markers, opening an “anabolic window,” defined as the time period when this drug is maximally anabolic.13–16 Intermittent doses enhance anabolic remodeling and modeling and thus significantly increase bone formation resulting in reduced risk of fractures.12,17 Within this window, PTH may initially induce modeling by activating the bone lining cells followed by increases in remodeling activity, which comprises the most significant aspect of the anabolic effect in humans.18–20
A number of clinical strategies have been evaluated for keeping the anabolic window open for extended periods of time with limited success. Briefly, coadministration of PTH with antiresorptives (e.g., bisphosphonates or raloxifene) failed to enhance the anabolic effect.21–23 The cyclical administration of PTH (alternating three months on/off) versus daily treatment did not extend the anabolic window.16 Furthermore, even a one-year PTH “drug holiday” did not improve the attenuated response to this hormone.24
The mechanisms regulating the extent of the PTH anabolic window are unknown, but we recently demonstrated that disabling the nucleocytoplasmic shuttling transcription factor Nmp4/CIZ in mice extends and enhances PTH anabolic action.25,26 Therefore, targeting the Nmp4/CIZ-pathway is a potential adjuvant treatment for enhancing anabolic remodeling-based osteoporosis therapy.27 This, however, first requires an understanding of how this novel restraining arm of bone anabolism works. Here, we review the present knowledge on Nmp4/CIZ within the context of a historical perspective and describe current areas of investigation.
II. HISTORICAL PERSPECTIVE: THE SEARCH FOR MECHANISMS THAT COUPLE CHANGES IN CELL MORPHOLOGY/ADHESION WITH GENE EXPRESSION
Two research groups independently discovered Nmp4/CIZ by asking distinct but ultimately related questions concerning the more mechanical aspects of signal transduction and gene expression. The late Hisamaru Hirai and colleagues first cloned CIZ from a screen for binding partners of the focal adhesion-associated adaptor protein p130Cas.28 As an adaptor protein, p130Cas controls several signaling events by undergoing changes in phosphorylation and associating with numerous effector proteins in multimolecular complexes.29,30 It is responsive to integrin stimulation and participates in the remodeling of actin stress fibers.31 As a primary mechanical sensor, the mechanical extension or unfolding of p130Cas during cell stretching favors its phosphorylation by c-Src, which in turn triggers downstream mechanotransduction signaling cascades.32 The p130Cas-signaling hub governs proliferation, cell survival, and adhesion/migration by regulating many signaling pathways including PI3K-Akt, GSK-3beta, Erk, and the nongenomic effects of ERalpha.29,30,33 This adaptor protein also plays a significant role in the fusion of osteoclast precursors and osteoclast adhesion.34–36 The Hirai study showed that CIZ shuttles between the cytoplasm and the nucleus and as a transcription factor binds to an AT-rich consensus sequence found in the gene regulatory regions of numerous matrix metalloproteinases.28
PTH induces changes in osteoblast shape,37,38 cytoskeletal organization,37,39 nuclear organization, 40,41 and the activity of focal adhesions.42 Thus, we inquired whether this shift in cell shape mechanically alters gene expression critical to its anabolic effect.43 Earlier work by Maniotis et al.,44 Dhawan et al.,45 and Thomas et al.46 had demonstrated physical and functional connections between integrin receptors, the cytoskeleton, nuclear shape, DNA, and gene expression. We proposed that as part of the substructure of the cell, nuclear matrix architectural transcription factors convert alterations in cell shape/adhesion into bends and twists of target genes, which in turn could alter protein-protein interactions between transacting factors that regulate activity.43 Consistent with this hypothesis, we characterized a PTH-responsive, nuclear matrix protein DNA-binding activity along the AT-rich regulatory region of the rat type I collagen gene that we designated as Nmp4.47 Binding to the minor groove of AT-rich DNA is the preferred consensus sequence for some architectural transcription factors,48 and we demonstrated that Nmp4 indeed binds to DNA in this manner and is capable of bending the type I collagen gene.47
Combining these two different pictures of Nmp4/CIZ led us to propose the concept of the mechanosome, the functional interaction between a mechanosensor associated with an adhesion complex and a shuttling architectural transcription factor.49,50 In this hypothetical scheme, the mechanosome converts the unfolding of p130Cas in the cytoplasm to Nmp4/CIZ-mediated DNA bending in the nucleus. This idea was derived in part from earlier studies on the role of beta-catenin/LEF-1 in mediating osteoblast response to mechanical load.51,52 LEF-1 is also an architectural transcription factor,53 and beta-catenin is part of a complex of proteins that constitute adherens junctions; LEF-1/beta-catenin is an integral component of the Wnt signaling pathway, which is a central regulator of skeletal modeling and remodeling.54
Although ubiquitously expressed,55 the underlying assumption was that the impact of Nmp4/CIZ on bone phenotype, and thus on the mechanosome, was specific to its control of the osteoblast.49,56 However, a recent study from our laboratory suggests that this protein regulates osteoclasts as well, and thus governs both the formation and resorption arms of bone remodeling.25 This suggests a need to expand the mechanosome hypothesis, which we take up again at the end of this review.
III. Nmp4/CIZ GLOBAL KNOCKOUT PHENOTYPE: NO GROSS ABNORMALITIES, NORMAL LIFE SPAN
A global “CIZ-deficient” mouse57 and a global Nmp4-knockout (KO) mouse26 were constructed independently by different homologous recombination strategies. The CIZ-deficient mouse was prepared by introducing β-galactosidase fused to the CIZ-N terminus and neo resistance gene into the second exon of the CIZ gene.57 The Nmp4-KO mouse was prepared by replacing exons 4–7 with the neo gene cassette.26 The description of the baseline phenotype of the Nmp4-KO mouse was determined from two colony populations: (i) the KO and wild-type (WT) F2 progeny of the mating of the first heterozygotes, which were a hybrid of 129SvEv and C57BL/6J, and (ii) these F2 mice that had been backcrossed six generations onto a C57BL/6J background (these N6 animals were compared to WT C57BL/6J mice).26 The baseline phenotype of the CIZ-deficient mouse was described by comparing WT and null mice on a DBA (25%) × C57BL/6 F2 background that had been derived from the original heterozygous cross.56,57
Nmp4/CIZ is ubiquitously expressed, yet the global Nmp4-KO and CIZ-deficient mice are healthy, have a normal lifespan, and are free of any gross morphological defects.26,56,57 Both the Nmp4-KO F2 and CIZ-deficient male and female mice show a modest (4–10%), but significant, decrease in body weight compared to their WT littermates, and this phenotype appears to be retained throughout adulthood.26,57 We have not observed this decreased body weight in the N6 Nmp4-KO mice.26 The male CIZ-deficient mice exhibit variable degrees of spermatogenic cell degeneration within the seminiferous tubules, and these animals suffer from sporadic infertility.57 Our anecdotal observations on the Nmp4-KO males are consistent with this aspect of the phenotype. Additionally, we have reported that the Nmp4-KO litters are modestly, but significantly, smaller than WT litters.26 Finally, the Mendelian frequencies expected from matings between Nmp4 heterozygous mice show a significantly diminished yield of KO pups, suggesting that some of the null embryos die in utero.26
IV. Nmp4/CIZ GLOBAL KNOCKOUT SKELETAL PHENOTYPE: MODESTLY ENHANCED BASELINE BONE MINERAL DENSITY, YET EVIDENCE FOR AN OVERACTIVE OSTEOCLAST
The baseline skeletal phenotypes of the Nmp4-KO and CIZ-deficient mice at eight weeks of age are similar with some important distinctions, which may be due to the differences in strain backgrounds.26,56 Both the Nmp4-KO and CIZ-deficient mice exhibited modest but significantly enhanced bone mineral density and bone mineral content throughout the skeleton. Additionally, we observed no significant difference in Nmp4-KO and WT femur lengths.26 Although significant differences in trabecular bone were not detected in the Nmp4-KO mice, the CIZ-deficient animals exhibited a 40% increase in femoral BV/TV and 15% increase in vertebral BV/TV. Furthermore, there was a modest, but significant, increase in matrix apposition rate and bone formation rate in the femoral cross sections evaluated in the CIZ-deficient mice compared to the WT animals.56
On the other hand, the Nmp4-KO mice exhibited an osteoclast phenotype,25 which was absent in the CIZ-deficient animals.56 Specifically, the Nmp4-KO mice showed elevated serum C-terminal telopeptides (CTXs), a marker for resorption over a seven-week time-course experiment (10–17 weeks of age).25 Additionally, the null marrow from eight-week-old mice yielded twofold more osteoclasts than did WT marrow, and the isolated Nmp4-KO osteoclasts exhibited an elevated resorption activity compared to the WT osteoclasts.25 Histomorphometric analysis revealed an elevated baseline number of tartrate-resistant acid phosphatase positive-staining cells (TRAP+ n/BS) in 17-week-old Nmp4-KO mice, which provides an estimate of the number of osteoclast precursors and mature osteoclasts normalized to the bone surface.25 This is consistent with our recent evidence showing that the null mice exhibited a modest but significant increase in CFU-GM cells (osteoclast precursors) compared to their WT littermates.58,59
V. Nmp4/CIZ GLOBAL KNOCKOUT SKELETAL PHENOTYPE: AN EXTENDED PTH ANABOLIC WINDOW, ENHANCED RESPONSE TO BMP2, AND RESISTANCE TO DISUSE-INDUCED BONE LOSS
The response of the Nmp4-KO mouse to intermittent doses of PTH demonstrates that the length and amplitude of the anabolic window has been expanded in these animals.25,26 In a typical experiment, female WT and null mice were sorted into four treatment groups and administered human PTH(1–34), 30μg/kg/day, or vehicle control for seven weeks (10–17 weeks of age). Serum analysis revealed that osteocalcin levels, a marker for bone formation and osteoblast number, in the WT animals significantly increased with PTH, peaked at three weeks of treatment, and declined to baseline levels by seven weeks of hormone challenge.25 In contrast, the PTH-induced increase in serum osteocalcin in the Nmp4-KO mice continued to rise throughout the duration of the seven-week regimen.25 Evaluation of both the raw and the percent change osteocalcin data using a two-way ANOVA indicated a strong interaction between the independent variables of genotype and treatment.25 As described above, the null mice showed elevated serum CTX compared to the WT mice and PTH stimulated a near-significant increase in both genotypes, but evaluation of both the raw data and the percent change data showed no interaction between the independent variables of genotype and treatment.25 At the end of seven weeks of treatment, the KO mice manifested a strikingly enhanced PTH-induced increase in trabecular bone while maintaining robust increases in cortical bone as compared to WT animals. For example, the null mice gained approximately 2.6-fold more femoral trabecular bone (BV/TV), 1.4-fold more L5 vertebral bone, and 1.1-fold higher tibial trabecular bone, all showing significant genotype x treatment interactions.25,26
Interestingly, the WT and Nmp4-KO mice exhibited equivalent PTH-induced gains in trabecular bone during the first two weeks of treatment, but at three weeks the null mice showed significantly more hormone-stimulated addition of bone than did the WT animals, which coincided with the serum osteocalcin profiles.25,59 The femoral bone histomorphometry measurements at the end of the seven-week treatment period showed no difference in bone formation rate (BFR) between the genotypes,25 but these experiments did not analyze vertebral or tibial histomorphmetry at this time point to determine the basis of the continued increase in serum osteocalcin. Further histomorphometric and bone microarchitecture analyses at seven weeks treatment and beyond are required to fully characterize this extended anabolic window.
The WT and KO femoral mRNA profiles harvested at two weeks and three weeks of treatment (12–13 weeks of age) represented a composite of multiple marrow and bone cell types, but provided clues as to the early events leading toward the development of the enhanced PTH-stimulated bone formation response in the null mice.25 Consistent with the two-week μCT data showing equivalent amounts of new bone added in this early phase of treatment, the WT and KO mRNA profiles showed similar PTH-induced increases in the expression of genes that support bone formation, e.g., Runx2, Osterix, Col1a1, Alpl (alkaline phosphatase), and Pthr1.25 However, there was a transient enhanced response of the AP-1 transcription factors c-fos and Fra-2 to PTH in the Nmp4-KO mice, and there was an elevated baseline expression of Nfatc1 at two weeks but not three weeks into the treatment regimen.25 These transcription factors have numerous important functional roles in bone physiology, but are particularly significant to the development of the osteoblast, osteoclast, and chondrocyte.60–62 The significance of the transient nature of this increase in gene expression remains to be determined, but may involve the enhanced hormone mobilization of both osteoprogenitors and osteoclast progenitors at the beginning of the anabolic window. Additionally, the null mice exhibited a significant decrease in the baseline expression of the proapoptotic gene Bax, irrespective of treatment, perhaps indicative of longer-lived osteoblasts supporting the enhanced bone formation. Finally, the Rankl/Opg ratio was diminished in the Nmp4-KO animals compared to WT mice at two and three weeks of treatment, which was significant at the later time point. This is consistent with a null bone microenvironment permissive for bone formation.25
In addition to its role extending the PTH anabolic window, Nmp4/CIZ also plays a role in BMP2-induced increase in bone formation.56 Briefly, eight-week-old female CIZ-deficient mice received rhBMP2 (5 μg) injections onto the parietal bones every other day for 10 days. Soft X-ray analysis demonstrated that the CIZ-deficient mice formed twofold more new bone than their WT counterparts during the same 10-day treatment period.56 In a related experiment from the same study, CIZ deficiency also significantly enhanced the formation of new bone during the recovery from surgical marrow ablation.56
Another remarkable aspect the CIZ-deficient skeletal phenotype is its resistance to disuse-induced bone loss.63 In these experiments, 10-week-old male CIZ-deficient and WT mice were subjected to hind limb unloading by tail suspension for two weeks. Unloading induced significant trabecular and cortical bone loss in the WT mice, but not in the null mice. Histomorphometric analysis showed that the unloaded WT limb exhibited decreases in mineral apposition rate (MAR), mineralizing surface per bone surface (MS/BS), and BFR in both the cancellous and cortical compartments, but these parameters were unaffected in the unloaded CIZ-deficient femur. Interestingly, bone marrow stromal cells (BMSCs) from unloaded WT mice showed a significantly decreased mineralization in culture as compared to cells from the loaded controls; however, this disparity was not observed in cultures from null mice.63 Finally, osteoclastic bone resorption parameters were similar in the genotypes irrespective of treatment. Quantification of osteoclast surface per bone surface (Oc.S/BS) and osteoclast number per bone surface (Oc.N/BS) in the cancellous bones of the tibia were not affected by unloading in either genotype. Also, the WT and CIZ-deficient bone marrow yielded equivalent numbers of osteoclasts in cultures generated from unloaded and loaded bones.63
VI. CELLULAR BASIS OF THE Nmp4/CIZ-KNOCKOUT SKELETAL PHENOTYPE: MORE BONE CELLS AND HEIGHTENED RESPONSIVENESS TO ANABOLIC STIMULI KEEPS THE ANABOLIC WINDOW OPEN
Recent data from our laboratory demonstrated that bone marrow from female Nmp4-KO mice yielded approximately fourfold more osteoprogenitor cells, as assessed by the CFU-FAlkPhos+ colony assay, than did the marrow from their WT littermates.58,59 Additionally, the null bone marrow contained twofold more CD8+ T cells than did the WT bone marrow, although there was no difference in the number of CD8+ T cells in the peripheral blood between the genotypes.58,59 Interestingly, the CD8+ T cells have been shown to support the PTH-induced anabolic response. Specifically, these cells express the PTH receptor (PTHR1) and secrete Wnt10b in response to PTH, which in turn enhances osteoblast proliferation, differentiation, and life span.64
The impact of Nmp4/CIZ on the baseline phenotype of osteoblasts and its expression during bone cell development has not been fully elucidated. BMSC-derived osteoblasts from the CIZ-deficient mice exhibited an enhanced alkaline phosphatase activity at 10 days in culture and formed more mineralized nodules than did WT cells.56 On the other hand, the calvarial-derived osteoblast-like cell line MC3T3-E1 also formed fewer mineralized nodules in culture when overexpressing CIZ.65 Consistent with these in vitro observations, mRNA expression of genes that support the osteoblast phenotype, including Col1a1, Osterix, and Opn (osteopontin), were modestly, yet significantly, elevated in the humeri of untreated CIZ-deficient mice, compared to their WT counterparts.56
Of note, we have been unable to reproducibly demonstrate a significant impact of Nmp4/CIZ on the baseline mature osteoblast phenotype using cells derived from the Nmp4-KO mice. We have not observed striking differences in population doubling times, alkaline phosphatase expression, or mineralization in primary cultures of BMSC-derived osteoblasts obtained from WT and Nmp4-null animals.26 Specifically, BMSC-derived WT and Nmp4-KO osteoblasts cultured under standard osteogenic conditions for 10–14 days66 showed no difference in basal mRNA expression of Runx2, Osterix, Alpl, Igf1, Mmp13, Rankl, Opg, osteocalcin (Oc), or Opn.26 Nor did we observe an enhanced expression of these genes in the femurs of untreated eight-week-old Nmp4-null animals save for a modest, near-significant increase in Col1a1.26 Finally, we did not discern any differences in these parameters between WT and null immortalized clonal cell lines.67
That said, osteoblasts derived from both the CIZ-deficient and Nmp4-KO mice showed a modestly heightened response to a variety of anabolic stimuli, and this characteristic likely contributes to the extended PTH anabolic window and underlies the augmented BMP2-stimulated bone formation. 65,67 As previously indicated, PTH-induced c-fos mRNA expression was transiently enhanced in Nmp4-KO femoral bone, compared to WT bone, one hour after the last hormone injection at two weeks of PTH treatment.25 Consistent with the in vivo observation, primary Nmp4-KO BMSC-derived osteoblasts and an Nmp4-null clonal cell line both showed a modest concentration-dependent enhancement in PTH-stimulated increases in c-fos mRNA expression as compared to that observed in WT bone cells.67
With regard to BMP2 responsiveness, CIZ-deficient BMSC-derived osteoblasts maintained in 100 ng/ml BMP2 for 10 days exhibited an enhanced alkaline phosphatase activity as compared to WT cells maintained under the same conditions.56 Additionally, overexpression of CIZ in MC3T3-E1 cells suppressed BMP2-enhanced mRNA expression of Alpl, Oc, and Col1a1.65 Similarly, we demonstrated enhanced responsiveness of Nmp4-KO osteoblasts to BMP2, but this sensitivity was dependent on the number of days the cells were in culture and the number of days in the presence of this growth factor.67 Specifically, the primary BMSC-derived Nmp4-null osteoblasts exhibited an enhanced BMP2-induced increase in mRNA Alpl expression after eight days in culture of which the last three days included treatment with this potent anabolic agonist. However, WT and Nmp4-KO BMSC-derived osteoblasts displayed equal increases in mRNA Alpl expression when evaluated after 10 days exposure to BMP2 and 15 days in culture.67
Nmp4/CIZ regulation of osteoblast responsiveness to anabolic stimuli does not appear to be limited to gene expression but also impacts cell signaling as well.68 As an example, primary Nmp4-KO calvarial-derived osteoblasts exhibited an enhanced oscillatory shear stress (OSS)–induced activation of the Wnt signaling pathway.68 OSS is the cellular analogue to mechanical loading, a strong anabolic agonist to bone.69 OSS induced an enhanced phosphorylation of ERK, Akt, and GSK-3beta in the Nmp4-KO osteoblasts compared to WT cells within minutes of challenge.68 Consistent with these observations, the null cells exhibited an increased OSS-stimulated beta-catenin nuclear translocation.68 Finally, in a potentially telling observation, we observed that OSS stimulated a more extensive reorganization of stress fibers and the formation of focal adhesions in the Nmp4-null cells than in the WT osteoblasts.68 This is consistent with Nmp4/CIZ binding to the focal adhesion-associated adaptor protein p130Cas.
Recent data from our laboratory indicate that Nmp4/CIZ-KO osteoblasts are not the only cell type with a heightened response to agonists that regulate bone remodeling.25 As detailed earlier, we reported that the Nmp4-KO mature osteoclasts exhibited an enhanced in vitro resorption activity in response to the cytokines RANKL/M-CSF compared to WT cells.25 This raises the question as to whether there is a common Nmp4/CIZ-driven pathway in both osteoblasts and osteoclasts, and other cell types, involved in cell response. For example, this may involve Nmp4/CIZ association with p130Cas as a central hub for regulating multiple signal transduction pathways and Nmp4/CIZ association with epigenetic remodeling proteins for regulating multiple gene targets; this universal and wide-ranging molecular mechanism could act to suppress a variety of cell type–specific responses to multiple agonists (see below for further discussion).
We propose that Nmp4 restricts the PTH anabolic window by repressing osteoprogenitor pool expansion and dampening cell sensitivity to anabolic stimuli. PTH recruits osteoprogenitors into the osteoblast differentiation pathway and enhances their survival,70–72 Thus, the observed three-week lag period before the Nmp4-KO mice exhibit enhanced hormone-stimulated bone formation and show a surge in serum osteocalcin compared to the WT animals25,59 may be due to the differential depletion of the osteoprogenitors between the genotypes as they are recruited into the anabolic window. The increased presence of the BM CD8+ T cells may bolster the expansion of the null osteoprogenitors as a consequence of enhanced secretion of Wnt10b (see Fig. 1), which, in turn, may surpass the modest elevation in bone resorption observed in the Nmp4-KO mice. Finally, the subtle increased sensitivity of the null osteoprogenitors/osteoblasts to anabolic stimuli (PTH, BMP2, load) may further augment and extend the PTH anabolic window. As mentioned previously, the discrepancies in cell phenotype (osteoblasts and osteoclasts) noted between the CIZ-deficient and Nmp4-KO mice may be due to strain differences between the mouse models. Alternatively, perhaps this marginally augmented bone-forming activity is difficult to reproduce in vitro outside the confines of the bone marrow microenvironment. The observation that the time in culture is significant to the sensitivity of the null cells to BMP2 suggests that Nmp4/CIZ impact on osteoblast phenotype may be specific to the stage of differentiation. If the disrupted association of Nmp4/CIZ and the mechanosensor p130Cas plays a significant role in the expression of the null osteoblast phenotype, then it is conceivable that subtle changes in cell culture density and adhesion could significantly impact the behavior of the cells. Clearly, more study is required on Nmp4/CIZ control of osteoblast and osteoclast phenotypes.
FIGURE 1.
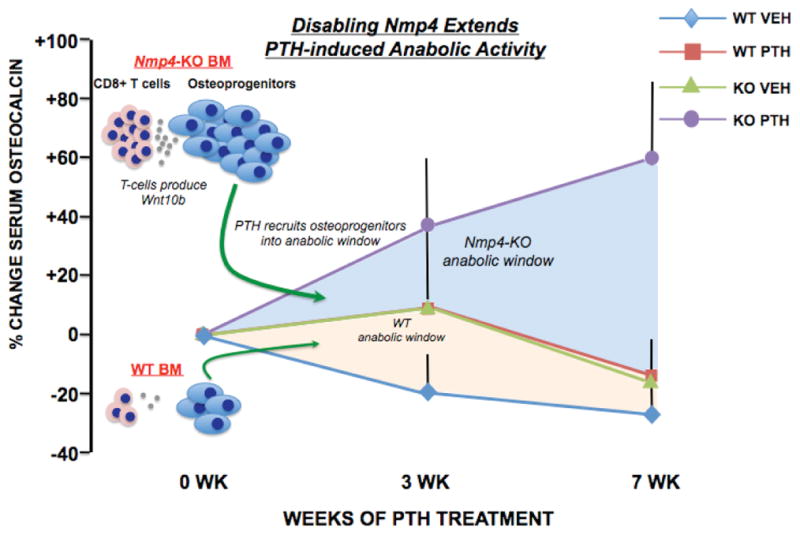
Disabing Nmp4 in mice extends the PTH anabolic window. The percent change of serum osteocalcin (a marker for bone formation and osteoblast number) in WT and Nmp4-KO mice treated with intermittent PTH or vehicle (VEH) for seven weeks (see Ref. 25 for details). Null mice treated with PTH showed an elevated and extended bone-forming period compared to WT-treated mice. We propose an expanded osteoprogenitor pool bolstered by CD8+ T cells that express Wnt10b supports this extended anabolic window.58,59
VII. Nmp4/CIZ MOLECULAR PROFILE: FUNCTIONAL DOMAINS FOR REGULATING CELL SIGNALING AND TRANSCRIPTION
Nmp4/CIZ is a nucleocytoplasmic shuttling protein, appears to be expressed in most if not all tissues, and is highly conserved between rodents and humans (~96%).55 This single-copy gene is located on mouse chromosome 6 band F1.73 The human ortholog (ZNF384) is located on chromosome 12p12 and was first identified as a partial cDNA, TNRC1 (CAGH1) from a human brain expression library.74 The rat gene gives rise to at least seven isoforms with molecular weights ranging from approximately 40 to 63 kDa, but it is unknown whether these proteins have distinct cellular functions.28,55
All Nmp4/CIZ isoforms have from five to eight Cys2His2 zinc fingers, e.g., the Nmp4-21H/CIZ6-2 isoform has six fingers (Fig. 2). The zinc fingers #2, #3, and #6 of this isoform bind to the minor groove of the unusual homopolymeric (dAdT) consensus site.75 The zinc finger domain mediates nuclear localization and association with the nuclear matrix76 (Fig. 2). The AT-hook domain of the Nmp4/CIZ protein weakly associates with the DNA major groove and may provide this protein with its capacity for bending DNA.47 The AT-hook is a common functional domain for certain architectural transcription factors such as HMGA1, proteins that regulate gene expression by bending or looping the DNA, thus altering interactions between other trans-acting proteins.48 Architectural transcription factors typically do not have a transacting domain, but numerous conventional transcription factors alter local DNA structure as part of their regulatory function. Nmp4/CIZ has context-dependent transactivation domains, i.e., their activity is dependent on the organization of the regulatory elements surrounding the Nmp4/CIZ consensus sequence75 (Fig. 2).
FIGURE 2.
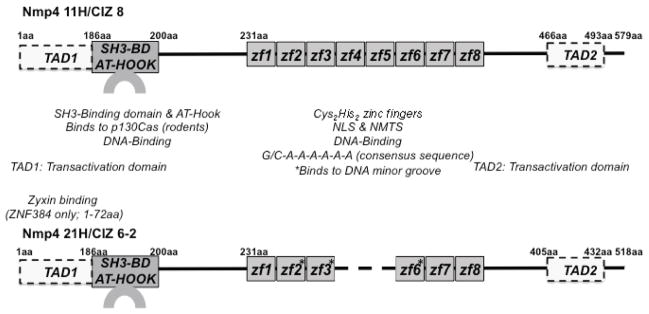
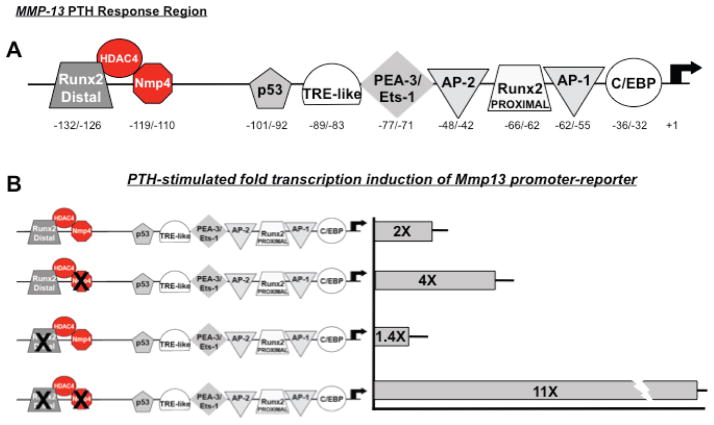
Nmp4/CIZ has the functional domains of a transcription factor and signaling molecule. Rat isoforms Nmp4 11H [CIZ 8] and Nmp4 21H [CIZ 6-2] are shown.28,55 The amino acid positions are indicated by numerals but are not positioned to scale. The Cys2His2 zinc fingers act as a nuclear localization signal (NLS) and a nuclear matrix-targeting signal (NMTS); for Nmp4 11H, the zinc fingers #4–#8 are minimally required for the NLS and NMTS.76 The Nmp4/CIZ SH3-binding domain (SH3-BD) associates with p130Cas in rodent cells; however, the absence of a single proline residue in the human ortholog ZNF384 obviates p130Cas association, but instead ZNF384 binds to zyxin.28,79 The transactivation and DNA-binding domains were mapped using Nmp4 21H.75 DNA binding to the minor groove of DNA is mediated by the zinc fingers #2, #3, and #6 (asterisks), whereas the AT-hook motif, which overlaps with the p130Cas SH3-binding sequence, weakly associates with the DNA major groove. There are context-dependent transactivation domains within the first 186aa (TAD1) of the protein and within a polyglutamine/alanine repeat (TAD2) (see Ref. 75 for full discussion).
As a transcription factor, Nmp4/CIZ often acts as a suppressor consistent with its blunting of bone response to PTH and BMP2.55,65,77 We have demonstrated that Nmp4/CIZ represses PTH-stimulated Mmp13 transcription induction.77 Mmp13 is significant to PTH-induced remodeling, and the human, rat, and mouse genes have a conserved PTH-response regulatory region. In the rat gene, this region spans nucleotides (nt) −148 to −38 and mediates the binding of multiple trans-acting proteins including Runx2, Ets-1, and cFos/c-Jun77 (Fig. 3). These proteins support hormone transcription induction but the mechanistic details with respect to their interactions have not been fully elucidated. Nmp4/CIZ binds to this PTH response region adjacent to the distal Runx2 site (Fig. 3), and to address the significance of this interaction to PTH-stimulated induction we made a series of null-binding mutations within this PTH response region, including mutating individually the Nmp4/CIZ consensus site and the Runx2 consensus site, as well as mutating both the Nmp4/CIZ and Runx2 sites in combination.77 These mutations were introduced into an Mmp13 promoter-reporter construct containing the first 1329nt of the 5′-regulatory region and transfected into UMR 106-01 osteoblast-like cells. Exposure of the cells to PTH induced a twofold increase in activity in the WT Mmp13 promoter-reporter, a fourfold increase in the construct containing the mutated Nmp4/CIZ site, a near twofold response in the construct containing the distal Runx2 binding site mutation, and an 11-fold increase in the Mmp13 promoter-reporter construct harboring both the Nmp4/CIZ and Runx2 binding site mutations77 (Fig. 3).
FIGURE 3.
Nmp4/CIZ is a repressive transcription factor that binds near an epigenetic remodeling site in the PTH response region of the Mmp13 promoter. (a) The region in the Mmp13 promoter that regulates response to PTH is conserved in mice, rats, and humans. Nmp4/CIZ binds to this region in a sequence-specific fashion between (−119/−110bp).77 The distal Runx2 site binds to HDAC4 and mediates suppression of Mmp13 transcription. Whether Nmp4 binds to Runx2 or HDAC4 is not yet known. PTH removes HDAC4 as part of the transcription induction mechanism. 78 (b) Introducing null-binding mutations in the Nmp4/CIZ-binding site within the Mmp13 promoter-reporter construct containing the first 1329nt of the 5′-regulatory region and transfected into UMR 106-01 osteoblast-like cells enhances PTH-stimulated transcription induction. Mutating the distal Runx2 site does not significantly alter PTH response, but mutating both the Runx2 and Nmp4 sites dramatically increases hormone-induced transcription (see Ref. 77).
Partridge and colleagues have recently showed that histone deacetylase 4 (HDAC4) interacts with Runx2, binds the Mmp13 promoter near the distal Runx2 consensus sequence adjacent to the Nmp4 binding site, and suppresses Mmp13 gene transcription in osteoblasts.78 PTH induced the rapid cAMP-dependent protein kinase-dependent release of HDAC4 from the promoter and subsequent transcription of Mmp13. Knockout of HDAC4 led to an increase in Mmp13 expression, and overexpression of HDAC4 decreased the PTH induction of Mmp13.78 Additionally, PTH stimulated HDAC4 gene expression and enzymatic activity at times corresponding to the reassociation of HDAC4 with the Mmp13 promoter and a decline in its transcription. These data, in conjunction with our previous study, raise the question as to whether Nmp4 plays a significant role in regulating epigenetic remodeling along this site (Fig. 3).
Nmp4/CIZ may also act as a signaling molecule. As previously described, Nmp4-KO cells exhibit an enhanced phosphorylation of ERK, Akt, and GSK-3beta within minutes of exposure68 suggesting that this nucleocytoplasmic shuttling protein has significant cytoplasmic activities. The adaptor protein p130Cas regulates these signaling pathways29,30,33 consistent with the Nmp4-p130Cas association.28 The sequence APPKPPR from the 186th amino acid residue in the rat Nmp4/CIZ ortholog corresponds to the p130Cas SH3-binding consensus sequence XXPXKPX, and it also resembles the p130Cas SH3-binding site of FAK (APPKPSR)28 (see Fig. 2). Nmp4/CIZ binding activity can distinguish between the SH3 domains of p130Cas, Abl, Ash/Grb2, Crk, Fyn, and Src as determined by far-Western screening.28 The human ortholog ZNF384 does not bind to p130Cas but instead binds to zyxin, which in turn binds to p130Cas.79 Although the rat Nmp4/CIZ and human ZNF384 are highly conserved, one proline residue in the p130Cas SH3-binding consensus sequence is absent in the latter, which prevents the human protein from binding to p130Cas directly, but instead mediates its association with zyxin and in turn zyxin binds to p130Cas in the human cell.79 Zyxin is also an adaptor protein that associates with focal adhesions.80 Interestingly, this SH3-binding functional domain overlaps with the AT-hook motif (Fig. 2). The functional significance of this juxtaposition, if any, remains to be elucidated but raises the question as to whether it mediates dual and distinct functions in the cytoplasm and nucleus.
VIII. SUMMARY: IS Nmp4/CIZ AN ADJUVANT THERAPY TARGET FOR KEEPING THE PTH ANABOLIC WINDOW OPEN?
The Nmp4/CIZ-KO mouse skeletal phenotype has potentially profound clinical significance in that it offers the first clues as to the nature of the regulation of the PTH anabolic window. Disabling this protein augments and extends PTH anabolic activity and thus might ultimately provide a novel target as the basis of an adjuvant therapy for improving this hormone’s clinical efficacy.27 An additional attractive aspect of the null skeletal phenotype is its resistance to disuse-induced bone loss since compromised mobility, common in the elderly population, often accompanies and exacerbates osteoporosis.81 Finally, disabling Nmp4 in a patient may not present problems with collateral damage since the global KO mice are healthy and long lived and appear not to suffer from the loss of this protein with the exception of sporadic male infertility.
The cellular basis of Nmp4’s control of the PTH anabolic window appears to involve its suppression of the size of the osteoprogenitor pool from which the hormone recruits osteoblasts and the repression of bone cell response to PTH and other anabolic stimuli. The modest elevation of bone resorption observed in the Nmp4-KO mice may contribute to anabolic bone turnover. Finally, the baseline increase in T cells from the knockout marrow may enhance bone gain through production of Wnt10b, a PTH response mediator.
Fully elucidating the molecular mechanisms of Nmp4/CIZ action will be of considerable importance for pursuing this molecule as a therapeutic target. WhetherNmp4/CIZ is significant to epigenetic remodeling is currently under study in our laboratory. Drugs targeting epigenetic processes, or “epidrugs,” are at the forefront of drug discovery. 82 The impact of Nmp4/CIZ on osteoblast cell signaling is intriguing, but whether its interaction with p130Cas is functionally significant to this phenomenon and the regulation of the PTH anabolic window is unclear. Finally, this question remains: Is there a single novel Nmp4/CIZ signaling-transcriptional pathway operative in both osteoblasts and osteoclasts and can this be used as the basis of a drug strategy for controlling the bone formation and bone resorption arms of the remodeling process?
Our original goal of understanding the connection between PTH-induced changes in osteoblast shape and the hormone’s anabolic effect on bone remains elusive. We have shown that Nmp4/CIZ has a significant repressive action on PTH-stimulated bone formation and that this protein has the capacity for bending DNA as an architectural transcription factor while localizing to the osteoblast nuclear matrix. As described above, others have demonstrated that Nmp4/CIZ associates with the focal adhesion-associated proteins p130Cas and zyxin; therefore, the function, location, and protein-protein associations of Nmp4/CIZ are all consistent with our original hypothesized function of carrying PTH-generated mechanical information from the membrane to the nucleus resulting in a change in bone formation. However, we still lack key evidence demonstrating that Nmp4/CIZ translates changes in cell morphology/adhesion into alterations in gene expression. In its current form, the mechanosome hypothesis is osteoblast-centric, and if the Nmp4 regulation of the PTH anabolic window involves multiple cell types, then the mechanosome hypothesis must be reevaluated in this broader context. Does Nmp4/CIZ play a similar role in many different kinds of cells within the bone marrow microenvironment and if so what is this novel function and how does it culminate in closing the PTH anabolic window?
Acknowledgments
Some of the data described in this review were supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, NIH), Grant No., DK053796 (to J.P.B.)
References
- 1.Centers for Disease Control and Prevention (CDC) Trends in aging—United States and worldwide. MMWR Morb Mortal Wkly Rep. 2003;52:101–4. 106. [PubMed] [Google Scholar]
- 2.Coles LS. Demographics of human supercentenarians and the implications for longevity medicine. Ann NY Acad Sci. 2004;1019:490–5. doi: 10.1196/annals.1297.090. [DOI] [PubMed] [Google Scholar]
- 3.Blacklow RS. Actuarially speaking: an overview of life expectancy. What can we anticipate? Am J Clin Nutr. 2007;86:1560S–2S. doi: 10.1093/ajcn/86.5.1560S. [DOI] [PubMed] [Google Scholar]
- 4.Brecher E. Osteoporosis-Breaking the silence. Univ Toronto Med J. 2010;88:44–50. [Google Scholar]
- 5.Karasik D. Osteoporosis: an evolutionary perspective. Hum Genet. 2008;124:349–56. doi: 10.1007/s00439-008-0559-8. [DOI] [PubMed] [Google Scholar]
- 6.Beard MK. Bisphosphonate therapy for osteoporosis: combining optimal fracture risk reduction with patient preference. Curr Med Res Opin. 2012;28:141–7. doi: 10.1185/03007995.2011.643296. [DOI] [PubMed] [Google Scholar]
- 7.Rizzoli R, Akesson K, Bouxsein M, Kanis JA, Napoli N, Papapoulos S, Reginster JY, Cooper C. Subtrochanteric fractures after long-term treatment with bisphosphonates: a European Society on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, and International Osteoporosis Foundation Working Group Report. Osteoporos Int. 2011;22:373–90. doi: 10.1007/s00198-010-1453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Saag KG, Curtis JR. Long-term safety concerns of antiresorptive therapy. Rheum Dis Clin North Am. 2011;37:387–400. doi: 10.1016/j.rdc.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Park-Wyllie LY, Mamdani MM, Juurlink DN, Hawker GA, Gunraj N, Austin PC, Whelan DB, Weiler PJ, Laupacis A. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA. 2011;305:783–9. doi: 10.1001/jama.2011.190. [DOI] [PubMed] [Google Scholar]
- 9.Park-Wyllie LY, Mamdani MM, Juurlink DN, Hawker GA, Gunraj N, Austin PC, Whelan DB, Weiler PJ, Laupacis A. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA. 2011;305:783–789. doi: 10.1001/jama.2011.190. [DOI] [PubMed] [Google Scholar]
- 10.Stroup J, Kane MP, Abu-Baker AM. Teriparatide in the treatment of osteoporosis. Am J Health Syst Pharm. 2008;65:532–9. doi: 10.2146/ajhp070171. [DOI] [PubMed] [Google Scholar]
- 11.Trivedi R, Goswami R, Chattopadhyay N. Investigational anabolic therapies for osteoporosis. Expert Opin Investigat Drugs. 2010;19:995–1005. doi: 10.1517/13543784.2010.501077. [DOI] [PubMed] [Google Scholar]
- 12.Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res. 2005;20:177–84. doi: 10.1359/JBMR.041114. [DOI] [PubMed] [Google Scholar]
- 13.Cusano NE, Bilezikian JP. Combination antiresorptive and osteoanabolic therapy for osteoporosis: we are not there yet. Curr Med Res Opin. 2011;27:1705–7. doi: 10.1185/03007995.2011.599837. [DOI] [PubMed] [Google Scholar]
- 14.Cusano NE, Bilezikian JP. Teriparatide: Variations on the theme of a 2-year therapeutic course. IBMS BoneKEy. 2010;7:84–7. [Google Scholar]
- 15.Bilezikian JP. Combination anabolic and antiresorptive therapy for osteoporosis: opening the anabolic window. Curr Osteoporos Rep. 2008;6:24–30. doi: 10.1007/s11914-008-0005-9. [DOI] [PubMed] [Google Scholar]
- 16.Cosman F, Nieves J, Zion M, Woelfert L, Luckey M, Lindsay R. Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med. 2005;353:566–75. doi: 10.1056/NEJMoa050157. [DOI] [PubMed] [Google Scholar]
- 17.Ma YL, Zeng Q, Donley DW, Ste-Marie LG, Gallagher JC, Dalsky GP, Marcus R, Eriksen EF. Teriparatide increases bone formation in modeling and remodeling osteons and enhances IGF-II immunoreactivity in postmenopausal women with osteoporosis. J Bone Miner Res. 2006;21:855–64. doi: 10.1359/jbmr.060314. [DOI] [PubMed] [Google Scholar]
- 18.Khosla S, Westendorf JJ, Oursler MJ. Building bone to reverse osteoporosis and repair fractures. J Clin Invest. 2008;118:421–8. doi: 10.1172/JCI33612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Compston JE. Skeletal actions of intermittent parathyroid hormone: effects on bone remodeling and structure. Bone. 2007;40:1447–52. doi: 10.1016/j.bone.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology. 1995;136:3632–8. doi: 10.1210/endo.136.8.7628403. [DOI] [PubMed] [Google Scholar]
- 21.Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2010;95:1838–45. doi: 10.1210/jc.2009-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cosman F, Wermers R, Recknor C, Mauck K, Xie L, Glass EV, Krege JH. Effects of teriparatide in postmenopausal women with osteoporosis on prior alendronate or raloxifene: differences between stopping and continuing the antiresorptive agent. J Clin Endocrinol Metab. 2009;94:3772–80. doi: 10.1210/jc.2008-2719. [DOI] [PubMed] [Google Scholar]
- 23.Finkelstein J, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003;349:1216–26. doi: 10.1056/NEJMoa035725. [DOI] [PubMed] [Google Scholar]
- 24.Finkelstein JS, Wyland JJ, Leder BZ, Burnett-Bowie SM, Lee H, Jüppner H, Neer RM. Effects of teriparatide retreatment in osteoporotic men and women. J Clin Endocrinol Metab. 2009;94:2495–501. doi: 10.1210/jc.2009-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Childress P, Philip BK, Robling AG, Bruzzaniti A, Kacena MA, Bivi N, Plotkin LI, Heller A, Bidwell JP. Nmp4/CIZ suppresses the response of bone to anabolic parathyroid hormone by regulating both osteoblasts and osteoclasts. Calcif Tissue Int. 2011;89:74–89. doi: 10.1007/s00223-011-9496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robling AG, Childress P, Yu J, Cotte J, Heller A, Philip BK, Bidwell JP. Nmp4/CIZ suppresses parathyroid hormone-induced increases in trabecular bone. J Cell Physiol. 2009;219:734–43. doi: 10.1002/jcp.21717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krane SM. Identifying genes that regulate bone remodeling as potential therapeutic targets. J Exp Med. 2005;201:841–3. doi: 10.1084/jem.20050354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamoto T, Yamagata T, Sakai R, Ogawa S, Honda H, Ueno H, Hirano N, Yazaki Y, Hirai H. CIZ, a zinc finger protein that interacts with p130(cas) and activates the expression of matrix metalloproteinases. Mol Cell Biol. 2000;20:1649–58. doi: 10.1128/mcb.20.5.1649-1658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Stefano P, Leal MP, Tornillo G, Bisaro B, Repetto D, Pincini A, Santopietro E, Sharma N, Turco E, Cabodi S, Defilippi P. The adaptor proteins p140CAP and p130CAS as molecular hubs in cell migration and invasion of cancer cells. Am J Cancer Res. 2011;1:663–73. [PMC free article] [PubMed] [Google Scholar]
- 30.Tikhmyanova N, Little JL, Golemis EA. CAS proteins in normal and pathological cell growth control. Cell Mol Life Sci. 2010;67:1025–48. doi: 10.1007/s00018-009-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abassi YA, Rehn M, Ekman N, Alitalo K, Vuori K. p130Cas couples the tyrosine kinase Bmx/Etk with regulation of the actin cytoskeleton and cell migration. J Biol Chem. 2003;278:35636–43. doi: 10.1074/jbc.M306438200. [DOI] [PubMed] [Google Scholar]
- 32.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–26. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cabodi S, Moro L, Baj G, Smeriglio M, Di Stefano P, Gippone S, Surico N, Silengo L, Turco E, Tarone G, Defilippi P. p130Cas interacts with estrogen receptor alpha and modulates non-genomic estrogen signaling in breast cancer cells. J Cell Sci. 2004;117:1603–11. doi: 10.1242/jcs.01025. [DOI] [PubMed] [Google Scholar]
- 34.Vives V, Laurin M, Cres G, Larrousse P, Morichaud Z, Noel D, Côté JF, Blangy A. The Rac1 exchange factor Dock5 is essential for bone resorption by osteoclasts. J Bone Miner Res. 2011;26:1099–110. doi: 10.1002/jbmr.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalo P, Guadamillas MC, Hernández-Riquer MV, Pollán A, Grande-García A, Bartolomé RA, Vasanji A, Ambrogio C, Chiarle R, Teixidó J, Risteli J, Apte SS, del Pozo MA, Arroyo AG. MT1-MMP is required for myeloid cell fusion via regulation of Rac1 signaling. Dev Cell. 2010;18:77–89. doi: 10.1016/j.devcel.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura I, Duong le T, Rodan SB, Rodan GA. Involvement of alpha(v)beta3 integrins in osteoclast function. J Bone Miner Metab. 2007;25:337–44. doi: 10.1007/s00774-007-0773-9. [DOI] [PubMed] [Google Scholar]
- 37.Egan JJ, Gronowicz G, Rodan GA. Parathyroid hormone promotes the disassembly of cytoskeletal actin and myosin in cultured osteoblastic cells: mediation by cyclic AMP. J Cell Biochem. 1991;45:101–11. doi: 10.1002/jcb.240450117. [DOI] [PubMed] [Google Scholar]
- 38.Matthews JL, Talmage RV. Influence of parathyroid hormone on bone cell ultrastructure. Clin Orthop Relat Res. 1981;156:27–38. [PubMed] [Google Scholar]
- 39.Zhang J, Ryder KD, Bethel JA, Ramirez R, Duncan RL. PTH-induced actin depolymerization increases mechanosensitive channel activity to enhance mechanically stimulated Ca2+ signaling in osteoblasts. J Bone Miner Res. 2006;21:1729–37. doi: 10.1359/jbmr.060722. [DOI] [PubMed] [Google Scholar]
- 40.Feister HA, Onyia JE, Miles RR, Yang X, Galvin R, Hock JM, Bidwell JP. The expression of the nuclear matrix proteins NuMA, topoisomerase II-alpha, and-beta in bone and osseous cell culture: regulation by parathyroid hormone. Bone. 2000;26:227–34. doi: 10.1016/s8756-3282(99)00269-0. [DOI] [PubMed] [Google Scholar]
- 41.Torrungruang K, Feister H, Swartz D, Hancock EB, Hock J, Bidwell JP. Parathyroid hormone regulates the expression of the nuclear mitotic apparatus protein in the osteoblast-like cells, ROS 17/2.8. Bone. 1998;22:317–24. doi: 10.1016/s8756-3282(97)00300-1. [DOI] [PubMed] [Google Scholar]
- 42.Davies J, Chambers TJ. Parathyroid hormone activates adhesion in bone marrow stromal precursor cells. J Endocrinol. 2004;180:505–13. doi: 10.1677/joe.0.1800505. [DOI] [PubMed] [Google Scholar]
- 43.Bidwell JP, Alvarez M, Feister H, Onyia J, Hock J. Nuclear matrix proteins and osteoblast gene expression. J Bone Miner Res. 1998;13:155–67. doi: 10.1359/jbmr.1998.13.2.155. [DOI] [PubMed] [Google Scholar]
- 44.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94:849–54. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhawan J, Lichtler AC, Rowe DW, Farmer SR. Cell adhesion regulates pro-alpha 1(I) collagen mRNA stability and transcription in mouse fibroblasts. J Biol Chem. 1991;266:8470–5. [PubMed] [Google Scholar]
- 46.Thomas CH, Collier JH, Sfeir CS, Healy KE. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc Natl Acad Sci U S A. 2002;99:1972–7. doi: 10.1073/pnas.032668799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alvarez M, Thunyakitpisal P, Morrison P, Onyia J, Hock J, Bidwell JP. PTH responsive osteoblast nuclear matrix architectural transcription factor binds to the rat type I collagen promoter. J Cell Biochem. 1998;69:336–52. doi: 10.1002/(sici)1097-4644(19980601)69:3<336::aid-jcb11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 48.Alvarez M, Rhodes SJ, Bidwell JP. Context-dependent transcription: all politics is local. Gene. 2003;313:43–57. doi: 10.1016/s0378-1119(03)00627-9. [DOI] [PubMed] [Google Scholar]
- 49.Bidwell JP, Pavalko FM. Mechanosomes carry a loaded message. Sci Signal. 2010;3:pe51. doi: 10.1126/scisignal.3153pe51. [DOI] [PubMed] [Google Scholar]
- 50.Pavalko FM, Norvell SM, Burr DB, Turner CH, Duncan RL, Bidwell JP. A model for mechanotransduction in bone cells: the load-bearing mechanosomes. J Cell Biochem. 2003;88:104–12. doi: 10.1002/jcb.10284. [DOI] [PubMed] [Google Scholar]
- 51.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–8. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 52.Norvell SM, Alvarez M, Bidwell JP, Pavalko FM. Fluid shear stress induces beta-catenin signaling in osteoblasts. Calcif Tissue Int. 2004;75:396–404. doi: 10.1007/s00223-004-0213-y. [DOI] [PubMed] [Google Scholar]
- 53.Giese K, Cox J, Grosschedl R. The HMG domain of lymphoid enhancer factor 1 bends DNA and facilitates assembly of functional nucleoprotein structures. Cell. 1992;69:185–95. doi: 10.1016/0092-8674(92)90129-z. [DOI] [PubMed] [Google Scholar]
- 54.Williams BO, Insogna KL. Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. J Bone Miner Res. 2009;24:171–8. doi: 10.1359/jbmr.081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thunyakitpisal P, Alvarez M, Tokunaga K, Onyia JE, Hock J, Ohashi N, Feister H, Rhodes SJ, Bidwell JP. Cloning and functional analysis of a family of nuclear matrix transcription factors (NP/NMP4) that regulate type I collagen expression in osteoblasts. J Bone Miner Res. 2001;16:10–23. doi: 10.1359/jbmr.2001.16.1.10. [DOI] [PubMed] [Google Scholar]
- 56.Morinobu M, Nakamoto T, Hino K, Tsuji K, Shen ZJ, Nakashima K, Nifuji A, Yamamoto H, Hirai H, Noda M. The nucleocytoplasmic shuttling protein CIZ reduces adult bone mass by inhibiting bone morphogenetic protein-induced bone formation. J Exp Med. 2005;201:961–70. doi: 10.1084/jem.20041097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakamoto T, Shiratsuchi A, Oda H, Inoue K, Matsumura T, Ichikawa M, Saito T, Seo S, Maki K, Asai T, Suzuki T, Hangaishi A, Yamagata T, Aizawa S, Noda M, Nakanishi Y, Hirai H. Impaired spermatogenesis and male fertility defects in CIZ/Nmp4-disrupted mice. Genes Cells. 2004;9:575–89. doi: 10.1111/j.1356-9597.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- 58.He Y, Childress P, Hood M, Jr, Alvarez M, Kacena M, Hanlon M, McKee B, Bidwell JP, Yang FC. Disabling Nmp4/CIZ enhances the number of osteoprogenitors, CD8+ T cells, and CFU-GM cells in murine bone mar-Critical row. 10th Annual Midwest Blood Club Symposium; Indianapolis. March 15–16, 2012; p. Abstract No. 68. [Google Scholar]
- 59.He Y, Childress P, Hood M, Jr, Alvarez M, Kacena MA, Hanlon M, McKee B, Bidwell JP, Yang FC. Nmp4/CIZ suppresses the parathyroid hormone anabolic window by restricting mesenchymal stem cell and osteoprogenitor frequency. Stem Cells Dev. 2012 doi: 10.1089/scd.2012.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner EF. Bone development and inflammatory disease is regulated by AP-1 (Fos/Jun) Ann Rheum Dis. 2010;69 (Suppl 1):i86–8. doi: 10.1136/ard.2009.119396. [DOI] [PubMed] [Google Scholar]
- 61.Negishi-Koga T, Takayanagi H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol Rev. 2009;231:241–56. doi: 10.1111/j.1600-065X.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 62.Takayanagi H. The role of NFAT in osteoclast formation. Ann NY Acad Sci. 2007;1116:227–37. doi: 10.1196/annals.1402.071. [DOI] [PubMed] [Google Scholar]
- 63.Hino K, Nakamoto T, Nifuji A, Morinobu M, Yamamoto H, Ezura Y, Noda M. Deficiency of CIZ, a nucleocytoplasmic shuttling protein, prevents unloading-induced bone loss through the enhancement of osteoblastic bone formation in vivo. Bone. 2007;40:852–60. doi: 10.1016/j.bone.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 64.Terauchi M, Li JY, Bedi B, Baek KH, Tawfeek H, Galley S, Gilbert L, Nanes MS, Zayzafoon M, Guldberg R, Lamar DL, Singer MA, Lane TF, Kronenberg HM, Weitzmann MN, Pacifici R. T lymphocytes amplify the anabolic activity of parathyroid hormone through Wnt10b signaling. Cell Metab. 2009;10:229–40. doi: 10.1016/j.cmet.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen ZJ, Nakamoto T, Tsuji K, Nifuji A, Miyazono K, Komori T, Hirai H, Noda M. Negative regulation of bone morphogenetic protein/Smad signaling by Cas-interacting zinc finger protein in osteoblasts. J Biol Chem. 2002;277:29840–6. doi: 10.1074/jbc.M203157200. [DOI] [PubMed] [Google Scholar]
- 66.Huang JC, Sakata T, Pfleger LL, Bencsik M, Halloran BP, Bikle DD, Nissenson RA. PTH differentially regulates expression of RANKL and OPG. J Bone Miner Res. 2004;19:235–44. doi: 10.1359/JBMR.0301226. [DOI] [PubMed] [Google Scholar]
- 67.Alvarez MB, Childress P, Philip BK, Gerard-O’Riley R, Hanlon M, Herbert BS, Robling AG, Pavalko FM, Bidwell JP. Immortalization and characterization of osteoblast cell lines generated from wild-type and Nmp4-null mouse bone marrow stromal cells using murine telomerase reverse transcriptase (mTERT) J Cell Physiol. 2012;227:1873–82. doi: 10.1002/jcp.22915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang Z, Bidwell JP, Young SR, Gerard-O’Riley R, Wang H, Pavalko FM. Nmp4/CIZ inhibits mechanically induced beta-catenin signaling activity in osteoblasts. J Cell Physiol. 2010;223:35–41. doi: 10.1002/jcp.22057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shivaram GM, Kim CH, Batra NN, Yang W, Harris SE, Jacobs CR. Novel early response genes in osteoblasts exposed to dynamic fluid flow. Philos Trans A. 2010;368:605–16. doi: 10.1098/rsta.2009.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–46. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang YH, Liu Y, Rowe DW. Effects of transient PTH on early proliferation, apoptosis, and subsequent differentiation of osteoblast in primary osteoblast cultures. Am J Physiol Endocrinol Metab. 2007;292:E594–E603. doi: 10.1152/ajpendo.00216.2006. [DOI] [PubMed] [Google Scholar]
- 72.Wang YH, Liu Y, Buhl K, Rowe DW. Comparison of the action of transient and continuous PTH on primary osteoblast cultures expressing differentiation stage-specific GFP. J Bone Miner Res. 2005;20:5–14. doi: 10.1359/JBMR.041016. [DOI] [PubMed] [Google Scholar]
- 73.Alvarez MB, Thunyakitpisal P, Rhodes SJ, Everett ET, Bidwell JP. Assignment of Nmp4 to mouse chromosome 6 band F1 flanked by D6Mit134 and D6Mit255 using radiation hybrid mapping and fluorescence in situ hybridization. Cytogenet Cell Genet. 2001;94:244–5. doi: 10.1159/000048824. [DOI] [PubMed] [Google Scholar]
- 74.Margolis RL, Abraham MR, Gatchell SB, Li SH, Kidwai AS, Breschel TS, Stine OC, Callahan C, McInnis MG, Ross CA. cDNAs with long CAG trinucleotide repeats from human brain. Hum Genet. 1997;100:114–22. doi: 10.1007/s004390050476. [DOI] [PubMed] [Google Scholar]
- 75.Torrungruang K, Alvarez M, Shah R, Onyia JE, Rhodes SJ, Bidwell JP. DNA binding and gene activation properties of the Nmp4 nuclear matrix transcription factors. J Biol Chem. 2002;277:16153–9. doi: 10.1074/jbc.M107496200. [DOI] [PubMed] [Google Scholar]
- 76.Feister HA, Torrungruang K, Thunyakitpisal P, Parker GE, Rhodes SJ, Bidwell JP. NP/NMP4 transcription factors have distinct osteoblast nuclear matrix subdomains. J Cell Biochem. 2000;79:506–17. [PubMed] [Google Scholar]
- 77.Shah R, Alvarez M, Jones DR, Torrungruang K, Watt AJ, Selvamurugan N, Partridge NC, Quinn CO, Pavalko FM, Rhodes SJ, Bidwell JP. Nmp4/CIZ regulation of matrix metalloproteinase 13 (MMP-13) response to parathyroid hormone in osteoblasts. Am J Physiol Endocrinol Metab. 2004;287:E289–96. doi: 10.1152/ajpendo.00517.2003. [DOI] [PubMed] [Google Scholar]
- 78.Shimizu E, Selvamurugan N, Westendorf JJ, Olson EN, Partridge NC. HDAC4 represses matrix metalloproteinase-13 transcription in osteoblastic cells, and parathyroid hormone controls this repression. J Biol Chem. 2010;285:9616–26. doi: 10.1074/jbc.M109.094862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Janssen H, Marynen P. Interaction partners for human ZNF384/CIZ/NMP4--zyxin as a mediator for p130CAS signaling? Exp Cell Res. 2006;312:1194–204. doi: 10.1016/j.yexcr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 80.Hirata H, Tatsumi H, Sokabe M. Zyxin emerges as a key player in the mechanotransduction at cell adhesive structures. Commun Integr Biol. 2008;1:192–5. doi: 10.4161/cib.1.2.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guadalupe-Grau A, Fuentes T, Guerra B, Calbet JA. Exercise and bone mass in adults. Sports Med. 2009;39:439–468. doi: 10.2165/00007256-200939060-00002. [DOI] [PubMed] [Google Scholar]
- 82.Schneider-Stock R, Ghantous A, Bajbouj K, Saikali M, Darwiche N. Epigenetic mechanisms of plant-derived anticancer drugs. Front Biosci. 2012;17:129–73. doi: 10.2741/3919. [DOI] [PubMed] [Google Scholar]