Abstract
The delivery of mechanical signals to the skeleton using vibration is being considered as a non-drug treatment of osteoporosis. Delivered over a range of magnitudes and frequencies, vibration has been shown to be both anabolic and anti-catabolic to the musculoskeletal tissues, yet caution must be emphasized as these mechanical signals, particularly chronic exposure to higher intensities, is a known pathogen to many physiological systems. In contrast, accumulating preclinical and clinical evidence indicates that low intensity vibration (LIV) improves bone quality through regulating the activity of cells responsible for bone remodeling, as well as biasing the differentiation fate of their mesenchymal and hematopoietic stem cell progenitors. In vitro studies provide insights into the biologic mechanisms of LIV, and indicate that cells respond to these low magnitude signals through a distinct mechanism driven not by matrix strain but acceleration. These cell, animal and human studies may represent the foundation of a safe, non-drug means to protect and improve the musculoskeletal system of the elderly, injured and infirm.
Keywords: Low Intensity Vibration, Mesenchymal Stem Cells, Hematopoietic Stem Cells, Mechanosensitivity, Nucleus Motion, Fluid Shear, Biomechanics, Sarcopenia, Osteopenia
INTRODUCTION
Treatment Options for Osteoporosis
Treatments for osteoporosis, a skeletal disorder characterized by low bone mineral density (−2.5 T-score) [1] and increased fracture risk, have been developed through the constant improvement of our understanding of the signals that control bone formation and resorption [2]. The risk of developing osteoporosis is increased in conditions including aging, hormonal deficiency (e.g., postmenopausal women and hypogondal men), chronic inflammation (e.g., rheumatoid arthritis, obesity and Crohns disease) and lack of mechanical challenge to the skeleton (e.g., immobility, chronic bed rest, extended spaceflight, disabling conditions such as cerebral palsy) [3–6]. The bone loss of osteoporosis reflects the disrupted balance of formation and resorption activities of remodeling, where catabolic removal of bone tissue driven by osteoclasts exceeds the anabolic capacity of osteoblasts to replace it. To protect and/or recover bone quantity and quality in osteoporotic patients, pharmacological agents have been developed which stimulate the anabolic activity of osteoblasts (e.g., intermittent parathyroid hormone therapy [7], sclerostin inhibitor [8]), or which suppress the resorptive actions of osteoclasts (e.g., bisphosphonate [9], selective estrogen receptor modulator (SERMs) [10]). Building on the recognized benefits of exercise on protecting and reinforcing the musculoskeletal system, the potential of whole body vibration (WBV) to serve as a surrogate for exercise is being investigated as a non-drug intervention for osteoporosis, as established in cell, animal and clinical studies.
Bone’s Sensitivity to Mechanical Signals
Typical daily activities, both mild and strenuous, deliver dynamic mechanical challenges to the skeleton. Regardless of animal species, the peak strain (i.e., deformation per unit length) experienced by load bearing bones during extreme activities ranges from 2000 to 3500 microstrain (µε) [11, 12], suggesting that bone cells adapt bone mass and morphology towards a certain specific range of mechanical signals. In addition to the very rare peak strains achieved during intense actions, high fidelity measurements of strain over long periods of time, made in dog, sheep and turkey [13] revealed that bone strain follows a power:law relationship (1/f), and indicate that low magnitude, high frequency strains (<10 µε arising between 20–50Hz, or cycles per second), such as those induced by regular muscle contraction to maintain balance during stance - although extremely low – were essentially omnipresent in the bone’s mechanical history, and could represent a predominant source of regulatory information to mechanically controlled bone remodeling. Importantly, for mechanical signals to be “relevant” in terms of bone formation, they must be dynamic (time varying); static mechanical signals, no matter the magnitude, are essentially ignored by the cells responsible for bone formation [14].
Whole body vibration (WBV) represents the means of delivering mechanical challenges to the weight-bearing skeleton without requiring locomotion. At one level perceived as a possible surrogate for exercise, WBV delivered via oscillatory platforms are being explored to improve bone quality in numerous preclinical and clinical conditions. WBV is most commonly administered to a subject standing on a vibrating plate that generates mechanical signals via vertical, horizontal, and/or pivotal accelerations [15]. The effects of various vibration protocols, as defined by their duration (i.e., exposure time), frequency (i.e., cycles per second, or Hz) and intensity (i.e., acceleration in g, where 1g = Earth’s gravitational force = 9.8ms−2), have been tested in athletes [16–19], bed-ridden healthy adult males [20], young females with low BMD [21], cerebral palsy children [22, 23], postmenopausal women [24–26] and Crohn disease patients [27]. It must be pointed out that many WBV devices and published work reports displacement of the device rather than intensity, but intensity – the principal component of vibration – is a complex product of frequency and displacement, and ‘simply’ reporting the degree of movement of the plate is insufficient to determine the actual intensity of the device. With this in mind, it must be noted that the safety of vibration protocols must be considered before application in clinical conditions, particularly when their use is considered for the elderly, injured or infirm.
Risks of WBV as a Treatment for Any Clinical Condition
As with any intervention, before WBV can be accepted as a reasonable means of reducing the risk of fractures due to osteoporosis, the risks of acute and/or chronic exposure to vibration must be considered relative to the potential benefits of treatment. Despite the growing number of studies which indicate that vibration delivered over a range of durations, frequencies and intensities can be anabolic and anti-catabolic to musculoskeletal system, caution must also be emphasized as vibration is a known pathogen to many physiologic systems, and at higher intensities may cause the very fracture it is intended to prevent. Thus, before WBV therapy might be considered as a treatment strategy for osteoporosis, it is essential to relate the mechanical parameters of WBV to the extensive ranges of studies which delineate the potential dangers of vibration [28].
A long history of the study of vibration in the workplace has shown that these mechanical signals can be extremely destructive to a host of tissues and systems. For example, vibrations have been shown to induce pathogenic responses such as low back pain in truck drivers [29] and circulatory disorders (e.g., Raynaud’s syndrome) in construction site workers who operate machinery [30]. The conditions extend to blurred vision, tinnitus, headaches, and joint pain. It is also critical to emphasize that vibrations delivered near the natural frequency of human body segments (i.e., less than 20Hz) may amplify their potential risk due to the resonance-induced amplification of the vibration intensity. Considering the hosts of pathologies that are associated with vibration, the International Safety Organization (ISO) has established a rigid threshold for human tolerance of vibration (ISO-2631), which provides strict duration guidelines for human exposure as a function of acceleration frequency and intensity, and certainly hospital review boards should consider the frequency and intensity parameters of WBV devices while evaluating the risk and safety of any WBV study. Indeed, while high intensity signals of 10g and greater – which far exceed ISO-2631 thresholds for even one minute of exposure per day, have been adopted as an unique exercise challenge for professional athletes [16], several studies emphasize that these high intensity signals should be avoided among the osteoporotic patients who are already prone to fracture [31]. Also, individuals who are pregnant, who have retinal detachment, fresh surgery wounds and recent implantation such as joint implants (e.g., hip and knee implants), pacemaker, cochlear implants should avoid vibration therapy.
Considering the Safety of Low Intensity Vibration as an Osteoporosis Intervention
In some contrast to high magnitude (>>1g) WBV, low magnitude (less than <<1g) vibration therapy, which generated less than 5µε on the periosteal surface of bone and are several orders of magnitude less than the strain level created by strenuous activities [12, 32], remains well within the limits of ISO guidelines. According to ISO-2631, exposure to low intensity vibration (LIV) induced at 0.3g at 30Hz would be considered safe for between four to eight hours each day, reducing concerns for LIV in terms of its potential as a treatment for osteoporosis. Furthermore, in a randomized, placebo-controlled, double-blinded 6-month study in elderly women, these subjects retained very high compliance (>90% adherence), with high satisfaction and no adverse experiences for the daily use of a 10-min vibrating platform [33].
VIBRATION THERAPY FOR OSTEOPOROSIS
Anabolic and Anti-Catabolic Effects of LIV on Normal and Pathologic Animal Models
A range of animal studies have been performed to explore the preclinical efficacy of LIV as an anabolic or anti-catabolic intervention for the musculoskeletal system. To gain better insights of the working mechanisms of LIV during normal and pathological conditions, animal models including turkey [34, 35], sheep [36–38], rat [32, 39, 40] and mouse [41–44] have been used, including systemic challenges such as aging, ovariectomy (OVX) [32, 39], disuse induced by hindlimb unloading [45, 46], and diet-induced obesity [47, 48].
One of the earliest reports of bone tissue response to LIV was a 1-year study of adult sheep subjected to low magnitude (0.3g) and high frequency (30Hz) vibrations for 20 minutes per day for 5 days a week. As compared to age-matched controls, the group of elderly (8y) sheep in the LIV groups showed that these extremely small strains (5µε) stimulated a significant increase of trabecular bone density (+34%) in the distal femur [49]. To establish the effects of LIV on the quality of the bone, finite element analysis of the trabecular bone revealed that LIV significantly increased the apparent tissue stiffness of trabecular bone in the three orthogonal directions and resulted in a more uniform stress and strain distribution in the off-axis loading direction under a given load [50]. These data indicated that trabecular bone adapted to LIV in a way that improved both the quality and quantity of bone relative to age matched controls.
While healthy animal models demonstrated the anabolic effects of LIV under normal conditions, various rodent models have been adopted to test whether LIV holds potential of mitigating the bone loss which arises in parallel with a range of pathological conditions. For example, a rat model of hindlimb unloading (HU) has been used to study the ability of LIV to protect bone from the catabolism which arises with immobility of bed-ridden patients or microgravity during spaceflight. Over four weeks, the removal of the normal functional loading environment by HU caused significant bone loss in female Sprague Dawley rats (6–8 months of age), a loss no different than that measured in HU rats allowed to simply walk around for ten minutes per day. In some contrast, in HU rats allowed to walk on a LIV device for ten minutes per day, these vibrations (0.25g, 90Hz, 10min/d) normalized the bone formation rate (BFR) of HU rats back to the age-matched control level [46].
Ovariectiomized (OVX) rodents, recognized as an established model for the study of osteoporosis in postmenopausal women [51], showed that LIV can exert an osteogenic effect in a pathological condition by modulating the activity of bone-forming osteoblasts. Histomorphometric analysis revealed that 4 weeks of LIV (0.3g, 90Hz, 10min/d) induced an increase in the BFR along with the increases in bone volume fraction (BV/TV) and trabecular thickness in OVX Sprague Dawley rats (6–8 months of age) [32]. Revealing the potential of LIV in improving fracture healing in osteoporotic bone, a 8-week study showed that, while OVX rats (9 months of age) exhibited inferior fracture healing than their sham-operated control, LIV (0.3g, 35Hz, 20min/d) augmented fracture healing compared to their osteoporotic control by enhancing callus formation, remodeling, and mineralization [40]. The ability to augment the healing of fractures was confirmed in a larger animal model, where the healing of a 3mm osteotomy in a sheep tibia was shown to markedly increase when subject to LIV delivered by an external fixator [52]. Using murine models, the anti-resorptive effect of LIV was demonstrated in a 3-week study in which LIV (0.3g, 45Hz, 15min/d) induced the reduction of osteoclastic activity along with the increase in BFR on female BALB/cByJ mice [42].
LIV Biases the Fate of the Progenitor of Bone-forming Osteoblast
To examine the mechanism of action of mechanical regulation of bone formation and resorption, many investigators have focused on how strain influences the proliferation and differentiation of osteoblasts and osteoclasts [53]. This emphasis has arisen from the presumption that mechanical influences on bone mass and morphology target the resident cell populations, including osteocytes. It is critical to remember, however, that the bone marrow which neighbors bone tissue, is the reservoir of not only bone cell progenitors, but also the stem cells which build the immune system, mesenchymal stem cell (MSCs) and hematopoietic stem cells (HSCs), respectively. Sharing the bone marrow environment, these cells are known to interact with each other and influence the bone remodeling activities. Importantly, recent animal studies indicated that in addition to the targeting of the terminally differentiated osteoblast and/or osteoclast, that LIV influences a range of cell types in the bone marrow niche.
Examining the potential influence of LIV on bone marrow MSCs, extremely low magnitude mechanical signals (0.2g, 90Hz, 15min/d) were applied to male C57BL/6J mice (7 weeks of age) during 3-week HU [54]. This study showed that the application of LIV during disuse preserved the marrow environment as demonstrated by the 30% greater osteogenic marrow stem cell population in LIV-treated HU mice as compared to age-matched control HU mice, where the progenitors appeared to already be committing to adipogenesis and their differentiation into adipocytes (i.e., fat cells). Further, after allowing for 3 weeks of re-ambulation, mice subject to LIV for the first 3 weeks showed an even greater (51%) marrow osteoprogenitor population along with 30% greater BV/TV relative to their control, indicating that these mechanical signals may have enhanced the recovery of bone morphology by retaining the osteogenic potential of bone marrow cells during disuse.
Interestingly, given that MSCs are the common progenitor of osteoblasts as well as adipocytes, another series of studies which focused on diet-induced obesity in mice demonstrated that while LIV promotes bone formation through the biasing of MSC towards osteoblastogenesis, these signals also appear to prevent obesity by reducing the number of MSC that commit to adipogenesis [48]. Following 12w of LIV, there was approximately 30% less subcutaneous and visceral adipose burden in these mechanically stimulated mice as compared to control. As reflected by flow cytometry and mRNA analyses of Runt-related transcription factor 2 (RUNX2) and peroxisome proliferator-activated receptor (PPARα) for osteogenesis and adipogenesis respectively, 6 weeks of LIV (0.2g, 90Hz, 15mins/d) increased the bone marrow MSC population (37%) and biased their differentiation towards osteoblastic and away from adipogenic lineage in male C57BL/6J mice. These data suggest that, by using mechanical signals to drive the MSC progenitor pool residing in the bone marrow towards the formation of bone and other higher order connective tissues, LIV may simultaneously suppress obesity and its sequelae by the inhibition of the formation of adipose tissue.
At the transcriptional level, the effects of LIV on gene expression within the whole bone marrow showed that, in a 4-week study comparing adult female BALB/cByJ mice subjected to either HU alone or HU and LIV, the application of LIV (0.3g, 45Hz, 10min/d) caused upregulation of genes involved in bone remodeling processes including metalloproteinase-2 (MMP-2), inducible nitric oxide synthase (iNOS) and receptor activator of the nuclear factor kappa-B ligand (RANKL) [55]. Notably, this study highlighted the temporal response of the coupling bone remodeling activities towards mechanical stimulation.
LIV Biases the Fate of the Progenitor of Bone-Resorbing Osteoclast
HSCs are closely related to MSCs, with MSCs essential to their niche and long-term growth. While previous studies focused on the effects of LIV on bone marrow MSCs and hence the bone formation side of the bone remodeling activities, a recent study showed that LIV may also influence the bone resorption side of this process, also achieved at the level of influencing stem cell fate [47].
Through a dichotomous early differentiation pathway, HSCs can give rise to both bone-resorbing osteoclasts via the myeloid lineage, as well as to the formation of immune cells such as B-cells and T-cells via the lymphoid lineage. To explore the effects of LIV on the fate of HSC differentiation, a mouse model of diet-induced obesity provided a means to study the dynamics between the HSC commitment towards either of these two lineages. As it is well known that obesity increases the risk of immune dysfunction, with a reduction of immune cell proliferation and function [56, 57], it implicates a potential impact of obesity on the HSCs differentiation less towards immune cells and more towards osteoclasts. In a 7-month study with male C57BL/6J mice, diet-induced obesity accelerated age-related loss of trabecular bone by 61% and reduced immune B-cell number in the marrow and blood by 52% and 36%, respectively, relative to control mice fed a regular diet. LIV (0.2g, 90Hz, 15min/d), applied in the final 4 months of the protocol, served to restore both bone structure and B & T cells to those levels measured in regular diet controls, in part, by reducing osteoclastic activity and biasing HSC differentiation towards the B & T-cell lineage and away from a osteoclastic lineage. In other words, these low magnitude mechanical signals influence bone quantity and quality by biasing the fate of both MSC and HSC progenitors, driving them away from fat (MSC) and osteoclast (HSC) formation, enabling their commitment to the formation of bone and a healthy immune system.
Mechanistic Insight Derived from In Vitro Cell Studies
While animal studies improve the understanding of the effects of vibration in general and LIV in particular on the complex challenges of in vivo systems, in vitro cellular studies permits a closer study of the biologic and physical mechanisms responsible for these outcomes, as well as an investigation of interactions between cells. Such LIV derived cellular responses have already demonstrated the importance of key molecules, such as bone morphogenic protein (Bmp) 2, in bone’s response to mechanical signals [58]. These studies highlight the dynamic interplay between bone cells and may lead to identification of new targets for drug development or a better understanding of the potential synergy between mechanical signals and drug based therapeutics for osteoporosis.
A spectrum of mechanical signals has been identified as central regulator of bone homeostasis [13], providing a dynamic mechanical environment in which within bone cells can sense and adapt [59]. When applied externally, osteoblasts, effector cells that lay down the bone matrix, respond to LIV by increasing early gene expression as well as matrix maturation markers such as osteopontin, alkaline phosphatase (ALP), and mineralization [58]. In osteocytes, terminally differentiated osteoblasts, LIV (0.15g) modulated the expression of receptor activator of RANKL in a frequency dependent manner [60], which in turn inhibited the recruitment of osteoclasts and their bone resorbing activity. These results suggest that LIV, directly or indirectly, has the potential to modulate bone cell metabolism.
As inferred from the animal studies described above, in addition to LIV’s influence on the differentiated bone cell populations, LIV also influences MSC metabolism and morphology [61–63]. Application of LIV directly modulates the proliferation [63], and osteogenic [62] or adipogenic [61] commitment of MSCs. Further, the architecture of the MSC cytoskeleton with its microfilamentous and microtubular network linking adhesion receptors to the cell nucleus play a role in perceiving small deformations and directly informing the nucleus [64, 65]. Application of strain to MSC induces focal adhesion assembly which amplifies force generated signaling [66], an attribute which can be amplified by separating the mechanical challenges by three hours, exploiting the refractory period of the MSC’s sensitivity to LIV signals to enhance its efficacy [61].
And in addition to isolated cell types, studies also highlight the dynamic interactions between a range of cell populations in the bone milieu. For example, in osteoblasts, LIV gives rise to functionally more robust extra cellular matrix which in turn increases the osteogenic potential of MSCs [67].
Unfortunately, the nature of the specific mechanical signals that drive the LIV response are poorly understood. Mainly because the mechanical information provided by vibration is extremely complex and must be represented by multitude of parameters in both time and space. Cells subject to LIV are not only subject to accelerations as a function of frequency and intensity, but also secondary mechanical signals such as vibration induced fluid shear. One strategy to better understand the effect of LIV on cells is to first identify the specific mechanical information established by these mechanical signals and then isolate those aspects of the mechanical signal that drive the cellular response.
Using a parametric approach, we have shown that osteoblasts favor higher frequency signals over lower frequencies [68, 69]. Increasing vibration frequency (10Hz to 100Hz) osteoblasts increase their transcriptional levels of cyclooxygenase-2 (COX-2), a mechanically inducible enzyme which is responsible from PGE2 mediated bone repair and MSC differentiation [70]. Interestingly, within the same acceleration, the increasing frequency is associated with decreasing strain magnitude due to decrease in peak velocity in each cycle [71, 72]. This inverse frequency/strain relationship combined with the osteoblast’s tendency to favor higher frequencies suggests that instead of directly responding to strains, cells respond to LIV in a more fundamental manner. Extrapolating this to the physical environment of the cell itself, and that the LIV response is larger at higher frequencies when the strain at the “outside” of the cell is smaller than that which is analytically projected to occur at the nucleus, supports the hypothesis that the cellular sensing of LIV is regulated from within the cell. These experiments provide support for the proposal that accelerations might drive cellular responses via nuclear motions [69], rather than matrix distortion of the cell. This notion suggests that cells essentially behave like a tunable “tuning fork” based on their internal structure (cytoskeleton) [73] and perhaps, rather than sensing deformations (i.e., “outside-in”), LIV can generate forces within the cell and initiate an “inside-out” cellular response. To a degree, this can be illustrated by the challenge of delivering mechanical information to the yolk of an egg taken by the refrigerator. Rather than risk cracking the shell by squeezing the egg to deliver mechanical challenges to the ‘nucleus,’ it is far more effective to simply shake the egg, efficiently stimulating the yolk with essentially zero distortion of the ‘membrane.’
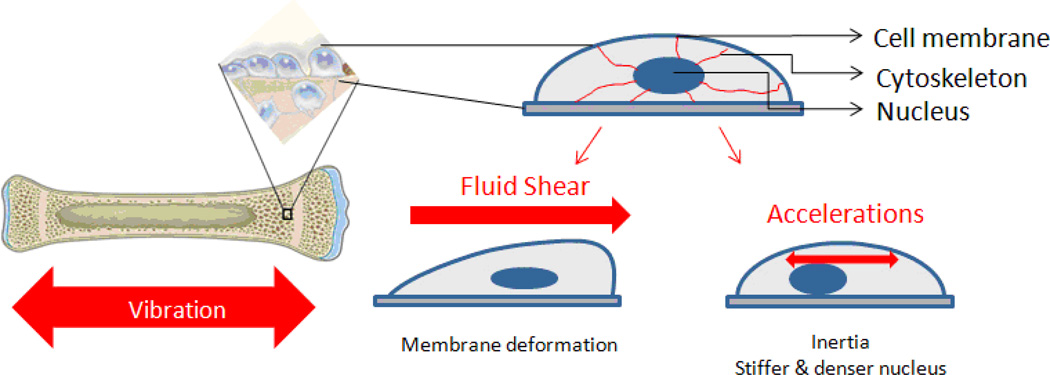
It is important to emphasize that LIV generates a range of mechanical challenges to the cell which go beyond strain and acceleration. During the application of LIV, cell populations within the bone are subject not only to acceleratory motions but also to fluid shear as a result of fluid-cell interactions (Fig. 1). Computational studies reveal that the bone surfaces that are in contact with the bone marrow will be subject to fluid shear stresses (0.5–5Pa) during LIV even as small as 0.1g [59, 74]. Changes in the magnitude of fluid shear during vibrations could modulate the function of resident bone cells and ultimately influence the mechanical adaptation of bone, at mechanical challenges thought far too minimal to have any physiologic relevance. Using computational and experimental methods (Fig. 1), the mechanical challenge of LIV was separated into acceleration and fluid shear. Although the exact role of fluid shear during application of LIV is yet to be identified, these studies showed that groups with lower fluid shear stress of 0.04Pa elicited higher responses when compared to groups with higher fluid shear of 2.63 Pa [72]. Fluid shear of 1Pa typically used in fluid shear experiments to elicit cellular response [75], it has been shown that bone cells can sense as low 0.2–0.5Pa [76, 77]. These results points to the fact that during LIV, cellular response does not require fluid shear, and certainly, that mechanical signals do not need to be large to be influential.
Figure 1.
Trabecular bone and endothelial surfaces, where progenitor populations and osteoblasts reside, will be subjected to accelerations as well as fluid shear during low magnitude high frequency vibrations, each of which is inseparable.
Clinical Evidence of a Role for Low Intensity Vibration for the Treatment of Osteoporosis
Double-blind, placebo controlled clinical studies of LIV indicate that the brief applications of low magnitude mechanical signals can help protect and even enhance the musculoskeletal system. In a 12-month study of young women (15–20 years of age) within the lowest quartile of BMD, application of LIV of 0.3g at 30Hz for 10min per day – reported as Intention to Treat data, increased the trabecular bone in the lumbar vertebrae (2.1%), cortical bone in the femoral midshaft (3.4%), and paraspinous musculature (4.9%), as compared to non-LIV controls [21]. In examining the response in a per protocol analysis, those women who used the device at least two minutes per day showed the musculoskeletal enhancement markedly increase, with trabecular bone of the spine up to 3.9%, cortical bone of the femur up to 2.9%, and paraspinous musculature up to 7.2%, as compared to controls combined with women who were non compliant with the device. In addition to emphasizing the ability of LIV to serve as anabolic signals to the skeleton, this study demonstrates that these low magnitude mechanical signals will also build up muscle in areas key to balance and posture.
In a 12-month study conducted with postmenopausal women (47–64 years of age, 3–8 years past the menopause), LIV at 0.2g at 30Hz for twenty minutes per day showed a relative benefit of 2.13% in femoral neck, 1.5% in spine and 3.5 % in overall BMD when compared to non-vibrated subjects [28]. Important to recognize, however, these signals served to protect bone from being lost in this relatively older group of subjects, as opposed to the increase in bone and muscle mass described in the young females, above.
In addition to the evidence of LIV’s effects on female subjects, effects of LIV on male subjects (25–40 years of age) experiencing bed rest-induced bone loss has demonstrated that, while 60 days of head-down tilt bed rest caused bone loss in multiple skeletal sites, five sessions of LIV (0.3g, 30Hz, 4 mins of LIV combined with a static resistive load (1.5g) + 1min rest period per session, 5 sessions/d) mitigated bone loss at sites including hip and distal tibia [20]. In a parallel study of 90d bedrest that did not investigate the combination of resistive loading, indicated that LIV during bed rest protected key characteristics of balance (e.g., peak sway velocity, peak sway magnitude) relative to sham controls, suggesting that those subject to chronic bed rest are at greater risk of falling due to poor balance, but the introduction of brief, daily LIV signals will help retain stability [78].
In a six-month study which examined the ability of LIV to protect the bone quality in children with cerebral palsy (4–19 years of age), it was shown that the volumetric trabecular BMD (vTBMD) of sham control subjects decreased by 12%, while LIV (0.3g, 90Hz, 10min/d) increased the vTBMD of the treated subjects by 6% [22]. This study is particularly encouraging, as it emphasizes that there is nothing really “wrong” with the cell population responsible for bone adaptation, they simply are not suitably stimulated to help protect and enhance bone morphology.
A subsequent study adopting the same LIV protocol showed that 6 months of LIV could also strengthen the cortical bone structure (cortical bone area and moment of inertia) in children with cerebral palsy (6–12 years of age) [23]. Very recently, LIV (0.3g, 30Hz, 10mins/d) was shown to significantly enhance the skeleton of young Crohns disease patients (8–21 years of age), as demonstrated by an increase in spine BMD (+0.27 Z-score) in LIV-treated patients relative to the placebo group in a 12-month study [27]. A similar LIV response was measured – using high-field MRI coils – in the distal tibia of patients with end-stage renal disease: six months of mechanical signals significantly enhanced the trabecular compartment in this region relative to sham controls [79].
This clinical work provides early insight into the potential for LIV to protect and even enhance the musculoskeletal system of humans, achieved without the use of drugs. Nevertheless, there remains a significant challenge in addressing the mechanism of action of this non-drug approach to the treatment of osteoporosis, a gap that must be addressed by further animal and cell studies. Further, while these studies have shown that both bone and muscle, as well as retention of balance, are benefitted by these low magnitude mechanical signals, none of these studies have yet shown a reduction in falls or fractures. If, on the other hand, LIV does improve bone quantity and quality, as well as muscle mass and function, with outcomes such as improved balance, such an approach addresses key risk factors for fracture that bone-specific drug strategies fail to target [21, 78].
Conclusion
Simplifying the complex nature of in vivo physiologic systems, in vitro cell studies illustrate that both osteoblasts and MSCs are sensitive to LIV, influencing their transcriptional activity, fate selection, attachment to the matrix and even their cytoskeletal architecture. Combined with computational models, these studies suggest that such exceedingly small mechanical signals may exert their regulatory influence not through matrix or cell deformation, but through acceleration-induced movement of the nucleus relative to the membrane.
Certainly, an improved understanding of the biologic mechanisms and clinical outcomes of vibration in the treatment of musculoskeletal disorders will help determine if these ‘surrogate’ mechanical signals can serve in the prevention and/or reversal of osteoporosis and sarcopenia. There is accumulating evidence that exceedingly low magnitude mechanical signals, delivered using low intensity vibration (less than 1 g) may ultimately represent a safe and effective non-drug-based strategy for the treatment of osteoporosis. It is as clear that high intensity vibration (greater than 1g) can cause permanent damage to a range of physiologic systems, and must be avoided, particularly in the elderly, injured or infirm. Clinical studies have demonstrated the osteoprotective and anabolic influences of LIV in various conditions including disuse, the menopause, cerebral palsy and Crohns disease, while animal models have provided unique insights into the biologic and physical mechanisms of LIV and the adaptive response of the musculoskeletal system. LIV has been shown to modulate not only the resident osteoblasts and osteoclasts that are directly involved in the bone remodeling activities, but key progenitors residing in the bone marrow niche, those that ultimately define the fate of the musculoskeletal system.
Notably, LIV modulates the differentiation and lineage commitment of bone marrow MSCs and HSCs, which are progenitors of the bone-forming osteoblasts and bone resorbing osteoclasts respectively, indicating that these mechanical signals may affect the musculoskeletal system in the developmental level. Considering recent evidence that LIV may well help to rescue immune T- and B-cell population and function damaged by obesity, it reinforces the importance of exercise in general, and mechanical signals in particular as a mean to treat other metabolic diseases such as obesity and type 2 diabetes.
Acknowledgments
Funding Sources
This work funded by National Institutes of Health Grants: AR-39278 & EB-14351
Footnotes
Disclosure
ME Chan: none; G Utzer: none; and CT Rubin is a founder of Marodyne Medical.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Organization, W.H. WHO technical report series. Geneva: World Health Organization; 1994. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. [PubMed] [Google Scholar]
- 2.Bilezikian JP, Matsumoto T, Bellido T, et al. Targeting bone remodeling for the treatment of osteoporosis: summary of the proceedings of an ASBMR workshop. J Bone Miner Res. 2009;24(3):373–385. doi: 10.1359/jbmr.090105. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki T, Nonoda Y, Ishii M. Long-term outcomes of children and adolescents who had cerebral palsy with secondary osteoporosis. Curr. Med. Res. Opin. 2012;28(5):737–747. doi: 10.1185/03007995.2011.645562. [DOI] [PubMed] [Google Scholar]
- 4.Smith SM, Wastney ME, O'Brien KO, et al. Bone Markers, Calcium Metabolism, and Calcium Kinetics During Extended-Duration Space Flight on the Mir Space Station. J. Bone Miner. Res. 2005;20(2):208–218. doi: 10.1359/JBMR.041105. [DOI] [PubMed] [Google Scholar]
- 5.Leblanc AD, Schneider VS, Evans HJ, et al. Bone mineral loss and recovery after 17 weeks of bed rest. J. Bone Miner. Res. 1990;5(8):843–850. doi: 10.1002/jbmr.5650050807. [DOI] [PubMed] [Google Scholar]
- 6.Vico L, Collet P, Guignandon A, et al. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. The Lancet. 2000;355(9215):1607–1611. doi: 10.1016/s0140-6736(00)02217-0. [DOI] [PubMed] [Google Scholar]
- 7.Compston JE. Skeletal actions of intermittent parathyroid hormone: effects on bone remodelling and structure. Bone. 2007;40(6):1447–1452. doi: 10.1016/j.bone.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Ominsky MS, Vlasseros F, Jolette J, et al. Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res. 2010;25(5):948–959. doi: 10.1002/jbmr.14. [DOI] [PubMed] [Google Scholar]
- 9.Boonen S, Kay R, Cooper C, et al. Osteoporosis management: a perspective based on bisphosphonate data from randomised clinical trials and observational databases. Int J Clin Pract. 2009;63(12):1792–1804. doi: 10.1111/j.1742-1241.2009.02206.x. [DOI] [PubMed] [Google Scholar]
- 10.Ohta H. Effects of SERMs on bone health. Evidence for the selective estrogen receptor modulator raloxifene: its evolving role in the treatment of osteoporosis. Clin Calcium. 2010;20(3):315–321. [PubMed] [Google Scholar]
- 11.Rubin CT, Lanyon LE. Limb mechanics as a function of speed and gait: a study of functional strains in the radius and tibia of horse and dog. J. Exp. Biol. 1982;101(DEC):187–211. doi: 10.1242/jeb.101.1.187. [DOI] [PubMed] [Google Scholar]
- 12.Rubin CT, Lanyon LE. Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling. J. Theor. Biol. 1984;107(2):321–327. doi: 10.1016/s0022-5193(84)80031-4. [DOI] [PubMed] [Google Scholar]
- 13.Fritton SP, McLeod KJ, Rubin CT. Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J. Biomech. 2000;33(3):317–325. doi: 10.1016/s0021-9290(99)00210-9. [DOI] [PubMed] [Google Scholar]
- 14.Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodelling. J Biomech. 1984;17(12):897–905. doi: 10.1016/0021-9290(84)90003-4. [DOI] [PubMed] [Google Scholar]
- 15.Pel JJ, Bagheri J, van Dam LM, et al. Platform accelerations of three different whole-body vibration devices and the transmission of vertical vibrations to the lower limbs. Med Eng Phys. 2009;31(8):937–944. doi: 10.1016/j.medengphy.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Issurin VB, Tenenbaum G. Acute and residual effects of vibratory stimulation on explosive strength in elite and amateur athletes. J Sports Sci. 1999;17(3):177–182. doi: 10.1080/026404199366073. [DOI] [PubMed] [Google Scholar]
- 17.Issurin VB, Liebermann DG, Tenenbaum G. Effect of vibratory stimulation training on maximal force and flexibility. J Sports Sci. 1994;12(6):561–566. doi: 10.1080/02640419408732206. [DOI] [PubMed] [Google Scholar]
- 18.Bosco C, Iacovelli M, Tsarpela O, et al. Hormonal responses to whole-body vibration in men. Eur J Appl Physiol. 2000;81(6):449–454. doi: 10.1007/s004210050067. [DOI] [PubMed] [Google Scholar]
- 19.Gerodimos V, Zafeiridis A, Karatrantou K, et al. The acute effects of different whole-body vibration amplitudes and frequencies on flexibility and vertical jumping performance. J Sci Med Sport. 2010;13(4):438–443. doi: 10.1016/j.jsams.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Wan Y, Tam KF, et al. Resistive vibration exercise retards bone loss in weightbearing skeletons during 60 days bed rest. Osteoporos Int. 2012;23(8):2169–2178. doi: 10.1007/s00198-011-1839-z. [DOI] [PubMed] [Google Scholar]
- 21.Gilsanz V, Wren TAL, Sanchez M, et al. Low-Level, High-Frequency Mechanical Signals Enhance Musculoskeletal Development of Young Women With Low BMD. J. Bone Miner. Res. 2006;21(9):1464–1474. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 22.Ward K, Alsop C, Caulton J, et al. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19(3):360–369. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 23.Wren TAL, Lee DC, Hara R, et al. Effect of High-frequency, Low-magnitude Vibration on Bone and Muscle in Children With Cerebral Palsy. Journal of Pediatric Orthopaedics. 2010;30(7):732–738. doi: 10.1097/BPO.0b013e3181efbabc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verschueren SMP, Roelants M, Delecluse C, et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: A randomized controlled pilot study. J. Bone Miner. Res. 2004;19(3):352–359. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- 25.Gusi N, Raimundo A, Leal A. Low-frequency vibratory exercise reduces the risk of bone fracture more than walking: a randomized controlled trial. BMC Musculoskelet Disord. 2006;7:92. doi: 10.1186/1471-2474-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwamoto J, Takeda T, Sato Y, Uzawa M. Effect of whole-body vibration exercise on lumbar bone mineral density, bone turnover, and chronic back pain in post-menopausal osteoporotic women treated with alendronate. Aging Clin Exp Res. 2005;17(2):157–163. doi: 10.1007/BF03324589. [DOI] [PubMed] [Google Scholar]
- 27.Leonard M, Shults J, Zemel B, et al. The American Society for Bone and Mineral Research. Minneapolis, Minnesota, USA: 2012. Effects of Low Magnitude Mechanical Signals (LMMS) on Bone Density and Structure in Pediatric Crohn Disease: A Randomized Trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin C, Recker R, Cullen D, et al. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: A clinical trial assessing compliance, efficacy, and safety. J. Bone Miner. Res. 2004;19(3):343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 29.Drerup B, Granitzka M, Assheuer J, Zerlett G. Assessment of disc injury in subjects exposed to long-term whole-body vibration. Eur Spine J. 1999;8(6):458–467. doi: 10.1007/s005860050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffin JJ. Handbook of Human Vibration. London, UK: Academic Press; 2001. [Google Scholar]
- 31.Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341(8837):72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 32.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40(6):1333–1339. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Hannan MT, Cheng DM, Green E, et al. Establishing the compliance in elderly women for use of a low level mechanical stress device in a clinical osteoporosis study. Osteoporos Int. 2004;15(11):918–926. doi: 10.1007/s00198-004-1637-y. [DOI] [PubMed] [Google Scholar]
- 34.Rubin C, Gross T, Qin YX, et al. Differentiation of the bone-tissue remodeling response to axial and torsional loading in the turkey ulna. J Bone Joint Surg Am. 1996;78(10):1523–1533. doi: 10.2106/00004623-199610000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Sun YQ, McLeod KJ, Rubin CT. Mechanically induced periosteal bone formation is paralleled by the upregulation of collagen type one mRNA in osteocytes as measured by in situ reverse transcript-polymerase chain reaction. Calcif Tissue Int. 1995;57(6):456–462. doi: 10.1007/BF00301950. [DOI] [PubMed] [Google Scholar]
- 36.Rubin C, Turner AS, Bain S, et al. Anabolism: Low mechanical signals strengthen long bones. Nature. 2001;412(6847):603–604. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 37.Rubin C, Turner AS, Mallinckrodt C, et al. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone. 2002;30(3):445–452. doi: 10.1016/s8756-3282(01)00689-5. [DOI] [PubMed] [Google Scholar]
- 38.Rubin C, Turner AS, Muller R, et al. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res. 2002;17(2):349–357. doi: 10.1359/jbmr.2002.17.2.349. [DOI] [PubMed] [Google Scholar]
- 39.Oxlund BS, Ortoft G, Andreassen TT, Oxlund H. Low-intensity, high-frequency vibration appears to prevent the decrease in strength of the femur and tibia associated with ovariectomy of adult rats. Bone. 2003;32(1):69–77. doi: 10.1016/s8756-3282(02)00916-x. [DOI] [PubMed] [Google Scholar]
- 40.Shi HF, Cheung WH, Qin L, et al. Low-magnitude high-frequency vibration treatment augments fracture healing in ovariectomy-induced osteoporotic bone. Bone. 2010;46(5):1299–1305. doi: 10.1016/j.bone.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 41.Judex S, Donahue LR, Rubin C. Genetic predisposition to low bone mass is paralleled by an enhanced sensitivity to signals anabolic to the skeleton. FASEB J. 2002;16(10):1280–1282. doi: 10.1096/fj.01-0913fje. [DOI] [PubMed] [Google Scholar]
- 42.Xie L, Jacobson JM, Choi ES, et al. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39(5):1059–1066. doi: 10.1016/j.bone.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Ozcivici E, Garman R, Judex S. High-frequency oscillatory motions enhance the simulated mechanical properties of non-weight bearing trabecular bone. J. Biomech. 2007;40(15):3404–3411. doi: 10.1016/j.jbiomech.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 44.Garman R, Gaudette G, Donahue LR, et al. Low-level Accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J. Orth. Res. 2007;25(6):732–740. doi: 10.1002/jor.20354. [DOI] [PubMed] [Google Scholar]
- 45.Judex S, Garman R, Squire M, et al. Genetically linked site-specificity of disuse osteoporosis. J Bone Miner Res. 2004;19(4):607–613. doi: 10.1359/JBMR.040110. [DOI] [PubMed] [Google Scholar]
- 46.Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J. 2001;15(12):2225–2229. doi: 10.1096/fj.01-0166com. [DOI] [PubMed] [Google Scholar]
- 47. Chan ME, Adler BJ, Green DE, Rubin CT. Bone structure and B-cell populations, crippled by obesity, are partially rescued by brief daily exposure to low-magnitude mechanical signals. The FASEB Journal. 2012 doi: 10.1096/fj.12-209841. While bone remodeling involves the coupling of bone formation and bone resorption activities, most previous studies focused on only the influence of LIV on the bone formation side. This study, however, explored the effect of LIV on the bone resorption aspect by evaluating not only the activity of bone-resorbing osteoclasts but also the differentiation fate of their progenitor HSCs, demonstrating that LIV restores both bone structure and immune B- and T-cells in obese animals through biasing the dichotomous early differentiation pathway of HSCs away from osteoclastogenesis and towards lymphopoiesis.
- 48. Luu YK, Capilla E, Rosen CJ, et al. Mechanical Stimulation of Mesenchymal Stem Cell Proliferation and Differentiation Promotes Osteogenesis While Preventing Dietary-Induced Obesity. J. Bone Miner. Res. 2009;24(1):50–61. doi: 10.1359/JBMR.080817. Looking beyond the effects of LIV on the terminally differentiated bone-forming osteoblasts that are directly involved in bone formation, this study shows that LIV promotes bone formation and prevents obesity by biasing the fate of their progenitor MSCs towards osteoblastogenesis and away from adipogenesis.
- 49.Rubin C, Turner AS, Bain S, et al. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412(6847):603–604. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 50.Judex S, Boyd S, Qin YX, et al. Adaptations of trabecular bone to low magnitude vibrations result in more uniform stress and strain under load. Ann Biomed Eng. 2003;31(1):12–20. doi: 10.1114/1.1535414. [DOI] [PubMed] [Google Scholar]
- 51.Jee WS, Yao W. Overview: animal models of osteopenia and osteoporosis. J Musculoskelet Neuronal Interact. 2001;1(3):193–207. [PubMed] [Google Scholar]
- 52.Goodship AE, Lawes TJ, Rubin CT. Low-magnitude high-frequency mechanical signals accelerate and augment endochondral bone repair: Preliminary evidence of efficacy. J. Orth. Res. 2009;27(7):922–930. doi: 10.1002/jor.20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ehrlich PJ, Lanyon LE. Mechanical strain and bone cell function: A review. Osteoporosis Int. 2002;13(9):688–700. doi: 10.1007/s001980200095. [DOI] [PubMed] [Google Scholar]
- 54. Ozcivici E, Luu YK, Rubin CT, Judex S. Low-level vibrations retain bone marrow's osteogenic potential and augment recovery of trabecular bone during reambulation. PLoS One. 2010;5(6):e11178. doi: 10.1371/journal.pone.0011178. Corroborating the positive result of LIV on the osteoblastogenesis of MSCs, this study further demonstrates that LIV preserves the osteogenic potential of bone marrow progenitor cells even during bone disuse, and that upon re-ambulation, the recovery of bone morphology is enhanced.
- 55.Judex S, Zhong N, Squire ME, et al. Mechanical modulation of molecular signals which regulate anabolic and catabolic activity in bone tissue. J Cell Biochem. 2005;94(5):982–994. doi: 10.1002/jcb.20363. [DOI] [PubMed] [Google Scholar]
- 56.O'Rourke RW, Kay T, Scholz MH, et al. Alterations in T-cell subset frequency in peripheral blood in obesity. Obes Surg. 2005;15(10):1463–1468. doi: 10.1381/096089205774859308. [DOI] [PubMed] [Google Scholar]
- 57.Sato Mito N, Suzui M, Yoshino H, et al. Long term effects of high fat and sucrose diets on obesity and lymphocyte proliferation in mice. J Nutr Health Aging. 2009;13(7):602–606. doi: 10.1007/s12603-009-0170-2. [DOI] [PubMed] [Google Scholar]
- 58.Patel MJ, Chang KH, Sykes MC, et al. Low Magnitude and High Frequency Mechanical Loading Prevents Decreased Bone Formation Responses of 2T3 Preosteoblasts. J. Cell. Biochem. 2009;106(2):306–316. doi: 10.1002/jcb.22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dickerson DA, Sander EA, Nauman EA. Modeling the mechanical consequences of vibratory loading in the vertebral body: microscale effects. Biomech. Model. Mechanobiol. 2008;7(3):191–202. doi: 10.1007/s10237-007-0085-y. [DOI] [PubMed] [Google Scholar]
- 60. Lau E, Al-Dujaili S, Guenther A, et al. Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone. 2010;46(6):1508–1515. doi: 10.1016/j.bone.2010.02.031. This in vitro study illustrated that LIV could influence bone remodeling activities through regulating the interplay between different bone cell types including osteoclast and osteocyte.
- 61.Sen B, Xie Z, Case N, et al. Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. J. Biomech. 2011;44(4):593–599. doi: 10.1016/j.jbiomech.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Y, Guan X, Zhu Z, et al. Osteogenic differentiation of bone marrow-derived mesenchymal stromal cells on bone-derived scaffolds: effect of microvibration and role of ERK1/2 activation. Eur Cell Mater. 2011;22:12–25. doi: 10.22203/ecm.v022a02. [DOI] [PubMed] [Google Scholar]
- 63.Tirkkonen L, Halonen H, Hyttinen J, et al. The effects of vibration loading on adipose stem cell number, viability and differentiation towards bone-forming cells. J. R. Soc. Interface. 2011;8(65):1736–1747. doi: 10.1098/rsif.2011.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. U. S. A. 1997;94(3):849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu SH, Chen JX, Butler JP, Wang N. Prestress mediates force propagation into the nucleus. Biochem. Biophys. Res. Commun. 2005;329(2):423–428. doi: 10.1016/j.bbrc.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 66.Sen B, Guilluy C, Xie Z, et al. Mechanically induced focal adhesion assembly amplifies anti-adipogenic pathways in mesenchymal stem cells. Stem Cells. 2011;29(11):1829–1836. doi: 10.1002/stem.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dumas V, Ducharne B, Perrier A, et al. Extracellular Matrix Produced by Osteoblasts Cultured Under Low-Magnitude, High-Frequency Stimulation is Favourable to Osteogenic Differentiation of Mesenchymal Stem Cells. Calcif. Tissue Int. 2010;87(4):351–364. doi: 10.1007/s00223-010-9394-8. [DOI] [PubMed] [Google Scholar]
- 68.Rosenberg N, Levy M, Francis M. Experimental model for stimulation of cultured human osteoblast-like cells by high frequency vibration. Cytotechnology. 2002;39(3):125–130. doi: 10.1023/A:1023925230651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bacabac RG, Smit TH, Van Loon JJWA, et al. Bone cell responses to high-frequency vibration stress: does the nucleus oscillate within the cytoplasm? FASEB J. 2006;20(7):858–864. doi: 10.1096/fj.05-4966.com. Through the study of bone cell responses to a wide frequency range, this study implicates that bone cells may sense and respond to high frequency LIV in a distinct mechanism driven by acceleration-induced nucleus oscillations.
- 70.Zhang X, Schwarz EM, Young DA, et al. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. The Journal of Clinical Investigation. 2002;109(11):1405–1415. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holguin N, Uzer G, Chiang FP, et al. Brief daily exposure to low-intensity vibration mitigates the degradation of the intervertebral disc in a frequency-specific manner. J Appl Physiol. 2011;111(6):1846–1853. doi: 10.1152/japplphysiol.00846.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Uzer G, Manske S, Chan M, et al. Separating Fluid Shear Stress from Acceleration during Vibrations In Vitro: Identification of Mechanical Signals Modulating the Cellular Response. Cellular and Molecular Bioengineering. 2012;5(3):266–276. doi: 10.1007/s12195-012-0231-1. To improve the understanding of the contribution of different mechanical signals induced by LIV, this study shows that during LIV treatment, mechanical information carried to the cells can be effectively separated to two components: acceleration and the fluid stress which is a function of the frequency, acceleration and fluid viscosity. Further, in an in-vitro the peak shear stress can be effectively separated from the peak acceleration by controlling the frequency, acceleration and/or fluid viscosity of LIV. Further, combining the information of mechanical signals with in vitro cell experiment, this study suggests that, rather than sensing deformation at the cell membrane from “outside-in”, bone cells may respond to LIV from “inside-out” through the acceleration-induced movement of the internal cellular structure.
- 73.Shafrir Y, Forgacs G. Mechanotransduction through the cytoskeleton. Am. J. Physiol.-Cell Physiol. 2002;282(3):C479–C486. doi: 10.1152/ajpcell.00394.2001. [DOI] [PubMed] [Google Scholar]
- 74.Coughlin TR, Niebur GL. Fluid shear stress in trabecular bone marrow due to low-magnitude high-frequency vibration. J. Biomech. 2012;45(13):2222–2229. doi: 10.1016/j.jbiomech.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 75.Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation - the role of RhoA, ROCKII and cytoskeletal dynamics. J. Cell Sci. 2009;122(4):546–553. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.KleinNulend J, Semeins CM, Ajubi NE, et al. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts - Correlation with prostaglandin upregulation. Biochem. Biophys. Res. Commun. 1995;217(2):640–648. doi: 10.1006/bbrc.1995.2822. [DOI] [PubMed] [Google Scholar]
- 77.Klein-Nulend J, van der Plas A, Semeins CM, et al. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 1995;9(5):441–445. doi: 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- 78.Muir J, Judex S, Qin YX, Rubin C. Postural instability caused by extended bed rest is alleviated by brief daily exposure to low magnitude mechanical signals. Gait Posture. 2011;33(3):429–435. doi: 10.1016/j.gaitpost.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rajapakse C. ASBMR. Minneapolis: 2012. Micro-MRI Based Biomechanics Indicates Strength and Stiffness of the Tibia are Improved by Brief Daily Exposure to Low Magnitude Mechanical Signals in Patients with End-Stage Renal Disease. [Google Scholar]