Summary
It remains controversial whether the highly-homologous ribosomal protein (RP) paralogs found in lower eukaryotes have distinct functions and this has not been explored in vertebrates. Here we demonstrate that despite ubiquitous expression, the RP paralogs, Rpl22 and Rpl22-like1 (Rpl22l1) play essential, distinct, and antagonistic roles in hematopoietic development. Knockdown of rpl22 in zebrafish embryos selectively blocks the development of T lineage progenitors after they have seeded the thymus. In contrast, knockdown of the rpl22 paralog, rpl22l1, impairs the emergence of hematopoietic stem cells (HSC) in the aorta-gonad-mesonephros by abrogating Smad1 expression and the consequent induction of essential transcriptional regulator, Runx1. Indeed, despite the ability of both paralogs to bind Smad1 RNA, Rpl22 and Rpl22l1 have opposing effects on Smad1 expression. Accordingly, circumstances that tip the balance of these paralogs in favor of Rpl22 (e.g., Rpl22l1 knockdown or Rpl22 overexpression) result in repression of Smad1 and blockade of HSC emergence.
Keywords: ribosomal protein, paralog, T cell development, hematopoietic stem cell, Rpl22, Rpl22l1
Introduction
Ribosomal proteins (RP) are critical structural components of the ribosome that are usually essential for life (Amsterdam et al., 2004). Even mono-allelic inactivation of some RP has been implicated in human diseases, including RPS14 in 5q- syndrome (Ebert et al., 2008) RPS19 in Diamond–Blackfan Anemia (Draptchinskaia et al., 1999). These and other ribosomopathies share defects in hematopoiesis and in many cases an elevated risk of developing hematologic malignancies (Narla and Ebert, 2010). While the molecular basis for the tissue restriction of the phenotypes of ribosomopathies remains unclear, the common feature of hematopoietic defects reveal critical roles of these RP in blood cell development and transformation.
Mutations in individual RP are also reported to cause distinct and tissue-restricted developmental abnormalities in model organisms (Kondrashov et al., 2011). The distinct phenotypes have been proposed to result from individual RP performing differing functions from within “specialized ribosomes” or, alternatively, through “extraribosomal functions” outside of the ribosome, that influence cell growth, senescence, apoptosis, DNA repair, transcription, mRNA processing, and translation (Sonenberg and Hinnebusch, 2009; Warner and McIntosh, 2009; Xue and Barna, 2012). Among the best-characterized extraribosomal functions is regulation of p53 activation (Deisenroth and Zhang, 2010; Zhang and Lu, 2009). Disruption of ribosome biogenesis activates p53 by inducing nucleolar stress, which releases Rpl5, Rpl11, and Rpl23 from the nucleolus, and enables them to bind MDM2 and block MDM2-mediated p53 degradation (Deisenroth and Zhang, 2010; Pestov et al., 2001; Zhang and Lu, 2009). Another well established, extraribosomal function is the translational regulation of mRNAs bearing GAIT elements by Rpl13a (Mukhopadhyay et al., 2008).
Gaining insight into the critical functions of RP in lower organisms has been complicated by highly homologous RP paralogs (59 of 78 RP in S. cerevisiae have paralogs). Loss-of-function analysis focused on growth defects in yeast revealed that most RP paralogs in yeast were able to cross-complement and were likely to be functionally redundant (Rotenberg et al., 1988). However, more recent analysis indicates that some RP paralogs may have unique functions (Haarer et al., 2007; Steffen et al., 2008). Analysis of the Rpl23aA/Rpl23aB paralogous pair in Arabidopsis revealed that while loss of Rpl23aA severely disrupted development, knockdown of Rpl23aB had no phenotype (Degenhardt and Bonham-Smith, 2008). Moreover, rpl22aΔ, but not rpl22bΔ mutants, exhibit a defect in bud site selection, which is not rescued by high copy number suppression with RPL22B (Komili et al., 2007). While the basis for these seemingly distinct functions remains unclear, these data support the notion that some RP paralogs can perform distinct functions.
The mammalian orthologs of yeast RPL22A and RPL22B are Rpl22 and Rpl22-like1 (Rpl22l1), respectively. Rpl22 is an RNA-binding protein component of the 60S ribosomal subunit that is dispensable for global protein synthesis, but can bind cellular and viral RNA, including telomerase RNA and Epstein-Barr Virus (EBV) EBER-1 RNA (Houmani et al., 2009). We have recently shown that despite ubiquitous expression, germline ablation of Rpl22 causes an exquisitely selectively defect in the development of αβ T lymphocytes (Anderson et al., 2007). The arrest is p53-dependent and results from translational de-repression of p53, rather than through the increased p53 stability that typically accompanies perturbed ribosome biogenesis (Anderson et al., 2007). Because p53 de-repression and developmental arrest are restricted to αβ T cells, we hypothesize that this might reflect compensation by the highly homologous paralog of Rpl22, RpL22l1 (Anderson et al., 2007). However, the function of RpL22l1 and its relationship to that of Rpl22 have not been explored in metazoans.
To address the function of Rpl22l1 in vertebrate development and its relationship to Rpl22, we utilized the zebrafish model (Goessling et al., 2007; Lieschke and Trede, 2009). We determined that the zebrafish orthologs of the mammalian Rpl22 and Rpl22l1 genes were widely expressed, but were enriched in hematopoietic stem and progenitor cells. Loss-of-function analysis revealed that these paralogs perform critical, tissue-restricted, distinct functions in hematopoiesis. Indeed, morpholino (MO) knockdown of Rpl22 caused a p53-dependent arrest in development of T cell progenitors after they have seeded the thymus. Conversely, knockdown of Rpl22l1 disrupted hematopoiesis in a distinct manner, by blocking the generation of Runx1-expressing, definitive hematopoietic stem cells (HSC) in the aorta gonad mesonephros (AGM) region. Importantly, the blockade of HSC emergence resulted from post-transcriptional repression of Smad1, which is controlled through the balance of the antagonistic actions of Rpl22 and Rpl22l1.
Results
Identification of zebrafish orthologs of Rpl22 and Rpl22l1
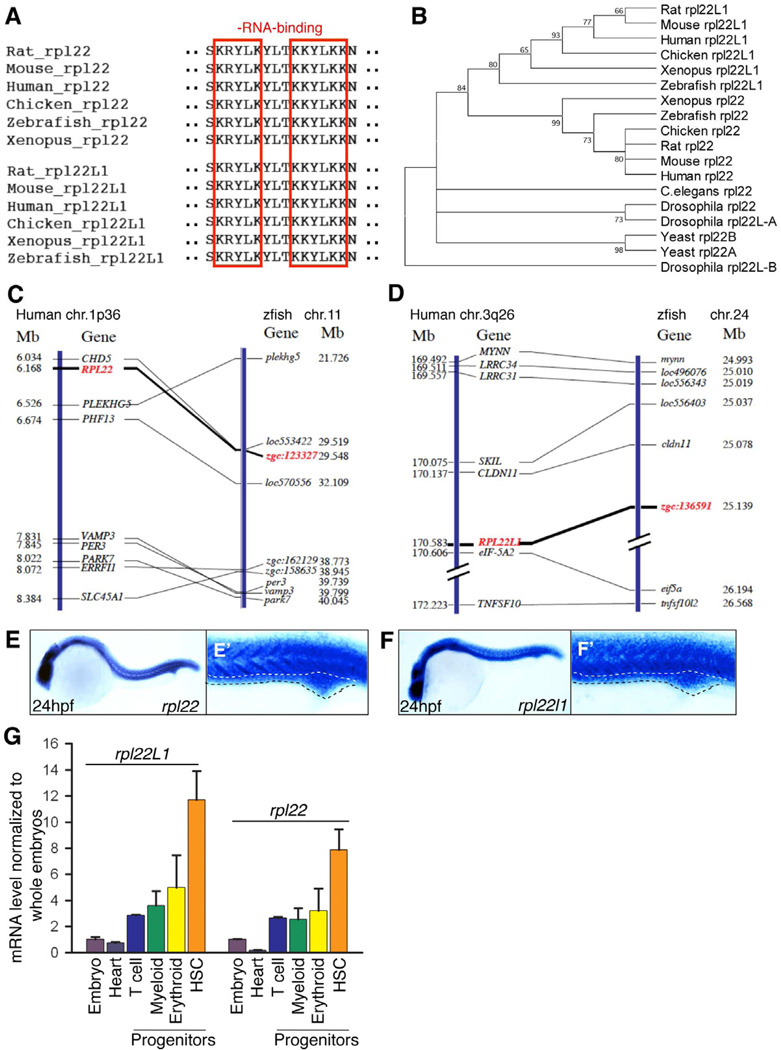
The zebrafish orthologs of human Rpl22 (zgc:123327) and Rpl22l1 (zgc:136591) were identified by homology. Rpl22 and Rpl22l1 are highly conserved from human to zebrafish, are 73% identical (Figure S1A), and share identical RNA-binding motifs (Figure 1A, red boxes), but their sequences diverge at the amino (N) and carboxy (C) termini (Figure S1A). Phylogenetic tree analysis revealed that the zebrafish rpl22 and rpl221 genes are closely related to their mammalian orthologs (Figure 1B). Finally, zgc:123327 and zgc:136591 are syntenic to the human RPL22 (1p36) and RPL22L1 (3q26) loci, respectively (Figure 1C,D).
Figure 1. Bioinformatic and expression analysis of zebrafish rpl22 and rpl22l1.
(A–D) Sequence alignment of RNA-binding domains, phylogenetic analysis, and synteny analysis. (A) Red-boxed area denotes RNA-binding motif. (B) Numbers on the branches of the phylogenetic tree are bootstrap values. (C) The human the human (1p36) and zebrafish rpl22 loci (LG11) are flanked by a common set of genes (CHD5, PLEKGH5, PHF13, VAMP3, PER3, PARK7, ERRF11, and SLC45A1). (D) Human RPL22L1 (3q26) and zebrafish loci (lg24) are flanked by a common set of genes (MYNN, LRRC34, LRRC31, SKIL, CLDN11, EIF-5A2, and TNFSF10). Orthologous RPL22 and RPL22L1 gene symbols are in red and bold.
(E–F’) Zebrafish rpl22 and rpl22l1 WISH analysis on 24 hpf embryos. (E,F) Lateral views with head to the left. (E’, F’) Insets depict the ICM region delimited by dashed lines.
(G) Real time PCR quantification of rpl22 and rpl22l1 mRNA levels in hematopoietic progenitor populations. Tg fish lines were employed to identify the following progenitors: 1) Heart, cmcl2:EGFP at 2dpf; 2) T cell, lck:EGFP at 5dpf; 3) Myeloid, mpo:EGFP at 5dpf; 4) Erythroid, gata1:dsRED2 at 5dpf; and 5) HSC, cd41:EFGP at 3.5dpf. GFP+ cells were isolated by flow cytometry, following which rpl22 and rpl22l1 expression was measured by real time PCR. Results of triplicate measurements were normalized to b-actin and whole embryo homogenates and represented graphically as the mean ±S.D.
The results were combined from at least 3 separate experiments with representative photographs depicting the observed phenotypes. Unless otherwise specified, all images represent lateral views with the head to the left. See also Figure S1.
Whole mount in situ hybridization (WISH) revealed that zebrafish rpl22 and rpl22l1 are expressed both maternally and zygotically throughout early development (Figure S1B,C). Moreover, both were incorporated into ribosomes and polysomes engaged in translation (Figure S1D). rpl22 and rpl22l1 were also ubiquitously expressed at 24 hours post fertilization (hpf) (Figure 1E,F), including in the intermediate cell mass (ICM), a site of primitive hematopoiesis (Figure 1E’,F’; ICM, dotted line). Quantification of rpl22 and rpl22l1 mRNA levels by real time PCR revealed that expression of both rpl22 and rpl22l1 was markedly enriched in HSC, with rpl22l1 expression being somewhat higher than rpl22 (Figure 1G). Their expression in T lymphoid, erythroid, and myeloid progenitors was lower than in HSC, but still enriched relative to that in the embryo as a whole or in heart (Figure 1G). Thus, while Rpl22 and Rpl22l1 are widely expressed in zebrafish, they are enriched in hematopoietic tissues.
Rpl22l1 knockdown arrests T cell development in a p53-dependent manner
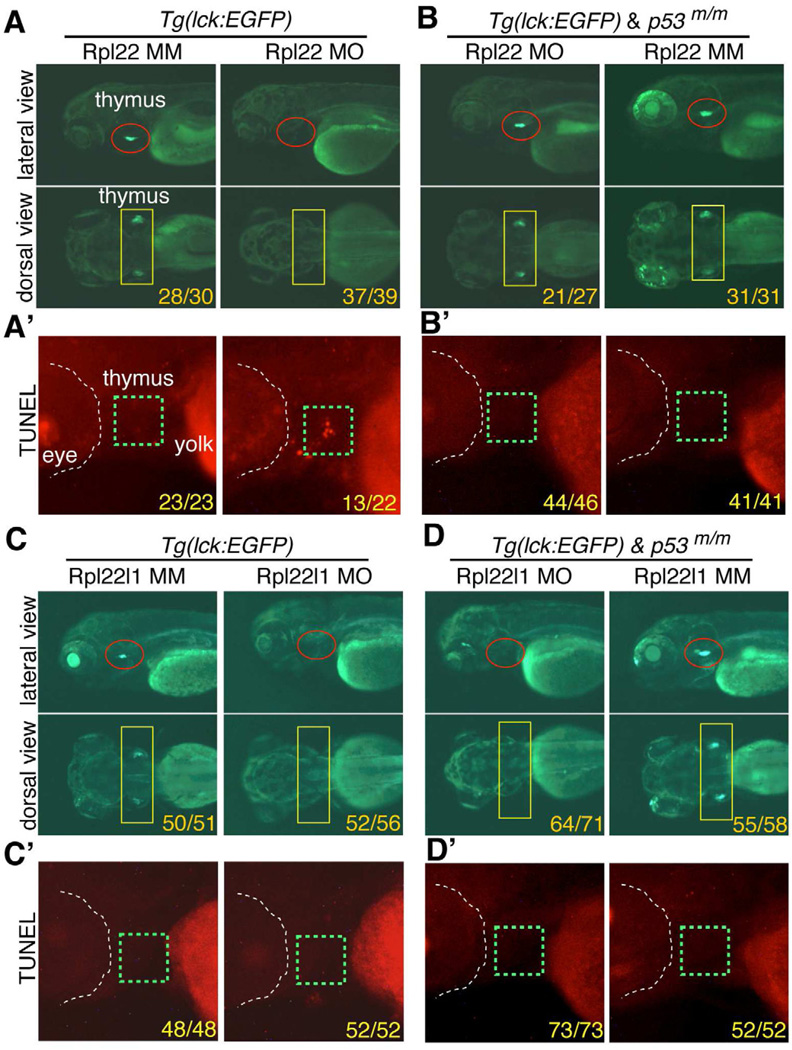
We reported previously that germline ablation of the widely expressed Rpl22 gene in mouse caused a highly-selective, p53-dependent arrest in αβ-lineage T cell development (Anderson et al., 2007). To determine if that was true for loss of Rpl22 and Rpl22l1 in zebrafish, we knocked down their expression and assessed the effect on development of T cells marked by an lck:EGFP transgene (Langenau et al., 2004). To do so, we employed a translational start site anti-sense MO for Rpl22 and a splice-site MO for Rpl22l1, both of which effectively reduced expression from 1dpf to 5dpf (Figure S2A–F). Neither rpl22 mopholino-injected (morphant) nor rpl22l1 morphant embryos exhibited gross developmental defects through 5 dpf (Figure S2G,H). Importantly, the morpholinos employed were specific, as neither reduced expression of the cognate paralog (Figure S2I,J). Surprisingly, both rpl22 and rpl22l1 morphants lacked GFP+ cells in the thymus, indicating a block in T cell development and suggesting that each RP played a distinct, but essential role in T cell development (Figure 2A,C). As was true in Rpl22-deficient mice, the block in T cell development in rpl22 morphants was associated with increased apoptosis as indicated by TUNEL staining, and was rescued by p53-deficiency (Figure 2A’B,B’) (Anderson et al., 2007). While disruption of ribosome biogenesis can trigger nucleolar stress and activate p53-dependent apoptosis, our analysis of Rpl22-deficient mice suggested that Rpl22-deficiency was not activating p53 by impairing ribosome biogenesis, since neither rRNA processing nor the polysome profile was altered (Anderson et al., 2007; Deisenroth and Zhang, 2010)(Data not shown). The absence of an effect on ribosome biogenesis presumably explains why rpl22 morphants do not exhibit the kind of gross morphologic defects associated with knockdown of other RP (Anderson et al., 2007; Chakraborty et al., 2009; Danilova et al., 2008). Like the rpl22 morphants, rpl22l1 morphants also exhibited a block in T cell development (Figure 2C), but it was not associated with increased apoptosis, nor was it rescued by p53-deficiency (Figure 2C–D’). Thus, both Rpl22 and its paralog, Rpl22l1, play essential roles in T cell development; however, they differ in induction of apoptosis and p53-dependence, suggesting that they perform distinct functions.
Figure 2. The arrest of thymocyte development in rpl22, but not rpl22l1, morphants is p53-dependent.
(A–D’) Knockdown of both Rpl22 and Rpl22l1 arrest T cell development, but the arrest in rpl22l1 morpants is p53-independent. Rpl22 and Rpl22l1 were knocked down in p53-sufficient (A,C) or p53-deficient (p53m/m; B,D) Tg(lck:EGFP) embryos by injection of 1ng of Rpl22 MO, Rpl22l1 MO, or MM control, following which T cell development was assessed microscopically by scoring for the loss of GFP-marked T cell progenitors at 5 dpf. (lateral view, red circles; dorsal view, yellow rectangles). Effects on apoptosis in the thymus (green dashed rectangles) were assessed at 3.5 dpf by TUNEL staining (A’,B’,C’,D’).
Images depict phenotypes representative of at least 3 separate experiments, with numbers referring to the fraction of morphants with the depicted phenotypes. See also Figure S2.
The functions of Rpl22 and Rpl22l1 are critical in distinct progenitor pools
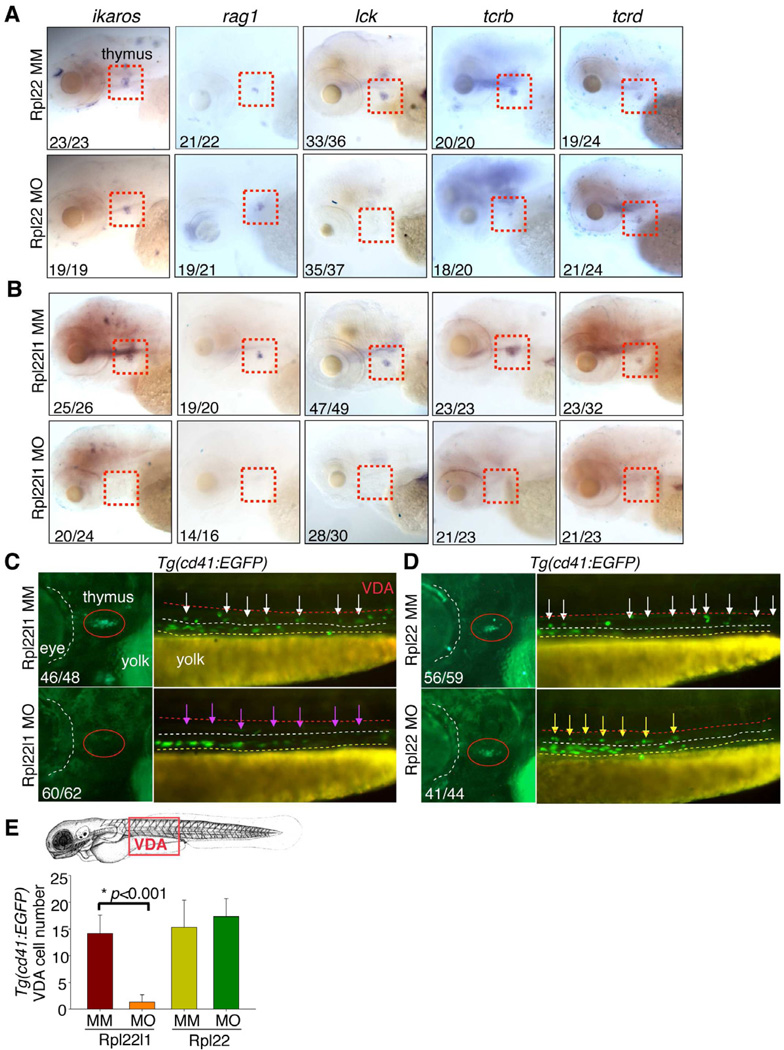
To gain insight into the T cell progenitor populations that might be differentially dependent upon Rpl22 and Rpl22l1, we performed WISH on morphants at 5 dpf using probes identifying important regulators of T cell development (Figure 3). These include: 1) FoxN1, a transcription factor required for generation of thymic epithelium (Su et al., 2003); 2) Ikaros, a DNA-binding protein required for lymphoid development (Georgopoulos et al., 1997); 3) Rag1, a lymphocyte-specific nuclease required for T cell antigen receptor gene rearrangement (TCR); 4) lck, a tyrosine kinase required for T cell development (Langenau et al., 2004); and 5) TCRβ (tcrb) and TCRδ (tcrd) subunits that identify αβ-lineage and γδ-lineage progenitors, respectively (Lee et al., 2010). WISH using probes marking T lineage progenitors (Figure 3A; ikaros, rag1) revealed that knockdown of Rpl22 did not impair seeding of the thymus; however, it did impair progenitor development and expansion as indicated by the reduced staining with probes that mark more mature progeny (Figure 3A; lck, tcrb). The block in T cell development in rpl22 morphants was selective for αβ T cells, since γδ progenitors were unaffected (Figure 3A; tcrd), as we observed previously in Rpl22-deficient mice (Anderson et al., 2007). In contrast, WISH analysis of the rpl22l1 morphants revealed that staining with all of the probes described above was dramatically reduced (Figure 3B). This suggested that unlike Rpl22, whose elimination selectively blocked αβ T cell development after thymus seeding, knockdown of Rpl22l1 blocked development of both αβ and γδ T cells, possibly by preventing thymus seeding. The absence of thymic progenitors in rpl22l1 morphants was not due to a perturbation of the vasculature or thymic stroma, since these structures were unchanged (Figure S3A,B). Thus, these data suggest that the arrest of T cell development in rpl22 and rpl22l1 morphants results from effects on distinct progenitor populations.)
Figure 3. Distinct, lineage-restricted defects in hematopoiesis in rpl22 and rpl22l1 morphants.
(A–B) WISH analysis of thymocyte development with the indicated probes in rpl22 (A) and rpl22l1 morphants (B) at 5 dpf. Thymus, red dashed rectangles.
(C–E) Evaluation of thymus colonization and HSC emergence in rpl22l1 and rpl22 morphants. Tg(cd41:EGFP) embryos were injected with Rpl22l1 MO, Rpl22 MO, or MM control, following which seeding of the thymus and HSC emergence was assessed at 3.5dpf by tracking the presence of GFP+ cells in the thymus (red circles) or VDA (between the red and white dashed lines). Emerging HSC are indicated by arrows. The area between the dashed white and yellow lines is the pronephric duct. The number of CD41-EGFPlow HSCs was quantified in 6 representative embryos per group and presented as mean ± SD. *, p < 0.001.
Images depict phenotypes representative of at least 3 separate experiments, with numbers referring to the fraction of morphants with the depicted phenotypes. See also Figure S3.
Rpl22l1 is required for HSC emergence and thymic seeding
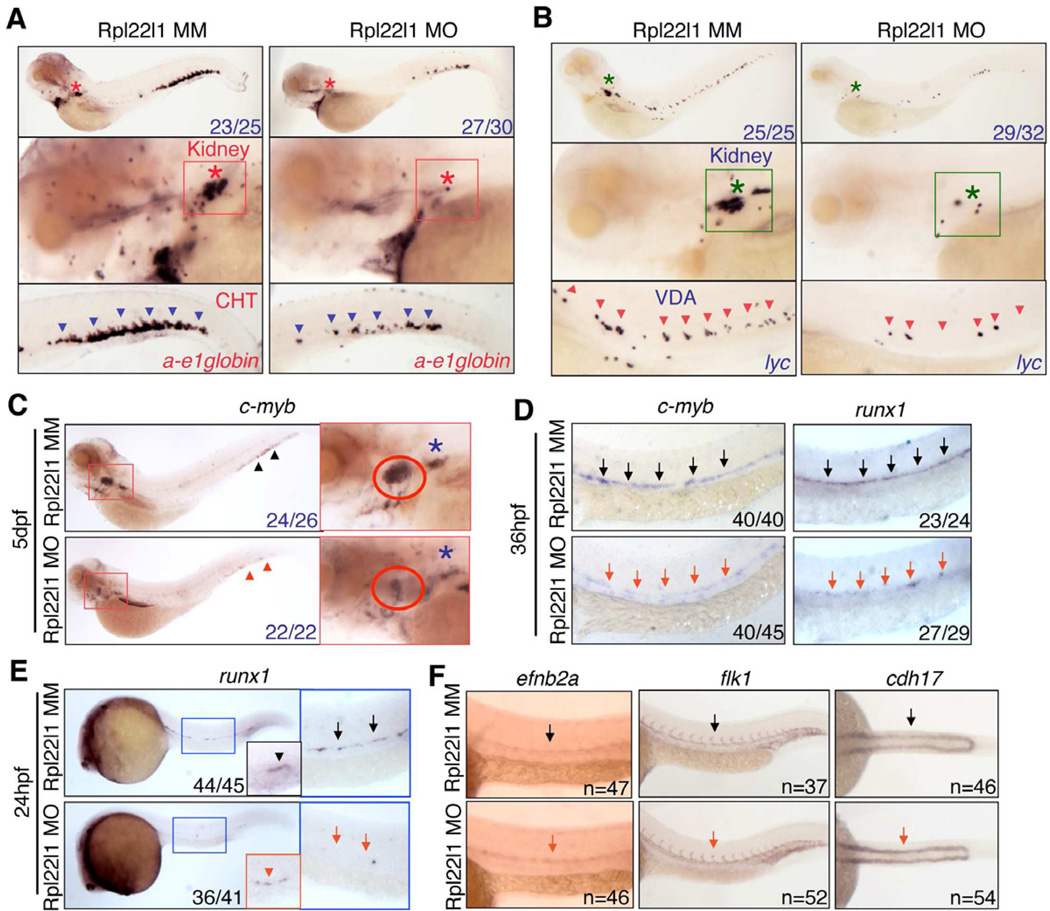
The loss of intrathymic progenitors in rpl22l1 morphants suggested that either their generation or their seeding was disrupted. T cell development at 2 dpf when the thymic rudiment is seeded by CD41+ progenitors, which derive from the CD41+Runx1+ definitive HSC that emerge from the ventral wall of the dorsal aorta (VDA) between 24 and 30 hpf (Boehm and Bleul, 2006; Jin et al., 2007; Lam et al., 2009). To investigate whether loss of Rpl22l1 interferes with thymic seeding, we knocked Rpl22l1 down in Tg(cd41:EGFP) reporter fish and determined if the accumulation of CD41+ progenitors in the thymus was impaired (Bertrand et al., 2008; Kissa et al., 2008). Indeed, loss of Rpl22l1 blocked the appearance of CD41-EGFPlow progenitors in the thymus at 3.5 dpf and 5 dpf (Figures 3C and S3C, red oval). The absence of CD41-EGFPlow progenitors in the thymus resulted from impaired HSC generation in the VDA, since CD41-EGFPlow HSCs were reduced in rpl22l1 morphants relative to control treated embryos (Figure 3C, purple arrows; Figure 3E). The reduction in CD41-EGFP marked cells was restricted to HSC, as non-hematopoietic CD41-EGFP+ cells in the pronephric tubules were not affected by Rpl22l1 knockdown (Figure 3C, between yellow and white dashed lines). Identical results were obtained using a second rpl22l1 morpholino that blocks Rpl22l1 translation (Figure S3E–H). CD41-EGFPHigh thrombocytes in the caudal hematopoietic tissue (CHT) were also markedly reduced by Rpl22l1 knockdown, an expected consequence of impairing the generation of HSC from which they derive (Figure S3C, purple arrowheads). In striking contrast, knockdown of Rpl22 did not cause any alterations in accumulation of CD41-marked progenitor cells in the thymus, VDA or CHT (Figures 3D,E and S3D), confirming that T progenitors in rpl22 morphants were able to seed the thymus, but failed to develop further. The impairment of HSC emergence by Rpl22l1 knockdown, also inhibited the development of definitive progenitors that arise from HSC. These included definitive erythroid progenitors, which were reduced in both CHT and kidney, where hematopoiesis occurs in the adult (Jin et al., 2007) (Figure 4A; ae1-globin), as well as, lyc+ definitive myeloid progenitors, which were reduced in the VDA and kidney at 5 dpf (Figure 4B). Thus, knockdown of Rpl22l1 and Rpl22 blocks T cell development by acting on different progenitor populations. Rpl22 knockdown blocks development of progenitors after thymic seeding. In contrast, Rpl22l1 knockdown blocks T cell development by preventing the generation of definitive HSC and their downstream progeny, including those of the T, erythroid and myeloid lineages.
Figure 4. Rpl22l1 knockdown blocks HSC emergence and definitive hematopoiesis.
(A,B) The effect of Rpl22l1 knockdown on development of definitive erythroid (A; a-e1globin) and myeloid (B; lyc) progenitors was assessed by WISH with the indicated probes at 5dpf in embryos injected with Rpl22l1 MO or MM control. Developing kidney, boxed area and asterisk; CHT, blue arrowheads; VDA, red arrowheads.
(C–E) Effect of Rpl22l1 knockdown on c-myb and Runx expressing progenitors. Embryos were injected with MO as above and evaluated by WISH at 5dpf (C), 36hpf (D), and 24hpf (E) with the indicated probes. Developing kidney (C; red boxed insert, blue asterisk); thymus (C; red boxed inset, red circle); AGM, (D; arrows); AGM (E; blue boxed area, arrows); and erythro-myeloid progenitors (E; EMP, red boxed inset, red arrowhead).
(F) Impact of Rpl22l1 knockdown on the vasculature. Dorsal aorta (efnb2a), vasculature (flk1) and pronephros (cdh17) were assessed by WISH in control and rpl22l1 morphant embryos as above with the indicated probes (arrows).
Images depict phenotypes representative of at least 3 separate experiments, with numbers referring to the fraction of morphants with the depicted phenotypes. See also Figure S4.
Rpl22l1 knockdown blocks production of Runx1+ progenitors
Having demonstrated that Rpl22l1 loss impaired the emergence of definitive HSC, we sought to understand the basis for this block by investigating the expression of transcriptional regulators of HSC specification (Lam et al., 2010). We found that expression of c-Myb, a marker of definitive HSC, was strikingly reduced in rpl22l1 morphants at 5 dpf in the thymus (Figure 4C; red circle), as well as in the kidney (Figure 4C; blue asterisk) (Jin et al., 2007; Murayama et al., 2006). Myb expression was also reduced in rpl22l1 morphants in the AGM at 36 hpf, suggesting that Rpl22l1 is required for the initiation and/or maintenance of definitive hematopoiesis (Figure 4D). The blockade in emergence of CD41+ Myb-expressing progenitors in rpl22l1 morphants closely resembles the phenotype resulting Runx1 knockdown (Kissa and Herbomel, 2010; Kissa et al., 2008; Wilkinson et al., 2009). Because Runx1 is essential for HSC emergence in both mouse and zebrafish (Bertrand et al., 2010; Bertrand et al., 2008; Boisset et al., 2010; Chen et al., 2009; Kissa and Herbomel, 2010; Lam et al., 2010), and because its expression is one of the earliest markers of HSC emergence (Bajoghli et al., 2004; Lam et al., 2010), we asked if Rpl22l1 knockdown interfered with Runx1 expression in the AGM. Indeed, rpl22l1 morphants exhibited reduced expression of Runx1 in the VDA at both 24hpf and 36hpf (Figure 4D; 4E, large inset). The reduced Runx1 expression did not result impaired specification of the dorsal aorta, since WISH analysis using efnb2a, a marker for dorsal aorta, revealed that it was unaffected in rpl22l1 morphants (Figure 4F). rpl22l1 morphants also had normal vasculature (Figure 4F, flk1,) and pronephros (Figure 4F, cdh17). Interestingly, the attenuation of Runx1 expression in rpl22l1 mophants was selective for definitive HSC, as Runx1 expression in primitive erythro-myeloid progenitors in the PBI was not as severely affected (Figure 4E, arrowheads and small insets). Likewise, other markers of primitive hematopoieisis were also unaffected in rpl22l1 morphants at 24hpf (Figure S4A,B), and in some cases were actually increased (Figure S4C–E; e.g., gata1, primitive erythroid progenitors). Thus, while Rpl22l1 is required for the emergence of Runx1+ definitive HSC, it is dispensable for primitive hematopoiesis. In contrast, Rpl22 knockdown caused no perturbations in the aforementioned markers of stem cell emergence, vasculature or primitive hematopoiesis (Figure S4F–H); however, rpl22 morphants did exhibit a slight reduction in definitive erythroid progenitors and a modest increase in definitive myeloid progenitors (Figure S4I,J). Importantly, these observations provide further support that Rpl22 and Rpl22l1 are performing distinct functions in distinct progenitor pools. It should be noted that he blockade in development of Runx1+ HSC in rpl22l1 morphants is not p53-dependent, as there was no increase in apoptosis in the VDA, and the block was not rescued by inactivation of p53 (Figure S5), suggesting that Rpl22l1 loss is not impairing HSC specification in a p53-dependent manner.
Rpl22 and Rpl22l1 are unable to reciprocally rescue the developmental arrest caused by loss of their paralog
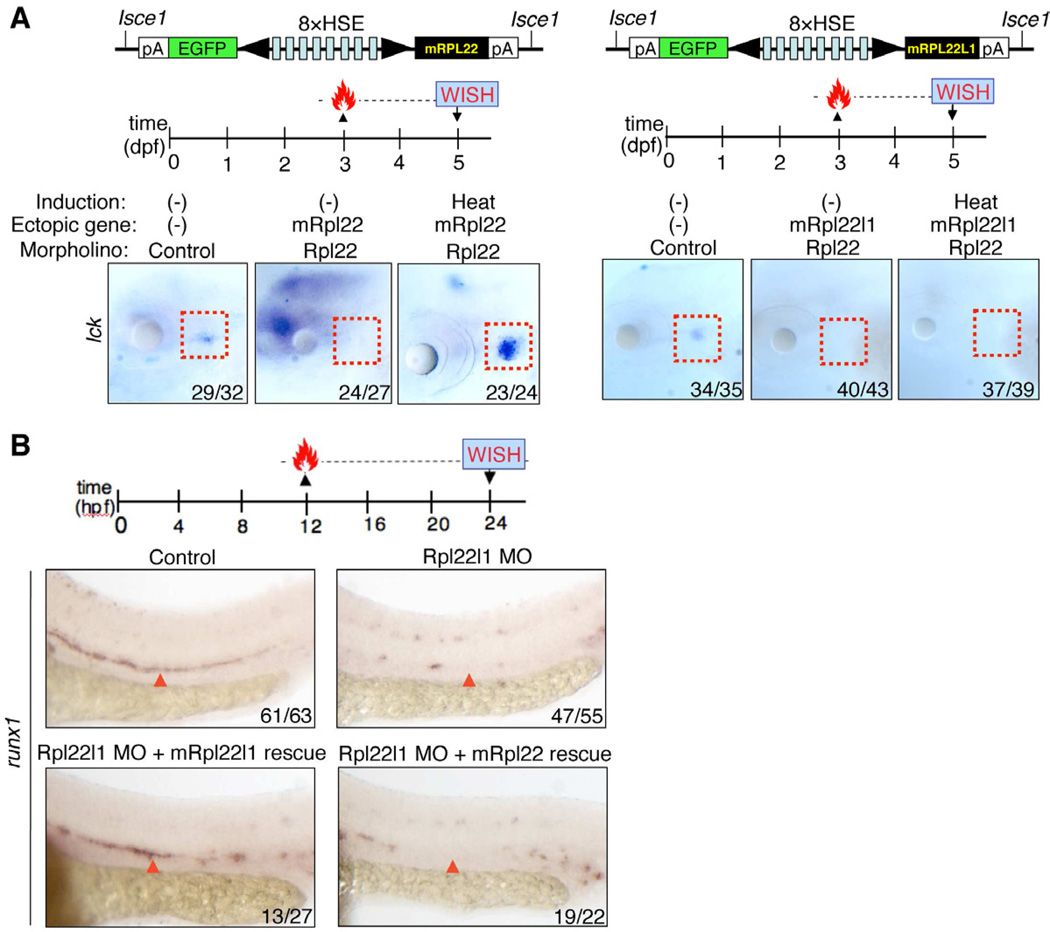
Rpl22 and Rpl22l1 knockdown might cause distinct developmental defects either because they perform distinct functions or because they exhibit different expression patterns. To distinguish these possibilities, we performed reciprocal rescue experiments in rpl22 and rpl22l1 morphants by enforcing the expression of MO-resistant forms of either Rpl22 or Rpl22l1 in a heat-inducible manner (Figures 5A and S5C,D)(Bajoghli et al., 2004). To assess the respective abilities or Rpl22 and Rpl22l1 to circumvent the block in T cell development caused by Rpl22 knockdown, rpl22 morphants were injected with heat-inducible expression constructs encoding morpholino-resistant murine Rpl22 and Rpl22l1, heat induced at 3dpf, and analyzed at 5dpf (Figure 5A, red rectangles). WISH analysis on EGFP+ embryos using an lck probe revealed that enforced expression of Rpl22 rescued thymocyte development in rpl22 morphants, in a heat-shock inducible manner (Figure 5A, left); however, ectopic expression of Rpl22l1 failed to do so (Figure 5A, right). To assess the respective abilities or Rpl22 and Rpl22l1 to circumvent the block in HSC emergence caused by knockdown of Rpl22l1, rpl22l1 morphants were injected with the same heat-inducible expression constructs, heat induced at 12hpf and analyzed by WISH at 24hpf using runx1 to mark emerging HSC (Figure 5B). Enforced expression of Rpl22l1 rescued HSC emergence in rpl22l1 morphants; however, enforced expression of Rpl22 was unable to do so (Figure 5B, red arrowheads). Collectively, the inability of each RP to rescue the developmental arrest caused by loss of its cognate paralog, demonstrates that despite their high degree of sequence identity, Rpl22 and Rpl22l1, perform critical, tissue-restricted roles in hematopoiesis that are distinct from one another, with Rpl22 being essential for development of thymic progenitors after arrival in the thymus, and Rpl22l1 acting much earlier to regulate the emergence of HSC in the AGM.
Figure 5. Rpl22 and Rpl22l1 are unable to cross-compensate defects caused by loss of their paralog.
(A–B) Cross-complementation analysis of the hematopoietic defects caused by knockdown of Rpl22 and Rpl22l1. Embryos injected with Rpl22 MO, Rpl22l1 MO, or MM control were co-injected with the indicated heat-inducible rescue plasmids. Expression of Rpl22/Rpl22l1 was restored by heating for 1h at 37°C at either 3dpf (A) or 12hpf (B) following which effects on T cell development were assessed by WISH at 5dpf with an lck probe (A) and effects on HSC emergence were assessed at 24hpf using a runx1 probe. Thymocytes (A; red dashed rectangles). HSC (B; red arrowheads).
Images depict phenotypes representative of at least 3 separate experiments, with numbers referring to the fraction of morphants with the depicted phenotypes. See also Figures S5.
Both Rpl22 and Rpl22l1 bind smad1 mRNA but have opposing effects on expression
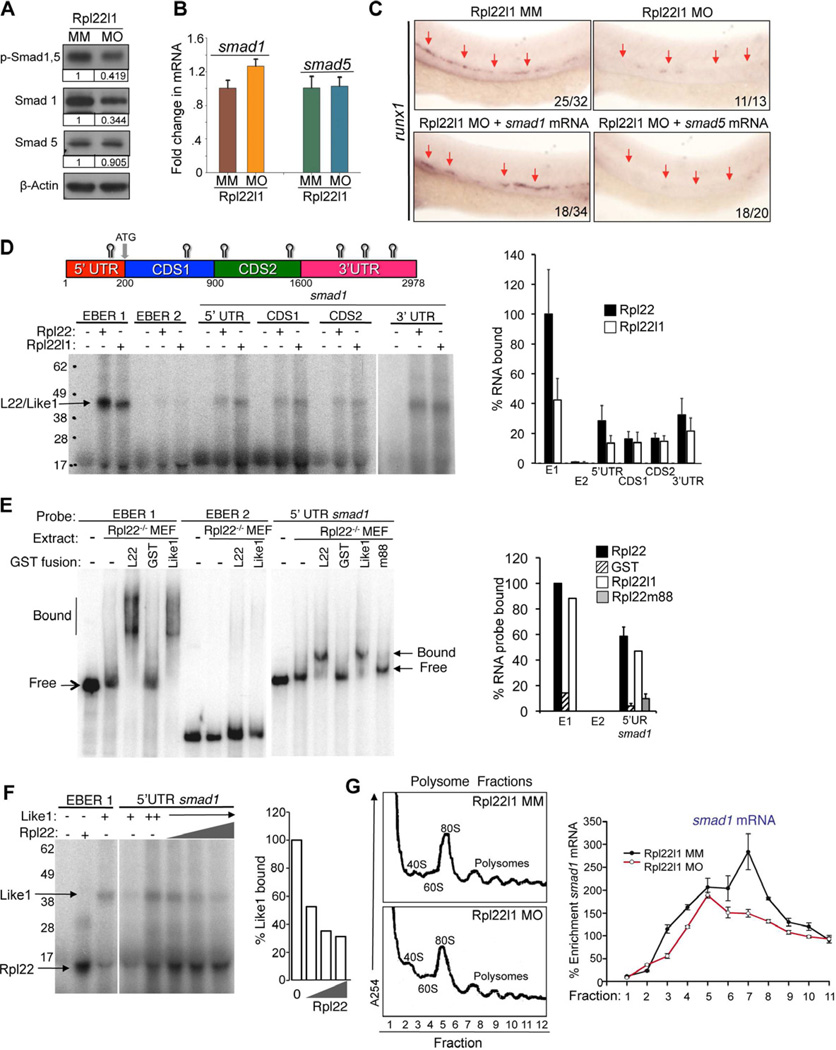
In zebrafish, the BMP4, Notch, hedgehog, and VEGF signaling pathways are all important for HSC emergence from the VDA (Wilkinson et al., 2009). Among these pathways, BMP signaling induces hematopoetic mesoderm through the downstream transcription factors, Smad1, 5 and 8 (McReynolds et al., 2007; Wilkinson et al., 2009), which can transactivate the runx1 promoter (Pimanda et al., 2007). Moreover, knockdown of either BMP4 or Smad1 blocks HSC emergence while sparing primative hematopoiesis, just as we observe in rpl22l1 morphants (Figure S4C–E) (McReynolds et al., 2007; Wilkinson et al., 2009). Accordingly, to determine if alterations in BMP signaling were responsible for the phenotype of rpl22l1 morphants, we asked whether Smad1/5 phosphorylation was altered (Tucker et al., 2008). Indeed, both Smad1/5 phosphorylation and Smad1 expression were decreased (Figure 6A), strongly suggesting that BMP/Smad signaling was impaired. While Smad1 protein levels were decreased to about 1/3 of the control expression level at 24hpf (Figure 6A), Smad1 mRNA levels were unchanged, indicating that the reduction in Smad1 protein was occurring post-transcriptionally (Figure 6B). To determine if the reduction in Smad1 expression was responsible for impairing HSC emergence in rpl22l1 morphants, we attempted to rescue development by ectopic expression of Smad1 and Smad5. Indeed, injection of smad1, but not smad5, mRNA partially rescued runx1 expression in the VDA of rpl22l1 morphants (Figure 6C, arrows). Together, these data indicate that Rpl22l1 promotes the emergence of Runx1+ HSC by supporting BMP signaling, through facilitating the expression of Smad1.
Figure 6. The blockade of HSC emergence in rpl22l1 morphants results from reduced Smad1 expression.
(A–B) Effect of Rpl22l1 knockdown on Smad expression. The expression levels and phosphorylation of Smad1 and 5 were measured by immunoblotting of controls and rpl22l1 morphants at 24hpf. β-actin was used as a loading control. Band intensity relative to control is indicated. (B) smad1 and smad5 mRNA levels were quantified by real time PCR and normalized to b-actin, following which the mean ±S.D. was depicted graphically.
(C) Complementation of the arrest in HSC emergence in rpl22l1 morphants by Smad1. 100pg of the indicated mRNAs were injected in rpl22l1 morphants following which the emergence of HSC was evaluated by WISH using a runx1 probe. Images depict phenotypes representative of at least 3 separate experiments, with numbers referring to the fraction of morphants with the depicted phenotypes.
(D–F) Analysis of binding of smad1 mRNA by Rpl22 and Rpl22l1. (D) Schematic depicting the location in Smad1 mRNA of Rpl22/Rpl22l1 binding sites predicted using M-Fold. The indicated radiolabeled mRNA probes were incubated with GST-hRpl22 (Rpl22) or GSTmRpl22l1 (Rpl22l1), following which binding was assessed by RPA. Binding was quantified by phosphorimagery, normalized to the level of Rpl22 binding to EBER1 (E1, positive control), and the mean ± S.D depicted graphically (right). EBER2 (E2) served as a negative control. (E) Binding of Rpl22, Rpl22l1, and RNA-binding mutant Rpl22 (m88) to the 5’ UTR of smad1 mRNA was assessed by EMSA in which detergent extracts of Rpl22−/− MEF were supplemented with the indicated fusion proteins. Positions of bound and free probes are marked. The fraction of bound probe was quantified by phosphorimagery and represented graphically as above. Results represent the mean ± S.D. of three experiments performed. (F) Rpl22 competes with Rpl22l1 for binding to the 5’UTR of smad1 mRNA. RPA analysis was performed with GST-Rpl22l1 as in D, except that a smaller His-tagged Rpl22 fusion protein was added in increasing amounts to determine if it could displace bound GST-Rpl22l1. The bands corresponding to GST-Rpl22l1 (Like1) and His-Rpl22 (Rpl22) are indicated. The fraction of Rpl22l1 bound to smad1 RNA in the presence of increasing quantities of Rpl22 was quantified and depicted graphically as a fraction of that bound in the absence of Rpl22.
(G) Polysome analysis of rpl22l1 morphants. Detergent extracts of 24hpf control (MM) or rpl22l1 morphant embryos were fractionated by ultracentrifugation on a sucrose gradient. The position of monosomes and polysomes was identified by monitoring O.D.254. smad1 RNA content of each fraction was quantified by real time PCR and normalized to gapdh as well as the level in unfractionated extract, which was defined as 100%. The mean of triplicate samples ± S.D. is depicted graphically on the right.
All results of representative of at least 3 experiments performed.
Rpl22 and Rpl22l1 have identical RNA-binding helices, but play distinct roles in hematopoiesis. Accordingly, we propose that these paralogs might do so by binding to a similar cohort of mRNA targets while having opposing effects on their expression, thereby functioning antagonistically in some contexts. Since Smad1 is critical for HSC emergence and its expression is reduced in rpl22l1 morphants, we asked if the reduction of Smad1 in rpl22l1 morphants results from repression by Rpl22. Previous analysis showed that Rpl22 binds the EBV latency RNA EBER1, and other targets, via a loosely defined stem-loop motif (Dobbelstein and Shenk, 1995). M-fold analysis predicted the presence of several Rpl22/Rpl22l1 binding sites in smad1 mRNA (Figure 6D). To assess whether smad1 mRNA could be bound by both Rpl22 and Rpl22l1, we performed RNAse protection analysis (RPA). RPA revealed that both Rpl22 and Rpl22l1 bound not only EBER1, but also bound to multiple domains of smad1 mRNA (Figure 6D). Rpl22 and Rpl22l1 binding to smad1 mRNA was confirmed by electrophoretic mobility shift assay (EMSA), using extracts from Rpl22−/− mouse embryonic fibroblasts supplemented with recombinant Rpl22 or Rpl22l1 (Figure 6E). Indeed, both Rpl22 and Rpl22l1 were able to induce a quantitative shift in the smad1 5’UTR mRNA and that binding was abrogated by mutating the RNA-binding domain of Rpl22 (m88; Figure 6E). Our model that Rpl22 and Rpl22l1 can bind a similar set of RNAs but have distinct effects on their expression suggests that they may compete for binding to smad1. In agreement, Rpl22l1 binding to the 5’ UTR of smad1 mRNA was partially displaced by increasing amounts of a smaller, His-tagged Rpl22 fusion protein (Figure 6F). Finally, if the post-transcriptional reduction of Smad1 expression in rpl22l1 morphants results from decreased translation, then smad1 mRNA in polysomes should be reduced. Sucrose gradient analysis of polysomal profiles of rpl22l1 morphants revealed that while the polysome trace was not significantly altered, smad1 mRNA was shifted away from the polysome fractions, consistent with a reduction in Smad1 translation (Figure 6G). Together, these observations support a model where both Rpl22 and Like1 bind smad1 mRNA, but have antagonistic effects on Smad1 protein levels, with Rpl22 acting to repress expression and Rpl22l1 acting to oppose that repression.
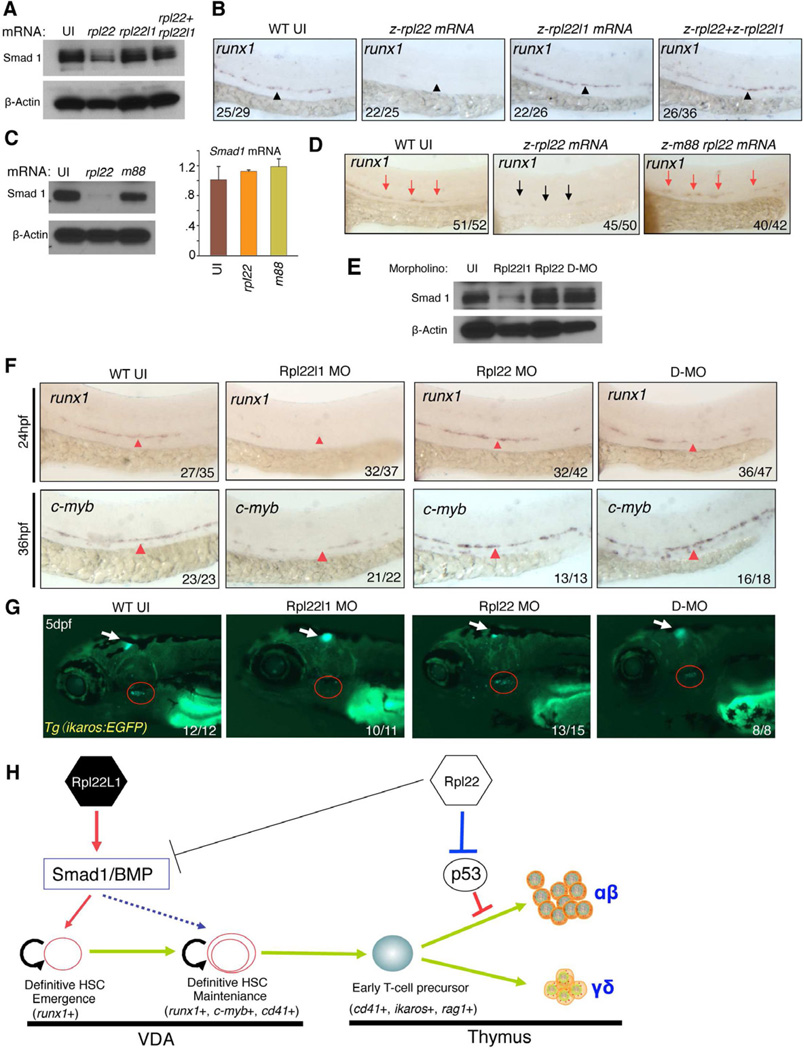
To test this model, we assessed how overexpression of Rpl22 and Rpl22l1 affected Smad1 protein levels. Injection of Rpl22 mRNA into zebrafish embryos repressed endogenous Smad1 protein levels and blocked the generation of Runx1+ HSC in the VDA at 24 hpf (Figure 7A,B). In contrast, injection of Rpl22l1 mRNA did not suppress Smad1 expression or impair HSC emergence. Importantly, however, co-injection of both Rpl22l1 and Rpl22 mRNA, antagonized the ability of Rpl22 to repress Smad1, and rescued the emergence of Runx1+ HSC (Figure 7A,B). The ability of Rpl22 to repress Smad1 was dependent upon its ability to bind RNA (Figure 7C,D; m88). Finally, as shown earlier, knockdown of Rpl22l1, but not Rpl22, reduced Smad1 protein expression and impaired the generation of Runx1+ and c-Myb+ HSC in the VDA, respectively (Figure 7E,F). This was also observed upon knockdown of Rpl22l1 using a second, translational start site morpholino (Figure S6A,B). Our antagonism model posits that Rpl22l1 knockdown reduces Smad1 levels because there is no Rpl22l1 to oppose Smad1 repression by Rpl22. Accordingly, Smad1 expression and HSC emergence should be restored in rpl22l1 morphants by knockdown of Rpl22. Indeed, in double morphants (rpl22l1 and rpl22), we found that Smad1 protein expression was restored to that observed in untreated embryos (Figure 7E; D-MO). Moreover, simultaneous knockdown of both Rpl22 and Rpl22l1 not only restored the development of Runx1+ and c-Myb+ HSC in the VDA at 24 and 36 hpf, respectively, but also restored thymic seeding by Tg(Ikaros-EGFP) marked progenitors at 5 dpf (Figure 7F,G). Importantly, knockdown of Rpl22 and Rpl22l1 in double morphants was as effective as in single morphants (Figure S6C). Taken together, these data demonstrate that Rpl22 and Rpl22l1 regulate HSC emergence through antagonistic effects on Smad1 expression.
Figure 7. Opposing roles of Rpl22 and Rpl22l1 in regulating Smad1 expression and HSC emergence.
(A,B) Effect of Rpl22 and Rpl22l1 overexpression on Smad1 protein levels and HSC emergence. 100pg of RNA encoding rpl22, rpl22l1, or both were injected into embryos and the effect on Smad1 protein levels assessed by western blotting at 24 hpf (A). β-Actin served as a loading control. The effect on HSC emergence in the AGM was assessed by WISH for runx1 at 24 hpf (B).
(C,D) Requirement of Rpl22 RNA binding ability in regulating Smad1 expression and HSC emergence. 75ng of mRNA encoding intact or RNA binding mutant rpl22 (m88) was injected into embryos and the effect on Smad1 protein levels was assessed by blotting at 24hpf, as above. The effect on smad1 mRNA levels was determined using real time PCR and the mean ± S.D was depicted graphically (C). The effect on HSC emergence was assessed by WISH as above (D).
(E–G) Rescue of developmental arrest in rpl22l1 morphants by knockdown of Rpl22. 1ng of MO targeting Rpl22, Rpl22l1, or the two together (D-MO) were injected into zebrafish embryos, following which Smad1 protein expression was assessed at 24hpf as above (E). The effect on HSC emergence was assessed by WISH at 24hpf (runx1) and 36 hpf (c-myb) and the effect on thymus seeding was assessed at 5dpf using the Tg(ikaros:EGFP) to mark thymic progenitors. VDA (F, red arrowheads); Thymus (G, red circle)
(H) Model of Rpl22 and Rpl22l1 function in hematopoiesis. Our analysis indicates that Rpl22l1 promotes runx1 induction and HSC emergence by facilitating Smad1 expression and BMP signaling. The promotion of Smad1 expression by Rpl22l1 is antagonized by Rpl22, perhaps through direct binding of Smad1 mRNA. In contrast, Rpl22 is dispensable for the emergence of HSC and consequent seeding of the thymus by progenitors; however, Rpl22 plays a critical role in supporting the development of thymic progenitors after seeding, by suppressing p53 expression in a lineage-restricted manner.
Images depict phenotypes representative of at least 3 separate experiments, with numbers referring to the fraction of morphants with the depicted phenotypes. See also Figure S6.
Discussion
We report here that despite their high sequence homology, Rpl22 and its paralog Rpl22l1 perform critical, distinct, tissue-restricted roles in hematopoiesis (Figure 7H). Indeed, while both Rpl22 and Rpl22l1 are essential for T lymphocyte development, they support this process through distinct mechanisms, operating in distinct progenitor pools. Rpl22 is dispensable for HSC emergence and thymic seeding, but is critical for the development of αβ T cell progenitors after seeding has occurred (Figures 2–3). In contrast, Rpl22l1 functions to promote the emergence of definitive HSC, which it supports by post-transcriptionally facilitating the expression of Smad1, a critical effector of the BMP4 signals upon which HSC emergence depends (Figures 6–7). The ability of Rpl22l1 to promote Smad1 expression and HSC emergence is directly opposed by Rpl22, which acts to repress Smad1 expression. Consequently, HSC emergence is critically regulated by the balance of Rpl22 and Rpl22l1, such that Rpl22l1 predominance promotes Smad1 expression and HSC emergence, while Rpl22 predominance represses them (Figure 7H).
While previous analysis in yeast and Arabidopsis suggests that some RP paralogs perform distinct functions (Degenhardt and Bonham-Smith, 2008; Komili et al., 2007; Steffen et al., 2008), ours is the first comparative analysis of RP paralog function in vertebrates. Two potential explanations have been advanced for the distinct phenotypes observed upon loss-of-function of RP paralogs. The first suggests that loss of RP paralogs causes distinct phenotypes by impairing ribosome biogenesis or translation to differing degrees, simply because processes differ in their sensitivity to reductions in protein synthesis (Komili et al., 2007; Lucioli et al., 1988). The alternative explanation is that some RP, and their paralogs, actually perform distinct functions either from within “specialized ribosomes” or in an “extraribosomal” capacity (Xue and Barna, 2012). Our findings support the latter interpretation, since Rpl22 and Rpl22l1 knockdown did not adversely affect protein synthesis (Figure S6D–F). More importantly, the rescue of HSC emergence in Rpl22/Rpl22l1 double morphants cannot be explained by differential effects on protein synthesis. It remains unclear whether Rpl22 and Rpl22l1 exert their distinct effects on development from within specialized ribosomes or extraribosomally; however, the recent 80S ribosome crystal structure indicates that Rpl22 is not located near the sites of mRNA entry or peptide exit (Ben-Shem et al., 2011; Klinge et al., 2011). This, coupled with the ability of Rpl22 and Rpl22l1 to bind Smad1 mRNA, suggests that these RP may be have functions outside of the ribosome.
Rpl22-deficiency blocks αβ T cell development in a p53-dependent manner (Figures 2,7H) (Anderson et al., 2007); however, the basis by which p53 induction is restricted to αβ T cell progenitors is unclear at present. Mutations in other RP have previously been linked to p53 induction through impaired ribosome biogenesis, which stabilizes p53 by blocking its degradation by MDM2 (Zhang and Lu, 2009). RP mutations that induce p53 in this manner cause widespread p53-induction, gross disruption of body morphology, and embryonic lethality in zebrafish (Chakraborty et al., 2009; Danilova et al., 2008). This seems an unlikely explanation for the induction of p53 upon Rpl22 knockdown, because p53 induction is restricted to αβ lineage thymocytes and is mediated by increased p53 translation (Anderson et al., 2007). Accordingly, we favor a model where Rpl22 acts extraribosomally to control p53 expression by either directly binding p53 mRNA or indirectly through controlling the expression of another regulator of p53 synthesis.
In contrast to Rpl22, Rpl22l1 plays an essential role in HSC emergence by facilitating Smad1 expression and consequently, the BMP4-mediated induction of Runx1 (Figures 5–7). Runx1 expression in the VDA is also impaired in zebrafish bearing mutations in other RP or factors involved in ribosome biogenesis (rps29, wdr43-like and nop14-like); however, these mutants exhibited gross morphologic abnormalities that were, in the one case examined (rps29), attributable to p53 activation (Figures 4,S3) (Burns et al., 2009; Taylor et al., 2012). The arrest of HSC emergence in rpl22l1 morphants is not accompanied by gross morphological defects and is p53-independent, instead resulting from reduced Smad1 expression, which disrupts BMP/Smad signaling. Consistent with this interpretation, knockdown of Bmp4 and Smad1 causes a similar, selective impairment in development of definitive HSC (McReynolds et al., 2007; Wilkinson et al., 2009). While Smad1 is clearly an important target of the regulation of HSC emergence by Rpl22 and Rpl22l1, other targets may also play a role. Specifically, we have recently shown that Rpl22 loss in murine thymocytes and MEF increased the expression of the stemness factor Lin28B, whose enforced expression can replicate certain aspects of fetal lymphoid development (Rao et al., 2012; Yuan et al., 2012). Notably, there is no evidence that Lin28B plays a role in HSC emergence or is regulated by Rpl22/Rpl22l1 antagonism; however, efforts are nevertheless underway to address these possibilities.
The mechanism by which Rpl22l1 knockdown represses Smad1 protein levels remains incompletely understood; however, our data (Figures 6–7) suggest that Smad1 expression is regulated by the antagonistic balance of Rpl22l1 and Rpl22, such that when Rpl22l1 predominates, Smad1 expression is facilitated, whereas Rpl22 predominance results in Smad1 repression. Both Rpl22 and Rpl22l1 can bind Smad1 mRNA, suggesting that the opposing effects they have on Smad1 expression, result from binding smad1 mRNA (Figures 6 and 7). Accordingly, the simplest mechanistic explanation for control of Smad1 expression by Rpl22 and Rpl22l1, is that both proteins bind smad1 mRNA and induce the assembly of ribonucleoprotein complexes that either decrease or increase, respectively, translation of smad1 mRNA. Several observations support this view. First, the ability of ectopically-expressed Rpl22 to repress Smad1 expression depends on its ability to bind RNA. Second, the representation of smad1 mRNA in polysomes is reduced in rpl22l1 morphants. Finally, Rpl22 is able to compete for Rpl22l1 binding to the 5’ UTR of smad1 mRNA. Nevertheless, neither the sites in smad1 mRNA bound by Rpl22 and Rpl22l1, nor the role of competition for binding in regulating Smad1 expression has been explored. Efforts to address these questions are currently underway.
How might homologous proteins like Rpl22 and Rpl22l1 exert opposing effects on targets? The RNA binding helices of Rpl22 and Rpl22l1 are identical, but these proteins are more divergent at their N- and C-termini (Figures 1, S1). Accordingly, an attractive model is that these paralogs bind to a largely overlapping spectrum of RNA targets; however, they exert distinct effects on those targets, because their N/C-terminal sequences mediate interactions with a distinct set of cofactors. Specifically, Rpl22 might interact with co-factors that negatively regulate target expression, while Rpl22l1 interacts with distinct co-factors that oppose this action. Similar explanations have been advanced to suggest that the paralog of RPS4, RPS4Y2, might perform distinct functions (Lopes et al., 2010). Greater insight into the molecular basis for the distinct functions of Rpl22 and Rpl22l1 will require addressing the importance of the N- and C-termini and identifying their interacting partners.
Genomic analysis suggests that Rpl22 and Rpl22l1 might play important roles not only in normal stem cells but also in cancer. Rpl22 is enriched in mouse neuronal, embryonic, hematopoietic and spermatogonial stem cell populations (Ramalho-Santos et al., 2002). Rpl22l1 is induced in HSC, perhaps by BMP signaling, as has been reported in ovarian germ stem cells in Drosophila, where its expression is inversely correlated with differentiation. (Kai et al., 2005). The antagonistic relationship between Rpl22 and Rpl22l1 may play a critical role in modulating stem cell production, which if unchecked could lead to malignancy. Indeed, it is interesting that the RPL22L1 gene resides in human 3q26 (Figure 1D), which is frequently amplified in a variety of human cancers, such as leukemia, ovarian, lung, head and neck, liver, and breast (Guan et al., 2004; Nanjundan et al., 2007; Shayesteh et al., 1999). Conversely, the locus encoding RPL22 (1p36) is frequently deleted in cancer and contains many tumor suppressors, including RPL22 (Figure 1C) (Bagchi et al., 2007; Rao et al., 2012). It is noteworthy that either RPL22 inactivation or RPL22L1 amplification would tip the balance in favor of Rpl22l1. Since both are associated with malignancy, careful control of antagonistic balance of Rpl22 and Rpl22l1 may be important not only for HSC emergence, but might also safeguard against malignancy.
Our data reveal critical, distinct, and antagonistic roles for the RP paralogs, Rpl22 and Rpl22l1, in regulating HSC emergence. Further studies are needed to gain insight into the molecular basis for their distinct functions and, importantly, whether they are exerted from within the ribosome or represent a growing list of RP that have been co-opted to function extraribosomally.
Methods
Zebrafish maintenance and lines
Zebrafish were housed at 28.5 °C under standard aquaculture conditions. Embryos were staged as described previously (Kimmel et al., 1995). The AB wild-type, mutant, and lineage marking transgenic fish were used for MO, mRNA and plasmid injections. The fish lines employed are listed in the Supplementary Experimental Procedures.
Bioinformatics
Genomic sequences were obtained by searching the UCSC and ENSEMBL databases. Multiple alignments of Rpl22 and Rpl22l1 amino acid sequences were obtained using Clustal W2 (http://www.ebi.ac.uk/Tools/clustalw2/index.html) and with the Neighbor Joining method to construct a phylogenetic tree using MEGA 4.0 software (Tamura et al., 2007). Synteny analysis of the zebrafish orthologs of rpl22 and rpl22l1 was performed as described previously (Liu et al., 2003).
Analysis of zebrafish morphants
Antisense MO oligonucleuotides (Gene Tools, LLC) were injected into 1- or 2-cell stage zebrafish embryos at the indicated concentrations. MO sequences are listed in Supplementary Experimental Procedures.
WISH analysis and apoptosis assay
WISH and whole-mount TUNEL assays were carried out as described (Bennett et al., 2001; Liu et al., 2003). The stained embryos were mounted in 2% methylcellulose and photographed using the Nikon SMZ1500 stereomicroscope equipped with DS-Fi1 digital camera and Nikon Ar imaging software.
Plasmid constructs, RNA production, and transient overexpression studies
RNAs injected into zebrafish embryos were produced by in vitro transcription. Heat shock-inducible expression of mouse Rpl22 and Rpl22l1 was accomplished using the heat-inducible pSGH2 vector and heating to 37°C for 1 h as described (Bajoghli et al., 2004). See Supplementary Experimental Procedures for details.
Protein expression and purification
Recombinant proteins were produced in E. coli as either GST or His-tagged fusions and purified using glutathione- or nickel-sepharose, respectively using standard methods. See Supplementary Experimental Procedures for details.
RNA Binding Assays
To assess RNA binding by Rpl22 and Rpl22l1, the indicated RNA targets were synthesized by in vitro transcription and incubated with recombinant fusion proteins, following which RNA binding was assessed by RPA. RNA binding was also evaluated by EMSA in which detergent extracts from Rpl22−/− MEF were supplemented with in vitro transcribed RNA targets and Rpl22 or Rpl22l1 fusion proteins. Band shifts were evaluated by non-denaturing gel electrophoresis. See Supplementary Experimental Procedures for details.
Real time PCR, protein extraction, and western blotting
Total RNA from pools of embryos was extracted using Trizol according to manufacturer’s suggestions and reverse transcribed, following which RNA targets were quantified using Taqman real-time PCR primer/probes (listed in Supplementary Experimental Procedures). The relative expression values were normalized to gapdh. Protein extraction and western blotting were carried out as described, with antibodies listed in Supplementary Experimental Procedures (Link et al., 2006).
Fluorescence-activated cell sorting (FACS)
Transgenic embryos were dissected with trypsin/EDTA (Life Technologies), following which a single-cell suspension was produced by passage through a 40-µm nylon mesh, prior to flow cytometric isolation using the FACSVantageSE (Becton Dickinson Immunocytometry Systems, San Jose, CA).
Polysome isolation and real time PCR quantification of smad1 mRNA
Detergent extracts of embryos were layered over a 17–50% sucrose gradient, following which polysomes were resolved by centrifugation at 37,000×g for 2h at 4°C. Gradient samples were harvested and RNA content was monitored using a UV flow cell at O.D.254. smad1 mRNA content of the gradient fractions was quantified by Real-time PCR and normalized to β-actin. See Supplementary Experimental Procedures for details.
Statistical Analysis
A Student’s t test was used in the experiments shown in Figure 3. Statistical significance was accepted when p < 0.05.
Supplementary Material
Highlights.
Rpl22 and Rpl22l1 perform distinct, tissue-restricted roles in hematopoiesis
HSC emergence is controlled by the antagonistic balance between Rpl22 and Rpl22l1
Rpl22 and Rpl22l1 bind smad1 mRNA but have opposing effects on Smad1 expression
Rpl22/22l1 control HSC emergence by post-transcriptional Smad1 regulation
Acknowledgments
We thank Drs. Dietmar Kappes, Brian Kennedy, Dmitri Pestov, Jackie Perrigoue, Jason Stadanlick, Randy Strich, Nikolaus Trede, and Alexei Tulin for critical comments on the manuscript, Dr. Thomas Boehm for providing the Tg(ikaros:EGFP) transgenic line, tcrb2, and tcrd probes, Drs. Trede, Roger Patient, Mary Mullins, Matthias Hammerschmidt, Pengfei Xu, Hao Yuan and Jun Zhu, for providing lck, c-myb, bmp4, smad1, smad5 probes, full-length cDNA, and pSGH2 vector, and Sang-Yun Lee, Darius Balciunas, Francis Coffey, Megan Fisher, Xingjun Liu, Qin Li, Mara Robu, Madhusmita Datta, Suzanne McDaniels, George Merkel, Jinhua Wu, and Bruce Young for technical assistance with the project. Finally, we gratefully acknowledge the assistance of the following core facilities of the Fox Chase Cancer Center: Flow Cytometry, DNA Sequencing, Imaging and Laboratory Animal/Zebrafish. This work was supported by NIH grants AI081814, AI073920, NIH core grant P01CA06927, Center grant #P30-DK-50306, and an appropriation from the Commonwealth of Pennsylvania.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson SJ, Lauritsen JP, Hartman MG, Foushee AM, Lefebvre JM, Shinton SA, Gerhardt B, Hardy RR, Oravecz T, Wiest DL. Ablation of ribosomal protein L22 selectively impairs alphabeta T cell development by activation of a p53- dependent checkpoint. Immunity. 2007;26:759–772. doi: 10.1016/j.immuni.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Bagchi A, Papazoglu C, Wu Y, Capurso D, Brodt M, Francis D, Bredel M, Vogel H, Mills AA. CHD5 is a tumor suppressor at human 1p36. Cell. 2007;128:459–475. doi: 10.1016/j.cell.2006.11.052. [DOI] [PubMed] [Google Scholar]
- Bajoghli B, Aghaallaei N, Heimbucher T, Czerny T. An artificial promoter construct for heat-inducible misexpression during fish embryogenesis. Dev Biol. 2004;271:416–430. doi: 10.1016/j.ydbio.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Ben-Shem A, Garreau de Loubresse N, Melnikov S, Jenner L, Yusupova G, Yusupov M. The structure of the eukaryotic ribosome at 3.0 A resolution. Science. 2011;334:1524–1529. doi: 10.1126/science.1212642. [DOI] [PubMed] [Google Scholar]
- Bennett CM, Kanki JP, Rhodes J, Liu TX, Paw BH, Kieran MW, Langenau DM, Delahaye-Brown A, Zon LI, Fleming MD, et al. Myelopoiesis in the zebrafish, Danio rerio. Blood. 2001;98:643–651. doi: 10.1182/blood.v98.3.643. [DOI] [PubMed] [Google Scholar]
- Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand JY, Kim AD, Teng S, Traver D. CD41+ cmyb+ precursors colonize the zebrafish pronephros by a novel migration route to initiate adult hematopoiesis. Development. 2008;135:1853–1862. doi: 10.1242/dev.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T, Bleul CC. Thymus-homing precursors and the thymic microenvironment. Trends Immunol. 2006;27:477–484. doi: 10.1016/j.it.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- Burns CE, Galloway JL, Smith AC, Keefe MD, Cashman TJ, Paik EJ, Mayhall EA, Amsterdam AH, Zon LI. A genetic screen in zebrafish defines a hierarchical network of pathways required for hematopoietic stem cell emergence. Blood. 2009;113:5776–5782. doi: 10.1182/blood-2008-12-193607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A, Uechi T, Higa S, Torihara H, Kenmochi N. Loss of ribosomal protein L11 affects zebrafish embryonic development through a p53-dependent apoptotic response. PLoS ONE. 2009;4:e4152. doi: 10.1371/journal.pone.0004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilova N, Sakamoto KM, Lin S. Ribosomal protein S19 deficiency in zebrafish leads to developmental abnormalities and defective erythropoiesis through activation of p53 protein family. Blood. 2008;112:5228–5237. doi: 10.1182/blood-2008-01-132290. [DOI] [PubMed] [Google Scholar]
- Degenhardt RF, Bonham-Smith PC. Arabidopsis ribosomal proteins RPL23aA and RPL23aB are differentially targeted to the nucleolus and are disparately required for normal development. Plant Physiol. 2008;147:128–142. doi: 10.1104/pp.107.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenroth C, Zhang Y. Ribosome biogenesis surveillance: probing the ribosomal protein-Mdm2-p53 pathway. Oncogene. 2010;29:4253–4260. doi: 10.1038/onc.2010.189. [DOI] [PubMed] [Google Scholar]
- Dobbelstein M, Shenk T. In vitro selection of RNA ligands for the ribosomal L22 protein associated with Epstein-Barr virus-expressed RNA by using randomized and cDNA-derived RNA libraries. J Virol. 1995;69:8027–8034. doi: 10.1128/jvi.69.12.8027-8034.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draptchinskaia N, Gustavsson P, Andersson B, Pettersson M, Willig TN, Dianzani I, Ball S, Tchernia G, Klar J, Matsson H, et al. The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat Genet. 1999;21:169–175. doi: 10.1038/5951. [DOI] [PubMed] [Google Scholar]
- Ebert BL, Pretz J, Bosco J, Chang CY, Tamayo P, Galili N, Raza A, Root DE, Attar E, Ellis SR, et al. Identification of RPS14 as a 5q- syndrome gene by RNA interference screen. Nature. 2008;451:335–339. doi: 10.1038/nature06494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos K, Winandy S, Avitahl N. The role of the Ikaros gene in lymphocyte development and homeostasis. Annu Rev Immunol. 1997;15:155–176. doi: 10.1146/annurev.immunol.15.1.155. [DOI] [PubMed] [Google Scholar]
- Goessling W, North TE, Zon LI. New waves of discovery: modeling cancer in zebrafish. J Clin Oncol. 2007;25:2473–2479. doi: 10.1200/JCO.2006.08.9821. [DOI] [PubMed] [Google Scholar]
- Guan XY, Fung JM, Ma NF, Lau SH, Tai LS, Xie D, Zhang Y, Hu L, Wu QL, Fang Y, et al. Oncogenic role of eIF-5A2 in the development of ovarian cancer. Cancer Res. 2004;64:4197–4200. doi: 10.1158/0008-5472.CAN-03-3747. [DOI] [PubMed] [Google Scholar]
- Haarer B, Viggiano S, Hibbs MA, Troyanskaya OG, Amberg DC. Modeling complex genetic interactions in a simple eukaryotic genome: actin displays a rich spectrum of complex haploinsufficiencies. Genes Dev. 2007;21:148–159. doi: 10.1101/gad.1477507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmani JL, Davis CI, Ruf IK. Growth-promoting properties of Epstein-Barr virus EBER-1 RNA correlate with ribosomal protein L22 binding. J Virol. 2009;83:9844–9853. doi: 10.1128/JVI.01014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Xu J, Wen Z. Migratory path of definitive hematopoietic stem/progenitor cells during zebrafish development. Blood. 2007;109:5208–5214. doi: 10.1182/blood-2007-01-069005. [DOI] [PubMed] [Google Scholar]
- Kai T, Williams D, Spradling AC. The expression profile of purified Drosophila germline stem cells. Dev Biol. 2005;283:486–502. doi: 10.1016/j.ydbio.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- Kissa K, Murayama E, Zapata A, Cortes A, Perret E, Machu C, Herbomel P. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood. 2008;111:1147–1156. doi: 10.1182/blood-2007-07-099499. [DOI] [PubMed] [Google Scholar]
- Klinge S, Voigts-Hoffmann F, Leibundgut M, Arpagaus S, Ban N. Crystal structure of the eukaryotic 60S ribosomal subunit in complex with initiation factor 6. Science. 2011;334:941–948. doi: 10.1126/science.1211204. [DOI] [PubMed] [Google Scholar]
- Komili S, Farny NG, Roth FP, Silver PA. Functional specificity among ribosomal proteins regulates gene expression. Cell. 2007;131:557–571. doi: 10.1016/j.cell.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrashov N, Pusic A, Stumpf CR, Shimizu K, Hsieh AC, Xue S, Ishijima J, Shiroishi T, Barna M. Ribosome-mediated specificity in Hox mRNA translation and vertebrate tissue patterning. Cell. 2011;145:383–397. doi: 10.1016/j.cell.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam EY, Chau JY, Kalev-Zylinska ML, Fountaine TM, Mead RS, Hall CJ, Crosier PS, Crosier KE, Flores MV. Zebrafish runx1 promoter-EGFP transgenics mark discrete sites of definitive blood progenitors. Blood. 2009;113:1241–1249. doi: 10.1182/blood-2008-04-149898. [DOI] [PubMed] [Google Scholar]
- Lam EY, Hall CJ, Crosier PS, Crosier KE, Flores MV. Live imaging of Runx1 expression in the dorsal aorta tracks the emergence of blood progenitors from endothelial cells. Blood. 2010;116:909–914. doi: 10.1182/blood-2010-01-264382. [DOI] [PubMed] [Google Scholar]
- Langenau DM, Ferrando AA, Traver D, Kutok JL, Hezel JP, Kanki JP, Zon LI, Look AT, Trede NS. In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc Natl Acad Sci U S A. 2004;101:7369–7374. doi: 10.1073/pnas.0402248101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Stadanlick J, Kappes DJ, Wiest DL. Towards a molecular understanding of the differential signals regulating alphabeta/gammadelta T lineage choice. Semin Immunol. 2010;22:237–246. doi: 10.1016/j.smim.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke GJ, Trede NS. Fish immunology. Curr Biol. 2009;19:R678–R682. doi: 10.1016/j.cub.2009.06.068. [DOI] [PubMed] [Google Scholar]
- Link V, Shevchenko A, Heisenberg CP. Proteomics of early zebrafish embryos. BMC Dev Biol. 2006;6:1. doi: 10.1186/1471-213X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TX, Howlett NG, Deng M, Langenau DM, Hsu K, Rhodes J, Kanki JP, D'Andrea AD, Look AT. Knockdown of zebrafish Fancd2 causes developmental abnormalities via p53-dependent apoptosis. Dev Cell. 2003;5:903–914. doi: 10.1016/s1534-5807(03)00339-3. [DOI] [PubMed] [Google Scholar]
- Lopes AM, Miguel RN, Sargent CA, Ellis PJ, Amorim A, Affara NA. The human RPS4 paralogue on Yq11.223 encodes a structurally conserved ribosomal protein and is preferentially expressed during spermatogenesis. BMC Mol Biol. 2010;11:33. doi: 10.1186/1471-2199-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucioli A, Presutti C, Ciafre S, Caffarelli E, Fragapane P, Bozzoni I. Gene dosage alteration of L2 ribosomal protein genes in Saccharomyces cerevisiae: effects on ribosome synthesis. Mol Cell Biol. 1988;8:4792–4798. doi: 10.1128/mcb.8.11.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds LJ, Gupta S, Figueroa ME, Mullins MC, Evans T. Smad1 and Smad5 differentially regulate embryonic hematopoiesis. Blood. 2007;110:3881–3890. doi: 10.1182/blood-2007-04-085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay R, Ray PS, Arif A, Brady AK, Kinter M, Fox PL. DAPK-ZIPK- L13a axis constitutes a negative-feedback module regulating inflammatory gene expression. Mol Cell. 2008;32:371–382. doi: 10.1016/j.molcel.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama E, Kissa K, Zapata A, Mordelet E, Briolat V, Lin HF, Handin RI, Herbomel P. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity. 2006;25:963–975. doi: 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Nanjundan M, Nakayama Y, Cheng KW, Lahad J, Liu J, Lu K, Kuo WL, Smith- McCune K, Fishman D, Gray JW, et al. Amplification of MDS1/EVI1 and EVI1, located in the 3q26.2 amplicon, is associated with favorable patient prognosis in ovarian cancer. Cancer Res. 2007;67:3074–3084. doi: 10.1158/0008-5472.CAN-06-2366. [DOI] [PubMed] [Google Scholar]
- Narla A, Ebert BL. Ribosomopathies: human disorders of ribosome dysfunction. Blood. 2010;115:3196–3205. doi: 10.1182/blood-2009-10-178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestov DG, Strezoska Z, Lau LF. Evidence of p53-dependent cross-talk between ribosome biogenesis and the cell cycle: effects of nucleolar protein Bop1 on G(1)/S transition. Mol Cell Biol. 2001;21:4246–4255. doi: 10.1128/MCB.21.13.4246-4255.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimanda JE, Donaldson IJ, de Bruijn MF, Kinston S, Knezevic K, Huckle L, Piltz S, Landry JR, Green AR, Tannahill D, et al. The SCL transcriptional network and BMP signaling pathway interact to regulate RUNX1 activity. Proc Natl Acad Sci U S A. 2007;104:840–845. doi: 10.1073/pnas.0607196104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. "Stemness": transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- Rao S, Lee SY, Gutierrez A, Perrigoue J, Thapa RJ, Tu Z, Jeffers JR, Rhodes M, Anderson S, Oravecz T, et al. Inactivation of ribosomal protein L22 promotes transformation by induction of the stemness factor, Lin28B. Blood. 2012;120:3764–3773. doi: 10.1182/blood-2012-03-415349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg MO, Moritz M, Woolford JL., Jr Depletion of Saccharomyces cerevisiae ribosomal protein L16 causes a decrease in 60S ribosomal subunits and formation of half-mer polyribosomes. Genes Dev. 1988;2:160–172. doi: 10.1101/gad.2.2.160. [DOI] [PubMed] [Google Scholar]
- Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen KK, MacKay VL, Kerr EO, Tsuchiya M, Hu D, Fox LA, Dang N, Johnston ED, Oakes JA, Tchao BN, et al. Yeast life span extension by depletion of 60s ribosomal subunits is mediated by Gcn4. Cell. 2008;133:292–302. doi: 10.1016/j.cell.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su DM, Navarre S, Oh WJ, Condie BG, Manley NR. A domain of Foxn1 required for crosstalk-dependent thymic epithelial cell differentiation. Nat Immunol. 2003;4:1128–1135. doi: 10.1038/ni983. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Humphries JM, White RM, Murphey RD, Burns CE, Zon LI. Hematopoietic defects in rps29 mutant zebrafish depend upon p53 activation. Exp Hematol. 2012;40:228–237 e225. doi: 10.1016/j.exphem.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JA, Mintzer KA, Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell. 2008;14:108–119. doi: 10.1016/j.devcel.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner JR, McIntosh KB. How common are extraribosomal functions of ribosomal proteins? Mol Cell. 2009;34:3–11. doi: 10.1016/j.molcel.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson RN, Pouget C, Gering M, Russell AJ, Davies SG, Kimelman D, Patient R. Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta. Dev Cell. 2009;16:909–916. doi: 10.1016/j.devcel.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S, Barna M. Specialized ribosomes: a new frontier in gene regulation and organismal biology. Nat Rev Mol Cell Biol. 2012;13:355–369. doi: 10.1038/nrm3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335:1195–1200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lu H. Signaling to p53: ribosomal proteins find their way. Cancer Cell. 2009;16:369–377. doi: 10.1016/j.ccr.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.