Abstract
Older adults are more vulnerable to a negative impact of irrelevant information on cognitive performance. We used a psychophysical approach to evaluate which aspects of distraction are altered in aging: susceptibility for attention to be captured by a distractor, or the timing of disengagement from processing a distractor. We found that younger and older adults were equally susceptible to a detrimental influence of attentional capture on target detection in the initial moments after distractor presentation, but older adults exhibited a longer time window for the negative effects of capture to resolve. As was recently shown in younger adults, the timing of disengagement from capture correlated with individual differences in visual working memory capacity in the older cohort. These results suggest that the larger impact by distraction on perceptual abilities in normal aging is not the result of a greater susceptibility to attentional capture by distraction, but rather prolonged processing of distractors.
Keywords: Older adults, perception, working memory, visual capture
Introduction
It is well established that the presence of irrelevant external stimuli can interfere with a diverse array of cognitive operations. For example, attention to environmental details is disrupted by the abrupt appearance of new visual information. The distracting influence of abruptly presented visual stimuli has been shown to ‘capture’ attention and decrease goal-directed perceptual performance (Theeuwes, 1994). Susceptibility to visual capture by distracting information reflects an inability to effectively filter irrelevant information, thereby leading to an allocation of processing resources to information irrelevant to task goals (Fukuda & Vogel, 2009; Vogel, McCollough, & Machizawa, 2005).
Cognitive abilities, such as visual search (Plude & Hoyer, 1986) and selective attention (Plude & Doussard-Roosevelt, 1989), as well as short-term memory capacity (Dobbs & Rule, 1989; Foos & Wright, 1992), have been shown to decline in normal aging. The attentional control necessary for these cognitive tasks is dependent upon top-down signals from the prefrontal cortex both to coordinate early selection processes (Noudoost, Chang, Steinmetz, & Moore, 2010), as well as suppress cortical processing of irrelevant sensory input (Chao & Knight, 1995, 1998; Minamoto, Osaka, & Osaka, 2010; Zanto, Rubens, Thangavel, & Gazzaley, 2011). A diminished ability to inhibit distracting information has come to be recognized as a behavioral hallmark of cognitive aging (Hasher, Zacks, & May, 1999), and neurophysiological evidence of age-related deficits in suppressing irrelevant visual information has supported this view (Clapp & Gazzaley, 2010; Gazzaley et al., 2008; Gazzaley, Cooney, Rissman, & D'Esposito, 2005). For example, it has been demonstrated that distracting stimuli negatively impact visual working memory performance in older adults more than younger individuals, and functional MRI (fMRI) and EEG measures have shown that older individuals inappropriately direct attention to processing visual stimuli that are irrelevant to task demands (Gazzaley et al., 2008; Gazzaley et al., 2005). Moreover, the degree that irrelevant information is processed has been shown to directly correlate with impaired visual working memory performance (Clapp & Gazzaley, 2010; Clapp, Rubens, & Gazzaley, 2010; Gazzaley et al., 2005).
The ability to suppress irrelevant information has been shown to be variable across the younger population, and this is thought to contribute to individual differences in many cognitive abilities (Fukuda & Vogel, 2009). For example, it has been shown that younger individuals who are less efficient at filtering irrelevant information exhibit lower visual working memory capacity (VWMC) (Fukuda & Vogel, 2009; Minamoto et al., 2010; Vogel et al., 2005). It has recently been shown that individual variability in the impact that distracting stimuli has on performance is not mediated by differences in the susceptibility to attentional capture across younger individuals, but rather the speed in which one recovers from capture; i.e., the disengagement from distraction. Recently, Fukuda and Vogel (2011) evaluated this by assessing the ‘release time’ from capture using a target detection task in which stimulus onset asynchronies (SOAs) between peripheral visual distractors and the presentation of the target array were parametrically jittered. Interestingly, peripheral visual distractors impacted perceptual performance equally in the initial moments of capture (i.e. shortest SOA between distractor and target) for individuals with both high and low VWMC, while prolonged ‘disengagement’ from visual capture occurred in individuals with lower VWMC (Fukuda & Vogel, 2011). Furthermore, the prolonged disengagement from visual capture in low VWMC individuals was specific to distractors that shared a common feature to the target stimulus; i.e. same color, ‘relevant distractors’ compared to different color, ‘irrelevant distractors’ (Fukuda & Vogel, 2011). That is, prolonged disengagement does not seem to occur for just any form of irrelevant distraction, but instead occurs for distracting information that shares some common features with the current task demands.
We hypothesized that age-related inhibitory deficits may not be due to a greater susceptibility of older adults being captured by distractors, but instead a longer lasting impact of distraction as a result of older adults' inability to rapidly disengage from processing irrelevant information. Garden-path sentence and passage completion tasks (Hartman & Hasher, 1991; Hamm & Hasher, 1992) and directed forgetting paradigms (Zacks, Radvansky, & Hasher, 1996) have demonstrated age-related impairments in the ability to inhibit or ‘delete’ previous experiences (Hasher et al., 1999). Similarly, older individuals display prolonged facilitation of spatial cues orienting to targets, as well as delayed inhibition of return (i.e. slower target detection for locations that have been previously cued) compared to younger participants (Castel, Chasteen, Scialfa, Pratt, 2003). This hypothesis is in line with recent neuro-imaging evidence that older adults exhibit a diminished ability to disengage from processing interrupting stimuli associated with a secondary task (Clapp, Rubens, Sabharwal, & Gazzaley, 2011). Here, we addressed this hypothesis by assessing younger and older participant's susceptibility to attentional capture and disengagement from capture by manipulating SOAs between relevant and irrelevant distractors and the onset of a target array, in a comparable manner to that performed by Fukuda and Vogel (2011). Furthermore, we evaluate if prolonged disengagement from visual capture of distracting information is associated with an age-related decline in VWMC.
Method
Recruitment
Individuals from two age groups were recruited to participate in this study. The younger group consisted of 31 participants recruited from an online advertisement (18-30 years, mean= 23.8, SD 3.96; Female 20). The older group included 27 healthy participants (60-80 years, mean = 69.07 SD 5.72; Female 16) recruited from an in-lab database of previous participants. All participants reported normal to corrected vision and were not using any medications known to affect cognitive functioning. Also, all participants reported that they did not have any significant neurological or psychiatric disorders. A pre-screen testing for color-blindness (S. Ishihara, Tests for color-blindness -Handaya, Tokyo, Hongo Harukicho, 1917) was conducted prior to the main experimental session to ensure all participants could detect color differences between stimuli in the current experiment. All participants provided written informed consent and were compensated at a rate of $15 per hour for their time.
Neuropsychological screening
Participants in the older age group underwent a comprehensive neuropsychological testing within at least 2 years of the experimental procedure reported here to ensure that they had intact executive and memory function. A total of 14 neuropsychological tests were used, including: MMSE (Folstein, Folstein, & McHugh, 1975), geriatric depression (Yesavage et al., 1982), visual- spatial function (copy of a modified Rey-Osterrieth figure), visual-episodic memory (memory for details of a modified Rey-Osterrieth figure), visual-motor sequencing (trail making test A and B (Klove & Reitan, 1958; Tombaugh, 2004), phonemic fluency (words beginning with the letter ‘D’), semantic fluency (animals), calculation ability (arithmetic), executive functioning (Stroop interference test; (Stroop, 1935), working memory and incidental recall (backward digit span and digit symbol, WAIS-R (Wechsler, 1981). Intact function was assessed as scores that were within two standard deviations of the norm on each of the aforementioned tests (see Table 1).
Table 1. Demographic and neuropsychological data.
| Neuropsychological Tests: Mean (SE) | Standard Deviation from Normative Values (SE) | ||
|---|---|---|---|
| Younger (SE) | Older (SE) | Older (SE) | |
| N | 31 | 27 | n/a |
| Mean Age (year) | 23.8 (3.96) | 69.07 (5.72) | n/a |
| Age Range | 18-30 | 60-82 | n/a |
| Percent Female | 64.50% | 59.20% | n/a |
| Education (years) | 12+ | 12+ | n/a |
| Tests of Cognitive Impairment: Mean (SE) | |||
| MMSE | n/a | 29.3 (1.2) | 0.0 (0.6) |
| GDS | n/a | 2.7 (0.5) | n/a* |
| Executive Composite | |||
| WAIS-R Digit Span (backward) | 5.5 (1.3) | +0.8 (1.0) | |
| DKEFS Number Letter to Sequence | 67.7s (21.4) | +1.2 (0.5) | |
| Semantic Fluency Test | 24.2 (6.6) | +0.3 (1.2) | |
| Phonemic Fluency Test | 15.8 (5.6) | − 2.0 (0.6) | |
| Stroop: color-word naming | 53.6 (13.6) | 0.0 (1.0) | |
| Memory Composite | |||
| CVLT: Trial 5 | |||
| Recall | 12.5 (3.4) | +0.7 (1.5) | |
| CVLT: short delay free recall | 11.5 (2.9) | +1.0 (1.1) | |
| CVLT: short delay cued recall | 12.6 (2.8) | +0.7 (1.0) | |
| CVLT: long delay free recall | 11.3 (3.0) | +0.5 (1.0) | |
| CVLT: long delay cued recall | 12.6 (2.8) | +0.7 (1.0) | |
| Memory for Modified Rey | 12.1 (3.4) | +0.1 (1.1) | |
| Processing Speed Component | |||
| DKEFS Number to Sequence | 30.1s (13.2) | +1.2 (0.9) | |
| WAIS Digit Symbol Test | 56.9 (9.6) | +0.3 (0.5) | |
| Stroop: color naming | 88.9 (13.6) | − 0.2 (2.0) | |
Neuropsychological and Cognitive Metrics.
Change detection task (Visual working memory capacity)
The first task was a change detection task used in previous studies (e.g. (Awh, Barton, & Vogel, 2007; Fukuda & Vogel, 2009), in order to assess individuals' visual working memory capacity (Cowan, 2001). This task consisted of stimulus arrays of either 2, 4, 6 or 8 colored squares presented briefly (150ms) on the computer screen. Participants were instructed to remember the stimulus array over a retention interval of 900ms while fixating on a grey cross-hair. After which, a single colored square was presented in the same location as one of the previous items from the stimulus array and participants were required to make a button press to indicate whether the color of the square was the same or different as the original item in that location. On half of the trials, the color of the square was the same as the original item in that location and the other half of the trials presented a colored square that did not match the original item. Individual accuracy on each array size was transformed into a K estimate using a standardized formula (Cowan, 2001) considered to be a robust measurement of individuals' visual working memory capacity. The formula is K = S(H−F), where K is the memory capacity, S is the size of stimulus array, H is the observed hit rate, and F is the false alarm rate (for a recent review of this measure see (Rouder, Morey, Morey, Cowan, 2011). Using this formula, we calculated the mean K for all stimulus array sizes (S) for each individual, resulting in a single visual working memory capacity measure for each participant (see Results).
Perceptual thresholding task
Participants performed a visual search task (Fukuda & Vogel, 2011) consisting of 4 blocks of 60 trials in order to determine each individual's perceptual threshold for detecting target stimuli (- see capture task below). Individuals were instructed to identify the orientation of a target Landolt “C” (0.8°×0.8°) presented in red among an array of three other distractor Landolt “C”s drawn in different colors (i.e., blue, green, and magenta). All stimuli of the search array appeared within four grey squares (1.6°×1.6°) serving as placeholders appearing prior (500 ms) to the search array and remained on the screen for the duration of the trial. The search array was then masked by a multicolored square pattern presented within each placeholder location and participants were asked to report the orientation of the gap (top, right, left, or bottom) on the target stimuli by pressing one of four arrow keys on a keyboard. The presentation length of the visual search array was titrated for each participant with a stair-casing procedure to find the duration at which performance was approximately 75% correct for each individual (same as the ‘No Flanker’ condition in capture task below). The target durations for the last 20 trials were averaged to estimate the baseline duration for each block, then averaged across all blocks resulting in a single perceptual threshold value for each participant. Younger participants had a mean perceptual threshold of 90.8 ± 45.3 milliseconds (ms), while the mean threshold for older participants was considerably longer (222.2 ± 95.5 ms).
Capture task
After estimating each individual's perceptual threshold, participants performed an onset capture variation of the visual search task. Participants were instructed to identify the orientation of a target Landolt “C” presented in red among an array of three other distractor Landolt “C”s drawn in different colors. Note that there were four response options, and therefore chance performance was 25%. On a third of the trials, a colored square (0.8°×0.8°) was presented for 50ms at a position that flanked the position to one of the placeholders prior the search array onset. The position of the flanker relative to placeholders was completely randomized, therefore the flanker appearance had no relation to which placeholder would contain the Landolt “C” target. All other trials were identical to the previous perceptual thresholding task where no flanker was presented. On flanker-present trials, there were four possible stimulus onset asynchronies (SOAs) between the flanker and the search array: 50ms, 150ms, 250ms, or 350ms. The flanker appeared either in the same red color as the target stimulus of the subsequent search array (relevant flanker) or in a distractor color of blue (irrelevant flanker) – see Figure 1A. The order of presentation for these different types of trials was randomized and counter-balanced across each block. Each participant performed 8 blocks of 160 trials each and was allowed to take short breaks between each block. It is important to note that the recorded values of the No-flanker condition were randomly assigned to ‘dummy variables’ of the same SOA lengths as the flanker conditions: 50ms, 150ms, 250ms, 350ms (same procedure as Fukuda and Vogel (2011)). This random assignment of dummy variables was done for each individual prior to analysis and was coded into the experimental paradigm in order to perform subsequent repeated-measures ANOVAs.
Figure 1.
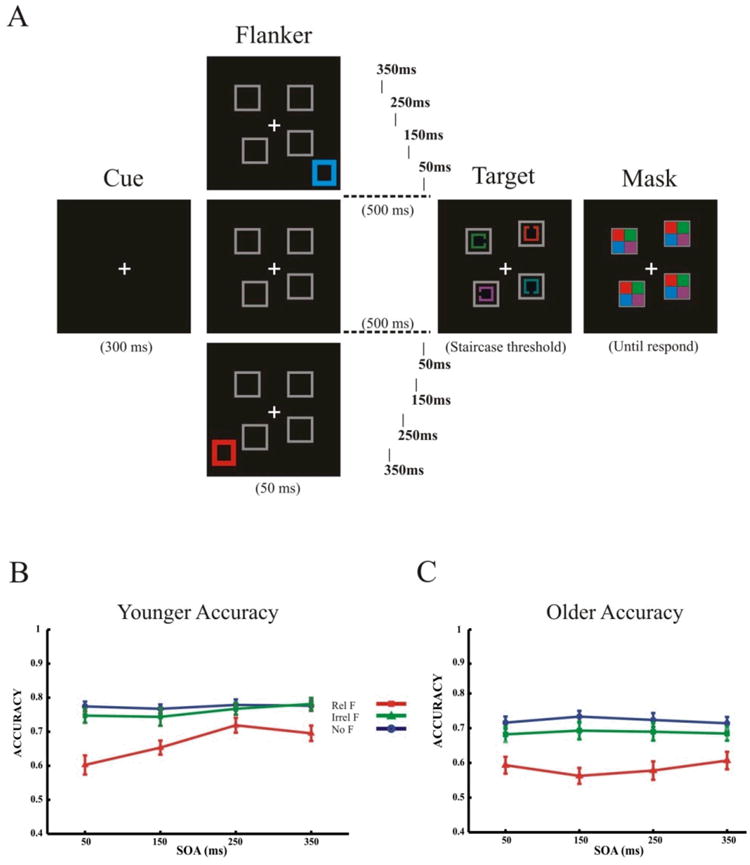
Capture task and accuracy for Older and Younger participants. (A) Participants were instructed to identify the orientation of a target Landolt “C” presented in red among an array of three other distractor Landolt “C”s drawn in different colors. On flanker-present trials, there were four possible stimulus onset asynchronies (SOAs) between the flanker and the search array: 50ms, 150ms, 250ms, or 350ms. The flanker appeared either in the same red color as the target stimulus of the subsequent search array (relevant flanker) or in a distractor color of blue (irrelevant flanker). (B) Younger participants' accuracy in the relevant flanker condition (‘Rel F’ – in red) steadily increased with longer SOAs between flanker and target stimuli until plateauing at 250ms. This effect of SOA on accuracy was not observed for the no flanker (‘No F’ – in blue) or irrelevant flanker condition (‘Irrel F’ – in green). (C) Older participants displayed no increase in accuracy for any of the conditions across SOAs.
Results
Visual Working Memory Capacity
Older participants displayed lower visual working memory accuracy for stimulus arrays of four items (older = 75.56 ± 8.05%; F(1,57) = 10.722; p = 0.002) and six items (older = 66.44 ± 7.20%; F(1,57) = 4.394; p = 0.041) compared to younger participants (four items younger = 82.45 ± 7.96% & six items younger = 70.77 ± 8.37%). There were no significant differences between younger and older participants for array sizes of two or eight items (Two items: older = 94.52 ± 4.14% & younger = 95.68 ± 3.43%; F(1,57) = 1.361; p = 0.248; Eight items: older = 62.52 ± 7.18% & younger = 63.10 ± 7.32%; F(1,57) = 0.092; p = 0.763). We computed individual K estimates using a standard formula (Cowan, 2001) to determine the VWMC of each participant (see Methods). This approach revealed significantly lower VWMC for older adults (K=2.01 ± 0.67) compared to younger adults (K=2.39 ± 0.71) (F(1,57)=4.573; p = 0.037).
Capture task accuracy
To evaluate capture task accuracy, we performed a three-way repeated measures ANOVA using the within-group factors of condition (relevant flanker, irrelevant flanker, no flanker) and SOA (50ms, 150ms, 250ms, 350ms), and a between-group factor of age (younger, older). This analysis revealed main effects of condition (F(2,112)=191.16; p=0.0001), SOA (F(3,168)=5.511; p=0.001) and age (F(1,56)=8.356; p=0.005), and 2-way interactions of SOA × age (F(3,168)=4.286; p=0.006) and SOA × condition (F(6,336)=2.571; p=0.019). Importantly, there was also a 3-way interaction of condition × SOA × age (F(6,336) = 3.49; p=0.002), suggesting a differential relationship of accuracy for SOAs of different conditions between younger and older participants (Figure 1B & C).
Subsequent paired t-tests revealed that for younger participants, accuracy in the relevant flanker condition steadily increased with longer SOAs between flanker and target stimuli (SOA: 50ms vs. 150ms, t(30)=2.299, p=0.029; 150ms vs. 250ms, t(30)=4.245, p = 0.001), until plateauing at 250ms (250ms vs. 350ms, p > 0.05) (Fig 1B). This effect of SOA on accuracy was not observed for the no flanker or irrelevant flanker conditions (all SOA comparisons, p > 0.05). For older participants, there was no increase in accuracy for any of the conditions across SOAs (all SOA comparisons, P>0.05) (Fig 1C). A direct comparison between age groups revealed that for the relevant flanker condition, younger (60.0 ± 7.76%) and older (59.17 ± 12.5%) accuracy did not differ at the shortest SOA (50ms), however, the younger adults' accuracy was significantly greater at all other SOAs compared to older adults (all, p <0.01) – see Table 2. Only the relevant flanker condition demonstrated this age-related relationship with SOA, suggesting that younger and older adults are impacted equivalently by distracting information when it appears just prior to the visual search task (i.e. SOA of 50 ms).
Table 2. Performance of Younger and Older adults.
| Younger | Older | Older Low | Older High | |
|---|---|---|---|---|
| N | 31 | 27 | 13 | 14 |
| WML 2 | 0.96 (0.03) | 0.95 (0.04) | 0.93 (0.04) | 0.96 (0.03) |
| WML 4 | 0.82 (0.08) | 0.76 (0.08) | 0.71 (0.06) | 0.80 (0.08) |
| WML 6 | 0.71 (0.08) | 0.66 (0.07) | 0.63 (0.05) | 0.70 (0.07) |
| WML 8 | 0.63 (0.07) | 0.63 (0.07) | 0.57 (0.05) | 0.67 (0.05) |
| mean K | 2.40 (0.71) | 2.01 (0.67) | 1.45 (0.32) | 2.52 (0.46) |
| range K | 1.31 - 3.60 | 0.91 - 3.41 | 0.91 - 1.97 | 2.00 - 3.41 |
| No F | 0.77 (0.01) | 0.72 (0.01) | 0.72 (0.09) | 0.73 (0.10) |
| Rel F 50 | 0.60 (0.15) | 0.59 (0.13) | 0.59 (0.12) | 0.59 (0.14) |
| Rel F 150 | 0.66 (0.11) | 0.56 (0.12) | 0.53 (0.10) | 0.59 (0.13) |
| Rel F 250 | 0.71 (0.12) | 0.58 (0.14) | 0.55 (0.13) | 0.60 (0.14) |
| Rel F 350 | 0.70 (0.13) | 0.60 (0.13) | 0.55 (0.14) | 0.65 (0.10) |
| Irrel F 50 | 0.74 (0.11) | 0.68 (0.11) | 0.66 (0.09) | 0.70 (0.13) |
| Irrel F 150 | 0.74 (0.14) | 0.69 (0.13) | 0.70 (0.12) | 0.68 (0.15) |
| Irrel F 250 | 0.77 (0.09) | 0.69 (0.13) | 0.71 (0.11) | 0.66 (0.15) |
| Irrel F 350 | 0.78 (0.10) | 0.68 (0.11) | 0.68 (0.12) | 0.69 (0.10) |
| Rel CC 50 | 0.17 (0.12) | 0.13 (0.09) | 0.13 (0.08) | 0.13 (0.10) |
| Rel CC 150 | 0.12 (0.07) | 0.16 (0.10) | 0.19 (0.08) | 0.13 (0.12) |
| Rel CC 250 | 0.06 (0.08) | 0.15 (0.08) | 0.16 (0.08) | 0.13 (0.08) |
| Rel CC 350 | 0.07 (0.08) | 0.12 (0.08) | 0.16 (0.08) | 0.07 (0.06) |
| Irrel CC 50 | 0.03 (0.07) | 0.03 (0.06) | 0.05 (0.04) | 0.02 (0.06) |
| Irrel CC 150 | 0.02 (0.11) | 0.04 (0.10) | 0.03 (0.08) | 0.05 (0.10) |
| Irrel CC 250 | 0.01 (0.06) | 0.03 (0.09) | 0.00 (0.06) | 0.06 (0.10) |
| Irrel CC 350 | 0.00 (0.06) | 0.03 (0.08) | 0.03 (0.09) | 0.03 (0.08) |
Mean performance of Younger and Older adults (visual WMC subgroups Older-low and Older-high). Visual working memory load (WML) and K estimates were derived from the change detection task (Cowan, 2001). Capture task accuracy for the no flanker condition (No F), relevant flanker condition (Rel F), irrelevant flanker condition (Irrel F) with corresponding SOAs (50-350ms). Capture costs scores are calculated by subtracting the no flanker from the relevant flanker accuracy (Rel CC) or the irrelevant flanker accuracy (Irrel CC). Standard deviations are in parentheses.
Capture Cost
In order to adjust for potential age-group differences in perceptual abilities and to further isolate the effects of visual capture by distractors, we subtracted each individuals' relevant and irrelevant flanker accuracy from their ‘baseline’ measure of no flanker accuracy, resulting in an index of capture cost for each flanker type (relevant capture & irrelevant capture). A 2 × 4 × 2 ANOVA of capture cost (relevant, irrelevant), SOA (50ms, 150ms, 250ms, 350ms) and age (younger, older) demonstrated that overall, older adults exhibited larger capture costs than younger adults (main effect of age (F(1,56) = 7.05; p = 0.010) and that relevant capture costs were greater than irrelevant capture costs for both age groups (main effect of capture cost (F(1,56) = 170.80; p = 0.001). Importantly, there was also an interaction of SOA × age (F(3,168) = 4.13; p=0.007) and a strong trend for a capture cost × SOA × age interaction (p = 0.062). Pairwise t-tests confirmed that these interactions were driven by relevant capture costs differentiating the age groups only at 250ms (t(56) = 3.929, P = 0.0001; all other SOAs p >0.05)(Figure 2). No group differences were found at any SOA for irrelevant capture costs (all p>0.05). These results reveal that for relevant capture, older participants exhibit the same degree of impact at 50ms as younger adults, but prolonged disengagement at 250ms compared to younger adults, while there is no significant difference between age groups at 350ms. Therefore, after adjusting for possible age-group differences in perceptual abilities using a measure of capture cost, older participants display an equivalent impact of relevant capture cost as younger participants when distractors appear just prior to the visual search task (i.e. SOA of 50 ms).
Figure 2.
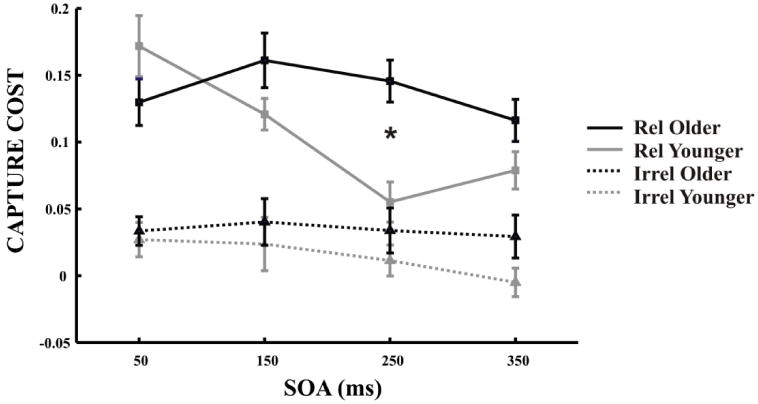
Capture cost in Older and Younger adults. Using an index of capture cost for relevant capture (solid lines) and irrelevant capture (dotted lines), both older adults (black lines) and younger adults (grey lines) are equally captured by relevant distracters at the shortest SOA of 50ms. However, older adults exhibited a prolonged capture cost for relevant distracters differentiating the age groups at 250ms (* p = 0.0001). No group differences were found at any SOA for irrelevant capture costs (all p>0.05).
Capture costs and VWMC in older participants
It has recently been documented that there is a relationship between capture release time and VWMC in younger adults (Fukuda & Vogel, 2011). To evaluate if this relationship is present in older adults and if diminished VWMC in aging is related to delays in disengagement from relevant flankers in older adults, the older participants were divided into high VWMC (n=14, mean K=2.5; range: 2.0 – 3.41) and low VWMC (n=13, mean K=1.45; range: 0.91 – 1.97) subgroups by use of a median split (Table 2). This resulted in a subgroup of older participants with significantly lower VWMC than younger participants (p=0.001), as well as, a subgroup of high VWMC older participants who were comparable in VWMC to younger participants (p>0.05) (Figure 3B). A 2×3×3 ANOVA of capture cost (relevant, irrelevant), SOA (50ms, 150ms, 250ms, 350ms) and group (Younger, Older-high VWMC, Older-low VWMC) revealed a capture cost × SOA × group interaction (F(6,165) = 2.45; p=0.027). Follow-up 2×3 ANOVAs of relevant capture costs (SOA (50ms, 150ms, 250ms, 350ms) and group (Younger, Older-high WMC, Younger-low WMC)) demonstrated an SOA × group interaction (F(6,165) = 3.86; p=0.001) (Figure 3A), while no such relationship is found for irrelevant capture costs (p>0.05) (Table 2).
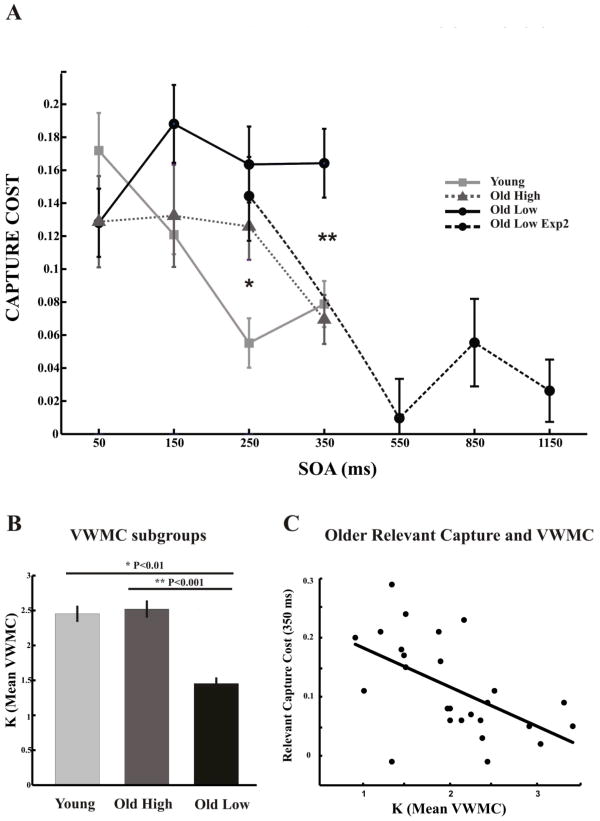
Figure 3.
Relevant capture cost and VWMC age groups. Older participants were divided into high VWMC (grey dotted line) and low VWMC (black solid line) subgroups by use of a median split. This resulted in a subgroup of older participants with significantly lower VWMC than younger participants (grey solid line), as well as, a subgroup of high VWMC older participants that was comparable in VWMC to younger participants (B). No differences for relevant capture between any of the groups were found at the shortest SOA of 50ms (A). The older high-VWMC exhibited an increased capture cost at 250ms (* p=0.013), however by 350ms there was no significant difference between the older high-VWMC subgroup and younger adults. While the older low-VWMC subgroup displayed greater relevant capture costs at all subsequent SOAs compared to younger adults, and deviated from the older high-VWMC subgroup at 350ms (**p = 0.001). The older high-VWMC subgroup was tested on a subsequent version of the capture task with an extended range of SOAs (black dotted line) where it was shown that this group's relevant capture cost returns to that of the other groups by 550ms. (C) Individual relevant capture cost performance at SOA of 350ms correlated with individual K estimates of VWMC in older adults.
Pairwise t-tests showed that at the shortest SOA (50ms), there were no differences for relevant capture between any of the groups (all p>0.05). However, the Older-low VWMC subgroup displayed greater relevant capture costs at all subsequent SOAs compared to younger adults: 150ms (t(42)=2.946, p=0.005), 250ms (t(42)=3.751, p=0.001), 350ms (t(42)=3.411, p=0.001), and at 350ms compared to the Older-high VWMC subgroup (t(25)=3.712, p=0.001) (Figure 3A). In contrast, the Older-high VWMC subgroup displayed no difference from younger adults at 150ms (p>0.05), but exhibited an increased capture cost at 250ms (t(43)=2.581, p=0.013). By 350ms there was no significant difference between the Older-high VWMC subgroup and younger adults (p>0.05) (Figure 3A). Interestingly, although the high VWMC older adults displayed a similar pattern of delayed disengagement from relevant capture as the overall older adult population (Figure 2), they showed no difference in VWMC from the younger group (Figure 3B). Furthermore, individual relevant capture cost performance at the SOA of 350ms correlated with individual K estimates of the older adults (r(27) = -0.5560, p = 0.0026)(Figure 3C), while K estimates did not correlate with any other SOA for relevant or irrelevant capture costs (all p > 0.05). Thus, the current data supports a relationship between VWMC and disengagement from relevant visual capture in older adults, but also suggests that age-related differences in disengagement from visual capture does not map directly onto differences in VWMC.
Since older participants with low VWMC displayed capture cost differences at 350ms in the relevant flanker condition (Figure 3A), this group was invited to return for additional testing on a version of the capture task with a range of SOAs of 250ms, 550ms, 850ms, 1150ms (12 of the original 13 participants returned). To evaluate capture cost in this subgroup of low VWMC older adults, a 2 × 4 repeated measures ANOVA of condition (relevant, irrelevant) and SOA (250ms, 550ms, 850ms, 1150ms) was performed, revealing main effects for condition (F(1,11)=36.9; p=0.0001), SOA (F(3,33)=3.38; p=0.030), and a strong trend for a condition × SOA interaction (F(3,33)=2.72; p=0.060). Similar to the original visit, capture cost assessments demonstrated a greater cost for relevant capture (0.14±0.09; p<0.0001) compared to irrelevant capture (0.0±0.09) at an SOA of 250ms. Furthermore, the relevant capture costs significantly decreased from an SOA of 250ms to 550ms (t(11)=2.856, p=0.016), after which relevant capture costs were stable for all subsequent SOAs (all p>0.05) (Figure 3A). In comparison, there were no differences in irrelevant capture costs with increasing SOA length (250ms: 0.0±0.09 vs. 550ms: 0.0±0.07; 550ms vs. 850ms: 0.0±0.07 & 850ms vs. 1150ms: 0.0±0.06; all p>0.05). Also, there was no difference in accuracy (1st: 55.5±13.5%; 2nd: 58.3±16.8%) or capture cost (1st: 0.16±0.08; 2nd: 0.14±0.09) for the relevant flanker condition at an SOA of 250ms between the two visits (p>0.05). This additional testing of the low-VWMC older adults reveals that this group of older adults does in fact eventually disengage from relevant distractors.
A median split for young participants' VWMC estimates resulted in no accuracy or capture cost differences (p>0.05) between high and low VWMC groups for any condition. One possible explanation for this incongruence between the results presented here for younger participants and those of Fukuda and Vogel (2011), is that the range of VWMC scores in our younger population (range of K: 1.31-3.60) was less than the older participants (range of K: 0.91-3.41) and to the younger participants (range of K: 1.0-4.2) reported by Fukuda and Vogel (2011). Additionally, the target stimulus used in our current study (identifying the orientation of a Landolt “C”) had a slightly larger gap than that used by Fukuda and Vogel (2011) in attempt to prevent any floor effects of performance in the older participants (gap width of 40 pixels vs. 25 pixels). This modification may have slightly changed the sensitivity of visual capture in the younger participants that is needed to differentiate between VWMC groups, yet was a robust measure for differing between older VWMC performance.
Discussion
In the current study, we assessed age-related differences in the susceptibility to, and disengagement from, attentional capture by manipulating the timing between flanker (distractor) stimuli and the onset of a target detection array. We found that younger and older adults demonstrated equivalent susceptibility to capture; i.e. the same attentional capture cost in the initial moments of distractor exposure (indicated by the shortest SOA of 50ms) for both relevant and irrelevant distractors. In contrast, older adults displayed a prolonged disengagement from relevant distractors compared to younger adults. Thus, the greater impact of distraction that accompanies normal aging does not seem to be the result of a greater susceptibility to attentional capture by distractors, but rather delayed disengagement from distractor processing.
It is well established that older individuals, in general, experience a more negative impact on cognitive performance by the presence of distracting visual information compared to younger adults (Hasher et al., 1999; Healey, Campbell, & Hasher, 2008). This has been associated with neural deficits in the top-down suppression of irrelevant stimuli (Gazzaley et al., 2008; Gazzaley et al., 2005). Similarly, it has been previously demonstrated that older adults are less efficient in using top-down information during visual search tasks, resulting in a greater impact of attentional capture compared to younger adults (Phillips & Takeda, 2010), and suggesting a possible etiology for age-related impairments in visual search (Plude & Hoyer, 1986) and selective attention (Plude & Doussard-Roosevelt, 1989). This capture effect has been shown to be exacerbated in older adults when information is presented just prior to the onset of a target (Pratt & Bellomo, 1999) and also when a distractor is presented peripherally to the target array (Lincort, Folk, & Hoyer, 1997). Such peripheral cueing paradigms have demonstrated that older adults exhibit a prolonged facilitation from spatial cueing and a delayed inhibition of return for previously cued locations (Lincort et al., 1997), suggesting that older adults have difficulty disengaging from spatial cues that reflexively orient attention.
However, the design of these previous studies did not permit a dissection of the processes of distractor engagement and disengagement. The current results suggest that the initial impact of distraction does not differ with age, but instead the time in which older adults disengage from processing distracting information is significantly prolonged. Recently it has been shown that older adults exhibit a prolonged time to disengage from interrupting visual stimuli that were presented as elements of a secondary task compared to younger adults, and this was related to a more negative impact on visual working memory (Clapp et al., 2011). These findings support previous reports of age-related deficits in directed forgetting experiments, where older adults display impairments in releasing information previously experienced (Zacks et al., 1996). Similarly, older adults have been shown to be impaired in RSVP (rapid serial visual presentations) paradigms, where serial items are rapidly presented and participants must ignore the first item and detect the second (single-item RSVP), again demonstrating difficulties in inhibiting task irrelevant information. In dual-task RSVP, this impairment is still evident, suggesting an inability to disengage from the previously relevant items in order to effectively engage attentional resources to the next item (Maciokas & Crognale, 2003). In combination, these results suggest an age-related ‘stickiness’ of processing, such that information not related to a primary task is processed for a longer time than it is in younger adults.
The presentation of a distractor initiates bottom-up processing due to stimulus-based external salience, yet recurrent top-down biasing contingent with the goals of the task influences the degree to which attention is captured by a distractor (for review see (Theeuwes, 2010), but also see (Folk & Remington, 2010)). As is clear in our data for both age groups, and the recent study by Fukuda & Vogel (2011), a relevant distractor has a larger impact, even at the 50ms SOA (Fig 2). Furthermore, the subsequent disengagement from bottom-up distractor processing is a more rapid process than releasing from distractors that are related to the top-down goals of the task in younger adults (Fukuda & Vogel, 2011; Kramer, Hahn, Irwin, & Theeuwes, 2000) and older adults in this current study. A previous report revealed that the impact of abruptly appearing salient distractors that are irrelevant to a task are comparable between younger and older adults (Lien, Gemperle, & Ruthruff, 2011); also this has been found to be true in terms of reflexive eye movements to this form of distraction (Kramer, Hahn, Irwin, & Theeuwes, 1999). This is consistent with our observation that age-related differences in disengagement from visual capture occurs only for relevant distractors, suggesting that prolonged stimulus disengagement in older adults may reflect age-related alterations in top-down attentional control. Similarly, it has been proposed that age-related decrements in cognitive performance are limited to those tasks requiring volitional processing, such as working memory, while more automatic tasks are relatively unaffected (Craik, Byrd, & Swanson, 1987; Rabbitt, Cumming, & Vyas, 1979; Rabbitt & Vyas, 1980).
Another goal of the study was to assess if age-related differences in VWMC account for differences in the timing of disengagement from relevant distractors. This hypothesis was based on recent findings of a relationship between VWMC and release time in younger adults (Fukuda & Vogel, 2011). Comparable to what was found in younger adults, higher relevant capture costs strongly correlated with lower VWMC estimates in older participants, such that individuals with lower VWMC have delayed disengagement (at SOA of 350ms). However, when older participants were split into subgroups with high and low VWMC, the high VWMC subgroup still displayed a prolonged impact on performance for relevant distraction at 250ms compared to younger participants, despite there being no difference in VWMC between them. Therefore, although older adults display a relationship between prolonged disengagement and VWMC comparable to previous reports in younger adults (Fukuda & Vogel, 2011), extended capture by distraction did not fully characterize age-related differences in VWMC. One possibility is that there may be a critical threshold that prolonged disengagement from processing a distractor needs to reach in order to negatively impact VWMC. Although causality cannot be inferred from these current results, the data may suggest that this threshold had not yet been reached in the Older-high VWMC adults tested here, but it had in the Older-low VWMC adults.
In conclusion, this study reveals that the greater impact of attentional capture in normal aging is not the result of a greater susceptibility to capture by distractors, but rather the delayed disengagement from distraction. Furthermore, prolonged processing of task-relevant distraction seems to be related to individual differences in VWMC for older adults, similar to what has been demonstrated previously in younger adults (Fukuda & Vogel, 2011). These results are in accordance with a growing body of evidence that older adults disengage slower from recently experienced events than younger adults (Hartman, & Hasher, 1991; Hamm, & Hasher, 1992, Maciokas & Crognale, 2003; Zacks et al., 1996) and that this prolonged disengagement may also influence long-term forms of learning (Campbell, Zimmerman, Healey, Lee, & Hasher, 2012; Campbell, Hasher, & Thomas, 2010). Overall, our results help to characterize an age-related ‘stickiness’ of processing which may be related to a broad range of inhibitory deficits in the older population (Hasher et al., 1999). Future research will help to better understand the etiology of this disengagement deficit and how the “stickiness” of processing in older adults is associated with other cognitive deficits, as well as the neural basis of this phenomenon.
Acknowledgments
This project was supported by National Institutes of Health Grant R01-AG30395 and AG030395-S1. We would also like to thank Jacqueline Boccanfuso and Joey Essoe for their valuable help coordinating the work presented here.
References
- Awh E, Barton B, Vogel EK. Visual working memory represents a fixed number of items regardless of complexity. Psychol Sci. 2007;18:622–628. doi: 10.1111/j.1467-9280.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Hasher L, Thomas RC. Hyper-binding: a unique age effect. Psychol Sci. 2010;21:399–405. doi: 10.1177/0956797609359910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Zimmerman S, Healey MK, Lee MM, Hasher L. Age differences in visual statistical learning. Psychol Aging. 2012 Jan 16; doi: 10.1037/a0026780. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel AD, Chasteen AL, Scialfa CT, Pratt J. Adult age differences in the time course of inhibition of return. J Gerontol B Psychol Sci Soc. 2003;58:256–259. doi: 10.1093/geronb/58.5.p256. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Human prefrontal lesions increase distractibility to irrelevant sensory inputs. Neuroreport. 1995;6:1605–1610. doi: 10.1097/00001756-199508000-00005. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Contribution of human prefrontal cortex to delay performance. J Cogn Neurosci. 1998;10:167–177. doi: 10.1162/089892998562636. [DOI] [PubMed] [Google Scholar]
- Clapp WC, Gazzaley A. Distinct mechanisms for the impact of distraction and interruption on working memory in aging. Neurobiol Aging. 2010;33:134–148. doi: 10.1016/j.neurobiolaging.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp WC, Rubens MT, Gazzaley A. Mechanisms of working memory disruption by external interference. Cereb Cortex. 2010;20:859–872. doi: 10.1093/cercor/bhp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp WC, Rubens MT, Sabharwal J, Gazzaley A. Deficit in switching between functional brain networks underlies the impact of multitasking on working memory in older adults. Proc Natl Acad Sci U S A. 2011;108:7212–7217. doi: 10.1073/pnas.1015297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Craik FI, Byrd M, Swanson JM. Patterns of memory loss in three elderly samples. Psychol Aging. 1987;2:79–86. doi: 10.1037/0882-7974.2.1.79. [DOI] [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychol Aging. 1989;4:500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington R. A critical evaluation of the disengagement hypothesis. Acta Psychol (Amst) 2010;135:103–105. doi: 10.1016/j.actpsy.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foos PW, Wright L. Adult age differences in the storage of information in working memory. Exp Aging Res. 1992;18:51–57. doi: 10.1080/03610739208253911. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Vogel EK. Human variation in overriding attentional capture. J Neurosci. 2009;29:8726–8733. doi: 10.1523/JNEUROSCI.2145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Vogel EK. Individual differences in recovery time from attentional capture. Psychol Sci. 2011;22:361–368. doi: 10.1177/0956797611398493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, D'Esposito M. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc Natl Acad Sci U S A. 2008;105:13122–13126. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Hartman M, Hasher L. Aging and suppression: memory for previously relevant information. Psychol Aging. 1991;4:587–594. doi: 10.1037//0882-7974.6.4.587. [DOI] [PubMed] [Google Scholar]
- Hamm VP, Hasher L. Age and the availability of inferences. Psychol Aging. 1992;7:56–64. doi: 10.1037//0882-7974.7.1.56. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT, May CP. Inhibitory control, circadian arousal, and age. Attention and Performance. 1999;17:653–675. [Google Scholar]
- Healey MK, Campbell KL, Hasher L. Cognitive aging and increased distractibility: costs and potential benefits. Prog Brain Res. 2008;169:353–363. doi: 10.1016/S0079-6123(07)00022-2. [DOI] [PubMed] [Google Scholar]
- Klove H, Reitan RM. Effect of dysphasia and spatial distortion on Wechsler-Bellevue results. AMA Arch Neurol Psychiatry. 1958;80:708–713. [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Irwin DE, Theeuwes J. Attentional capture and aging: implications for visual search performance and oculomotor control. Psychol Aging. 1999;14:135–154. doi: 10.1037//0882-7974.14.1.135. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Irwin DE, Theeuwes J. Age differences in the control of looking behavior: do you know where your eyes have been? Psychol Sci. 2000;11:210–217. doi: 10.1111/1467-9280.00243. [DOI] [PubMed] [Google Scholar]
- Lien MC, Gemperle A, Ruthruff E. Aging and involuntary attention capture: electrophysiological evidence for preserved attentional control with advanced age. Psychol Aging. 2011;26:188–202. doi: 10.1037/a0021073. [DOI] [PubMed] [Google Scholar]
- Lincort A, Folk C, Hoyer J. Effects of aging on voluntary and involuntary shifts of attention. Aging, Neuropsychology and Cognition. 1997;4:290–303. doi: 10.1080/13825589708256654. [DOI] [PubMed] [Google Scholar]
- Maciokas JB, Crognale MA. Cognitive and attentional changes with age: evidence from attentional blink deficits. Exp Aging Res. 2003;29:137–153. doi: 10.1080/03610730303715. [DOI] [PubMed] [Google Scholar]
- Minamoto T, Osaka M, Osaka N. Individual differences in working memory capacity and distractor processing: possible contribution of top-down inhibitory control. Brain Res. 2010;1335:63–73. doi: 10.1016/j.brainres.2010.03.088. [DOI] [PubMed] [Google Scholar]
- Noudoost B, Chang MH, Steinmetz NA, Moore T. Top-down control of visual attention. Curr Opin Neurobiol. 2010;20:183–190. doi: 10.1016/j.conb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S, Takeda Y. Frontal-parietal synchrony in elderly EEG for visual search. Int J Psychophysiol. 2010;75:39–43. doi: 10.1016/j.ijpsycho.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Plude DJ, Doussard-Roosevelt JA. Aging, selective attention, and feature integration. Psychol Aging. 1989;4:98–105. doi: 10.1037/0882-7974.4.1.98. [DOI] [PubMed] [Google Scholar]
- Plude DJ, Hoyer WJ. Age and the selectivity of visual information processing. Psychol Aging. 1986;1:4–10. doi: 10.1037//0882-7974.1.1.4. [DOI] [PubMed] [Google Scholar]
- Pratt J, Bellomo C. Attentional capture in younger and older adults. Aging, Neuropsychology and Cognition. 1999;26:19–31. [Google Scholar]
- Rabbitt P, Cumming G, Vyas S. Modulation of selective attention by sequential effects in visual search tasks. Q J Exp Psychol. 1979;31:305–317. doi: 10.1080/14640747908400729. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Vyas SM. Selective anticipation for events in old age. J Gerontol. 1980;35:913–919. doi: 10.1093/geronj/35.6.913. [DOI] [PubMed] [Google Scholar]
- Rouder J, Morey R, Morey C, Cowan N. How to measure working memory capacity in the change detection paradigm. Psychon Bull Rev. 2011;18:324–330. doi: 10.3758/s13423-011-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop J. Studies of interference in serial verbal Reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Theeuwes J. Stimulus-driven capture and attentional set: selective search for color and visual abrupt onsets. J Exp Psychol Hum Percept Perform. 1994;20:799–806. doi: 10.1037//0096-1523.20.4.799. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Top-down and bottom-up control of visual selection. Acta Psychol (Amst) 2010;135:77–99. doi: 10.1016/j.actpsy.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale—Revised. Psychological Corporation; New York: 1981. [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zacks RT, Radvansky G, Hasher L. Studies of directed forgetting in older adults. J Exp Psychol Learn Mem Cogn. 1996;22:143–156. doi: 10.1037//0278-7393.22.1.143. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci. 2011;14:656–661. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]