Abstract
The human embryo is not a feasible experimental system for the detailed study of implantation and early placentation, so surrogate systems have been sought for investigating the determination of the trophectoderm lineage, its differentiation into trophoblasts of the early implantation site, and subsequently the morphogenesis of the definitive placenta. An alternative to the use of embryos for studying early placental development was revealed by work with human embryonic stem cells (hESC), demonstrating BMP2/4-stimulated trophoblast differentiation, and spontaneous formation from embryoid bodies (EBs). These cells display a trophoblastic transcriptome, as well as a placental protein and steroid hormone secretory profile, and invasive and chemotactic behavior resembling human placental trophoblasts. With EB-derived trophoblasts, two-dimensional and three dimensional paradigms and other modifications of the culture environment, including extracellular matrix and aggregation with placental fibroblasts, impact on trophoblast differentiation. Recent studies have questioned the identity of the trophoblasts directed by BMP treatment of hESC, and careful attention to culture conditions is needed to interpret different results among research groups. Although the precise placental counterpart of the hESC-derived trophoblast remains unclear, hESC-derived trophoblasts remain an intriguing platform for modeling early implantation.
Keywords: embryonic stem cells, trophoblast, embryoid body
1. Introduction
The specification of the trophoblast lineage and the subsequent formation of the placenta are among the earliest differentiation events to take place in mammalian development. In vivo, trophectoderm formation is heralded by the onset of compaction at the morula stage, which occurs approximately 4–5 days after fertilization, although molecular events are likely to be put into motion well before this event [1,2]. Following blastocyst formation, the pathway of subsequent embryo development, implantation, and placentation and placental morphogenesis diverges significantly among mammals, encompassing filamentous embryonic elongation, as in ruminants, intrauterine embryonic diapause, as in carnivores, and invasive blastocyst intrusion and invasion into the receptive decidua, as in the initiation of human pregnancy [3]. For the modeling of human placentation, model systems have been developed ranging from immortal cell lines from placental trophoblast-derived tumors or targeted immortalization, to human placental explant tissue, to isolation of individual cells, all with the goal of developing approaches to interrogate this most important time in our development. Recent excellent reviews have broadly covered the range of options and resources in this area and the reader is referred to those papers for a more comprehensive perspective [4–6]. The current review is focused on the use of human embryonic stem cells to model these earliest stages of our development. We will first present a historical perspective on the recognition that nonhuman primate and then human embryonic stem cells provided an opportunity to study trophoblast differentiation. The majority of this review will then present the model for hESC-derived trophoblast differentiation using a specific paradigm, the formation of embryoid bodies, as the platform for these endeavors. Finally, recent developments in the field which have called into question one of the models for BMP4-driven trophoblast differentiation will be discussed, and directions for future research will be proposed.
2. Primate ESC differentiate to trophoblasts
In 1995 Thomson et al [7] reported the derivation of ES-like cells from rhesus monkey preimplantation blastocysts derived from natural mating and nonsurgically flushed from the uterus. These cells had key characteristics of ESC, including the expression of surface markers characteristic of pluripotent cells, and the ability to form structures derived from all three embryonic lineages when teratomas were formed following injection into immunodeficient mice. It was also determined that upon spontaneous differentiation in culture, there was evidence of trophoblast differentiation, including secretion of chorionic gonadotropin (CG), and expression of the CG alpha and beta subunit mRNAs [7].
This demonstrated a novel attribute of these cells, in that mouse ESC do not possess the ability to spontaneously form trophoblasts in the absence of exogenous genetic manipulation. The significance of this observation was reinforced when the Thomson lab [8] reported the derivation of hESC, and commented that trophoblast differentiation was evidenced again by the secretion of hCG into the culture medium upon spontaneous differentiation. Surprisingly, however, our lab has not been able to demonstrate the production of trophoblast-specific protein expression in teratomas from either rhesus monkey or human ESC (unpublished data) and perhaps this is due to some limitation of the murine environment, or an undefined inhibitory effect thereof, on trophoblast differentiation.
The pluripotency of hESC was further strengthened in 2002 by Xu et al [9] who demonstrated that remarkably, when hESC were treated with BMP-4 or related agonists, there was consistent differentiation to a uniform population of cells with an epithelial morphology, and upon analysis by microarray, the population of transcription factors, secreted proteins and other key markers supported the conclusion that the cells were trophoblasts. This was corroborated by the secretion of hCG, as well as progesterone (P4) and estradiol-17β (E2), the combination of which is a unique characteristic of primate trophoblasts. Although it remains unclear whether BMPs play a role in in vivo trophoblast differentiation [10], this model has been adopted by a number of researchers, and has been a valuable and reliable paradigm for the induction of trophoblast differentiation from pluripotent hESC, including definition of the ontogeny of gene expression in these cells [11], demonstration of the role of “physiologically low” oxygen culture conditions in the balance between pluripotency and differentiation [12], and comparison of BMP-induced trophoblasts with human embryonic trophectoderm [13]. There have been comprehensive reviews of the cell biology of the BMP response [6, 14, 15] so we will only return to this model later to discuss recent controversial findings.
3. An alternative model for trophoblast differentiation: embryoid body formation
Reasoning that pluripotent hESC could serve as embryonic surrogates, our lab investigated whether the formation of embryoid bodies (EBs) by suspension culture of undifferentiated hESC colonies could support trophoblast differentiation. EBs from human and primate ESC will undergo differentiation to all three germ layers, although not in an organized way reflecting in vivo embryonic development [7,8]. We first simply addressed whether there was evidence of trophoblast formation in suspension-cultured EBs. This was confirmed by the detection of consistent (although modest) secretion of hCG, P4 and E2 into the culture medium within 48 hr after initiation of suspension culture [16]. Immunohistochemistry (IHC) confirmed the formation of cytokeratin- and hCG-positive cells, typically at the periphery of the suspension EBs [16].
Recognizing that the trophoblast-like cells on the surface of the EBs might be able to be recovered for more detailed analysis upon adherent culture of the suspension structures, we found that within several weeks of allowing the EBs to adhere to the culture surface, there was amplification of hCG secretion, in association with outgrowth of cells with a trophoblast morphology [16]. Further evidence supporting a trophoblast identity of these cells included their expression of hCG and cytokeratin 8/18 as detected by flow cytometric staining of a monocellular suspension of these “outgrowths”, and their ability to respond to 8-bromo-cAMP with an increase in hCG production, a classic hallmark of trophoblast identity [17] (data in preparation, Gerami-Naini et al). Nonetheless, hormone secretion declined in culture with time [16].
Additional paradigms were implemented in efforts to further define the differentiation potential of the ESC-derived trophoblasts. First, we devised a three-dimensional culture system, with the hypothesis that the presence of an extracellular matrix (Matrigel) will support an implantation-like environment, and foster the formation of villous structures, recapitulating the formation of the placenta by the implanting embryo. We were able to show dramatic effects of this three-dimensional environment. Cells detached from the surface of the EBs when embedded into Matrigel [16], and hormone secretion by trophoblastic outgrowths was substantially higher, and sustained for longer periods of time, in the 3 dimensional vs. 2 dimensional environment [18]. We have not pursued the mechanisms or regulation of this outgrowth into the Matrigel, and this is an area that deserves further investigation, in comparison with mechanisms indentified in other in vitro trophoblast systems [19] and hypothesized to be important for in vivo implantation. In addition, the 2D vs 3D cellular signaling pathways which control hormone secretion are likely to be very important, and we attempted to implement a rigid vs. flexible collagen gel paradigm to study this question [20]. However, extensive digestion of the collagen gel matrix by (presumably) secreted matrix metalloproteinases prevented the use of this platform (Giakoumopoulos and Golos, unpublished).
4. Further characterization of the 2-dimensional trophoblast outgrowths
Further characterization has been ongoing for the cells which arise as 2-dimensional outgrowths from adherent embryoid bodies. First, we evaluated whether Matrigel itself, or specific extracellular matrix components thereof, was promoting the differentiation of trophoblasts in the 2-dimensional paradigm. Fig. 1 illustrates that culture on a variety of purified extracellular matrix proteins (e.g., fibronectin, laminin, collagen IV) which comprise important elements of Matrigel all were sufficient at supporting the development of hormonal secretory capability not significantly different from Matrigel itself. We also have conducted an initial exploration of the transcriptome of the 2-dimensional EB outgrowths and have confirmed that secreted factors, transcription factors, and other trophoblast-associated mRNAs are substantially up-regulated in the EB-derived cells (Table 1).
Fig. 1.
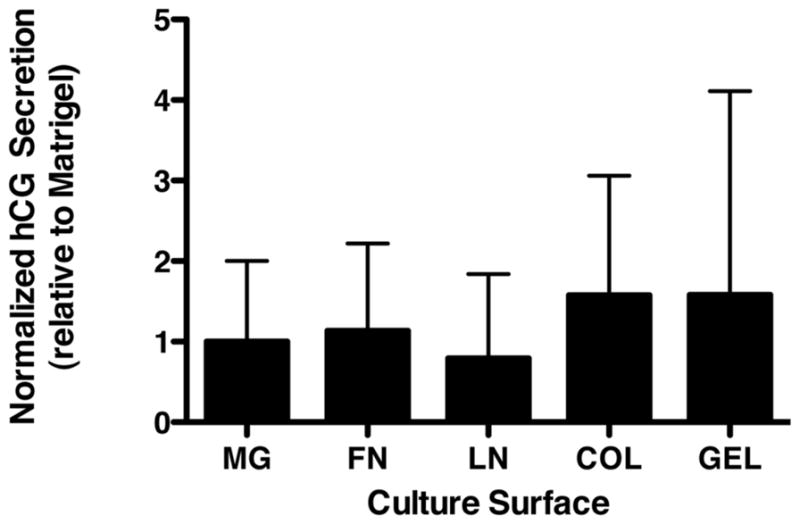
Effect of extracellular matrix growth surface on secretion of hCG by EB-derived trophoblast outgrowths. Representative data from three experiments is shown at 10 days post-plating. Secretion of hCG was determined by radioimmunoassay, and was normalized to metabolic activity determined by WST-8 assay (Dojindo Molecular Technologies, Rockville, MD).
Table 1.
Key markers of trophoblast identify in 2D-EB outgrowths
| Endocrine factors (fold-increase) | Transcription factors (fold-increase) | Other trophoblast markers (fold-increase) |
|---|---|---|
| hCGα 8194 | AP2A (614) | KRT7 (66) |
| HSD3β (1130) | Hey1 (203) | EGFR (305) |
| hCGβ648 | GATA3 (2431) | |
| CYP11A1 (253) | MSX2 (191) | |
| PGF (107) | HAND1 (356) | |
| CRH (35) | ||
| PSG1-9 (9–80) |
Selected markers for trophoblast differentiation are presented with their fold-induction over undifferentiated cells as determined in Affymetrix microarrays. The means of 5 separate undifferentiated RNA samples and 5 samples of 2-dimensional EB outgrowths are presented (Gerami-Naini, Giakoumopoulos et al, in preparation). Note that not expressed in either hESC or differentiated cells were hPL, Cdx2, TBR2, Eomes, or Brachury mRNAs.
One complexity of the 2-dimensional EB-derived trophoblast model (and indeed, the BMP-derived trophoblast model) is that it is not clearly understood which trophoblast within the in vivo placenta is represented by these hESC derivatives. Our studies with the EB model, and the work of other investigators with the BMP-derived paradigm, have shown the formation of multinuclear structures (Fig. 2), often at the periphery of colonies, implying an acceleration of differentiation with distance from less differentiated cells within the EB or ESC colony [12]. These multinuclear structures are typically described as representing villous syncytiotrophoblasts, although if this were true, a villous cytotrophoblast population would be a prerequisite for their formation. However, when bona fide villous CTB are isolated from first trimester [21] or term [22] placental tissue, there is widespread (>90%) formation of syncytia. This is not the outcome with ESC-derived trophoblasts. However, there are several possibilities which could account for this observation. First, the outgrowths could represent a mixed population of cells, only some of which represent fusion-competent cytotrophoblasts. Alternatively the multinuclear cells could represent, at least in part, human multinucleated extravillous trophoblasts (sometimes referred to as giant cells) also seen within the decidua [23]. Further careful identification of markers for these giant cells is needed. Finally, it may be that since the ESC likely are more representative of early gestation, these cells represent a developmentally earlier stage of placental development that precedes the accelerated syncytiotrophoblast differentiation which is seen in mature villi of later gestation. This is an area for further study with the ESC models.
Fig. 2.
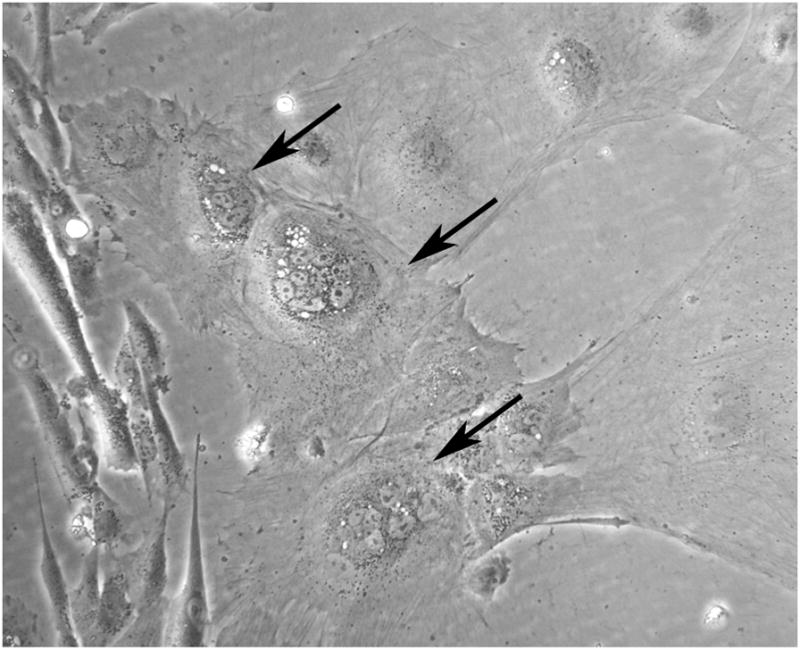
Representative appearance of multinuclear cells (arrows) within the migrating outgrowths from hESC-derived EBs after 16 days of culture.
Other characteristics of trophoblasts are readily observed with the EB outgrowths. Invasion of matrix and migration in a Transwell migration chamber are typical characteristics of invasive extravillous trophoblasts [24], and the EB outgrowths readily penetrate a Matrigel surface and migrate through the culture surface filter (Fig. 3). In addition, Fig. 3 confirms that essentially all of the migrated EB-derived cells are cytokeratin-positive, again confirming a trophoblast identity.
Fig. 3.
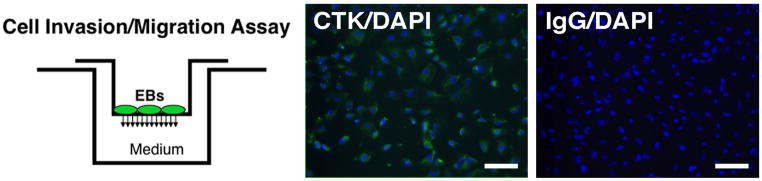
Migration of EB-derived trophoblasts through Matrigel-coated Transwell filters. Left panel: schematic representation of migration chamber. Cells which migrate through the filter are then analyzed by immunostaining. Center panel: Migratory cell immunofluorescent staining for cytokeratin (CTK, green) counterstained with DAPI (blue nuclei), confirming that virtually every cell migrated through the Transwell filter is cytokeratin positive (staining with a pan-cytokeratin antibody (Sigma, clone C-11), which recognizes a range of cytokeratins, including 8 and 18). Right image, immunofluorescent staining with a nonspecific primary antibody.
5. Molecular confirmation of trophoblast identity
Preliminary microarray analysis of these outgrowths has been done, at their peak of hCG secretion (2 weeks after EB plating). Table 1 provides a summary of altered gene expression in comparison with the undifferentiated H1 hESC line. A more detailed analysis (Gerami-Naini et al) is currently in preparation; however it is clear that secreted products and transcription factors which are up-regulated in the EB outgrowths point towards a trophoblast phenotype. However, because of the plethora of factors expressed, and the possibility that the population of EB outgrowths is a heterogeneous population, it is not possible to assign a specific identity to these cells, with regard to the precise trophoblast niche in the placenta that they represent. In addition, assignment of identity is complicated by the fact that the trophoblasts derived from EBs may not represent a type of trophoblast thus far isolated and analyzed from human primary tissues, since nearly all tissue evaluated in human trophoblast studies are derived from placentas from at least 6–8 weeks of gestation. In the mature placenta, the STB are clearly the primary source of hCG expression. However, most proliferating trophoblast cell lines also secrete hCG, and analysis of early human placental tissues [25] has shown that hCG is also expressed by the EVT including multinucleated trophoblasts found in the placental bed [27]. Thus, transcriptomic profiles of selected subpopulations of early pregnancy placental trophoblasts may be needed in order to be able to determine the developmental niche that the EB-derived trophoblasts represent.
For another example, we have determined that the primary connexin expressed by EB trophoblasts is Cxn43 (GJA1). In the in vivo human placenta, Cxn43 is primarily expressed in the villous CTB, but also in the multinucleated extravillous trophoblasts of the placental bed [26]. IHC of EB outgrowths demonstrates that there is widespread expression of Cxn43 throughout the cell population (Fig. 4). However, this is inconsistent with the secretion by EB outgrowths of hCG, P4, and E2, which are not appreciably synthesized in villous cytotrophoblasts. Thus, we suggest that the EB outgrowths may represent a trophoblast phenotype found in the early implantation site, which is hormonally potent, expressing hCG, as well as the enzymes which underlie the capacity for steroidogenesis.
Fig. 4.
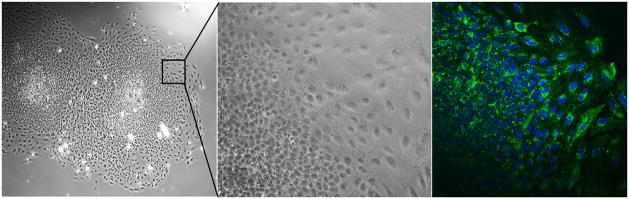
Connexin 43 expression in hESC-derived EB outgrowths. Left and middle panels: bright-field photomicrographs of hESC-derived EBs in adherent culture, with the middle panel showing a higher magnification image view of the indicated field. The right panel shows a confocal image of a similar field stained with and anti-Connexin43. Virtually all cells throughout the outgrowths are Cxn43-positive; some cells (lower left) are out of the focal plane in this image.
6. Paradigm for placental morphogenesis: an area for further research
One disappointment with the 3-dimensional EB differentiation paradigm [16] was that we did not observe the appearance of villous morphogenesis in these specimens. There was the occasional multilaminar trophoblast formation at the surface of the embryoid body; however we could not be confident that these cells provided any evidence of syncytium formation. The failure to form mature STB is corroborated by the fact that we do not observe the expression of chorionic somatomammotropin mRNA expression in the 2D outgrowth microarray data (Gerami-Naini et al, in preparation). It is possible that this reflects the likely early developmental stage at which these trophoblasts are evaluated in our studies. Our published studies which endeavored to create a more “villous” environment by incorporating placental fibroblasts into the EBs during suspension culture showed that while these EBs indeed had enhanced secretion of hCG [27], there was no enhanced formation of STB-like structures on the surfaces of these EBs. Thus, we feel an important area for further study is to investigate the role of other “effector” cells, or specific morphogens, on villus formation from the EB during trophoblast differentiation. A better understanding of the factors that direct appropriate early villous morphogenesis could provide therapeutic approaches for placental insufficiency or other dysfunction in early placental formation. Interestingly, the same placental fibroblasts, which enhanced hCG secretion in 3D aggregates, inhibited ESC differentiation in a 2-dimensional co-culture paradigm and instead sustained the pluripotent state, acting as effective feeder layers [27]. These data underscore the importance of the in vitro culture paradigm used for differentiation, including feeder cells, growth factor supplements, and extracellular matrix environments.
7. Conflicting results with ESC-derived trophoblasts
While the BMP-treated ESC paradigm is widely held to result in trophoblast differentiation, this phenomenon remained poorly understood on several levels. First, does this represent a physiologically relevant paradigm for in vivo placental development? In addition, what is the mechanism by which BMP drives the formation of trophoblasts? In response to the first question, it is not clear what population of trophoblasts is represented by this paradigm, so the context of this differentiation event remains to be fully defined in the actual implantation site. As for the second query, Yu et al [10] in seeking to understand how BMP curtails the pluripotency of the undifferentiated ESC, have recently demonstrated that the effect of BMP is dependent on the presence or absence of FGF2, an essential component of the maintenance of pluripotency in hESC (in distinction to mouse ESC, in which LIF plays this role). Trophoblast differentiation in response to BMPs depends on the absence of FGF2: in the presence of both FGF2 and BMP4, rather than direct trophoblast formation, the differentiation of mesendoderm is driven [10]. Intriguingly, Pera et al [28] also concluded that with the HES-2 or HES-3 hESC lines, there were only infrequent patches of trophoblast differentiation seen with BMP treatment, and rather, extraembryonic endoderm was formed in response to BMPs. Recently, Bernardo et al [29] reported that BMP-derived cells also express selected mesodermal genes (e.g., brachyury), but do not produce HLA-G, and only express low ELF5. They have concluded that these results “raise doubts that the BMP-induced cells are trophoblasts”, and that they “instead are mesodermal in nature”. It should be recognized that HLA-G is actually not expressed in most trophoblasts of the mature placenta [30], and ELF5 likewise is not ubiquitously expressed throughout the implantation site [31]. It has also been shown that brachyury, as well as other markers of mesoderm and embryonic and extraembryonic endoderm, are differentially expressed among the different hESC used among these studies, and are not absolutely inconsistent with a trophoblast phenotype [6]. In addition to the possibility of differential responses to BMP4 among cell lines, a recent study [32] also has demonstrated that BMP4 treatment directs the formation of multiple distinct progenitor populations, as revealed by screening with a monoclonal antibody library. Thus, it is possible that these diverse outcomes are multifactorial and reflect the characteristics of different hESC lines, culture conditions, and analytical approaches. With ESC, culture conditions are paramount, and Supplemental Table 1 summarizes the culture medium formulations used and the cell lines analyzed in these cited studies. It seems important to conduct head-to-head comparisons to reconcile the apparently different outcomes with different culture conditions, as regards the trophoblast phenotype of BMP-driven differentiation.
8. Conclusions and areas for further study
In the preceding sections we have presented and described data that demonstrate that much remains to be addressed with available systems for promoting trophoblast differentiation. We summarize important areas for future study in Table 3. Approaches are needed to dissect the impact of the 2D vs 3D Matrigel culture environment for hormone secretion, which we hypothesize reflects differential signaling through integrin-mediated and other mechanisms integrating extracellular adhesion effects on cell function. New paradigms are needed that incorporate effector cells, including placental mesenchyme as well as decidual components, which we hypothesize will effect placental morphogenesis and villous formation. Our understanding of the significance of hESC and iPSC differentiation [33] will be enhanced by better definition of the potential heterogeneity of trophoblast populations which arise from hESC differentiation paradigms. Recently, methods to derive a trophoblast stem/progenitor population from the chorionic plate have been reported [36]. While the role of these cells in this chorionic plate niche remains to be further elucidated, a direct comparison of these chorion-derived cells with hESC-derived trophoblasts will also be very informative.
Convincing data for the differentiation of trophoblasts from hESC has been presented by a number of labs. However, recent important questions have been raised which, at least, demonstrate that there is likely to be an impact of culture conditions in giving rise to disparate outcomes from different laboratories. Precise description of culture conditions, and cross-laboratory testing of different hESC lines will likely provide further insight into the current controversial findings. While BMP4-induced trophoblast differentiation could be considered a more manageable system than the preparation and culture of EBs, we suggest that trophoblast differentiation from EBs is a useful alternative to BMP treatment. Further studies are ongoing to ascertain the ontogeny of gene expression with time after plating of EB outgrowths, and whether BMP4-derived cells are significantly different from EB-derived trophoblasts. An area that does require attention in such studies is the spatial differences noted by most investigators in morphology of trophoblasts in different areas of the hESC colony when treated with BMP4 [e.g., 12]. It is possible that these spatial morphological differences underlie some aspects of the heterogeneity of differentiating hESC derivatives recently detected with antibody library testing of the cell surface markers of BMP4-treated hESC [32]. Selective analysis of specific populations of cells obtained, for example, by laser capture microdissection, will address these questions and extend our understanding of differentiation events with EBs serving as a surrogate system for embryonic trophoblast differentiation.
Supplementary Material
Table 2.
Research areas proposed for future study
| Impact of culture medium selection on BMP4-driven trophoblast differentiation |
| Functional/regional/spatial differences in the trajectory and outcome of EB trophoblast differentiation, including multinuclear cell phenotype/identity |
| Development of new paradigms incorporating effector cells to impact chorionic villus formation |
| Identification of 2D vs. 3D signaling mechanisms which result in differential hormone secretion |
| Comparison of the phenotypes of BMP- vs. EB- induced trophoblasts |
Acknowledgments
Many lab members contributed to the studies in the Golos lab discussed in this paper, particularly Oksana Dovzhenko, Mark Garthwaite and Maureen Durning. We thank the Assay Services staff of the WNPRC for hormone assays, Kim Keen for assistance with confocal microscopy, the James Thomson and Igor Slukvin labs for ongoing advice and assistance, and R. Michael Roberts (University of Missouri) for helpful discussion. This work was supported by NIH grants RR021876, HD38843, HD34215 and HD53925 to T.G.G., and P51OD011106/P51RR000167 to the WNPRC. M. Giakoumopoulos and B. Gerami-Naini were supported in part by T32 HD041921. This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Conflict of interest statement
We confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Home P, Saha B, Ray S, Dutta D, Gunewardena S, Yoo B, et al. Altered subcellular localization of transcription factor TEAD4 regulates first mammalian cell lineage commitment. Proc Natl Acad Sci USA. 2012;109:7362–7367. doi: 10.1073/pnas.1201595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yagi R, Kohn MJ, Karavanova I, Kaneko KJ, Vullhorst D, DePamphilis ML, Buonanno A. Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. Development. 2007;134:3827–3836. doi: 10.1242/dev.010223. [DOI] [PubMed] [Google Scholar]
- 3.Benirschke K, Kaufmann P, Baergen RN. Pathology of the human placenta. New York: Springer; 2006. Placental types; pp. 30–41. [Google Scholar]
- 4.Hannan NJ, Paiva P, Dimitriadis E, Salamonsen LA. Models for study of human embryo implantation: choice of cell lines? Biol Reprod. 2010;82:235–245. doi: 10.1095/biolreprod.109.077800. [DOI] [PubMed] [Google Scholar]
- 5.James JL, Carter AM, Chamley LW. Human placentation from nidation to 5 weeks of gestation. Part II: Tools to model the crucial first days. Placenta. 2012;33:327–334. doi: 10.1016/j.placenta.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Ezashi T, Telugu BP, Roberts RM. Model systems for studying trophoblast differentiation from human pluripotent stem cells. Cell Tissue Res. 2012;349:809–24. doi: 10.1007/s00441-012-1371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson JT, Kalishman J, Golos TG, Durning M, Becker R, Harris C, Hearn JP. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci USA. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 9.Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 10.Yu P, Pan G, Yu J, Thomson JA. FGF2 sustains NANOG and switches the outcome of BMP4-induced human embryonic stem cell differentiation. Cell Stem Cell. 2011;8:326–334. doi: 10.1016/j.stem.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchand M, Horcajadas JA, Esteban FJ, McElroy SL, Fisher SJ, Giudice LC. Transcriptomic signature of trophoblast differentiation in a human embryonic stem cell model. Biol Reprod. 2011;84:1258–71. doi: 10.1095/biolreprod.110.086413. [DOI] [PubMed] [Google Scholar]
- 12.Das P, Ezashi T, Schulz LC, Westfall SD, Livingston KA, Roberts RM. Effects of fgf2 and oxygen in the bmp4-driven differentiation of trophoblast from human embryonic stem cells. Stem Cell Res. 2007;1:61–74. doi: 10.1016/j.scr.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aghajanova L, Shen S, Rojas AM, Fisher SJ, Irwin JC, Giudice LC. Comparative transcriptome analysis of human trophectoderm and embryonic stem cell-derived trophoblasts reveal key participants in early implantation. Biol Reprod. 2012;86:1–21. doi: 10.1095/biolreprod.111.092775. [DOI] [PubMed] [Google Scholar]
- 14.Roberts RM, Fisher SJ. Trophoblast stem cells. Biol Reprod. 2011;84:412–421. doi: 10.1095/biolreprod.110.088724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin G, Martins-Taylor K, Xu RH. Human embryonic stem cell derivation, maintenance, and differentiation to trophoblast. Methods Mol Biol. 2010;636:1–24. doi: 10.1007/978-1-60761-691-7_1. [DOI] [PubMed] [Google Scholar]
- 16.Gerami-Naini B, Dovzhenko OV, Durning M, Wegner FH, Thomson JA, Golos TG. Trophoblast differentiation in embryoid bodies derived from human embryonic stem cells. Endocrinology. 2004;145:1517–1524. doi: 10.1210/en.2003-1241. [DOI] [PubMed] [Google Scholar]
- 17.Strauss JF, III, Kido S, Sayegh R Sakuragi N, Gafvels ME. The cAMP signalling system and human trophoblast function. Placenta. 1992;13:389–403. doi: 10.1016/0143-4004(92)90047-w. [DOI] [PubMed] [Google Scholar]
- 18.Golos TG, Pollastrini LM, Gerami-Naini B. Human embryonic stem cells as a model for trophoblast differentiation. Sem Reprod Med. 2006;24:314–321. doi: 10.1055/s-2006-952154. [DOI] [PubMed] [Google Scholar]
- 19.Knofler M, Pollheimer J. IFPA award in placentology lecture: Molecular regulation of human trophoblast invastion. Placenta. 2012;3326(Supplement A):555–562. doi: 10.1016/j.placenta.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wozniak MA, Keely PJ. Use of three-dimensional collagen gels to study mechanotransduction in T47D breast epithelial cells. Biol Proced Online. 2005;7:144–161. doi: 10.1251/bpo112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golos TG, Handrow RR, Durning M, Fisher JM, Rilling JK. Regulation of chorionic gonadotropin alpha and chorionic somato-mammotropin mRNA expression by 8-bromo-cAMP and dexamethasone in cultured rhesus monkey syncytiotrophoblasts. Endocrinology. 1992;131:89–100. doi: 10.1210/endo.131.1.1612035. [DOI] [PubMed] [Google Scholar]
- 22.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., III Purification, characterization, and in vitro differentiaton of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 23.Benirschke K, Kaufmann P, Baergen RN. Pathology of the human placenta. New York: Springer; 2006. Nonvillous parts and trophoblast invasion; pp. 191–312. [Google Scholar]
- 24.Harris LL, Baker PN, Brenchley PEC, Aplin JD. Trophoblast-derived Heparanase is not required for invasion. Placenta. 2008;29:332–337. doi: 10.1016/j.placenta.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Handschuh K, Guibourdence J, Tsatsaris V, Guesnon M, Laurendeau I, Evain-Brion D, et al. Human chorionic gonadotropin expression in human trophoblasts from early placenta: comparative study between villous and extravillous trophoblastic cells. Placenta. 2007;28:175–84. doi: 10.1016/j.placenta.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Malassine A, Cronier L. Involvement of gap junctions in placental functions and development. Biochim Biophys Acta. 2005;1719:117–124. doi: 10.1016/j.bbamem.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Giakoumopoulos M, Siegfried LM, Dambaeva SV, Garthwaite MA, Glennon MC, Golos TG. Placental-derived mesenchyme influences chorionic gonadotropin and progesterone secretion of human embryonic stem cell-derived trophoblasts. Reprod Sci. 2010;17:798–808. doi: 10.1177/1933719110371853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pera MF, Andrade J, Houssami S, Reubinoff B, Trounson A, Stanley EG, et al. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci. 2004;117:1269–1280. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- 29.Bernardo AS, Faial T, Gardner L, Niakan KK, Ortmann D, Senner CE, et al. BRACHYURY and CDX2 mediate BMP-induced differentiation of human and mouse pluripotent stem cells into embryonic and extraembryonic lineages. Cell Stem Cell. 2011;9:144–155. doi: 10.1016/j.stem.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt JS, Petroff MG, Morales P, Sedlmayr P, Geraghty DE, Ober C. HLA-G in reproduction: studies on the maternal-fetal interface. Human Immunol. 2000;61:1113–1117. doi: 10.1016/s0198-8859(00)00195-6. [DOI] [PubMed] [Google Scholar]
- 31.Hemberger M, Udayashankar R, Tesar P, Moore H, Burton GJ. ELF5-enforced transcriptional networks define an epigenetically regulated trophoblast stem cell compartment in the human placenta. Hum Mol Genet. 2010;19:2456–2467. doi: 10.1093/hmg/ddq128. [DOI] [PubMed] [Google Scholar]
- 32.Drukker M, Tang C, Ardehali R, Rinkevich Y, Seita J, Lee AS, et al. Isolation of primitive endoderm, mesoderm, vascular endothelial and trophoblast progenitors from human pluripotent stem cells. Nat Biotechnol. 2012;30:531–542. doi: 10.1038/nbt.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mali P, Ye Z, Hommond HH, Yu X, Lin J, Chen G, Zou J, Cheng L. Improved effiiency and pace of generating induced pluripotent stem cells from human adult and fetal fibroblasts. Stem Cells. 2008;26:1998–2005. doi: 10.1634/stemcells.2008-0346. [DOI] [PubMed] [Google Scholar]
- 34.Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Michen ER, Frane JL, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 35.Johansson BM, Wiles MV. Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesodern and hematopoietic development. Mol Cell Biol. 1995;15:141–151. doi: 10.1128/mcb.15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genbacev O, Donne M, Kapidzic M, Gormley M, Lamb J, Gilmore J, et al. Establishment of human trophoblast progenitor cell lines from the chorion. Stem Cells. 2008;29:1427–1436. doi: 10.1002/stem.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


