Abstract
PURPOSE
Maintenance of exercise after completing Phase II cardiac rehabilitation (CR) is challenging for many patients. We offered a telephone-based maintenance intervention and found improvement in exercise participation in the intervention group at 12 months post-CR discharge. We examined the effects of the intervention on psychosocial outcomes.
METHODS
The effects of a home-based exercise maintenance intervention on psychosocial outcomes among patients who had completed Phase II CR vs. contact control was evaluated in a randomized controlled trial. Data were collected in 2005–2010 and analyzed in 2011. One hundred thirty patients (mean age=63.6 years [SD=9.7], 20.8% female) were randomized to exercise counseling (Maintenance Counseling group, n=64) or contact control (Contact Control group, n=66). Maintenance Counseling group participants received exercise counseling (based on the Transtheoretical Model and Social-Cognitive Theory) delivered via telephone for 6 months, as well as print materials and feedback reports. Assessments of depression, quality of life and mental health were conducted at baseline, 6, and 12 months.
RESULTS
The Maintenance Counseling group reported statistically significant higher quality of life than the Contact Control group at 6 (b=0.29, se=0.08, P<.001) and 12 months (b=0.27, se=0.09, P=.002). Intervention effects on depressive symptoms were significant at 12 months (b=−6.42, se=2.43, P=.009). Effects on overall mental health were nonsignificant at both followups. No significant moderators of treatment effects were found.
CONCLUSION
A telephone-based intervention that helped to maintain exercise showed statistically significant improvements in quality of life and reduced depressive symptoms in this patient population.
Key words or phrases: Exercise maintenance, cardiac rehabilitation, psychosocial outcomes
There have been numerous epidemiologic studies reporting that mood, and particularly, depression is associated with both the development1–4 and recurrence5–8 of coronary heart disease (CHD). Aerobic exercise has been recognized as a successful option to manage depressive symptoms with improvements in depression following participation in an exercise-based cardiac rehabilitation (CR) program.9–11 While these studies provide encouraging evidence for the benefit of CR on depressive symptoms, the extent to which these benefits are sustained after discharge from CR was not addressed. Additionally, while exercise has proven to help with depression, depression can also be a barrier to exercise participation.12
Maintenance of exercise and psychosocial benefits following participation in CR remains an important challenge. Approximately 50% to 75% of patients fail to adhere to their prescribed exercise program in the year following completion of a Phase II CR program.13–15 We examined the efficacy of a telephone-based intervention to enhance maintenance of exercise participation among patients who had recently completed a Phase II CR program. Participants in the maintenance counseling (MC) group reported significantly higher exercise participation at 12 months (12M) than the contact control group (CC, 80 minutes difference, 95% CI=22, 137).16 While group differences in exercise participation at 6 months (6M) were no significantly different from the control condition, the maintenance intervention significantly increased the probability of participants exercising at or above physical activity guidelines and attenuated regression in motivational readiness compared to the control group at 6M and 12M.
Given the promising results of this telephone-based maintenance intervention, we examined whether exercise maintenance would translate to improvements in self-reported depressive symptoms, quality of life and mental health. The goals of this paper were to examine group differences in depressive symptoms, quality of life and mental health at 6M and 12M. Additionally, we explored whether treatment effects on these psychosocial outcomes were moderated by baseline exercise, gender, income, cardiac risk status, adherence to CR and anti-depressant usage. These variables were chosen because they represent subgroups that respond differentially to exercise interventions (eg, gender, income) or are related to the psychosocial outcomes (eg, antidepressant use). We hypothesized that the group that received the telephone intervention to support exercise maintenance would report better quality of life and mental health and reduced depressive symptoms at 6M and 12M.
METHODS
In this randomized controlled trial, 130 patients who had completed a Phase II CR program received brief advice from their CR case managers to maintain exercise and were then randomized to either the MC or CC group. Assessments were conducted at baseline, 6M and 12M. Institutional Review Board approval was obtained. Data were collected in 2005–2010.
Recruitment
Case managers distributed study invitations to patients who were scheduled to complete Phase II programs. A Research Assistant (RA) conducted a telephone screen among patients who responded positively to the invitation. If the patient was eligible and interested, the RA obtained patient written informed consent.
Patient Eligibility Criteria
Men and women aged ≥40 years who (1) were participating in supervised Phase II CR (generally a 12-week program that includes exercise training 3 times/week for about 90 min/session), (2) were scheduled to complete Phase II CR in the next 4 weeks, 3) were able to read and speak English, 4) provided consent for medical chart review to extract disease and treatment variables, 5) were able to walk unassisted, and 6) had access to a telephone. The study was powered to detect effects on the primary outcome of minutes of exercise of at least moderate-intensity. A sample size of 180 was required but due to recruitment difficulties, this goal was not met.
Five hundred forty-eight patients were invited to participate: 249 were screened for eligibility, 158 were not interested, and 141 did not respond prior to CR discharge16 (Figure 1). Of the 249 screened, 56 were not interested/eligibility unknown, 39 were not eligible, and 154 were eligible. Of the 154 eligible participants, 130 (84.4%) were randomized using a stratified scheme that ensure balance across strata defined by age (<65 years vs. ≥65 years), gender, and cardiovascular risk (low, intermediate, or high per AACVPR guidelines).17 Of the 64 participants assigned to the MC group, 20 dropped out by 12M (31%), and of the 66 participants in the CC group, 14 dropped out by 12M (21%).
Figure 1. Consort figure (CR, cardiac rehabilitation).
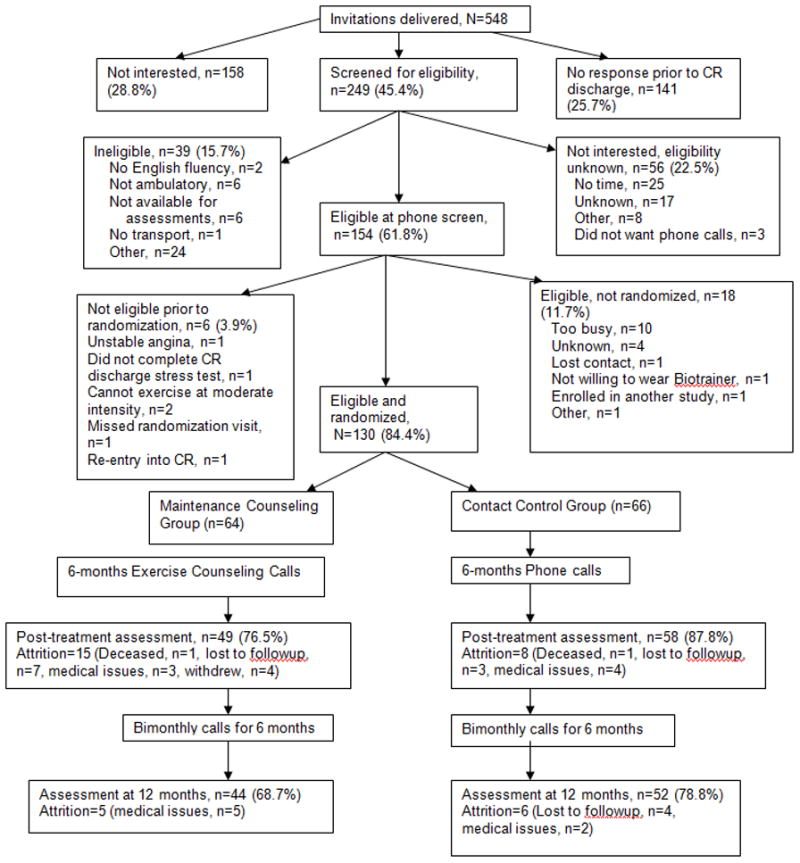
Reprinted with permission (Elsevier) from Pinto BM, Goldstein MG, Papandonatos GD, et al. Maintenance of exercise after phase II cardiac rehabilitation: A randomized controlled trial. Am J Prev Med. 2011;41:274–283.
Procedures
After baseline assessments were completed, case managers were cued by a prompt placed on patient medical charts to deliver brief advice on the importance of adhering to their individualized exercise prescription (based on a maximal stress test) received at CR discharge. Case managers had been trained on a brief motivational counseling protocol (based on the 5As counseling strategy).18,19 After receiving brief advice from the case managers, participants were randomized.
Maintenance Counseling Group (MC)
Following randomization, the Intervention Coordinator reviewed the patient individualized exercise prescription received at CR discharge. The MC intervention aimed at ensuring that patients adhered to their exercise prescription. The participant was given home logs to monitor exercise participation and a pedometer (Digiwalker, Yamax Corporation, Tokyo, Japan) to wear during exercise activities that involved walking or running. Each participant received calls over 6M (weekly over the first 2 months, biweekly for the next 2 months, and monthly for the last 2 months, a total of 14 calls) from the Intervention Coordinator to promote adherence to their prescribed aerobic exercise plan. Activity counseling was based on the Transtheoretical Model 20 and Social Cognitive Theory21 and tailored to each participant’s motivational readiness.22 Action stage was defined as exercising at levels consistent with the exercise prescription provided at CR discharge. Specific components from motivational interviewing23 were also included in the calls. Participants reported on the exercise recorded on home logs and received feedback. After the 6M intervention, bimonthly phone calls were provided to prompt and reinforce regular exercise. Participants were mailed informational tip sheets on exercise and on cardiovascular health during the 6-month program. Finally, personalized feedback letters summarizing the participant exercise progress and supporting motivation were sent at weeks 4, 8, 12, 16, and 20.
Contact Control Group (CC)
To control for frequency of contact with the 2 groups, these participants also received calls from the Intervention Coordinator at the same intervals as MC participants over the entire study period. During these calls, the Symptom Questionnaire24 was administered to monitor general health problems. The group also received tip-sheets on cardiovascular health (the same as those provided to MC participants). After completing the 12M assessment, participants received the exercise tip-sheets.
Intervention Delivery
All telephone calls to participants were audiotaped and 25% of these tapes were reviewed by the principal investigator and a coinvestigator to ensure fidelity to intervention content and process.
Measures
At baseline, demographic information was obtained from the participants and disease and treatment variables were obtained from medical records. Participants received $20 for completing the assessments at baseline, 6M, and 12M. At baseline, 6M, and 12M, in addition to assessments of physical activity, they completed the following psychosocial measures:
Cardiac Depression Scale
This valid and reliable 26-item scale was developed and validated among cardiac outpatients.25 Participants were asked to complete each item as it applied to them over the past month using a 7-point rating scale. Scores range from 26 to 182, with higher scores indicating higher levels of depression (cutoff score for major depression is ≥9526).
MacNew Heart Disease Health-Related Quality of Life Questionnaire
The MacNew Questionnaire is a self-administered quality of life assessment that was designed to evaluate the impact of treatment for CHD and has been shown to be valid and reliable.27–29 It assesses 3 domains: emotional, physical, social, and global quality of life. We analyzed the global score (range of 1–7) with higher scores indicating better quality of life. A change of 0.5 points suggests minimal to moderately important clinical change.30
MOS 36-Item Short Form Health Survey (SF-36)
The SF-36 assesses 8 health concepts (eg, physical functioning, bodily pain).31 Here, we analyzed the mental health component score that is derived from the responses to all the subscales. The SF-36 has been found to be valid and reliable among patients with coronary artery disease.32 Higher scores indicate better mental health status. The normative mean score for the mental health component is 50 (SD=10).
Analyses
Between-group differences in baseline demographics as well as number of calls were assessed using analysis of variance (ANOVA) for continuous variables and χ2 for categorical variables. Unadjusted means (standard deviations) for cardiac depression, quality of life and the SF-36 mental health component scale were summarized for each time point by study arm. All analyses were conducted in SAS 9.3 and significance level set apriori at .05. Using analysis of variance (ANOVA), we tested for between-group differences in baseline values of the outcomes. Finally, using ANOVA, we tested whether there were differences in the percentage of calls delivered to MC versus CC arms.
Using a mixed effects longitudinal regression model, we assessed whether there were between group differences in mean outcomes at both 6M and 12M. Models included random intercepts to account for the within subject correlation between repeated outcomes over time. Models were adjusted for baseline values of the outcome, as well as potential confounders of the treatment effect (gender and age). Although these potential confounders were balanced across treatment arms, they were chosen as potential cofounders of the treatment effect apriori and hence controlled for in all subsequent analyses. All analyses were conducted on the intent-to-treat sample and thus, included all participants randomized at baseline. Mixed effects models use a likelihood-based approach to estimation and therefore make use of all available data without directly imputing values of missing outcomes.
Using a similar set of longitudinal models, we assessed whether key baseline variables chosen apriori (gender, baseline levels of exercise, income, adherence, cardiac risk status, and antidepressant medication use) were moderators of the treatment effect on outcome (cardiac depression, quality of life, and the SF-36 mental health component). Models included the main effects of treatment and the potential moderator as well as the interaction between them.
RESULTS
Baseline demographics are presented in Table 1. There were no significant between-group differences in the baseline demographic variables. Amongst those randomized to the MC arm, 92% of scheduled phone calls were delivered versus 94% in the CC arm. There were no significant between-group difference in the frequency of calls delivered (P>.05). Unadjusted mean values of cardiac depression, quality of life, and the SF-36 mental health component over time are reported in Table 2. At baseline, MC participants reported average scores of 65.4 on the Cardiac Depression Scale (CC mean score = 68.0), 5.9 on the MacNew (CC mean score = 5.9) and 53.9 on the SF-36 mental health component scale (CC mean score = 53.7). There were no significant between-group differences in baseline values of these outcomes (P>.05). At baseline, 11.7 % of MC participants reported a score of ≥95 (cut-off for major depression) on the Cardiac Depression Scale vs. 12.3% of CC participants. Corresponding percentages at 6M and 12M were: 8.2% vs. 8.6% and 9.1% vs. 15.4%.
Table 1.
Baseline demographics by study arm
| MC Group (n=64) | Contact Control Group (n=66) | Overall (N=130) | |
|---|---|---|---|
|
| |||
| Age, M (SD) | 62.9 (9.3) | 64.3 (10.0) | 63.6 (9.7) |
|
| |||
| Gender, % male | 80.3% | 78.1% | 79.2% |
|
| |||
| Race/Ethnicity, % Non-Hispanic White | 95.3% | 92.4% | 93.8% |
|
| |||
| Marital status, % married/living with partner | 73.4% | 77.3% | 75.4% |
|
| |||
| Education, % with ≥ some college | 78.1% | 66.7% | 72.3% |
|
| |||
| Income, % ≥ $40,000 | 70.0% | 70.5% | 69.7% |
|
| |||
| Body mass index | 29.0 (SD=5.3) | 29.2 (SD=5.1) | 29.1 (5.2) |
|
| |||
| Baseline exercise, min/wk (SD) | 233.4 (199.1) | 198.6 (138.0) | 215.8 (171.1) |
|
| |||
| Cardiac Risk Status | |||
| 1 | 53.1% | 57.6% | 55.4% |
| 2 | 34.4% | 33.3% | 33.8% |
| 3 | 12.5% | 9.1% | 10.8% |
|
| |||
| Adherence to Phase II sessions | 91.1% | 93.2% | 92.2% |
|
| |||
| Antihypertensive medication | |||
| Prescribed | 84.4% | 84.8% | 85.8% |
| Not prescribed | 5.6% | 15.2% | 14.6% |
|
| |||
| Type 2 diabetes | |||
| Yes | 23.4% | 27.3% | 25.4% |
| No | 76.6% | 72.7% | 74.6% |
|
| |||
| Antidepressant use | 10.9% | 6.1% | 8.5% |
Table 2.
Unadjusted Mean Values of the Psychosocial Variables Over Time
| Baseline M (SD) | 6 Months M (SD) | 12 Months M (SD) | |
|---|---|---|---|
|
| |||
| Cardiac Depression | |||
| Maintenance Counseling | 65.4 (22.3) | 61.7 (21.4) | 65.1 (18.9) |
| Contact Control | 68.0 (26.0) | 66.1 (24.8) | 71.5 (26.8) |
|
| |||
| MacNew (QOL) | |||
| Maintenance Counseling | 5.9 (0. 8) | 6.3 (0.5) | 6.2 (0.6) |
| Contact Control | 5.9 (0.8) | 6.0 (0.7) | 5.9 (0.7) |
|
| |||
| SF-36 Mental Health Component | |||
| Maintenance Counseling | 53.9 (7.8) | 54.5 (5.8) | 54.4 (7.0) |
| Contact Control | 53.7 (8.8) | 53.1 (9.6) | 52.4 (10.4) |
QOL, quality of life
Longitudinal models suggest a significant between-group difference in mean cardiac depression at 12M, such that those randomized to the MC arm reported lower cardiac depression scores on average (b=−6.42, se=2.43, t=−2.64, P=.009). Additionally, similar analyses on the percentage of participants with scores indicating major depression showed a trend favoring the MC group at 12M (P=.18). Significant between-group differences in quality of life (MacNew) were evident at both followups such that those randomized to MC reported higher average quality of life compared to CC (b=0.29, se=0.08, t=3.48, P<.001 at 6M and b=0.27, se=0.09, t=3.14, P=.002 at 12M). Finally, there was a trend towards significance in the between-group difference in the SF-36 mental health component at 12M, such that those in the MC arm reported higher mental health component scores on average (b=2.32, se=1.38, t=1.69, P=.09) compared to CC. Adjusted mean differences between MC and CC participants with respect to each of the outcomes at 6M and 12M are seen in Table 3.
Table 3.
Adjusted Estimates of Mean Difference in Psychosocial Outcomes Between Groups over Time
| 6 Months M (SD) | 12 Months M (SD) | |
|---|---|---|
| Cardiac Depression | −3.2 (2.3) | −6.4 (2.4)a |
| MacNew (QOL) | 0.3 (0.1)a | 0.3 (0.1)a |
| SF-36 Mental Health Component | 1.1 (1.0) | 2.3 (1.1)a |
P<.05; Note that estimates represent between-group differences (Maintenance Counselinga vs. Contact Control) in adjusted mean values at each time.
Analyses did not suggest any significant moderators of the treatment effect on the psychosocial outcomes at 6M or 12M. In all cases, interactions between treatment arm and the potential moderator were not statistically significant (P>.05).
DISCUSSION
Our goal was to examine change in psychosocial outcomes among individuals who participated in a telephone-based exercise counseling program that targeted maintenance of exercise participation after CR. We hypothesized that the intervention group (MC) would report reduced depressive symptoms and enhanced quality of life and mental health at 6M and 12M relative to the controls (CC). Our results support reduced depressive symptoms in the MC group at 12M, and positive effects on quality of life at 6M and 12M while effects on mental health were nonsignificant at both time-points.
At study entry (CR discharge), the sample mean depression scores at baseline were low relative to normative data25 that may reflect the favorable effects of a multicomponent Phase II CR program on depression. Nonetheless, we found positive effects of the MC intervention on depressive symptoms at 12M. There have been a number of studies that indicate an association between depression and the onset of and recurrence of CHD.4–7 Additionally, depression impacts adherence to medication regimens, healthy food intake, exercise and stress reduction behaviors among those with CHD.33 Therefore, it is of utmost importance to understand both how to reduce the incidence of depression and to minimize its impact on other health habits among those who do become depressed prior to or following the diagnosis of CHD. Exercise can help manage depressive symptoms and is a critically important part of a maintenance regimen following CR. However, long-term exercise maintenance is extremely difficult for both healthy populations and those recovering from health conditions such as CHD.34,35 The results of our study are promising in that the intervention not only led to improved exercise maintenance at 12M16 but also attenuated depressive symptoms at 12M.
The telephone-based intervention that focused on exercise maintenance did lead to improved QOL at both followups. However, the group differences do not appear to be clinically significant as normative data suggests that a difference of 0.5 points implies important change.30 It is interesting that the change in quality of life was statistically significantly different between MC and CC at both 6M and 12M, but the change in cardiac depression was only significantly different between groups at 12M. We did report previously that the overall difference in exercise participation favored the MC group at 12M but not at 6M.16 There were no significant baseline differences between MC and CC on the Cardiac Depression scale or the MacNew scale. Therefore, it is unclear why there was not a significant difference between groups in depression until 12 months. We might speculate that there may be lingering protective effects of participating in CR on depressive symptoms and these can sustain individuals for the first 6M after rehabilitation. However, without the extra benefit of ongoing participation in a regular program of aerobic activity, it is possible that these protective effects on mood cannot be sustained.
Other studies that targeted exercise maintenance in this population have produced mixed effects on psychosocial outcomes. While an intervention that provided self-regulatory skills training for exercise found reduced depression in the intervention vs. control group,36 other interventions have demonstrated improvements in distress and in quality of life over time within the intervention group but nonsignificant group differences.37,38 Exercise consultations followed by exercise information vs. exercise information alone did not affect quality of life, anxiety, and depression at 6 or 12M in another trial.39
We do note that intervention effects were detected on measures of psychosocial outcomes that were developed for a cardiac population (Cardiac Depression Scale and the MacNew QOL measure) but not a generic assessment of mental health (SF-36). These results suggest that in future studies it will be important to use instruments that have been specifically developed for and/or validated on CR populations. Using only such instruments will both allow better precision, in comparison to using more global measures and will also help to minimize patient burden as many investigators administer both global and behavior/population-specific psychosocial measures.
As noted previously, the strengths of the study include a home-based, theoretically-grounded intervention that was directed towards exercise maintenance, a conservative comparison group that controlled for frequency of contact, and the use of well-established measures of depression, quality of life and mental health. Our sample was relatively homogenous (race/ethnicity and education) thereby limiting the generalizability of results. As is typical of CR populations, female representation in the sample was low (20%) and this may have limited our ability to detect the potential moderator effect of gender. Similarly, the study was not powered to identify significant moderators of treatment effects including baseline exercise, income, cardiac risk status, adherence to CR, and antidepressant usage. Additionally, 54.5% of those who received a study invitation did not respond or were not interested. This may suggest a bias in the results since it is possible that nonrespondents may have been more depressed and/or had lower quality of life. Finally, our intervention was fairly intensive and may have led to higher attrition in the MC group.
Findings from the current trial reveal that a non-face-to-face approach is effective at helping patients maintain exercise for 1 year posttreatment as well as to keep depressive symptoms at bay and improve quality of life during this critical first year after rehabilitation. In the absence of longer followup (>12M), it is not known whether the group differences we observed would change as time from CR discharge increased. Addressing patient commitment to long-term exercise maintenance and providing interventions to support such maintenance given the psychosocial benefits requires continued attention by healthcare providers. The gains these at-risk patients accrue from their participation in Phase II CR programs and their adoption of regular exercise should be supported by efforts to sustain behavior change over the long-term. Examining the costs of such efforts would also be important to address questions about cost-effectiveness.
Acknowledgments
We gratefully acknowledge the contributions of Michael Goldstein, M.D., Peter Tilkemeier, M.D., George Papandonatos, Ph.D., Lisa Breault, B. A., Kelly Greenwood, B. A., Cary Garcia, MBA, Jennifer Correia, B.A., Carl Robitaille, B.S., Loren Stable, M.S. and the case managers at the Cardiac Fitness Center at the Miriam Hospital (RI) and Dr. Mark Gabry and the case managers at the Cardiac Fitness Center, St. Anne’s Hospital (MA). We thank the National Heart, Lung and Blood Institute (HL 76734, Dr. Pinto) for funding the study.
References
- 1.Anda R, Williamson D, Jones D, et al. Depressed affect, hopelessness, and the risk of ischemic heart disease in a cohort of U.S. adults. Epidemiology. 1993;4:285–294. doi: 10.1097/00001648-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample. Circulation. 1996;93:1976–1980. doi: 10.1161/01.cir.93.11.1976. [DOI] [PubMed] [Google Scholar]
- 3.Aromaa A, Raitasalo R, Reunanen A, et al. Depression and cardiovascular diseases. Acta Psychiatr Scand Suppl. 1994;377:77–82. doi: 10.1111/j.1600-0447.1994.tb05807.x. [DOI] [PubMed] [Google Scholar]
- 4.Todaro JF, Shen BJ, Niaura R, Spiro A, 3rd, Ward KD. Effect of negative emotions on frequency of coronary heart disease (The Normative Aging Study) Am J Cardiol. 2003;92:901–906. doi: 10.1016/s0002-9149(03)00967-6. [DOI] [PubMed] [Google Scholar]
- 5.Carney RM, Rich MW, Freedland KE, et al. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med. 1988;50:627–633. doi: 10.1097/00006842-198811000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Frasure-Smith N, Lesperance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91:999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- 7.Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA. 1993;270:1819–1825. [PubMed] [Google Scholar]
- 8.Barefoot JC, Helms MJ, Mark DB, et al. Depression and long-term mortality risk in patients with coronary artery disease. Am J Cardiol. 1996;78:613–617. doi: 10.1016/s0002-9149(96)00380-3. [DOI] [PubMed] [Google Scholar]
- 9.Lavie CJ, Milani RV, Cassidy MM, Gilliland YE. Effects of cardiac rehabilitation and exercise training programs in women with depression. Am J Cardiol. 1999;83:1480–1483. A1487. doi: 10.1016/s0002-9149(99)00127-7. [DOI] [PubMed] [Google Scholar]
- 10.Milani RV, Lavie CJ. Prevalence and effects of cardiac rehabilitation on depression in the elderly with coronary heart disease. Am J Cardiol. 1998;81:1233–1236. doi: 10.1016/s0002-9149(98)00121-0. [DOI] [PubMed] [Google Scholar]
- 11.Milani RV, Lavie CJ, Cassidy MM. Effects of cardiac rehabilitation and exercise training programs on depression in patients after major coronary events. Am Heart J. 1996;132:726–732. doi: 10.1016/s0002-8703(96)90304-x. [DOI] [PubMed] [Google Scholar]
- 12.Lichtman JH, Bigger JT, Jr, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association. Circulation. 2008;118:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 13.Ades PA, Savage PD, Tischler MD, Poehlman ET, Dee J, Niggel J. Determinants of disability in older coronary patients. Am Heart J. 2002;143:151–156. doi: 10.1067/mhj.2002.119379. [DOI] [PubMed] [Google Scholar]
- 14.Bock BC, Carmona-Barros RE, Esler JL, Tilkemeier PL. Program participation and physical activity maintenance after cardiac rehabilitation. Behav Modif. 2003;27:37–53. doi: 10.1177/0145445502238692. [DOI] [PubMed] [Google Scholar]
- 15.Hellman EA. Use of the stages of change in exercise adherence model among older adults with a cardiac diagnosis. J Cardiopulm Rehabil. 1997;17:145–155. doi: 10.1097/00008483-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Pinto BM, Goldstein MG, Papandonatos GD, et al. Maintenance of exercise after Phase II cardiac rehabilitation. Am J Prev Med. 2011;41:274–283. doi: 10.1016/j.amepre.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wenger NK, Forelicher RN, Smith LK. Cardiac Rehabilitation as Secondary Prevention (AHCPR Publication No. 96–0673) Rockville, MD: U.S. Department of Health and Human Services; 1995. Clinical Practice Guidelines for Clinicians: No. 17. [Google Scholar]
- 18.Goldstein MG, Pinto BM, Marcus BH, et al. Physician-based physical activity counseling for middle-aged and older adults: a randomized trial. Ann Behav Med. 1999;21:40–47. doi: 10.1007/BF02895032. [DOI] [PubMed] [Google Scholar]
- 19.Pinto BM, Goldstein MG, Ashba J, Sciamanna CN, Jette A. Randomized controlled trial of physical activity counseling for older primary care patients. Am J Prev Med. 2005;29:247–255. doi: 10.1016/j.amepre.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 21.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 22.Marcus BH, Simkin LR. The stages of exercise behavior. J Sports Med Phys Fitness. 1993;33:83–88. [PubMed] [Google Scholar]
- 23.Rollnick S, Mason P, Butler C. Health Behavior Change: A Guide for Practitioners. Edinburgh: Churchill Livingstone; 2000. [Google Scholar]
- 24.Winningham M. Developing the Symptom Activity 27: an instrument to evaluate perception of symptom effects on activity. Oncol Nurs Forum. 1993;20:330. [Google Scholar]
- 25.Hare DL, Davis CR. Cardiac Depression Scale: validation of a new depression scale for cardiac patients. J Psychosom Res. 1996;40:379–386. doi: 10.1016/0022-3999(95)00612-5. [DOI] [PubMed] [Google Scholar]
- 26.Shi WY, Stewart AG, Hare DL. Major depression in cardiac patients is accurately assessed using the cardiac depression scale. Psychother Psychosom. 2010;79:391–392. doi: 10.1159/000320897. [DOI] [PubMed] [Google Scholar]
- 27.Lim LL, Valenti LA, Knapp JC, et al. A self-administered quality-of-life questionnaire after acute myocardial infarction. J Clin Epidemiol. 1993;46:1249–1256. doi: 10.1016/0895-4356(93)90089-j. [DOI] [PubMed] [Google Scholar]
- 28.Oldridge N, Guyatt G, Jones N, et al. Effects on quality of life with comprehensive rehabilitation after acute myocardial infarction. Am J Cardiol. 1991;67:1084–1089. doi: 10.1016/0002-9149(91)90870-q. [DOI] [PubMed] [Google Scholar]
- 29.Valenti L, Lim L, Heller RF, Knapp J. An improved questionnaire for assessing quality of life after acute myocardial infarction. Qual Life Res. 1996;5:151–161. doi: 10.1007/BF00435980. [DOI] [PubMed] [Google Scholar]
- 30.Dixon T, Lim LL, Oldridge NB. The MacNew heart disease health-related quality of life instrument: reference data for users. Qual Life Res. 2002;11:173–183. doi: 10.1023/a:1015005109731. [DOI] [PubMed] [Google Scholar]
- 31.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 32.Failde I, Ramos I. Validity and reliability of the SF-36 Health Survey Questionnaire in patients with coronary artery disease. J Clin Epidemiol. 2000;53:359–365. doi: 10.1016/s0895-4356(99)00175-4. [DOI] [PubMed] [Google Scholar]
- 33.Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med. 2000;160:1818–1823. doi: 10.1001/archinte.160.12.1818. [DOI] [PubMed] [Google Scholar]
- 34.U.S. Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Promotion. U.S. Government Printing Office; 1996. [Google Scholar]
- 35.United States Department of Health and Human Services. [Accessed August 25, 2009];2008 physical activity guidelines for Americans. Available at: www.health.gov/paguidelines.
- 36.Scholz U, Knoll N, Sniehotta FF, Schwarzer R. Physical activity and depressive symptoms in cardiac rehabilitation: long-term effects of a self-management intervention. Soc Sci Med. 2006;62:3109–3120. doi: 10.1016/j.socscimed.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 37.Butler L, Furber S, Phongsavan P, Mark A, Bauman A. Effects of a pedometer-based intervention on physical activity levels after cardiac rehabilitation: a randomized controlled trial. J Cardiopulm Rehabil Prev. 2009;29:105–114. doi: 10.1097/HCR.0b013e31819a01ff. [DOI] [PubMed] [Google Scholar]
- 38.Arrigo I, Brunner-LaRocca H, Lefkovits M, Pfisterer M, Hoffmann A. Comparative outcome one year after formal cardiac rehabilitation: the effects of a randomized intervention to improve exercise adherence. Eur J Cardiovasc Prev Rehabil. 2008;15:306–311. doi: 10.1097/HJR.0b013e3282f40e01. [DOI] [PubMed] [Google Scholar]
- 39.Hughes AR, Mutrie N, Macintyre PD. Effect of an exercise consultation on maintenance of physical activity after completion of phase III exercise-based cardiac rehabilitation. Eur J Cardiovasc Prev Rehabil. 2007;14:114–121. doi: 10.1097/HJR.0b013e3280116485. [DOI] [PubMed] [Google Scholar]


