Abstract
Estrogens have been found to improve memory and reduce risk of dementia, although conflicting results such as failure of estrogen replacement therapy for treatment of Alzheimer’s disease (AD) also has been reported. Only recently, our published human brain studies showed a depletion of brain estrogen in women with Alzheimer’s disease, while other studies have demonstrated cognitive impairment believed to be caused by inhibition of endogenous estrogen synthesis in females. To investigate whether the shortage of brain estrogen alters the sensitivity of response to estrogen replacement therapy, we have used genetic and surgical animal models to examine the response of estrogen treatment in AD neuropathology. Our studies have shown that early treatment with 17β-estradiol (E2) or genistein could reduce brain amyloid levels by increasing Aβ clearance in both APP23 mice with genetic deficiency of aromatase (APP/Ar+/−), in which the brains contains non-detectable levels of estrogen, and in APP23 mice with an ovariectomy (APP/OVX), in which the brains still contain certain levels of estrogen. However, only APP/Ar+/− mice showed a great reduction in brain amyloid plaque formation after E2 or genistein treatment along with downregulation of beta secretase (BACE1) mRNA and protein expression. Our results suggest that early and long-term usage of E2 and/or genistein may prevent AD pathologies in a dependent manner on endogenous brain estrogen levels in aged females.
Keywords: brain estrogen, hormone therapy, Alzheimer’s disease, BACE1, Aβ deposition
Women are diagnosed with Alzheimer’s disease (AD) at a greater rate than men, which is partially associated with a sharp reduction in estrogen levels during menopause in addition to the fact that women live longer than men in general (1–2). Studies have demonstrated a better verbal memory in women with longer endogenous estrogen exposure (3–4), while blockage of estrogen syntheses impairs cognitive function in breast cancer patients that have been treated with an aromatase inhibitor (5). Similarly, postmenopausal women treated with 17β-estradiol (E2), a natural form of estrogen, showed improvement on learning and memory functions (6–7), while conjugated equine estrogen (CEE), a synthetic form of estrogen, caused reduction of cognitive activities and increased risk of dementia as reported in various clinical trials, including the Women’s Health Initiative (WHI) studies (8–11). As E2 is a natural form of estrogen that exists in the human body while CEE is not, it is unknown whether the E2 response is influenced by endogenous estrogen levels, especially brain estrogen in aged females.
AD pathology is characterized by deposition of β-amyloid peptide (Aβ), which is generated from amyloid precursor protein (APP) by β-secretase (BACE1) and γ-secretase activity (12–14). While elevated BACE1 activity has been found in AD patients (15–17), our published studies have demonstrated elevated BACE1 activity associated with brain estrogen depletion in female AD patients and in a transgenic mouse model of AD (18).
Aβ can be cleared/degraded by insulin-degrading enzyme (IDE) and neprilysin (NEP). Overexpression of IDE in APP transgenic mice reduces Aβ levels and Aβ plaque density in the brain (19–20), and meanwhile, brain estrogen deficiency inhibits IDE activity in female APP transgenic mice (18). Likewise, other studies have shown that estrogen can also promote NEP, a key amyloid-degrading enzyme in the brain (21–22), and ameliorate the reduced NEP activity in the brain of ovariectomized rats (23–24).
Since clinical trials started to test the effects of estrogen on cognition (25–28), various forms of natural estrogens have been available for treatment. Some of the most common are 17α-estradiol, an estrogen that exhibits relatively low activity; genistein, a soybean-based estrogen receptor agonist/antagonist (29–30); and black cohosh, another phytoestrogen that displays selective estrogen receptor modulator properties without binding to estrogen receptors (31). Here, we examined whether endogenous brain estrogen levels alter the sensitivity in response to estrogen treatment, which may help to prevent or delay AD neuropathology.
Results
Brain Estrogen Levels and Plaque Contents Are Different Between in APP/OVX and APP/Ar+/− Female Mice
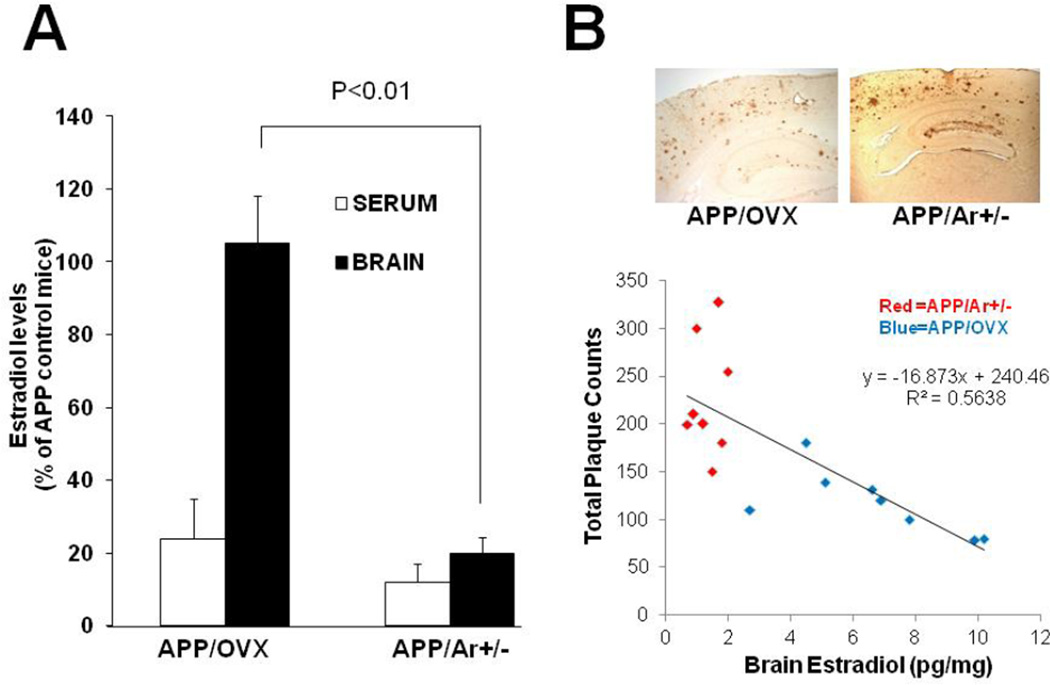
Serum and brain samples from a total of 26 female mice (APP/OVX n=8, APP/Ar+/− n=8, and APP n=10) at an age of 12 months were examined for estradiol levels. Compared to APP mice, both APP/OVX and APP/Ar+/− mice showed a great reduction of estradiol levels in the serum with t-test analysis (p=0.046 and 0.039, respectively). However, genetic partial deletion of aromatase, the key synthase for estrogen, not only reduced peripheral circulation of estradiol levels, but also decreased brain estrogen synthesis compared to APP and APP/OVX mice. The total brain estradiol levels were significantly reduced in APP/Ar+/− mice compared to APP and APP/OVX mice (p=0.008 and 0.006, respectively). There is no difference in brain estradiol level between APP/OVX mice and APP mice at same age (Figure 1A). The levels of estradiol in serum and brain from APP control mice at 12 months (n=10) are 109±15 pg/ml and 6.4±1 pg/mg, respectively. The serum levels of estradiol detected by RIA in this study are in the range of 49–228 pg/ml as reported in the literature (39, 40). The difference in the brain estradiol is correlated with amyloid plaque formation found in the brain of APP/OVX and APP/Ar+/− mice at an age of 12 months (Figure 1B). APP mice with normal brain estrogen levels (APP/OVX) showed less plaque formation than those lacking brain estrogen. Our data are again consistent with the hypothesis that brain estrogen deficiency is a key risk factor for AD in females.
Figure 1.
Estrogen levels and plaque formation in APP/OVX and APP/Ar+/− mice. (A) The levels of estradiol were measured by RIA from serum and brain of 12 month old APP/OVX (n=8) or APP/Ar+/− (n=8) mice. The serum and brain estradiol levels in APP23 mice at age of 12 months (n=10) are 109±15 pg/ml and 6.4±1 pg/mg protein, respectively. (B) The total number of Aβ plaques were detected and analyzed by immunohistochemical assays in the cortex of APP/OVX and APP/Ar+/− mice at 12 months of age. There is a correlation between brain estrogen level and number of plaques as shown in B.
Early, but Not Late Treatment with E2 or Genistein Prevents Plaque Formation in APP23/Ar+/− Female Mice
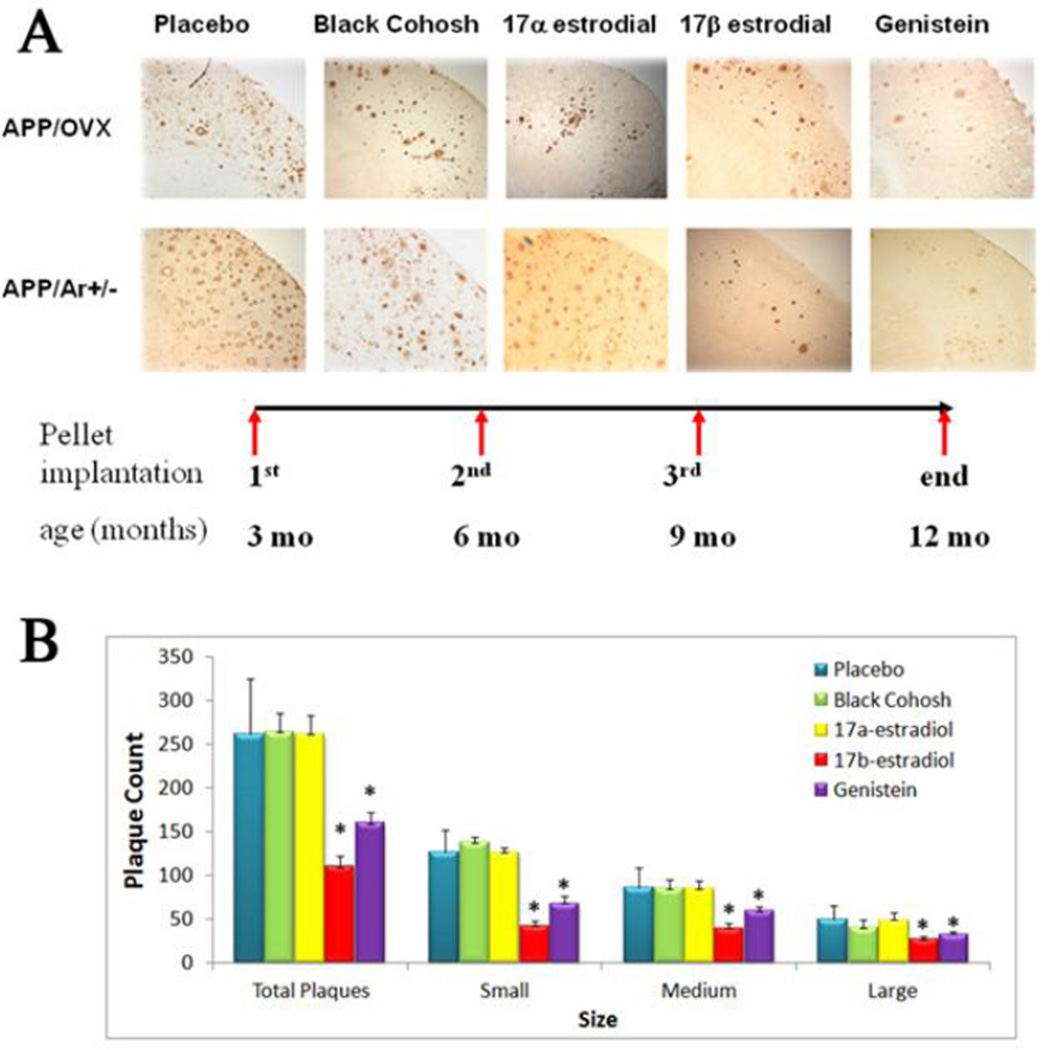
As our previous studies have shown, APP/Ar+/− female mice developed more severe brain plaques at an earlier timepoint than APP control or APP/OVX female mice (18). Here we tested whether the APP/Ar+/− mouse or APP/OVX mouse response to treatment is altered by treatment with different estrogen analogs. For early treatment, at an age of 3 months, animals were treated with various estrogens at selective dosages based on our previous experiments (data not published), such as E2 (18.9ug/day), 17α-estradiol (18.9ug/day), genistein (26ug/day), black cohosh (26ug/day) or placebo for 9 months. At an age of 12 months, all animals were sacrificed and tissues were harvested. As plaque formation in the brain is one of the hallmarks for AD pathology, we detected the effects of estrogen treatments on plaque formation using immunohistochemistry analysis. As shown in Figure 2, at 12 months APP/OVX and APP/Ar+/− mice treated with placebo, black cohosh or 17α-estradiol developed extensive plagues, while the APP/Ar+/− animals that received E2 or genistein treatments developed fewer plaques than placebo treated mice. With a total of 8 APP/Ar+/− mice for each treatment, 50% of the mice with E2 treatment developed no plaques and the remaining showed reduced plaque formation. For the genistein treatment, 5 of the 8 APP/Ar+/− mice (62%) showed reduced plaque density compare to the placebo treated mice. The size of Aβ plaques also indicates the severity of Aβ pathology. We used morphometric analyses on the brain sections immuno-stained with Aβ antibody 6E10. Results showed that large (>20 µm diameter), medium (10–20 µm diameter) and small (<10 µM) sized Aβ plaques in the entorhinal cortex (Fig. 2B) were significantly reduced in APP23/Ar+/− mice treated with E2 or genistein, indicating that in E2 and genistein treatment, not only reduces overall Aβ plaque number, but also decreases plaque size.
Figure 2.
Effect of early and long term treatment of various estrogens on brain plaque formation. At an age of 3 months old, female mice were treated with black cohosh (26ug/day), 17α-estradiol (18.9ug/day), 17β-estradiol (18.8ug/day), genistein (26ug/day) or placebo treatment for 9 months. At an age of 12 months, brain tissues were harvested. (A) Immunostaining for Aβ plaques as shown in dark brown in the images. (B) Statistical analyses show early E2 and genistein treatments reduced the total number of plaques in the cortex of both APP/OVX and APP/Ar+/− mice as well as in all sizes as large (>20 µm diameter), medium (10–20 µm diameter) and small (>10 µm diameter) analyzed by Image-pro Plus Analysis. * P<0.05 compared to placebo group.
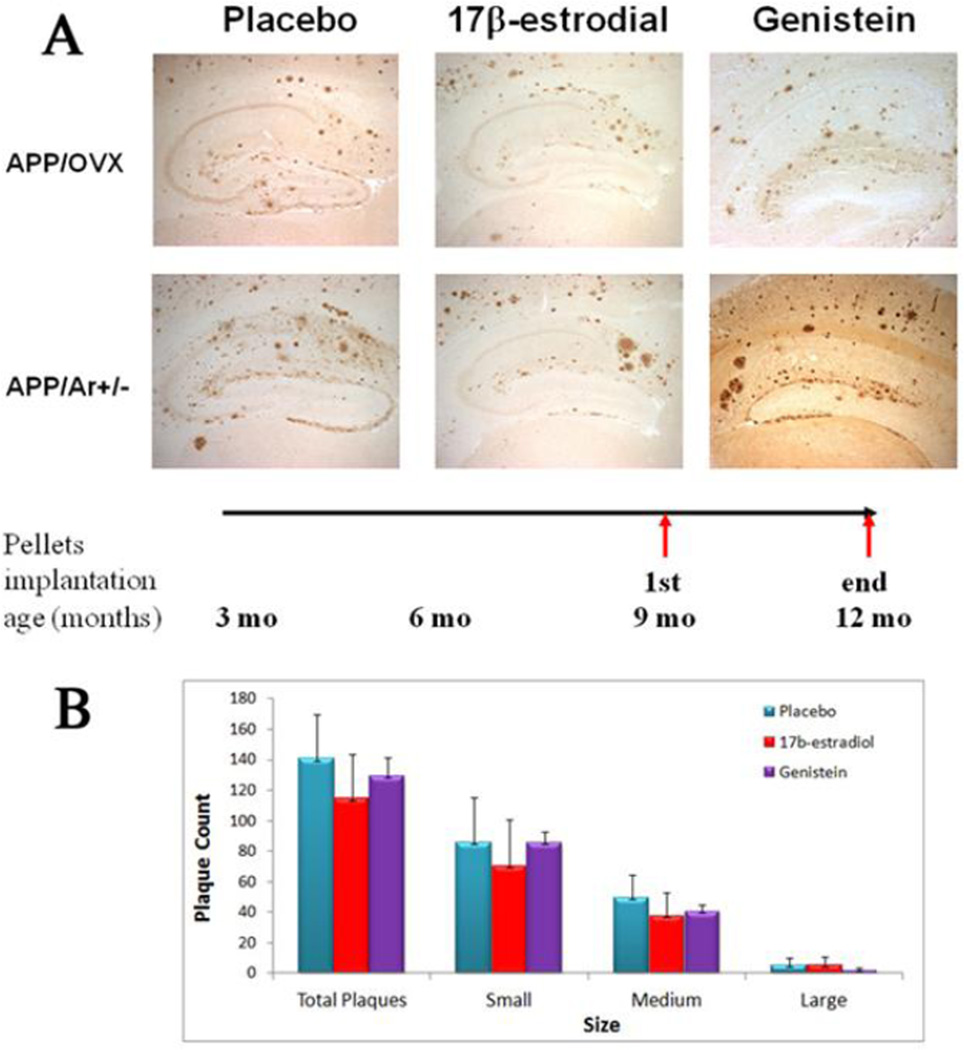
For late treatments, animals received the same treatment as described above, but started at a later age (9 months) when extensive plaques had already begun to form in the brain. There is no reduction of plaque formation was found in any group. As shown in figure 3B, late treatment of E2 or genistein did not alter the total number of plaques in APP/OVX or APP/Ar+/− mice. However, as shown in figure 3A, E2 treatment reduced the number of small plaques in the hippocampal CA3 region while genistein treatment made no reduction of plaques in any region of hippocampus.
Figure 3.
Effect of late and short term treatment of E2 and genistein on brain plaque formation. At an age of 9 months old, female mice were treated with 17β-estradiol (18.8ug/day), Genistein (26ug/day) or placebo for 3 months. At an age of 12 months, brain tissues were harvested. (A) Immunostaining of Aβ plaques as shown in dark brown in the images. (B) Plaque count was performed by Image-pro Plus Analysis (media Cybernetics) with three sizes as large (>20 µm diameter), medium (10–20 µm diameter) and small (>10 µm diameter). * P<0.05 compared to placebo group.
Early Treatment with E2 or Genistein Reduces Aβ1–42 Concentration in Brain
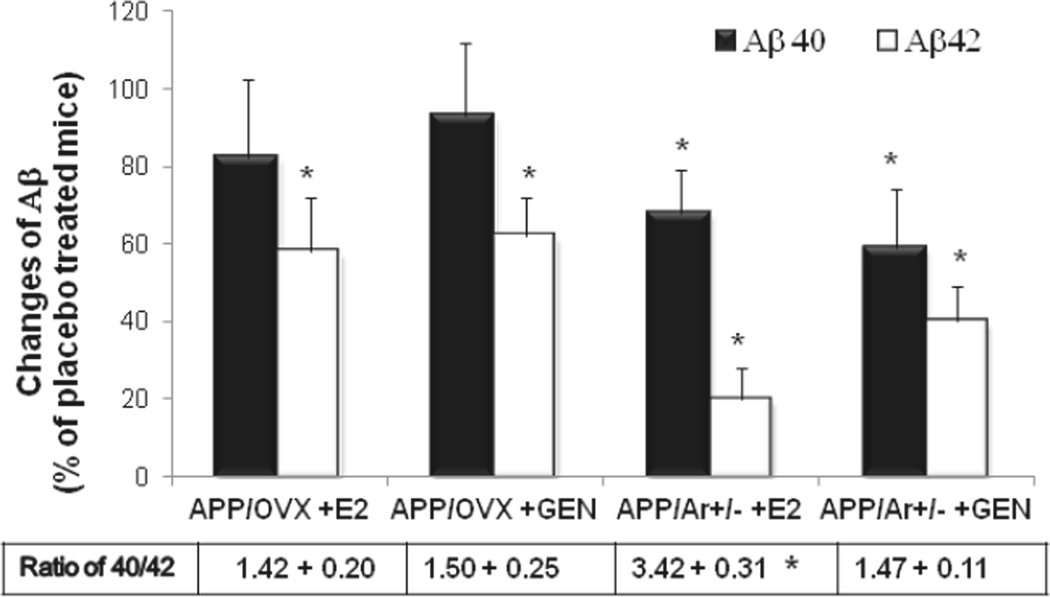
To examine whether the E2 and/or genistein administration would alter plaque-associated Aβ peptide expression, we examined levels of Aβ40 and Aβ42 in the brain. Both E2 and genistein treatments in APP/OVX and APP/Ar+/− mice lowered Aβ42 levels 80% and 60% compared to the placebo treated mice (Figure 4), while only APP/Ar+/− mice showed a reduction of both Aβ40 and Aβ42 after treatment. In addition, E2 treatment in APP/Ar+/− mice showed a significant increase in the ratio of Aβ40/Aβ42 in comparison with placebo treated APP/Ar+/− mice (3.45 ± 0.30 vs. 1.33 ± 0.25, p<0.05). Again, there was no change in the ratio of Aβ in the animals that received 17α-estradiol or black cohosh treatment in age-matched APP/OVX and APP/Ar+/− mice (data not shown).
Figure 4.
Level of Aβ in APP/OVX and APP/Ar+/− mice with estrogen treatment. Experimental mice received continuous treatment with placebo, E2 or genistein (Gen) from 3 months old and tissue was harvested at age of 12 months as described in the method. Brain tissue from total of 48 mice (n=8 each treatment group) were processed and measured for Aβ40 and Aβ42 by ELISA kits. Data presented as percentage of placebo treated APP/OVX or APP/Ar+/− mice. * indicates P< 0.05 compared to placebo treated mice.
Effect of Early Treatment with E2 or Genistein on BACE1 Protein mRNA Expression, as well as Enzyme Activities in Brains of APP23/Ar+/− Mice
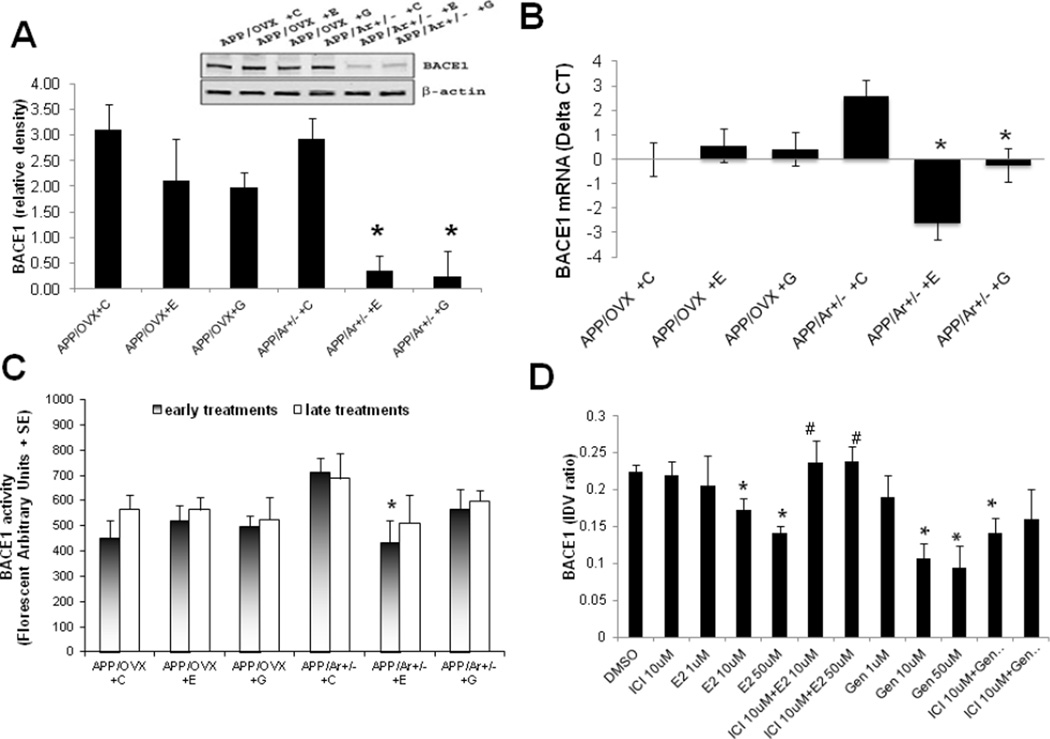
One of our recent studies demonstrated that BACE1 contributes to the early formation of plaques in APP/Ar+/− mice (18,34). Therefore, we examined whether estrogen treatment can inhibit expression of BACE1 found in APP/Ar+/− mice. Levels of BACE1 protein, mRNA, and enzyme activity were measured in both APP/OVX and APP/Ar+/− mice after early or late estrogen treatments. Placebo-treated APP/Ar+/− mice displayed higher levels of BACE1 protein expression compared to APP/OVX treated with placebo (Figure 5A). Furthermore, early treatment with E2 or genistein reduced BACE1 protein expression in APP/Ar+/− female mice, but not in APP/OVX mice that received the same treatment. Treatment with E2 or genistein also reduced BACE1 mRNA levels in comparison to APP/OVX that showed no change (Figure 5B). A similar reduction in BACE1 activity was also observed in APP/Ar+/− mice that received long-term E2 treatment from an age of 3 months (P<0.05) while a smaller decrease was observed in response to genistein treatment (Figure 5C). Black cohosh or 17α-estradiol treatment did not alter BACE1 expression or activity (data not shown).
Figure 5.
Effect of 17β-estradiol treatments on β secratase (BACE1) in vivo and in vitro. For in vivo study, APP/OVX (n=5 each treatment) and APP/Ar+/− (n=6 each treatment) mice received 17β-estradiol (E), genistein (G) or placebo (C) at an age of 3 months until tissue was harvested at age of 12 months, and BACE1 protein and mRNA levels and activities were measured as described in methods section. (A) Effects of early treatments on BACE1 protein expression by Western blot. (B) Inhibitory effect of early treatments on mRNA levels of BACE1 by real-time PCR. (C) Effect of early and late treatments on BACE1 activity expressed by florescent unite per minute (FU/min). (D) For in vitro study, BACE1 protein expression (mean ratios of BACE1/β-actin integrated optical density values, IDV) in the APP transfected 293 cells treated with 17β-estradiol (E2) and genistein (Gen) for 48 hours with or without estrogen receptor antagonist ICI 182789. * indicates p< 0.05 compared to placebo treated APP/Ar+/− mice or DMSO treated APP transfected 293 cells. # indicates P<0.05 compared to E2 10uM and 50uM treated cells.
To further study the dosage effect of E2 and genistein in terms of estrogen receptor dependency, we performed additional in vitro experiments as shown in figure 5D. Stable APP transfected 293 cells were treated with E2 or genistein at 1, 10 and 50uM for 48 hours, respectively. As shown in figure 5D, our data demonstrated a dose-response curve of E2 and genistein treatment on downregulation of BACE1 protein expression. A significant reduction of BACE1 protein levels were only found in high dosages of E2 and genistein (10, 50uM). In addition, we also co-treated the stable APP transfected 293 cells with estrogen receptor antagonist (ICI 182780, 10uM) with E2 or genistein for 48 hours and found that the E2-induced down regulation of BACE1 can be reversed by ICI 182780, suggesting an estrogen receptor-dependent mechanism. However, the genistein-induced down regulation of BACE1 failed to be blocked by ICI 182780. This may suggest that E2- and genistein-induced down regulation of BACE1 in vitro is mediated by different mechanisms.
Effects of E2 or Genistein Treatment on Aβ Degradation by Altering in NEP Activity in Brains
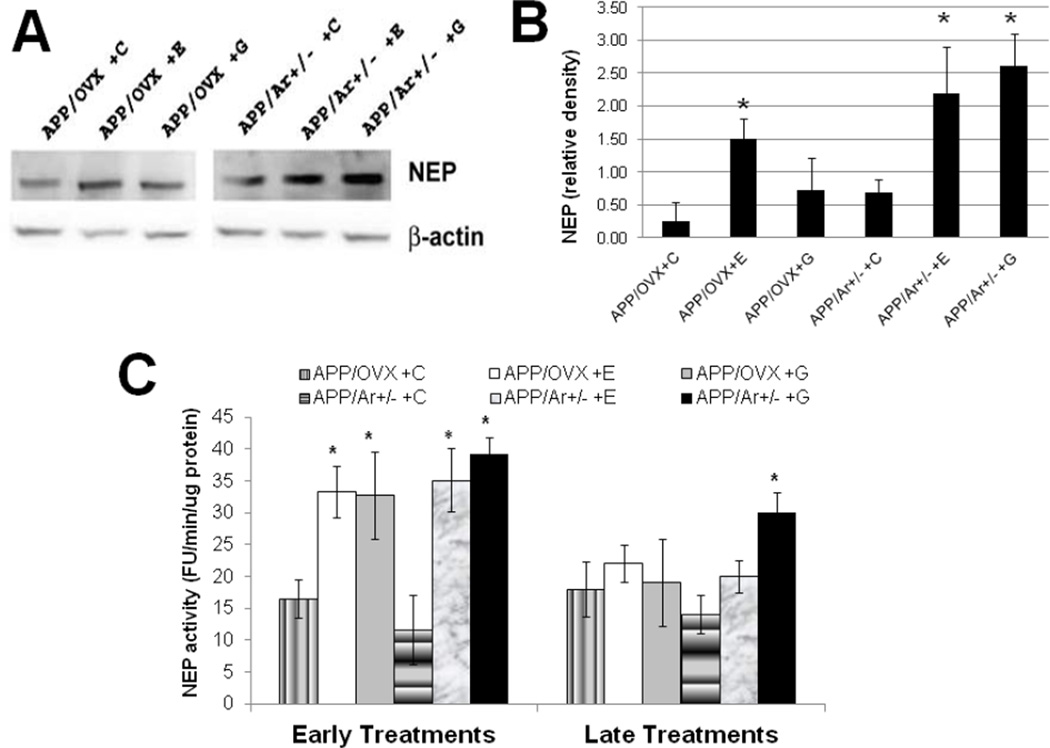
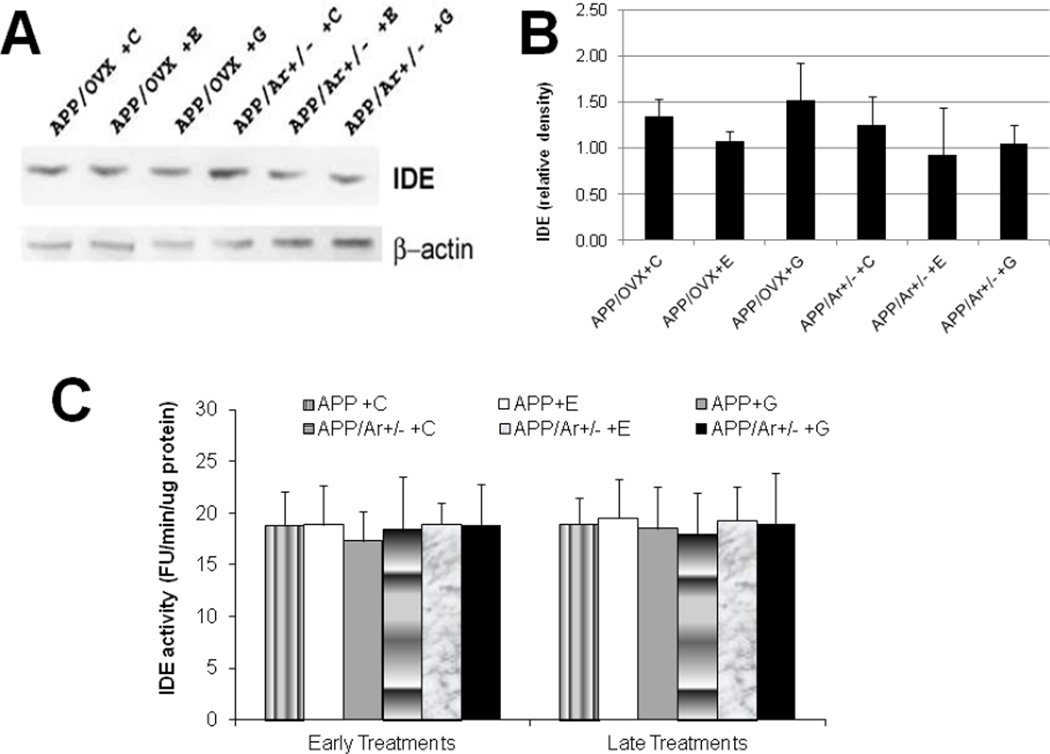
In addition to altered Aβ production, we also examined the action of E2 or genistein treatment on Aβ clearance. As shown in Figure 6A&B, early treatment with either E2 or genistein increases NEP protein expression in both APP/OVX (98–102% increase compared to APP/OVX control) and APP/Ar+/− (201–235% increase compare to APP/Ar+/− control) mice. In addition, NEP activity was also enhanced by late treatment with genistein in APP/Ar+/− mice by 114% (Figure 6C). In contrast, no significant changes in IDE protein expression or enzyme activity were found after any estrogen treatments in any group (Figure 7).
Figure 6.
Effect of estrogen treatments on Neprilysin (NEP) protein expression and enzyme activities in APP/OVX and APP/Ar+/− mice. All mice received 17β-estradiol (E), genistein (G) or placebo (C) as described in the methods. (A) the NEP Western blot images are shown, (B) the corresponding densitometry analysis of the western blot image is shown, (C) NEP enzyme activities are shown.* indicates p< 0.05 compared to placebo treated mice. # indicates P<0.05 compared to APP/OVX placebo treated mice.
Figure 7.
Effect of estrogen treatment on insulin degradation enzyme (IDE) protein expression and enzyme activities in APP/OVX and APP/Ar+/− mice. All mice received 17β-estradiol (E), genistein (G) or placebo (C) as described in the methods. (A) The IDE western blot image is shown, (B) the corresponding densitometry analysis of the western blot image is shown, and (C) IDE enzyme activities are shown. No significant differences were found among all treatments and genotypes.
Discussion
Our studies are the first to show that endogenous brain estrogen levels alter the sensitivity of response to estrogen replacement therapy in female AD transgenic mice. Potential mechanisms involve regulation of both BACE1 and NEP activity. While some conflicting reports in hormone replacement therapy from human and animal studies persist, increasing evidence suggest that endogenous estrogen levels are associated with cognitive function. For example, proestrus female rats and mice (with high endogenous estrogen level) express less anxiety and better cognitive function than at other phases of the estrous cycle (with lower endogenous estrogen level) (41). Studies have also reported that women with longer durations of reproductive years, and thus have a greater exposure to endogenous estrogen, show deceleration of cellular aging and reduced risk of cognitive decline (42). Furthermore, women taking aromatase inhibitors experience very low levels of endogenous estrogen, and develop cognitive dysfunction, especially short and long-term memory, which is correlated with estrogen deficiency (43,44). Moreover, a large scale clinical study showed a significant correlation between endogenous estrogen level and cognitive function in naturally postmenopausal women (4), suggesting that endogenous estrogen promotes cognitive function in aged females. Our recent studies have shown that women with lower brain estrogen levels may have a higher risk in developing AD, suggesting that brain estrogen could be an important preventative factor (18). However, it is unknown whether some of the conflicting reports on estrogen replacement therapy were related to the variation of brain estrogen levels in the studies.
In this study, we used a unique double transgenic/knockout APP/Ar+/− mice, that exhibits reduced endogenous estrogen levels and aggravated AD pathology, to study the effects of various exogenous estrogen treatments on APP processing and plaque formation. First, we demonstrated that reduction of endogenous estrogen synthesis in APP/Ar+/− mice reduces endogenous brain estrogen compared to that in APP/OVX mice (see figure 1A). We found that low brain estrogen levels correlated to more extensive plaque formation in the brain in APP/Ar+/− mice developed than age-matched APP/OVX mice (see figure 1B). Our data suggest that lower brain estrogen increases the risk of AD in females and therefore, must be evaluated in studies of AD in females. Estrogens are primarily synthesized in the gonads and reach the brain via circulation during the reproductive period, but local endogenous synthesis of estrogen in the brain is enhanced as a compensatory mechanism after menopause to maintain physiological levels (45–47). In some cases the brain may fail to maintain estrogen levels locally after menopause, which has been reported in female patients with AD (18, 46). Our APP/Ar+/− female mice with lower brain estrogen and more extensive AD neuropathology serve as a hormone sensitive animal model for the study of estrogen therapy and its potential mechanisms in preventing AD.
One of the major arguments regarding the WHI study is the starting time of estrogen treatment, soon after menopause vs. delayed treatment (10). To determine whether the starting time plays a role, we divided APP/OVX and APP/Ar+/− animals into two major groups; one that received treatment at an age of 3 months (early), and the other that received treatment at an age of 9 months (late). For the early treatment group, at an age of 12 months, APP/Ar+/− mice showed a great reduction of plaque formation after E2 and genistein treatment compared to placebo treated mice, while no significant change to plaque density was found in the APP/OVX mice that received the same treatment. No change in the plaque formation was observed in either APP/OVX or APP/Ar+/− mice that received 17α-estradiol or black cohosh treatments. Our findings suggest that brain estrogen levels may determine the response of some exogenous estrogen treatment in AD. However, there is difference in response to later treatment regardless the level of brain estrogen as shown in figure 3. Therefore, our data support the hypothesis that beginning estrogen treatment at an earlier age might be more beneficial to prevent AD.
We also studied the effect of estrogen treatment on APP processing in APP/OVX and APP/Ar+/− mice. As shown in figure 2, early treatment with E2 or Gen in APP/Ar+/− mice caused a greater reduction of Aβ40 and Aβ42, and a greater reduction of plaque formation than that in APP/OVX mice. In contrast, 17α-estradiol or black cohosh treatment did not have a significant effect on estrogen levels or Aβ and plaque formation (data not shown). This suggests brain estrogen deficiency enhances the response to the E2 and genistein treatments by reducing Aβ production in APP/Ar+/− mice.
To further understand the effect of brain estrogen level on APP processing in response to estrogen treatment, we examined BACE1, one of the major enzymes involved in APP processing. As shown in figure 5A, BACE1 protein levels were significantly decreased in APP/Ar+/− mice that received early treatment of E2 and genistein. The effect of E2 and genistein treatments on BACE1 expression was also found at the transcriptional level as measured by real time PCR in the APP/Ar+/− mice (figure 5B). Moreover, enzyme activity of BACE1 was only attenuated significantly by early treatment with E2 in the APP/Ar+/− mice as shown in figure 5C. To investigate whether the effects of E2 and genistein were mediated through estrogen receptors, we also treated the stable APP transfected HEK293 cells with E2 (1, 10 50uM) and genistein (1, 10, 50uM) for 48 hours and found that E2 (10, 50uM) and genistein (10, 50uM) treatments induced a significant down regulation of BACE1 protein levels (figure 5D). However, pretreated cells with an estrogen receptor antagonist blocked the reduction of BACE1 levels by E2 treatment, but not by genistein treatment (figure 5D). Our results suggest that E2-induced down-regulation of BACE1 is likely mediated through estrogen receptors, but that genistein-induced down-regulation is mediated through different means. Studies showed that estrogen receptors might be partially responsible for the differences seen between the various types of estrogen used for treatment. Genistein binds to estrogen receptor β with low affinity and plays both estrogen-like and antiestrogen roles in various biological events, including similar neuroprotective actions in animal studies (30,48,49). Although more vigorous investigations are needed to further examine these mechanisms, studies suggest that other receptors such as brain-derived neurotrophic factor (BDNF), may be responsible for the action of genistein (50–52). Our data suggest that brain estrogen deficiency might increase BACE1 sensitivity to estrogen treatment, followed by a protective mechanism that inhibits Aβ production and plaque formation in the brain.
To examine whether endogenous brain estrogen level alters the response of estrogen treatment on Aβ clearance, we measured NEP and IDE expression and enzyme activities. As figure 6 shows, early E2 and genistein treatments increase NEP protein expression and activity in both APP/OVX and APP/Ar+/− mice, while late treatment of genistein shows a more moderate increase in NEP enzyme activity compared to placebo treatment only in APP/Ar+/− mice. Because both APP/OVX and APP/Ar+/− mice showed an elevation of NEP expression and activities after the early E2 and Gen treatments, our data suggests that NEP activity might not be responsible for the brain estrogen-related sensitivity to early estrogen treatment. However, since late genistein treatment increased NEP activity only in APP/Ar+/− mice, it may suggest that lower brain estrogen level enhances sensitivity to genistein treatment at older ages via upregulation of NEP activity. In contrast, no effects of estrogen treatment were found on IDE in neither APP/OVX nor APP/Ar+/− mice (Figure 7). Together, our data suggest that early treatment with E2 or genistein-induced attenuation of Aβ42 in APP mice with normal levels of brain estrogen (APP/OVX) is mainly mediated through an increase of Aβ clearance by an up-regulation of NEP activity. In contrast, the same treatment in APP mice with lower levels of brain estrogen (APP/Ar+/−) has the additional effect of down-regulating BACE1 content and activity. Interestingly, our study showed differences in APP processing between E2 and genistein. Both E2 and genistein are potent inhibitors of BACE1 protein and mRNA expression and activity (see figure 5), but genistein has a greater effect on NEP activity, especially for late treatment (see figure 6). This distinction might be important since E2 may be more beneficial to preventing AD while genistein could be considered as both prevention and treatment for AD. However, since late administration of genistein in APP/Ar+/− mice still developed extensive brain plaque formation, the promotion of NEP activity by genistein treatment at a later time point does not appear to be sufficient enough to remove preexisting Aβ aggregates (Figure 3).
In summary, our studies have shown that early E2 and genistein treatment, not late treatment, prevents AD-like neuropathology via regulation of Aβ production and degradation in the brain, and that lower levels of endogenous brain estrogen increase the sensitivity of response to estrogen treatment in APP transgenic mice.
Materials and Methods
Animals
All mice were maintained in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Four genetic or surgical animal models were generated for these studies: sham wild type (WT), sham APP (APP), ovariectomized APP (APP/OVX), and APP with genetic deficiency of aromatase (APP/Ar+/−). Generation of the B6, D2-TgN (Thy1-APP23Swe) line of APP transgenic mice and the aromatase gene knockout (Ar−/−) line of mice on a C57BL/6 background have been described previously (18). APP transgenic mice were crossed with aromatase knockout mice, and the resulting APP/Ar+/− offspring were intercrossed through brother-sister mating to obtain litter matched genotypes. Bilateral ovariectomies were performed on a total of seventy 3-month-old female APP mice, and sham surgery (the same surgical procedure except without removing the ovaries as a surgical control) was performed on a total of twenty APP and WT mice, each. In brief, the ovariectomy was performed by creating a longitudinal incision on the lower back in the midline at the level of the last rib. The uterine horns were tied with surgical silk and the ovaries were cut and removed. The skin incision was closed by interrupted sutures.
Estrogen Treatments
For early treatment, at an age of 3 months old, APP/OVX mice (n=40) or APP/Ar+/− female mice (n=40) were anaesthetized with Pentobarbital Sodium, 50 mg/kg; IP injection, implanted subcutaneously with a sterilized E2 pellet (1.7mg or 18.9ug/day, n=8 each group), a 17α-estradiol pellet (1.7mg or 18.9ug/day, n=7 each group), a genistein pellet (24mg or 26ug/day, n=8 each group), a black cohosh pellet (24mg or 26ug/day, n=7 each group) or placebo pellet (n=10 each group). A total of 10 APP mice received the placebo treatments at the same age as control groups. All pellets were made for 90-day release by Innovative Research of America (Sarasota, FL). Pellets were re-implanted every 3 months in order to maintain hormone levels until tissue harvest at an age of 12 months. For late treatment, a total of 60 animals (APP/OVX n=30 and APP/Ar+/− n=30) were implanted with the same pellets at 9 months of age (n=5 for each treatment) until tissue harvest at 12 months of age. A total of 10 APP mice received placebo treatment at the same age as controls. For in vitro studies, stable APP transfected HEK293 cells were treated with E2 or genistein at 1, 10 and 50uM for 48 hours, respectively. To study the estrogen receptor dependency, some cells were also co-treated with estrogen receptor antagonist ICI 182780 at 10uM.
Tissue Preparation and Immunohistochemistry
Mice were anesthetized and their brains quickly removed and bisected. One hemisphere of brain tissue was fixed with 4% paraformaldehyde for immunohistochemistry, and the other half was frozen at −80°C for biochemistry and molecular biological analysis. The fixed tissue was serially sectioned (15–30µm in thickness) in the sagittal plane with a Leica CA 1900 cryostat. Eight to ten sections (about 120µm apart) were immunostained for Aβ (rabbit anti-Aβ-peptide; 1:250; Zymed, Carlsbad, CA). The images were digitally documented, then processed with a Leica DMLS complementary software package (MagnaFire SP), and analyzed by Image-pro Plus Analysis (media Cybernetics) as previously described (32).
Western Blot Analysis
To extract total protein, brain samples were homogenized in buffer containing 1% Nonidet P-40, 0.1% SDS, 50mM Tris (pH 8.0), 50mM NaCl, 0.05% deoxycholate, and protease inhibitors (Boehringer Mannheim, Indianapolis, IN). Extracts (40µg protein) were subjected to polyacrylamide gel electrophoresis, and separated proteins were transferred onto nitrocellulose membranes, which were then immunostained with the following primary antibodies: monoclonal anti-BACE (1:100; CalBiochem, San Diego, CA), polyclonal anti-IDE (1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and anti-NEP (1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The membranes were incubated with peroxidase-conjugated secondary antibodies (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and immunoreactive bands were visualized with an ECL system.
ELISA
Brain tissue from APP, APP/OVX, APP/Ar+/−, and wild-type mice were homogenized in homogenization buffer (250mM sucrose, 20mM Tris-HCl, pH 7.4, 1mM EDTA, and 1mM EGTA). An aliquot of the homogenate was dissolved in formic acid and neutralized with a neutralization buffer (1mM Tris and 0.5M Na2HPO4). Protein concentration was measured by a protein assay (Bio-Rad Laboratories). For the total Aβ ELISA, the capture antibody was monoclonal anti-Aβ antibody 4G8 (Chemicon), and the detection antibody was biotinylated monoclonal antibody anti-Aβ 6E10 (Serotec). Aβ40 and Aβ42 were measured with an Aβ40 and Aβ42 ELISA kit (Invitrogen). This ELISA system has been extensively tested and no cross-reactivity between Aβ40 and Aβ42 was observed. Data are presented as means ± SD of four experiments.
BACE1, IDE, and NEP activity
An aliquot of brain homogenate was further lysed in lysis buffer (10mM Tris-HCl, pH 7.4, 150mM NaCl, 1mM EDTA, 1mM EGTA, 1mM Na3VO4, 10% glycerol, and 0.5% Triton X-100). BACE1 enzymatic activity assays were performed as previously reported (Li et al. 2004) using synthetic peptide substrates containing BACE1 cleavage site (MCA-Glu-Val-Lys-Met-Asp-Ala-Glu-Phe-[Lys-DNP]-OH; Biosource International). BACE1 substrate was dissolved in DMSO and mixed with reaction buffer (50mM HAc and 100-mM NaCl, pH 4.1). An equal amount of protein was mixed with 100µl of substrate, and fluorescence intensity was measured with a microplate reader (BioTek) at an excitation wavelength of 320nm and an emission wavelength of 390nm (33–34).
IDE enzyme activity was measured as described previously (35). In brief, brains were homogenized in 50mM potassium phosphate buffer, pH 7.3, containing 200µm PMSF and a protease inhibitors (Sigma-Aldrich). Samples were centrifuged and the supernatant was used for IDE activity measurement. The hydrolysis of fluorogenic substrate peptides (2µm Abz-G G F L R K H G Q E D -Dnp in 20mM potassium phosphate buffer, pH 7.3) was measured by following an increase in fluorescence (excitation at 318nm and emission at 419nm) that occurred upon peptide bond cleavage. The max velocity of IDE activity was calculated within the first 20 min and indicated as fluorescence unit/min/microgram protein (36).
For the in vitro NEP activity assay, mouse brains were homogenized in 100mM MES buffer (pH 6.5) with protease inhibitors (Sigma-Aldrich). Homogenate was centrifuged at 20,000 g for 45 min to separate the membrane fraction and the supernatant was removed. The membrane pellet was resuspended in MES buffer before performing an NEP activity assay as previously described (37).
Quantitative analysis of BACE1 mRNA
Real time quantitative PCR (RT-qPCR) were performed as previously described (38). Briefly, reactions contained 2 µL of cDNA (20 ng), 10 µL of the 2× Master Mix and 0.5 µL of 20 µM of each BACE-1 primer (ttcatcaacggctccaact and ctccagggagtcgtcagg), 250 nM of BACE-1 gene specific probe, 500 nM reference (HPRT) gene primer mix (cgtgattagtgatgatgaaccag and cgagcaagacgttcagtcct), 250nM reference HPRT gene probe and DEPC-treated water to a final volume of 20µl. The reaction protocol started with a 2-min activation step at 50°C, a 10 min template denaturation step at 95°C, followed by 50 cycles of 95°C for 15 sec and 60°C for 20 sec, BACE-1 mRNA fold change (relative to the hypoxanthine-phosphoribosyl-transferase (HPRT) mRNA was calculated as previously described (38).
Estrogen radioimmunoassay (RIA)
The total estradiol levels from brain or serum were examined as previously described (18). In brief, two hundred microliters of serum or brain homogenate extract were incubated with 100µl of anti-serum for 4 hours at 4°C. [I125]-estradiol was added and incubated for 24 hours at 4°C. Proteins were precipitated and separated after centrifugation and radiation was counted by a gamma counter. This assay is based on a competitive radioimmunoassay method. The basic principle of radioimmunoassay is that there is competition between a radioactive and a non-radioactive antigen for a fixed number of antibody binding sites. The amount of [I-125]-labeled estradiol bound to the antibody is inversely proportional to the concentration of unlabeled estradiol present. The separation of free and bound antigen is achieved by using a double antibody system. A standard curve was produced using five serum standards, for which the estradiol concentration is known. The estradiol values of the samples were directly read against this standard curve. The sensitivity of the test for estradiol was 3.0 pg/ml.
Statistics
Statistical analyses were performed by two-way ANOVA with interactions followed by least significant difference post hoc analysis (multiple comparisons). Independent variables in the ANOVAs were genotype (APP/OVX, APP/Ar+/−), form of estrogen treatment (vehicle, 17α-estradiol, 17β-estradiol, black cohosh, genistein), and treatment types (early vs. late) with post hoc comparisons. Pearson’s correlation coefficients were used for correlation analyses. The level of significance was set at P< 0.05.
Acknowledgements
This work was supported by grants from the Alzheimer’s Association IIRG-07-59510, American Health Assistance Foundation Grant G2006-118, NIH R01AG032441, NIH R01AG025888. We thank Mr. Alex Bishop for editing and prove reading the manuscript.
References
- 1.Schäfer S, Wirths O, Multhaup G, Bayer TA. Gender dependent APP processing in a transgenic mouse model of Alzheimer's disease. J Neural Transm. 2007;114:387–394. doi: 10.1007/s00702-006-0580-9. [DOI] [PubMed] [Google Scholar]
- 2.Carroll JC, Rosario ER, Kreimer S, Villamagna A, Gentzschein E, Stanczyk FZ, Pike CJ. Sex differences in β-amyloid accumulation in 3×Tg-AD mice: role of neonatal sex steroid hormone exposure. Brain Res. 2010;17(1366):233–245. doi: 10.1016/j.brainres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan J, Carrière I, Scali J, Ritchie K, Ancelin M-L. Life-time estrogen exposure and cognitive functioning in later life. Psychoneuroendocrinol. 2009;34(2):287–298. doi: 10.1016/j.psyneuen.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Heys M, Jiang C, Cheng KK, Zhang W, Au Yeung SL, Lam TH, Leung GM, Schooling CM. Life long endogenous estrogen exposure and later adulthood cognitive function in a population of naturally postmenopausal women from Southern China: the Guangzhou Biobank Cohort Study. Psychoneuroendocrinology. 2011;36(6):864–873. doi: 10.1016/j.psyneuen.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Tralongo R, Mari AD. Cognitive impairment, aromatase inhibitors, and age. J Clin Oncol. 2005;23(18):4243. doi: 10.1200/JCO.2005.01.1304. [DOI] [PubMed] [Google Scholar]
- 6.Wroolie TE, Kenna HA, Williams KE, Powers BN, Holcomb M, Khaylis A, Rasgon NL. Differences in verbal memory performance in postmenopausal women receiving hormone therapy: 17β-estradiol versus conjugated equine estrogens. Am J Geriatr Psychiatry. 2011;19(9):792–802. doi: 10.1097/JGP.0b013e3181ff678a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valen-Sendstad A, Engedal K, Stray-Pedersen B, Strobel C, Barnett L, Meyer N, Nurminemi M ADACT Study Group. Effects of hormone therapy on depressive symptoms and cognitive functions in women with Alzheimer disease: a 12 month randomized, double-blind, placebo-controlled study of low-dose estradiol and norethisterone. Am J Geriatr Psychiatry. 2010;18(1):11–20. doi: 10.1097/JGP.0b013e3181beaaf4. [DOI] [PubMed] [Google Scholar]
- 8.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 9.Grady D, Yaffe K, Kristof M, Lin F, Richards C, Barrett-Connor E. Effect of postmenopausal hormone therapy on cognitive function: the Heart and Estrogen/progestin Replacement Study. Am J Med. 2002;113:543–548. doi: 10.1016/s0002-9343(02)01270-6. [DOI] [PubMed] [Google Scholar]
- 10.Taylor HS, Manson JE. Update in hormone therapy use in menopause. J. Clin. Endocrinol. Metab. 2011;96:255–264. doi: 10.1210/jc.2010-0536. [DOI] [PubMed] [Google Scholar]
- 11.Silverman DH, Geist CL, Kenna HA, Williams K, Wroolie T, Powers B, Brooks J, Rasgon NL. Differences in regional brain metabolism associated with specific formulations of hormone therapy in postmenopausal women at risk for AD. Psychoneuroendocrinology. 2011;36(4):502–513. doi: 10.1016/j.psyneuen.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 13.Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratman NC, Mathews WR, Buhl AE, Carter DB, Tomasselli AG, Parodi LA, Heinrikson RL, Gurney ME. Membrane-anchored aspartyl protease with Alzheimer's disease beta-secretase activity. Nature. 1999;402(6761):533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 14.De Strooper B, Vassar R, Golde T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol. 2010;6(2):99–107. doi: 10.1038/nrneurol.2009.218. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L-B, Lindholm K, Yan R, Citron M, Xia W, Konishi Y, Yang XL, Beach T, Sue L, Wang P, Price D, Li R, Shen Y. Elevated β-secrtase expression and enzymatic activity detected in Sporadic Alzheimer's brains. Nature Medicine. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- 16.Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer's disease. Ann Neurol. 2002;51(6):783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- 17.Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59(9):1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 18.Yue X, Lu M, Lancaster T, Cao P, Honda S-I, Harada N, Staufenbiel M, Zhong ZY, Shen Y, Li R. Brain estrogen deficiency accelerates Aβ plaque formation in Alzheimer's animal model. Proc Natl Acad Sci. 2005;102(52):19198–19203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ertekin-Taner N, Allen M, Fadale D, Scanlin L, Younkin L, Petersen RC, Graff-Radford N, Younkin SG. Genetic variants in a haplotype block spanning IDE are significantly associated with plasma Abeta42 levels and risk for Alzheimer disease. Hum Mutat. 2004;23:334–342. doi: 10.1002/humu.20016. [DOI] [PubMed] [Google Scholar]
- 20.Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40(6):1087–1093. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- 21.Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Aβ by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- 22.Xiao ZM, Sun L, Liu YM, Zhang JJ, Huang J. Estrogen regulation of the neprilysin gene through a hormone-responsive element. J Mol Neurosci. 2009;39(1–2):22–26. doi: 10.1007/s12031-008-9168-1. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Guan H, Booze RM, Eckman CB, Hersh LB. Estrogen regulates neprilysin activity in rat brain. Neurosci Lett. 2004;367(1):85–87. doi: 10.1016/j.neulet.2004.05.085. [DOI] [PubMed] [Google Scholar]
- 24.Yang HQ, Sun ZK, Jiang QH, Shang Q, Xu J. Effect of estrogen-depletion and 17beta-estradiol replacement therapy upon rat hippocampus beta-amyloid generation. Zhonghua Yi Xue Za Zhi. 2009;89:2658–2661. [PubMed] [Google Scholar]
- 25.Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279:688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- 26.Gibbs RB. Estrogen and nerve growth factor-related systems in brain. Effects on basal forebrain cholinergic neurons and implications for learning and memory processes and aging. Ann N Y Acad Sci. 1994;743:165–196. doi: 10.1111/j.1749-6632.1994.tb55792.x. [DOI] [PubMed] [Google Scholar]
- 27.Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA MIRAGE Study Group. Postmenopausal hormone therapy and Alzheimer's disease risk: interaction with age. J Neurol Neurosurg Psychiatry. 2005;76:103–105. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitmer RA, Quesenberry CP, Zhou J, Yaffe K. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol. 2011;69:163–169. doi: 10.1002/ana.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang CN, Chi CW, Lin YL, Chen CF, Shiao YJ. The neuroprotective effects of phytoestrogenss on amyloid beta protein- induced toxicity are mediated by abrogating the activation of caspase cascade in rat cortical neurons. J Biol Chem. 2001;276:5287–5295. doi: 10.1074/jbc.M006406200. [DOI] [PubMed] [Google Scholar]
- 30.Zhao L, Mao Z, Brinton RD. A select combination of clinically relevant phytoestrogens enhances estrogen receptor beta-binding selectivity and neuroprotective activities in vitro and in vivo. Endocrinology. 2009;150(2):770–783. doi: 10.1210/en.2008-0715. [DOI] [PubMed] [Google Scholar]
- 31.Seidlova-Wuttke D, Hesse O, Jarry H, Christoffel V, Spengler B, Becker T, Wuttke W. Evidence for selective estrogen receptor modulator activity in a black cohosh (Cimicifuga racemosa) extract: comparison with estradiol-17beta. Eur J Endocrinol. 2003;149(4):351–362. doi: 10.1530/eje.0.1490351. [DOI] [PubMed] [Google Scholar]
- 32.He P, Zhong Z, Lindholm K, Berning L, Lee W, Lemere C, Staufenbiel M, Li R, Shen Y. Deletion of tumor necrosis factor death receptor inhibits amyloid beta generation and prevents learning and memory deficits in Alzheimer's mice. J Cell Biol. 2007;178(5):829–841. doi: 10.1083/jcb.200705042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li R, Lindholm K, Yang LB, Yue X, Citron M, Yan R, Beach T, Sue L, Sabbagh M, Cai H, Wong P, Price D, Shen Y. Amyloid β peptide load is correlated with increased β-secretase activity in sporadic Alzheimer's disease patients. Proc Natl Acad Sci. 2004;101:3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McAllister C, Long J, Bowers A, Walker A, Cao P, Honda S-I, Harada N, Staufenbiel M, Shen Y, Li R. Genetic targeting aromatase in male amyloid precursor protein (APP) transgenic mice down-regulates beta-secretase (BACE1) and prevents Alzheimer-like pathology and cognitive impairment. Journal of Neuroscience. 2010;30:7326–7334. doi: 10.1523/JNEUROSCI.1180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song ES, Juliano MA, Juliano L, Hersh LB. Substrate activation of insulin-degrading enzyme (insulysin). A potential target for drug development. J Biol Chem. 2003;278(50):49789–49794. doi: 10.1074/jbc.M308983200. [DOI] [PubMed] [Google Scholar]
- 36.Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J Biol Chem. 1998;273(49):32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- 37.Li C, Hersh LB. Neprilysin: assay methods, purification, and characterization. Methods Enzymol. 1995;48:253–263. doi: 10.1016/0076-6879(95)48018-8. [DOI] [PubMed] [Google Scholar]
- 38.Ait-Ghezala G, Mathura VS, Laporte V, Quadros A, Paris D, Patel N, Volmar CH, Kolippakkam D, Crawford F, Mullan M. Genomic regulation after CD40 stimulation in microglia: relevance to Alzheimer's disease. Brain Res Mol Brain Res. 2005;(1–2):73–85. doi: 10.1016/j.molbrainres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Haisenleder DJ, Schoenfelder AH, Marcinko ES, Geddis LM, Marshall JC. Estimation of estradiol in mouse serum samples: evaluation of commercial estradiol immunoassays. Endocrinology. 2011;152(11):4443–4447. doi: 10.1210/en.2011-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanczyk FZ, Cho MM, Endres DB, Morrison JL, Patel S, Paulson RJ. Limitations of direct estradiol and testosterone immunoassay kits. Steroids. 2003;68(14):1173–1178. doi: 10.1016/j.steroids.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Ter Horst JP, de Kloet ER, Schächinger H, Oitzl MS. Relevance of Stress and Female Sex Hormones for Emotion and Cognition. Cell Mol Neurobiol. 2012;32(5):725–735. doi: 10.1007/s10571-011-9774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin J, Kroenke CH, Epel E, Kenna HA, Wolkowitz OM, Blackburn E, Rasgon NL. Greater endogenous estrogen exposure is associated with longer telomeres in postmenopausal women at risk for cognitive decline. Brain Res. 2011;1379:224–231. doi: 10.1016/j.brainres.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips KA, Ribi K, Fisher R. Do aromatase inhibitors have adverse effects on cognitive function? Breast Cancer Research. 2011;13:203. doi: 10.1186/bcr2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sherwin BB. Estrogen and cognitive functioning in women. Endocr Rev. 2003;24:133–151. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- 45.Hojo Y, Murakami G, Mukai H, Higo S, Hatanaka Y, Ogiue-Ikeda M, Ishii H, Kimoto T, Kawato S. Estrogen synthesis in the brain--role in synaptic plasticity and memory. Mol Cell Endocrinol. 2008;290(1–2):31–43. doi: 10.1016/j.mce.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 46.Butler HT, Warden DR, Hogervorst E, Ragoussis J, Smith AD, Lehmann DJ. Association of the aromatase gene with Alzheimer's disease in women. Neurosci Lett. 2010;468(3):202–206. doi: 10.1016/j.neulet.2009.10.089. [DOI] [PubMed] [Google Scholar]
- 47.Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24(26):5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Froyen EB, Steinberg FM. Soy isoflavones increase quinone reductase in hepa-1c1c7 cells via estrogen receptor beta and nuclear factor erythroid 2-related factor 2 binding to the antioxidant response element. J Nutr Biochem. 2011;22(9):843–848. doi: 10.1016/j.jnutbio.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Trivella DB, Bleicher L, Palmieri Lde C, Wiggers HJ, Montanari CA, Kelly JW, Lima LM, Foguel D, Polikarpov I. Conformational differences between the wild type and V30M mutant transthyretin modulate its binding to genistein: implications to tetramer stability and ligand-binding. J Struct Biol. 2010;170(3):522–531. doi: 10.1016/j.jsb.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Pan M, Han H, Zhong C, Geng Q. Effects of genistein and daidzein on hippocampus neuronal cell proliferation and BDNF expression in H19-7 neural cell line. J Nutr Health Aging. 2012;16(4):389–394. doi: 10.1007/s12603-011-0140-3. [DOI] [PubMed] [Google Scholar]
- 51.Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92(24):11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nature Medicine. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]