Abstract
The suprachiasmatic nucleus (SCN) of the mammalian hypothalamus is the central pacemaker for peripheral and organismal circadian rhythms. The development of this hypothalamic structure depends on genetic programs throughout embryogenesis. We have investigated the role of the homeodomain transcription factor Six6 in the development of the SCN. We first showed that Six6 mRNA has circadian regulation in the mouse SCN. We then characterized the behavioral activity patterns of Six6-null mice under various photoperiod manipulations and stained their hypothalami using SCN-specific markers. Six6-null mice display abnormal patterns of circadian behavior indicative of SCN abnormalities. The ability of light exposure to reset rhythms correlates with the presence or absence of optic nerves, but all Six6-null mice show irregular rhythms. In contrast, wild-type mice with crushed optic nerves maintain regular rhythms regardless of light exposure. Using immunohistochemistry for arginine vasopressin (AVP), vasoactive intestinal polypeptide (VIP), and β-galactosidase, we demonstrated the lack of these SCN markers in all Six6- null mice regardless of the presence of optic nerve or partial circadian rhythms. Therefore, Six6 is required for the normal development of the SCN, and the Six6-null mouse can mount independent, although irregular, circadian rhythms despite the apparent absence of a histochemically defined SCN.
Keywords: homeodomain protein, suprachiasmatic nucleus, circadian rhythm, Six6, development, optic nerve
The suprachiasmatic nucleus (SCN) of the mammalian hypothalamus is a central pacemaker of circadian rhythms. Physical ablation of this nucleus in rodents leads to behavioral and physiological arrhythmicity (Stephan and Zucker, 1972) and impaired fertility (Wiegand and Terasawa, 1982). Light input from photosensitive ganglion cells in the eye, via a retino-hypothalamic tract in the optic nerve, entrains the SCN’s approximately 24-h activity patterns to advance or delay the phase of rhythm such that the physiology of the animal is appropriately timed to its environmental context (Panda et al., 2002).
Six6, a murine homeobox gene orthologous to Drosophila optix (Jean et al., 1999), has been shown to play important roles in the developing eye, brain, and pituitary. It is expressed as early as 8 days post-conception in the mouse. Throughout development, it is expressed in the hypothalamus, the pituitary, and the olfactory placode and is later expressed in the optic vesicle, the optic stalk, and the retina (Jean et al., 1999). In adult, it remains expressed in the hypothalamus, as well as cells of the eye and pituitary (Conte et al., 2005). Deletion of the mouse Six6 gene leads to defects in precursor cell proliferation in the retina and pituitary (Li et al., 2002).
Six3 is a close homolog of Six6, and the two share partially overlapping expression patterns (Jean et al., 1999). Six3 and Six6 were among several transcription factors recently identified as being expressed early in development and strongly in the postnatal SCN (VanDunk et al., 2011). Neuronal deletion of Six3 was accomplished by breeding a LoxP-flanked Six3 allele-bearing mouse line (Liu et al., 2006) with a mouse line expressing Cre recombinase under the control of a neuron-specific enhancer from the Nestin gene (Tronche et al., 1999). Mice with complete neuronal deletion of Six3 lacked a number of markers for the SCN. This was in contrast to the presence of normal SCN in mice lacking functional RORα (Hamilton et al., 1996), a gene also expressed strongly in the SCN and early in development. However, the role of Six3 or Six6 in the postnatal mouse, either behaviorally or anatomically, has not yet been investigated.
Recently, Six6 was shown to play a critical role in reproduction (Larder et al., 2011). Gonadotropin-releasing hormone– (GnRH-) secreting neurons in the hypothalamus control the pituitary gonadotrope cells that, in turn, control reproduction. In a screen to identify genes important for GnRH neuron function, Six6 expression was 100-fold greater in a cell model of mature (GT1-7 line) versus immature (GN11 line) GnRH neurons. Female mice lacking Six6 showed a profound infertility phenotype; only 1 Six6-null female out of an experimental cohort of 8 was able to deliver a single litter over 3 months. Also, both male and female Six6-null mice had significantly reduced numbers of GnRH neurons (90% reduced). In addition, an altered pattern of tyrosine hydroxylase expression in the hypothalamus suggested a more general role for Six6 in the development of the hypothalamus.
Given the developmental and spatial expression pattern of Six6 and the role of its homolog Six3 in the formation of the SCN, interesting questions remained concerning the role of Six6 in the brain. Does Six6 expression follow a circadian rhythm, and is Six6 a target or component of the molecular circadian clock? Do mice lacking Six6 also lack a normal SCN, as observed in the neuron-specific Six3-null mice, or is the SCN of Six6-null mice intact, as observed for the RORα-null mice?
While the developmental and spatial expression pattern of Six6 has been reported in the mouse (VanDunk et al., 2011), its expression in the SCN over circadian time is not known. We demonstrated a circadian temporal expression pattern of Six6 in the adult hypothalamus and altered circadian behavioral phenotypes of Six6-null mice. The mouse SCN is characterized by a vasoactive intestinal polypeptide–(VIP-) positive core region at its ventral base and an arginine vasopressin– (AVP-) positive shell region surrounding the core dorsally, and staining for these 2 proteins has been used extensively to characterize the SCN (Leak and Moore, 2001). The SCN of the Six6-null mouse was characterized using immunohistochemical staining for these 2 SCN markers, as well as morphological analysis, indicating that the SCN fails to fully develop in the Six6-null mouse.
MATERIALS AND METHODS
Animals and Housing
Six6-null mice were obtained from Dr. Xue Li (Children’s Hospital of Boston, Harvard Medical School, Boston, MA) and were maintained in accordance with protocols approved by the University of California, San Diego, and the Salk Institute for Biological Studies Institutional Animal Care and Use Committees. Animals were housed under 12 h:12 h light:dark lighting conditions, except as noted below, and were given ad libitum access to food and water. Our colony was maintained on a mixed 129/Sv (original) and C57BL/6 (3-generation backcross) background strain.
Quantitative Reverse-Transcriptase Polymerase Chain Reaction
Male mice heterozygous for the Six6 deletion allele were euthanized via overdose of inhaled isoflurane anesthetic at zeitgeber time 20 (ZT20), 4 h prior to lights on (in the dark, n = 4) and at ZT8, 4 h prior to lights off (n = 4). Hypothalami were dissected and transferred to Trizol reagent (Life Technologies, Carlsbad, CA). RNA was extracted according to the manufacturer’s instructions. cDNA was prepared using a SuperScript III first-strand cDNA synthesis kit (Life Technologies). Quantitative polymerase chain reaction (PCR) was performed using a BioRad (Hercules, CA) iQ5 system and B-R-SYBR Green (Quanta Biosciences, Gaithersburg, MD). Gene-specific PCR primers (Suppl. Table S1) were used to determine the expression levels of mouse Six3, Six6, Bmal (Arntl), and Per2, along with the control genes Gapdh, H2afz, and Ppia. Known quantities of reference DNA plasmids each containing a gene of interest were used to prepare standard curves, against which the hypothalamic expression levels were measured. Expression of each gene of interest was normalized to the geometric average of the expression of the three control genes at each time point (Vandesompele et al., 2002).
Photoperiod Manipulations
Six6-null mice (n = 6) and wild-type (WT) littermate controls (n = 6) were raised in a 12 h:12 h light:dark photoperiod until 2 to 4 months of age. Mice were then introduced into cages containing running wheels equipped with magnets and monitored using magnetic switches. All cages were contained within a light-tight cabinet with programmable fluorescent lighting. The previous photoperiod was maintained, and the mice were given 7 days to acclimate to the new environment. Wheel turns were recorded for each mouse in 6-min bins and analyzed offline using the Clocklab (Actimetrics, Wilmette, IL) suite of Matlab (Mathworks, Natick, MA) plugins. Actograms were plotted using the above software, Microsoft Excel, and an online plotting program produced by Roberto Refinetti (http://www.circadian.org/actogram.html, accessed 2011)). Four different photoperiod manipulations were performed: (1) a 12 h:12 h light:dark schedule, (2) complete darkness, (3) a 1 h:11 h L:D skeleton photoperiod, and (4) a 6 h:6 h:6 h:6 h L:D:L:D, bifurcated light schedule. The final schedule included illumination with dim, narrowband green light (555 ± 23 nm; <0.1 lux) throughout all scotophases as shown previously to facilitate bifurcated entrainment in rodents (Gorman et al., 2003).
Hypothalamic Immunohistochemical and Cytological Staining
Mice were cardiac perfused with a phosphate-buffered saline (PBS) solution followed by a 4% formalin solution of PBS. Brains were dissected from the skull and postfixed for 16 h in a 4% formalin PBS solution. Brains were then transferred to a 50% sucrose solution of PBS for 12 h and then embedded in O.C.T. compound (Tissue-Tek; Sakura Finitek USA, Torrance, CA) and frozen at −80 °C. Brains were sectioned at 20 µm and thaw mounted onto PermaFrost plus glass slides. Brain sections were stained using 1 of 3 primary antibodies: polyclonal rabbit anti-VIP (ImmunoStar, Hudson, WI), rabbit antivasopressin (ImmunoStar), or rabbit antityrosine hydroxylase (Pel-Freez, Rogers, AR). For the β-galactosidase staining, brains were dissected, placed on dry ice, and stored at −80 °C until being sectioned at 20 µm. These sections were then stained using polyclonal chicken anti-β-galactosidase (Abcam, Cambridge, UK). Slides were then processed and stained with a peroxidase substrate chromogen kit (Vector VIP; Vector Labs, Burlingame, CA) according to the manufacturer’s recommendations. Following further rinses and an alcohol dehydration series, slides were air dried and mounted with cover-slips.
Cresyl violet Nissl staining was performed on 20-µm-thick cryostat brain sections. Slides were rinsed and then exposed to a 0.5% cresyl violet solution for 30 min and then processed as above for final coverslipping.
Optic Nerve Crush Surgery
The circadian wheel-running activity of 8-week-old male C57BL/6J mice was collected and analyzed as described (Siepka and Takahashi, 2005). After 10 days of baseline activity under a standard 12 h:12 h light:dark photoperiod, mice were anesthetized with ketamine and xylazine. The optic nerves of both eyes were exposed intraorbitally. Using a No. 5 forceps, the optic nerve, approximately 1 mm distal to the optic disc, was crushed for several seconds (for control mice, optic nerves were exposed but not crushed). After recovery, the mice were returned to the wheel-running cages, and their activity was monitored. Subsequent photoperiod manipulations included LD, DD (constant darkness), and LL (constant light).
RESULTS
Diurnal Expression Pattern of Six6 mRNA
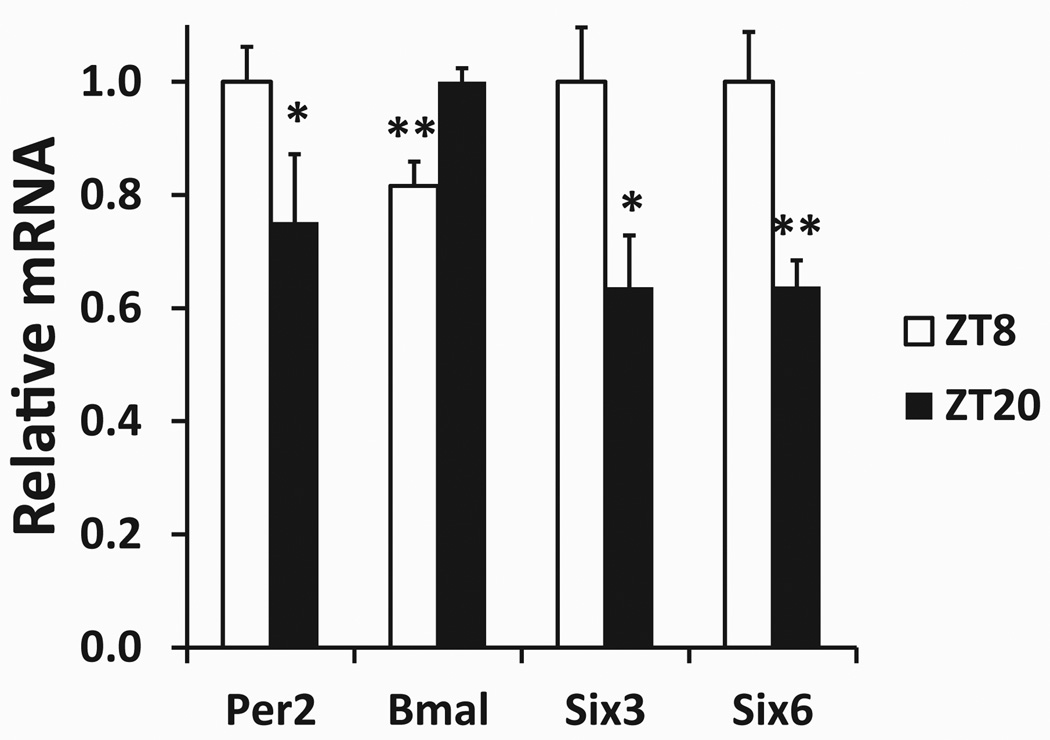
Quantitative reverse-transcription PCR was used to measure the expression levels of Six3 and Six6 at 2 zeitgeber time points. The 2 time points chosen are near the expected times of maximal and minimal expression of the known clock genes Bmal1 and Per2. These time points are also near the times of maximal and minimal expression of Six3 (VanDunk et al., 2011). As expected, at ZT8, the hypothalamic expression of Bmal is significantly lower and expression of Per2 is significantly higher than their expression at ZT20 (Fig. 1). Expression of Six3 mRNA is significantly lower at ZT20 than at ZT8, and this finding is consistent with a previous report (VanDunk et al., 2011). Six6 mRNA expression was significantly lower at ZT20 than at ZT8, indicating that Six6 expression appears to also be diurnally regulated (Fig. 1).
Figure 1.
Hypothalamic gene expression at 2 points in zeitgeber time. Gene expression data obtained via quantitative reverse-transcription PCR. Each gene’s expression has been normalized to the geometric mean of 3 control genes (see the Materials and Methods section) and is expressed relative to the peak average value between the 2 groups, ZT8 (n = 4) and ZT20 (n = 4). *Statistically significant difference in expression (p ≤ 0.05 via Student t test) between the ZT8 and ZT20 groups for each gene. **p ≤ 0.01.
Abnormal Photoperiod Entrainment of the Six6-Null Mouse
WT mice entrained normally to the 12 h:12 h photoperiod, as assessed by their wheel-running activity (Fig. 2). WT mice showed robust wheel running during the dark portion of the photoperiod, almost complete plete absence of activity during the light portion of the photoperiod, and evidence of negative masking at the light/dark transitions. For reference, Supplemental Figure S1 includes the activity profile for the 2 WT and 6 Six6-null mice for the entire period of observation. Because the Six6-null mice showed a wide variety of activity patterns, qualitative and descriptive analyses of the behavioral rhythms were used in addition to quantitative analyses. Two of the 6 Six6-null mice did not appear to have any differences in wheel-running behavior between light and dark portions of the photoperiod (Six6 KO C; Fig. 2). Other Six6-null mice showed greater activity during the dark portion of the photoperiod than the light portion, but the activity bouts did not show the characteristic sharp onset and gradual offset of activity during the dark; rather, there appeared to be 2 separate bouts of activity following lights-off and prior to lights-on (Six6 KO A). Finally, 2 of the Six6-null mice showed nearly normal entrainment to the L:D photoperiod (Six6 KO B).
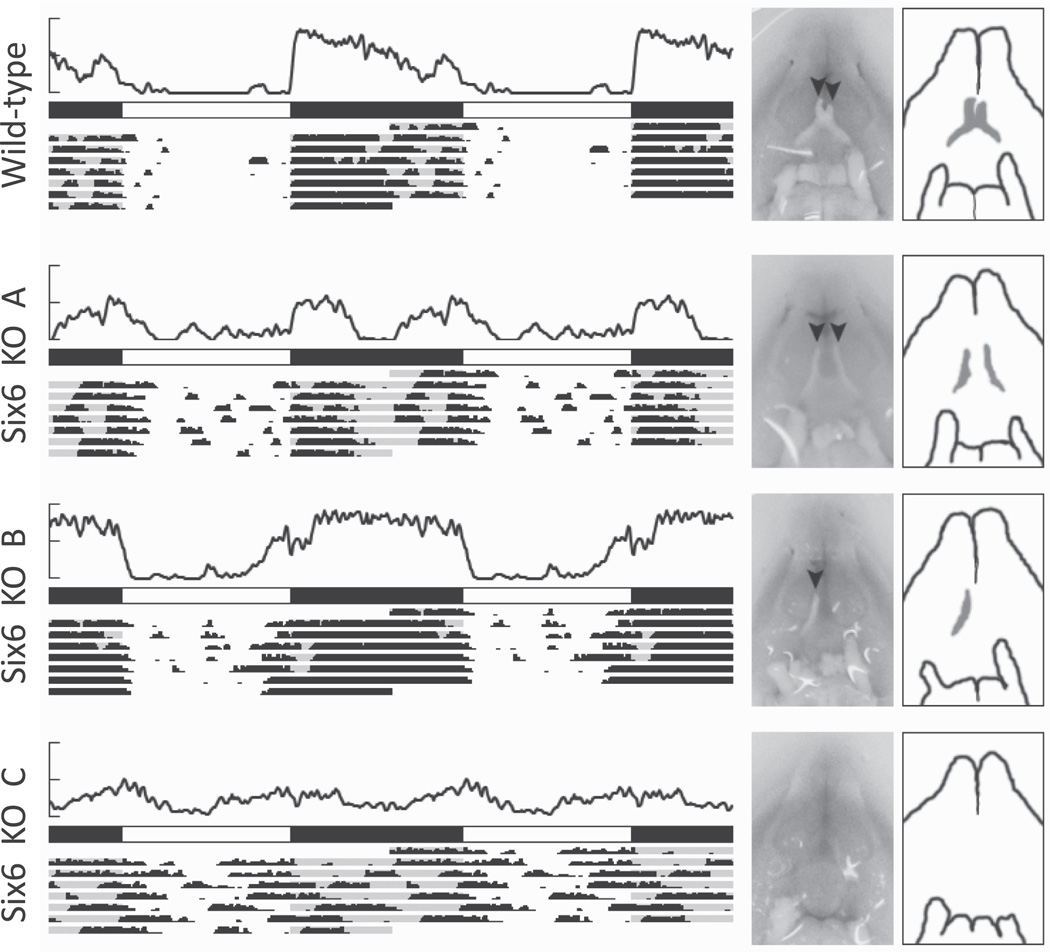
Figure 2.
Wheel-running activity and optic nerve phenotypes of 4 mice under L:D 12 h:12 h. For each mouse, an average activity plot (top; y-axis maximum = 500 revolutions per 6 min) is shown above photoperiod (filled = dark, open = light) and actograms representing 7 days of wheel-running data (each actogram is double-plotted such that 48 h of data are shown for each row). To the right of the activity are photographs and schematic drawings of each mouse’s optic nerve phenotype. The ventral surface of each brain is shown, with the location of the optic chiasm centered. Black arrowheads represent the presence of optic nerves.
Variable Optic Nerve Phenotype in Six6-Null Mice
The presence of the optic nerve is variable in Six6-null mice (Li et al., 2002). WT and null animals were sacrificed and dissected. As the brain was dissected, care was taken to note the size and presence of each optic nerve. All WT mice had normal optic nerves, but Six6-null mice had 1 of 3 optic nerve phenotypes (Fig. 2). Half of the Six6-null mice studied (n = 7/14) had no optic nerve or threadlike, severely atrophied, or poorly developed optic nerves, with no visible optic chiasm (e.g., Six6 KO C). Interestingly, the eyes of these Six6-null mice appeared relatively normal, with no obvious atrophy or lid closure. A second phenotype (n = 5/14) of Six6-null mice was characterized by the presence of either the right or the left optic nerve, with the other completely missing (e.g., Six6 KO B). A third Six6-null phenotype (n = 2/14) was characterized by the presence of both optic nerves but a lack of chiasm (e.g., Six6 KO A). In these mice, the optic nerves appear to project to the thalamus without forming a chiasm. It is unclear whether all or some of the retinohypothalamic tract is missing in these mice.
Abnormal Free-Running Activity of the Six6-Null Mouse
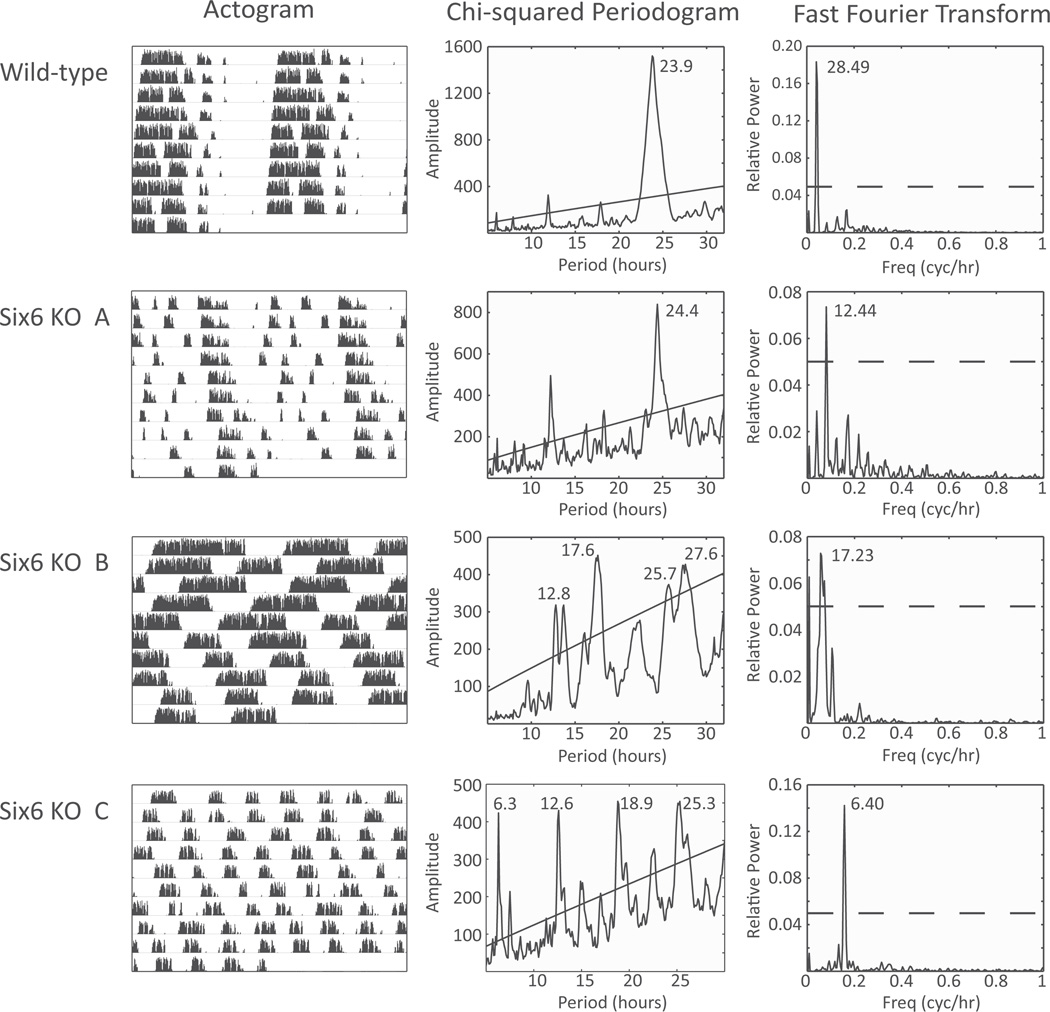
Following photoperiod entrainment, WT and Six6- null mice were released into constant darkness. With no environmental cues indicative of day length, WT mice (n = 6) retained robustly rhythmic activity patterns with a period of τ = 23.7 ± 0.16 h. However, Six6-null mice displayed a range of activity patterns in constant darkness (Fig. 3). Six6-null mice with single or double intact optic nerves showed a range of free-running activity patterns, with some showing strong circadian periodicity (n = 3, τ = 24.40 ± 0.50, and 1 showing weak circadian periodicity (multiple weak χ2 periodogram peaks; 6 KO B from Fig. 3). Neither of the 2 Six6-null mice without optic nerves had strong circadian rhythms, but 1 had strong ultradian periodicity (fast Fourier transform [FFT] peak = 6.40 h, relative power = 0.12; Fig. 3), while the other had weak ultradian periodicity (FFT peak = 7.00 h, relative power = 0.04).
Figure 3.
Wheel-running activity during constant darkness. (Left) Wild-type and Six6-null mouse wheel-running actograms are shown for 10 days of activity in constant darkness. (Middle) χ2 periodogram analysis of the actogram data. Diagonal line represents p = 0.05 significance threshold. (Right) Fast Fourier transform of the actogram data. Peak period listed; dotted line represents 0.05 relative power. Knockout mice A, B, and C are the same individual animals as in Figure 2.
Abnormal Skeleton Photoperiod Activity of the Six6-Null Mouse
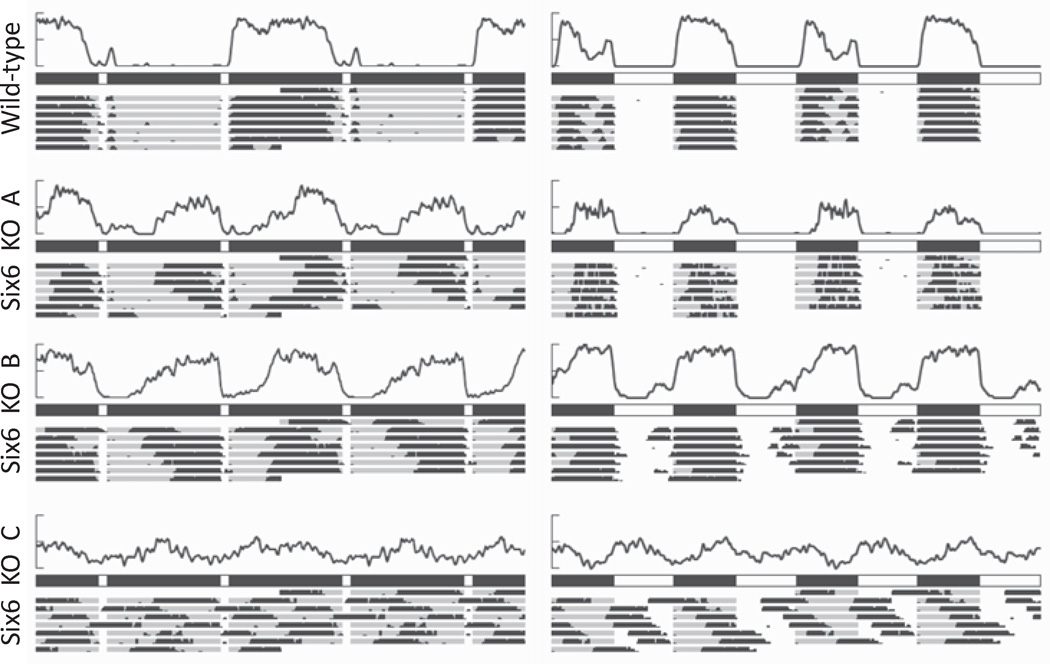
Given the abnormal and variable activity patterns of the Six6-null mice during standard 12 h:12 h photoperiods and under constant darkness, we subjected WT and Six6-null mice to 2 additional alternative photoperiods. Rodents are capable of entraining to brief, 1-h pulses of light given every 12 h (Pittendrigh and Daan, 1976). Robust activity is typically consolidated in 1 of the 2 long dark intervals between light pulses (subjective night) with inactivity in the opposite dark phase (subjective day). Wild-type mice behaved as expected, with all 6 wild-type mice displaying unambiguous subjective night and subjective day activity patterns (Fig. 4). There was no clear demarcation of subjective day and night for 3 of the 4 Six6-null mice with at least 1 optic nerve; their activity was distributed equally between the light pulses. The Six6-null mice with no optic nerves continued to exhibit only an unentrained ultradian pattern of activity.
Figure 4.
Wheel-running activity during skeleton and bifurcation photoperiods. (Left) Wild-type and knockout mouse average activity (top) and actogram (bottom) during 7 days of activity in a skeleton photoperiod of 1 h:11 h L:D. (Right) Wild-type and knockout mouse average activity (top), photoperiod (middle), and actogram (bottom) during 7 days of activity in a bifurcated 6 h:6 h:6 h:6 h L:D:L:D photoperiod. As in Figure 2, each activity average and actogram is double-plotted over 48 h of activity. Knockout mice A, B, and C are the same individual animals as in Figures 2 and 3.
We then subjected WT and Six6-null mice to a bifurcated, 6 h:6 h:6 h:6 h, L:D:L:D photoperiod to investigate whether the behavioral activity patterns would be different with an increased frequency of dark onset. In a bifurcated photoperiod with 4 light/dark transitions per 24 h, Six6-null mice with intact optic nerves showed strong activity during the dark portion of the photoperiod, as did WT mice (Fig. 4).
Six6-Null Mouse SCN Phenotype
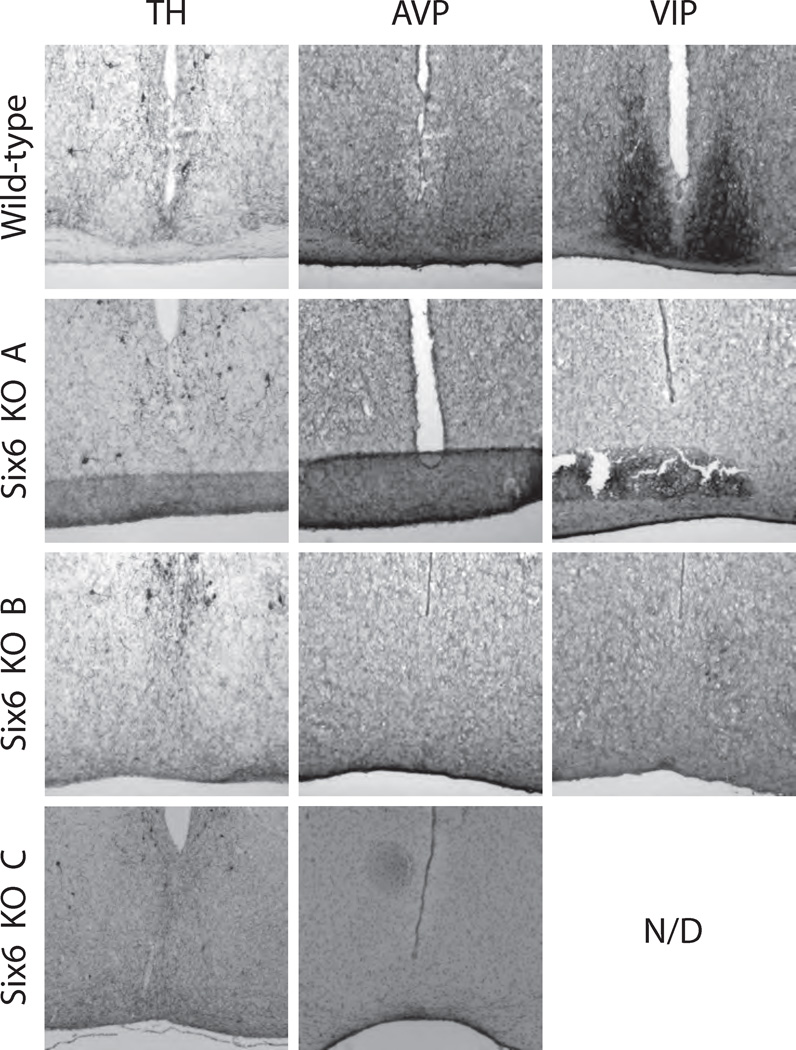
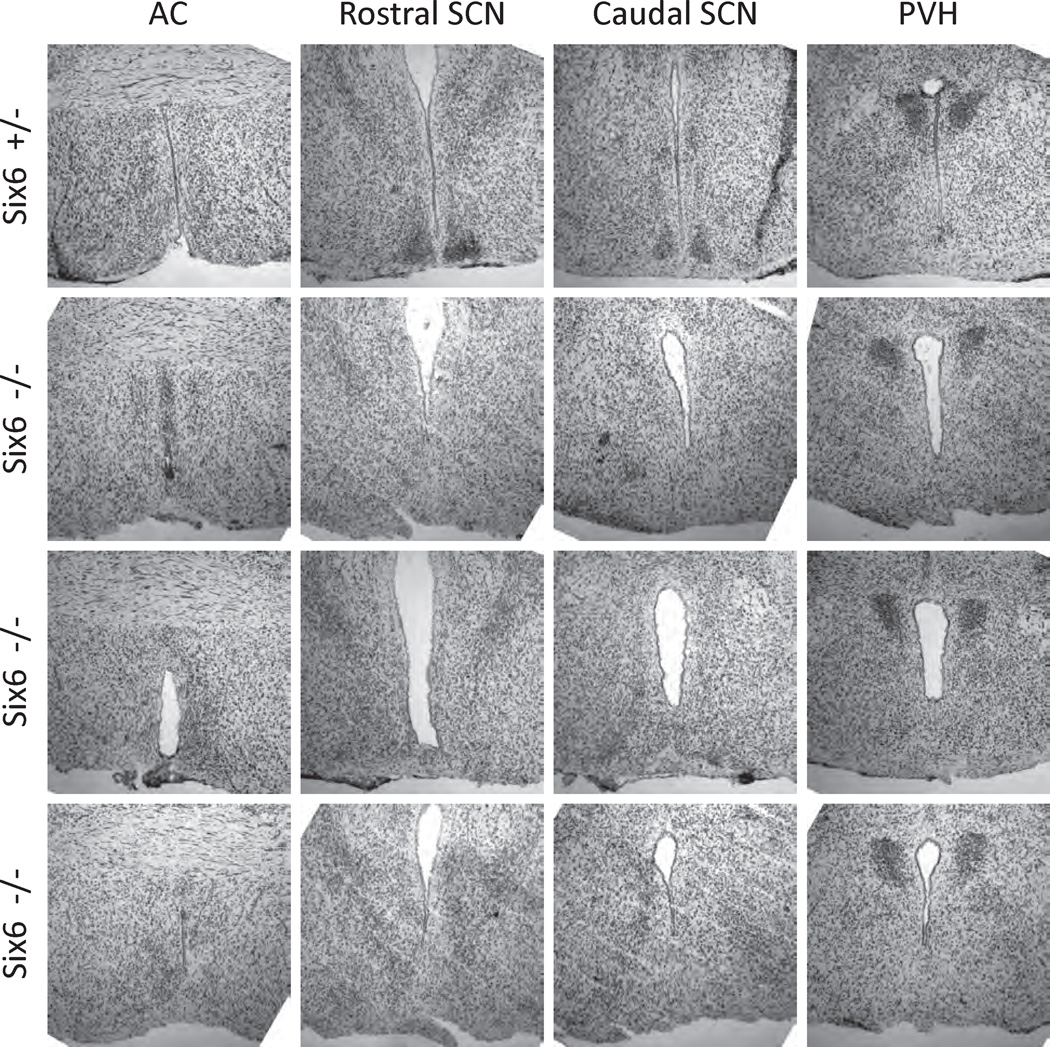
WT and the 3 types of Six6-null mouse hypothalami were sectioned and stained for the SCN markers AVP and VIP as described in the Materials and Methods section. Given that the range of circadian phenotypes varied along with optic nerve phenotypes, we hypothesized that the SCN of Six6-null mice would similarly vary. All WT mice had discrete, bilateral SCN morphologically identifiable through the dense ventral hypothalamic region of small cell bodies characteristic of the SCN, prominent following immunohistochemical or cytological staining (Fig. 5; also, see Supplemental Online Material for a full, section-matched, rostral-caudal staining series for WT and Six6-null mice). However, no Six6-null mouse, regardless of optic nerve phenotype, showed evidence for a histochemically (AVP and VIP) defined SCN. Moreover, general cytological staining (cresyl violet Nissl) did not reveal morphologically distinct SCN nuclei in any Six6-null mice (Fig. 6). As described previously for the conditional Six3-null mice, other hypothalamic nuclei with AVP-positive neurons (e.g., the supraoptic and paraventricular nuclei; VanDunk et al., 2011) were present in Six6-null mice (Suppl. Fig. S2). Interestingly, the AVP-positive supraoptic nuclei are present in Six6-null mice lacking optic nerves. The inability to morphologically or histochemically identify SCN in any of the Six6-null mice studied, regardless of optic nerve phenotype, indicates that Six6 plays a role in the development of the SCN independent of the defects seen in the optic nerve. Thus, Six6 itself is required for the full development of the SCN.
Figure 5.
SCN of 1 wild-type (WT) and 3 Six6-null mice used in the behavioral tests. Tyrosine hydroxylase (TH) and arginine vasopressin (AVP), and vasoactive intestinal polypeptide (VIP) immunohistochemical staining of WT and Six6-null (KO) mouse hypothalami at the level of the SCN. Supplemental Figure S2 includes these data along with neighboring rostral and caudal coronal sections for all image series. Knockout mice A, B, and C are the same individual animals as in Figures 2 to 4.
Figure 6.
SCN of heterozygous and Six6-null mice: cresyl violet Nissl staining. Mice heterozygous for the Six6-null allele (Six6 +/−) display normal SCN morphology, assessed qualitatively by dense nuclei of cell bodies (n = 3, 1 shown). Six6-null mice (n = 3) do not show normal SCN morphology, despite normal histology at the level of the anterior commissure (AC; leftmost column) and apparently normal paraventricular nuclei (PVH; rightmost column).
In addition, we performed immunohistochemistry for the marker β-galactosidase. The Six6-null allele contains an IRES-LacZ reporter gene in place of the 6 domain and the homeodomain portions of Six6 (Li et al., 2002) such that β-galactosidase staining indicates the pattern of Six6 gene expression in the SCN. Mice heterozygous for the null allele (Six6 +/−) showed strong β-galactosidase staining focused within the SCN, and Six6-null mice (Six6 −/−) showed a scattered pattern of β-galactosidase staining but an absence of the focused pattern found in the normal SCN (Suppl. Fig. S3). These results indicate that the Six6 promoter is active in some neurons despite the apparent lack of normal SCN morphology.
Requirement for the Optic Nerves for Photoperiod Entrainment but Not Circadian Rhythmicity
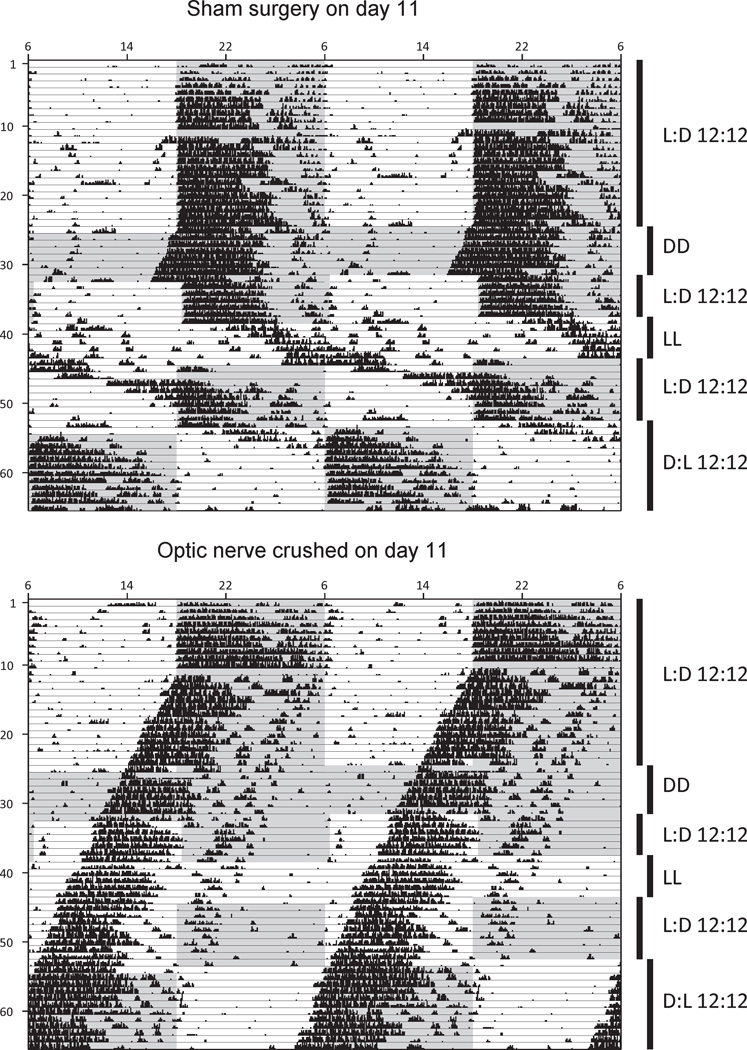
The optic nerves relay light information to multiple brain targets. These targets include the SCN, via the retinohypothalamic tract portion of the optic nerves, where light information is capable of resetting (entraining) the circadian clock to match external conditions (photoperiod). In genetically WT adult mice, we confirmed that the inputs from the optic nerve were necessary for the maintenance of circadian rhythmicity of wheel-running behavior. WT mice were given either a sham surgery or an optic nerve crush surgery as described in the Materials and Methods section, and wheel-running activity was monitored under various photoperiods (Fig. 7). The sham surgery resulted in normal entrainment behavior under LD (standard photoperiod), DD (constant darkness), and LL (constant light). A 12-h shift in the LD photoperiod also resulted in normal, robust reen-trainment to the new photoperiod within 4 or 5 days. The optic crush surgery resulted in a total loss of entrainment. However, circadian rhythmicity remained intact, with a strong, stable onset of wheel-running activity throughout the postsurgical activity monitoring. As expected, the lack of optic nerves in the WT adult mouse had no effect on circadian rhythmicity, although entrainment was lost.
Figure 7.
Circadian behavior following optic nerve crush. Behavioral actograms of wild-type mice receiving either sham optic nerve surgery (top) or optic nerve crush surgery (bottom) on day 11 of activity monitoring. Six different photoperiod manipulations were performed as shown (DD = constant darkness, LL = constant light), with the gray background indicating lights-off and white background indicating lights-on. Data are double-plotted across each row of the actogram (48 h total).
DISCUSSION
The developmental origins of the mouse SCN have been examined using BrdU labeling (Kabrita and Davis, 2008) as well as transcription factor expression profiling (VanDunk et al., 2011). Beginning around embryonic day 12 (Kabrita and Davis, 2008), the first cells of the SCN undergo terminal differentiation in the core region that will eventually become VIP positive (Shimogori et al., 2010). On embryonic days 13.5 to 14, the cells of the shell terminally differentiate. This developmental process likely shares many of the general principles of neurodevelopment, with transcription factors, signaling events, and environmental cues playing a role.
Prior to the birth of the earliest SCN cells, the retinal ganglion cells send their axons (embryonic day 13) to their thalamic targets (Hinds and Hinds, 1974; Lund and Blunt, 1976). However, the retinohypothalamic tract does not innervate the rat SCN until after birth (Mason et al., 1977; Stanfield and Cowan, 1976) and innervates the mouse SCN postnatally (McNeill et al., 2011), after the SCN has formed and begun expression of the markers VIP and AVP. Since the formation of the optic nerves precedes the formation of the SCN and lies in such close proximity to it, the SCN may, in some part, rely on signaling from the optic nerves for its formation. However, the variability of the Six6-null mouse optic nerve phenotype indicates that the optic nerve may not be sufficient to initiate formation of the SCN.
In rodents blinded via orbital enucleation, the morphology of the SCN is altered. In rats enucleated (bilaterally or unilaterally) at 4 weeks of age, the optic chiasm becomes thinner, SCN is displaced rostrally, and the number of cells in the typical location of the SCN is decreased (Nagai et al., 1992). However, the overall number of cells in the SCN was not affected by enucleation. The enucleation experiments were performed in rats at 4 weeks of age and thus reflect changes to the SCN after its initial development.
Previous work on transgenic and mutant mouse lines has explored the retinohypothalamic tract and its role in SCN development and physiology. Early work on the eyeless mouse (strain ZRDCT-AN [Chase and Chase, 1941] later confirmed to possess a single nucleotide polymorphism removing an alternate start codon of the Rx/rax gene [Tucker et al., 2001]) revealed hypothalamic and optic tract deficiencies (Silver, 1977; Laemle et al., 2002) in these mice. The SCN of the eyeless mouse strain is variably atypical, with 70% showing normal SCN morphology and cell densities and the remaining mice showing laterally asymmetric deficiencies in SCN size and cell number (Silver, 1977). Seventy-five percent of anopthalmic mice studied (Laemle and Ottenweller, 1998) showed stable circadian activity rhythms, 10% showed unstable rhythms, and 15% were behaviorally arrhythmic. In contrast to the Six-null mice reported here, the anopthalmic arrhythmic mice did not show any predominant ultradian periodicity. Whether these SCN and behavioral phenotypes are due to developmental or genetic variability is unknown, but this phenotypic variability is shared with the Six null mice.
The SCN phenotype in Six6-null mice is not likely due to a lack of light input via the retinohypothalamic tract to the SCN. Mice lacking rod and cone cells and the melanopsin (Opn4) gene display robust circadian rhythmicity, although this rhythmicity is not entrainable by light (Panda et al., 2003). From in utero through adulthood, these mice have no functional light input to the SCN, suggesting that this input is not necessary for SCN development and function. The optic nerves and optic chiasm in those mice, however, are intact.
The activity patterns of the Six6-null mice share some similarity to the activity patterns of mice in which the genes for VIP and peptide histidine leucine (PHI) were deleted (Colwell et al., 2003). These 2 SCN signaling molecules are capable of phase shifting the circadian clock when administered exogenously. Inactivation of these 2 genes led to mice with altered or arrhythmic behavior in constant darkness, similar to that seen in Six6-null mice. In addition, VIP/PHInull mice under a 1 h:11 h skeleton photoperiod did not display consolidated subjective days and nights; this is also similar to the activity patterns observed for the Six6-null mice. Another similarity that the Six6-null mice share with VIP/PHI-null mice is an increased number of activity bouts, with increased activity during subjective day in constant darkness. In constant darkness, the ultradian activity patterns of the Six6-null mice lacking optic nerves are qualitatively similar to VIP/PHI-null mice.
Whether or not the optic nerves of Six6-null mice form properly during development and then atrophy, or do not form at all, remain interesting questions. While further studies are needed to explore the relationship between optic nerve formation and SCN development, using morphological and histochemical approaches, we were not able to identify SCN in any Six6-null mice. This finding likely rules out the obvious hypothesis that the SCN phenotype of the Six6-null mice is secondary to the optic nerve phenotype.
However, Six6-null mice with partially spared optic nerves show some behavioral circadian rhythmicity while lacking apparent SCN, including the absence of AVP and VIP staining and morphological clustering of cells. While the role Six6 plays in the molecular clock—if any—is currently unknown, it is possible that Six6-null mice have a functioning cellular circadian clock in the apparent absence of normal SCN. If the molecular clock is functional in other brain regions (e.g., SCN neurons not located in a cytologically distinct hypothalamic nucleus or present only in small numbers) or peripheral organs (e.g., the liver), this may explain some of the residual behavioral rhythmicity observed in the Six6-null mice (Fig. 3, Supp. Fig. S1). The Six6-null mouse therefore serves as an excellent model in which this possibility may be explored.
In the present study, SCN morphology and expression of 2 SCN neuropeptides was characterized in WT and Six6-null mice. Other important neuropeptides are present in the SCN and may retain WT levels of expression in the absence of Six6, including GRP, PRK2, and Nms, and immunohistochemical characterization of these peptides in Six6-null mice is one future direction for this line of research. Another future approach to fully characterize the SCN—or lack thereof—in Six6-null mice would be to measure the embryonic and adult expression of genes such as Lhx1, Six3, Rorα, Gad67, and Calbindin.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Xue Li (Children’s Hospital of Boston, Harvard Medical School, Boston, MA) for providing Six6-null mice, Sunamita Leming for technical assistance, and Rachel Larder for helpful discussions. This work was supported by supported by NIH grants R01 DK044838, R01 HD072754, and R01 HD020377 (to P.L.M.), NIH grant R01 HD036460 (to M.R.G.), NIH grant R01 EY016807 (to S.P.), Japan Society for the Promotion of Science (JSPS) fellowship (to M.H.), and by NICHD/NIH through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (P.L.M.). P.L.M. was supported in part by P30 DK063491, P30 CA023100, and P42 ES010337. D.D.C. was supported in part by T32 GM008666 and T32 DK007541 and was a student in the University of California, San Diego, Biological Sciences Graduate Program.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
NOTE
Supplementary online material is available on the journal’s website at http://jbr.sagepub.com/supplemental.
REFERENCES
- Chase HB, Chase EB. Studies on an anophthalmic strain of mice I. Embryology of the eye region. J Morphology. 1941;68:279–301. [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R939–949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- Conte I, Morcillo J, Bovolenta P. Comparative analysis of Six 3 and Six 6 distribution in the developing and adult mouse brain. Dev Dyn. 2005;234:718–725. doi: 10.1002/dvdy.20463. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Elliott JA, Evans JA. Plasticity of hamster circadian entrainment patterns depends on light intensity. Chronobiol Int. 2003;20:233–248. doi: 10.1081/cbi-120018576. [DOI] [PubMed] [Google Scholar]
- Hamilton BA, Frankel WN, Kerrebrock AW, Hawkins TL, FitzHugh W, Kusumi K, Russell LB, Mueller KL, van Berkel V, Birren BW, et al. Disruption of the nuclear hormone receptor RORalpha in staggerer mice. Nature. 1996;379:736–739. doi: 10.1038/379736a0. [DOI] [PubMed] [Google Scholar]
- Hinds JW, Hinds PL. Early ganglion cell differentiation in the mouse retina: an electron microscopic analysis utilizing serial sections. Dev Biol. 1974;37:381–416. doi: 10.1016/0012-1606(74)90156-0. [DOI] [PubMed] [Google Scholar]
- Jean D, Bernier G, Gruss P. Six6 (Optx2) is a novel murine Six3-related homeobox gene that demarcates the presumptive pituitary/hypothalamic axis and the ventral optic stalk. Mech Dev. 1999;84:31–40. doi: 10.1016/s0925-4773(99)00068-4. [DOI] [PubMed] [Google Scholar]
- Kabrita CS, Davis FC. Development of the mouse suprachiasmatic nucleus: determination of time of cell origin and spatial arrangements within the nucleus. Brain Res. 2008;1195:20–27. doi: 10.1016/j.brainres.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemle LK, Hori N, Strominger NL, Tan Y, Carpenter DO. Physiological and anatomical properties of the suprachiasmatic nucleus of an anophthalmic mouse. Brain Res. 2002;953:73–81. doi: 10.1016/s0006-8993(02)03272-9. [DOI] [PubMed] [Google Scholar]
- Laemle LK, Ottenweller JE. Daily patterns of running wheel activity in male anophthalmic mice. Physiol Behav. 1998;64:165–171. doi: 10.1016/s0031-9384(98)00045-6. [DOI] [PubMed] [Google Scholar]
- Larder R, Clark DD, Miller NL, Mellon PL. Hypothalamic dysregulation and infertility in mice lacking the homeodomain protein Six6. J Neurosci. 2011;31:426–438. doi: 10.1523/JNEUROSCI.1688-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak RK, Moore RY. Topographic organization of suprachiasmatic nucleus projection neurons. J Comp Neurol. 2001;433:312–334. doi: 10.1002/cne.1142. [DOI] [PubMed] [Google Scholar]
- Li X, Perissi V, Liu F, Rose DW, Rosenfeld MG. Tissue-specific regulation of retinal and pituitary precursor cell proliferation. Science. 2002;297:1180–1183. doi: 10.1126/science.1073263. [DOI] [PubMed] [Google Scholar]
- Liu W, Lagutin OV, Mende M, Streit A, Oliver G. Six3 activation of Pax6 expression is essential for mammalian lens induction and specification. EMBO J. 2006;25:5383–5395. doi: 10.1038/sj.emboj.7601398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund RD, Blunt AH. Prenatal development of central optic pathways in albino rats. J Comp Neurol. 1976;165:247–264. doi: 10.1002/cne.901650209. [DOI] [PubMed] [Google Scholar]
- Mason CA, Sparrow N, Lincoln DW. Structural features of the retinohypothalamic projection in the rat during normal development. Brain Res. 1977;132:141–148. doi: 10.1016/0006-8993(77)90711-9. [DOI] [PubMed] [Google Scholar]
- McNeill DS, Sheely CJ, Ecker JL, Badea TC, Morhardt D, Guido W, Hattar S. Development of melanopsin-based irradiance detecting circuitry. Neural Dev. 2011;6:8. doi: 10.1186/1749-8104-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai N, Nagai K, Nakagawa H. Effect of orbital enucleation on glucose homeostasis and morphology of the suprachiasmatic nucleus. Brain Res. 1992;589:243–252. doi: 10.1016/0006-8993(92)91283-k. [DOI] [PubMed] [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents IV: entrainment: pacemaker as clock. J Comp Physiol A. 1976;106:291–331. [Google Scholar]
- Shimogori T, Lee DA, Miranda-Angulo A, Yang Y, Wang H, Jiang L, Yoshida AC, Kataoka A, Mashiko H, Avetisyan M, et al. A genomic atlas of mouse hypothalamic development. Nat Neurosci. 2010;13:767–775. doi: 10.1038/nn.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepka SM, Takahashi JS. Methods to record circadian rhythm wheel running activity in mice. Methods Enzymol. 2005;393:230–239. doi: 10.1016/S0076-6879(05)93008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J. Abnormal development of the suprachiasmatic nuclei of the hypothalamus in a strain of genetically anophthalmic mice. J Comp Neurol. 1977;176:589–606. doi: 10.1002/cne.901760409. [DOI] [PubMed] [Google Scholar]
- Stanfield B, Cowan WM. Evidence for a change in the retino-hypothalamic projection in the rat following early removal of one eye. Brain Res. 1976;104:129–136. doi: 10.1016/0006-8993(76)90652-1. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Tucker P, Laemle L, Munson A, Kanekar S, Oliver ER, Brown N, Schlecht H, Vetter M, Glaser T. The eyeless mouse mutation (ey1) removes an alternative start codon from the Rx/rax homeobox gene. Genesis. 2001;31:43–53. doi: 10.1002/gene.10003. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDunk C, Hunter LA, Gray PA. Development, maturation, and necessity of transcription factors in the mouse suprachiasmatic nucleus. J Neurosci. 2011;31:6457–6467. doi: 10.1523/JNEUROSCI.5385-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.