Abstract
The placenta is a large, highly vascularized hematopoietic tissue that functions during embryonic and foetal development of eutherian mammals. Although recognized as the interface tissue important in the exchange of oxygen, nutrients and waste products between the foetus and mother, the placenta has increasingly become a focus of research concerning the ontogeny of the blood system. Here, we describe recent data showing the intrinsic hematopoietic potential and appearance of hematopoietic cells in the mouse and human placenta, and probe the biological rationale behind its hematopoietic function. As a rest tissue that contains potent hematopoietic stem cells, the human placenta could represent (in addition to umbilical cord blood cells) an accessible supplemental source of cells for therapeutic strategies.
Keywords: human stem cells, placenta, hematopoietic stem cells, NOD-SCID, development
Early placenta development
The placenta is a mysterious tissue that has been known since the earliest times as the “alter ego” or “external soul” of the foetus. Aristotle recognized it as a tissue from which the embryo receives its nourishment, and Leonardo da Vinci and Vesalius depicted it as a disc at the maternal-foetal interface in the uterus 1. It was only in the mid-1500s that it gained its name placenta (from Latin), meaning flat cake. Proposed to be the liver and lungs of the foetus, only in 1734 was it established that the foetal and maternal vasculature of the placenta were not continuous 2. Although this aspect of placental anatomy remained controversial for many years (awaiting improved microscopic techniques and molecular markers), the function of the placenta was accepted to be facilitation of nutrient and waste exchange between the mother and foetus, provision of immunoprotection for the foetus and production of factors and hormones for foetal growth 3.
The development of the placenta has been extensively studied in the mouse conceptus 4. At embryonic day (E) 8.5, the allantois, a mesodermal outgrowth from the posterior end of the embryo, contacts the chorionic epithelium in the extraembryonic part of the conceptus (Figure 1A). This event is called chorio-allantoic fusion. Thereafter, a network of vasculature is generated from the allantois and grows into the chorionic plate. These vessels undergo extensive branching. The endothelial cells lining these vessels are in direct apposition with syncytiotrophoblasts and it is in the small spaces surrounding these structures that the maternal blood bathes the foetal villi. A diagram of the anatomical structure of the mouse placenta is provided in Figure 1B. Instead of the compact, maze-like villi in the labyrinth layer found in the mouse, the villous structures in the human placenta are less branched and the intervillous space is more open. Placenta architecture varies between species, but there are general similarities across evolution that include placental cell types, functions, and gene and protein expression reflecting developmental and regulatory conservation 5, 6.
Figure 1.
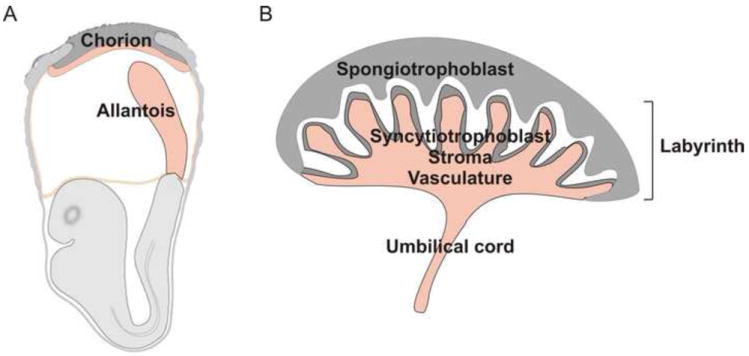
Placenta structure at different times in development (a) Early stages of chorio-allantoic placenta formation. The placenta is formed from the fusion of the allantois with the chorion. The allantois is a mesodermal outgrowth emanating from the posterior primitive streak. It elongates and upon contact with the chorionic mesoderm gives rise to the labyrinth of the placenta. (b) Mouse placenta structure. The placenta consists of the several cell types and layers. The side of the placenta facing the fetus is the labyrinth. It consists of a vascular network contiguous with the umbilical cord. The network of vessels in the labyrinth form branched villous structures that are surrounded by mesenchymal stromal and syncytiotrophoblast cells. The maternal vessels run through the spongiotrophoblast region (facing the mother) to open into the intervillous space (white area) where physiologic exchange between mother and fetus occurs.
The placenta is connected to the embryo through the umbilical cord. The umbilical artery is contiguous with the dorsal aorta, the main artery of the embryo (Figure 2A). Initially the connection is at the caudal aspect of the dorsal aorta, but following vascular remodelling, the connection becomes abdominal 7. Blood circulation throughout the embryo and extraembryonic tissue is established at E8.25 in the mouse (Figure 2B) and beginning between weeks 3 and 4 of gestation in the human conceptus. Following the development and growth of the placenta at these early stages, maternal circulation flows through the intervillous spaces beginning at approximately E10.5 and about the 11th week of gestation in the mouse and human placentas, respectively 8. It is at this time that the placenta becomes functional in its role as the exchange chamber between mother and foetus.
Figure 2.
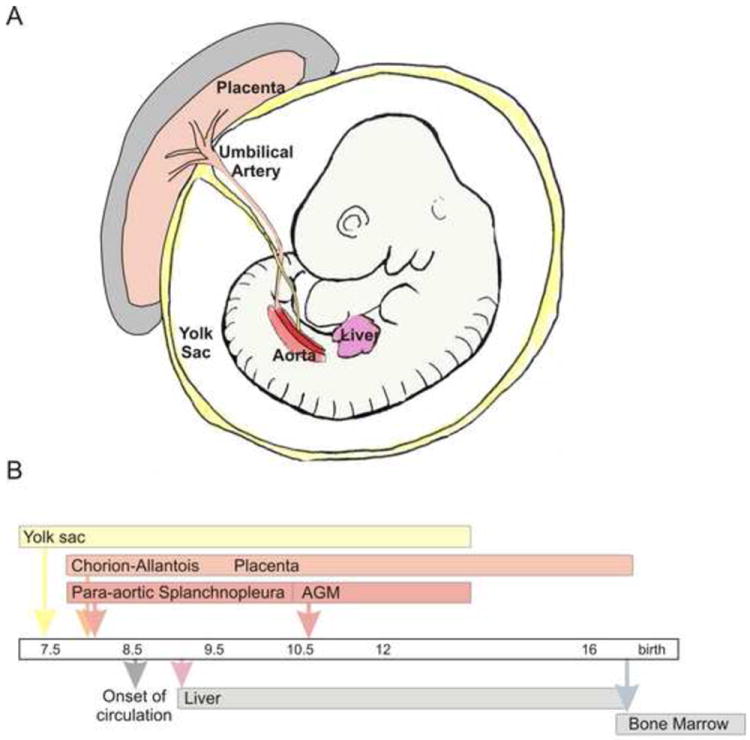
Hematopoiesis in the mouse embryo and placenta. (a) Sites of hematopoiesis in the midgestion (E10.5) mouse conceptus. Extraembryonic hematopoietic territories include the chorio-allantoic placenta and the yolk sac. Hematopoietic territories within the embryo body are the aorta (hematopoietic part of the AGM region) and the liver. The yolk sac, placenta and AGM are sites of de novo hematopoietic cell generation, whereas the liver is colonized by exogenously generated hematopoietic cells. Temporal appearance of hematopoietic cells in different tissues (from E 7.5 until birth).
The placenta and hematopoiesis
As compared to its generally recognized functions, the appreciation of the placenta as a potent hematopoietic site is relatively recent. Hematopoietic activity was initially observed in the mouse placenta in the 1960s and 1970s, but these findings were not immediately pursued (reviewed in 9). Studies in human placental villi suggest that already at day 21 postconception, macrophage-like cells and hemangioblastic cords arise from mesenchymal cells 10. Tissue grafting studies in the avian embryo model by Dieterlen-Lievre reveal that cells derived from the avian allantois contribute to adult haematopoiesis 11. Subsequent studies by this group established that the mouse placenta harbours a wide range of clonogenic hematopoietic progenitors beginning around E9 12. Although this is slightly later than the time such cells appear in the embryo proper or the yolk sac (Figure 2B), the placenta contains the most progenitors of any site up until E12 when the foetal liver surpasses it. The continued presence of hematopoietic progenitors in the mouse placenta throughout gestation demonstrates that the placenta it is a highly potent hematopoietic site.
Hematopoietic stem cells (HSCs) are found in highly vascularized tissues including the mouse placenta (reviewed in 13-15). HSCs are the basis of the adult hematopoietic hierarchy that produces all the blood lineages throughout adult life. Putative HSCs are tested by the stringent transplantation assay in which the donor cells are challenged to provide complete, long-term hematopoietic repopulation of adult irradiated (HSC-depleted) normal recipients. Using allelic or transgene markers to distinguish foetal-derived cells, HSCs are detectable in the mouse placenta at early E11 and HSC numbers increase dramatically up to E12.5 16, 17. Thereafter, HSC numbers in the placenta are superseded by the fetal liver, and after E15.5, very few or no HSCs are found in the placenta 16. Placenta HSCs express many of the same surface marker proteins as adult bone marrow and foetal liver HSCs, including CD34 and c-kit 16. Also, all placental HSCs express Ly6A (Sca-1) GFP (Stem cell antigen-1, green fluorescent protein) 17. Interestingly, Ly6A GFP expressing cells localize within the vasculature of the placental labyrinth and the umbilical vessel, and most of these cells express CD34. Histologic analyses show that the midgestation mouse placenta expresses important hematopoietic transcription factors such as Gata-2, Gata-3 and Runx1 17. Gata-2 is expressed in some endothelial cells and cells surrounding the vessels within the labyrinth, whereas Gata-3 is restricted to a few cells at the maternal-foetal interface. Runx1 is expressed in cells within the vascular lumen and the endothelium as well as cells surrounding the vasculature of the labyrinth 17, 18. The patterns of Gata-2 and Runx1 expression strongly suggest HSCs and progenitors are localized within the labyrinth and near the chorionic plate.
Human placenta hematopoiesis
Throughout development, the human placenta similarly contains a wide variety of hematopoietic cells, as well as mature and immature hematopoietic progenitors and HSCs (Figure 3). Primitive erythroblasts that morphologically resemble those in the yolk sac fill the placental vessels beginning around day 24 19. These cells express glycophorin-A, GATA-2 and c-KIT, but are not positive for CD34 or CD45. As measured by in vitro colonogenic activity, mature and immature hematopoietic progenitors are found as early as week 6 in gestation through week 17, and at term 20-23. These progenitors are multipotent and produce erythroid and myeloid lineage cells, including granulocytes and macrophages. The progenitors are initially in both CD34- and CD34+ fractions, but by week 15, all progenitors are CD34+ 20-22. Leukocytes begin to express CD45 at 12-14 weeks in gestation and at term.
Figure 3.
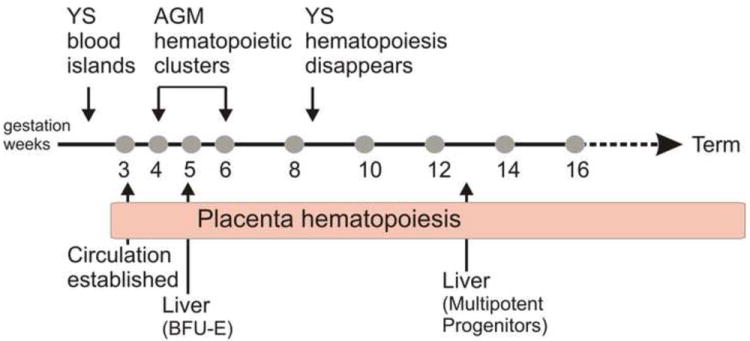
Hematopoiesis in the human conceptus and placenta Temporal appearance of hematopoietic cells in the yolk sac (YS), AGM region, liver and placenta of the human conceptus from gestational weeks in the first and second trimesters through term. BFU-E (burst forming unit-erythroid) represents the earliest erythroid progenitors. Multipotent progenitors can produce erythroid and myeloid lineage cells.
As a hematopoietic territory, the appearance of hematopoietic cells in the early gestational stage human placenta is slightly delayed as compared to the other hematopoietic sites (Figure 3). The human yolk sac begins generating blood at day 16 with the production of primitive erythroid cells, and at day 19, the intra-embryonic splanchnopleura (aorta region) becomes hematopoietic. The emergence of multipotent progenitors, HSCs and clusters of cells closely adherent to the ventral wall of the dorsal aorta, starts at around day 27 in the developing splanchnopleura/aorta-gonad-mesonephros (AGM) region 24. Beginning at day 30 and week 13, the first erythroid progenitors and multilineage progenitors respectively, colonize the liver. Thereafter, the bone marrow becomes hematopoietic 25. Thus, sequential waves of hematopoietic activity in the human conceptus and placenta are similar to those in the mouse conceptus.
Using the NOD-SCID (non-obese diabetic-severe combined immunodeficient) mouse transplantation assay (the surrogate transplantation assay for human HSC identification), potent multilineage, high-level repopulating HSCs have been found in the human placenta 22. Donor human placental cells contribute to high percentages of B lymphocytes and myeloid cells in the bone marrow and spleen of NOD-SCID recipients 10 weeks postinjection. HSCs can be found in the human placenta beginning at week 6 of gestation, throughout trimesters 1 and 2, and at the time of delivery (term). Considering the almost total absence of HSCs in the mouse term placenta 16, the discovery of HSCs in the human term placenta was unexpected. Human placental HSCs were verified as being foetal-derived by human forensic identity PCR analysis, indicating that the human placenta still functions to maintain, expand and/or produce the most immature of foetus-derived hematopoietic cells 22.
Interestingly, cell extraction methods allowing the isolation of cells from the placenta vasculature reveal that many potent HSCs are either closely adherent to the endothelium or are within the niches of this compartment 22. At week 6 in gestation, HSCs are found both in the CD34+ and CD34- fractions. It is as yet uncertain whether at later stages of development, placenta HSCs are retained in both these fractions, as they are in umbilical cord blood 26, 27. If so, the vascular endothelium might be either considered as the cellular source of HSCs or provide an important HSC growth supportive microenvironment.
Microenvironment of the placenta
Hematopoietic tissues harbour hematopoietic progenitors and stem cells in specific microenvironments or niches 28. Studies of adult bone marrow show that the microenvironment is a complex structure composed of mesenchymal stromal cells, endothelial cells, osteoblasts, adipocytes, extracellular matrix, growth factors, cytokines and adhesive molecules that provide support and regulatory functions for hematopoietic progenitor or stem-cell homing, self renewal, maintenance and differentiation. A close relationship exists between HSCs and the endosteal and endothelial niches in the bone marrow 29, 30. The hematopoietic niche of the placenta within the labyrinth is likely to be similar, consisting of endothelial, perivascular and mesenchymal cells but also placenta-specific syncytiotrophoblast cells.
Interestingly, throughout mouse development, the temporal and spatial distribution of mesenchymal stromal cells (MSC) correlates with hematopoietic territories such as the AGM, foetal liver and neonatal bone marrow 31. MSC lines have been derived from these tissues and many of these cell lines can provide support for hematopoietic cells in vitro 32, 33, underlining the role that stromal cells play in the proliferation and/or survival of HSCs. Such MSCs have osteogenic, adipogenic, chondrogenic, and/or myogenic differentiation potential 34, 35. Since the mouse placenta is a potent hematopoietic territory it is expected that this tissue will also yield hematopoietic supportive MSC lines and exhibit similar differentiation potentials.
MSC lines have already been isolated from human placenta and amnionic and chorionic fetal membranes (reviewed in 36). At the developmental stages tested, ranging from week 3 to term, human MSC lines express classical mesenchymal markers as well as markers of pericytes, CD146 and NG2 22, 37, and after gestational week 6, they possess the typical mesenchymal lineage potentials (osteogenic, adipogenic and/or endothelial) 22, 36-46. Also, some of these placental MSC lines constitute a potent feeder layer for in vitro maintenance and/or expansion of human umbilical cord blood CD34+ cells and progenitors 22, primate and human embryonic stem (ES) cells 23, 47, 48, and long-term-culture-initiating cells 23. Immunostaining of human placental sections localize such mesenchymal cell types within this tissue. Moreover, localized expression of CD146 and NG2 in the perivasculature of the placenta suggests that placenta mesenchymal cells are pericytes 22, 37. Interestingly, a MSC line derived [ad1]from the maternal part of a gestation week 3 human placenta provided potent in vitro maintenance and expansion of human umbilical cord blood CD34+ cells and an 8-fold increase in immature hematopoietic progenitors compared to to the input number in the sorted CD34+ cord blood population 22, suggesting that maternal cells contribute to the early hematopoietic supportive microenvironment by promoting the growth of the placenta as a highly vascular and hematopoietic territory. Thus, the hematopoietic inductive/supportive microenvironment of the placenta could be unique as compared to the other foetal and adult hematopoietic territories, and it most likely consists of the vascular endothelial, mesenchymal and syncytiotrophoblast cells that develop in parallel in the villi.
The coordinated development of microenvironment within the labyrinth requires vessel generation, invasion, branching morphogenesis and syncytiotrophoblast differentiation 4. Genetic studies in mutant mice have identified a panel of genes important for some of these processes 5. For example, the transcription factor encoded by Gcm1 is a pivotal molecule in the initiation of morphogenesis and syncytiotrophoblast differentiation. The germline deletion of Gcm1 (Glial cells missing 1) in mice is lethal at midgestation owing to a failure to develop the placenta labyrinth layer 49. Originally identified in Drosophila, Gcm is involved in macrophage-like cell development 50. In the mouse it is almost exclusively expressed in the placenta 51. Similarly, deletion of Esx1, a homeobox gene, results in failure to develop the labyrinth layer 52. PPARγ is required for the invasion of foetal vessels into the presumptive labyrinth at E9.5, as are the developmental factors such Wnt2 and EphB4/ephrin B2 4. It will be interesting to determine how the placental hematopoietic microenvironment and hematopoiesis are affected in these mouse models, and whether hematopoiesis occurs normally in other hematopoietic sites.
Generator or storage tissue for hematopoietic cells?
The hematopoietic system originates in the mesodermal germ layer of the conceptus. Histologic observation of yolk sac blood island anatomy linked the development of vascular endothelial and hematopoietic cells 53, 54. These studies suggested a common mesodermal precursor for these two lineages of cells, and the precursor cell was given the name hemangioblast. Other histologic studies proposed that the precursors of hematopoietic cells in the embryonic dorsal aorta are hemogenic endothelial cells, as clusters of hematopoietic cells are closely associated with the ventral endothelium of the dorsal aorta at early stages of development 13. Cell tracing 55, 56in mouse models in conjunction with vital imaging of mouse ES cell hematopoietic differentiation cultures and in vitro cultured early posterior primitive streak cells 57, 58show that hematopoietic cells transit through an endothelial cell stage before taking on hematopoietic fate. In vivo real-time imaging of the intact midgestational dorsal aorta shows the emergence of hematopoietic progenitor/stem cells from endothelial cells lining this vessel 59. Because the vasculature and other cells of the placenta originate from the allantoic mesoderm, which arises from the primitive streak at early stages of development, the allantoic mesoderm is postulated to be hemogenic.
Indeed, the allantois of the developing chick can produce hematopoietic cells 11, 60. Blood-island-like clusters of hematopoietic cells are found in the prevascularized allantois, and upon engraftment into the coelom of host embryos, the cells arising from the donor allantois contribute to adult blood. Results in mouse embryo grafting experiments examining this question are less clear. After engraftment and a short culture period, donor allantoic cells contribute to the endothelial lineage but only very rarely to a small number of erythroid cells 61. More recently, others have found that the mouse prefusion allantois and chorion tissues both possess intrinsic hematopoietic potential 62, 63; they give rise to clonogenic haematopoietic progenitors following a 48-hour organ explant culture period 63. Moreover, definitive hematopoietic markers such as Runx1 (a pivotal hematopoietic transcription factor 64) and Ly6A GFP (HSC marker 65) are expressed in the early allantois and chorion, indicating the hematopoietic potential 63. Thus, before it becomes vascularized, the early stage placenta is intrinsically hemogenic.
At a slightly later developmental stage, the mouse placenta generates hematopoietic progenitors. Embryos deficient for the Ncx1 gene 66 lack a heart beat and blood circulation, which is normally established between the embryo body and the extraembryonic tissues at E8.25. If in the absence of circulation a tissue such as the yolk sac, placenta or the body of the embryo contains hematopoietic cells, then the hematopoietic cells must be generated intrinsically 67. Indeed, erythro-myeloid and lymphoid progenitors are detected in the Ncx1-/- embryo body, yolk sac and placenta 18, 67, indicating their intrinsic hemogenic/hematopoietic capacity.
Because Ncx1-/- embryos die before E11, the time at which HSC activity is first detected in the placenta, it is as yet uncertain whether the placenta can generate HSCs. Quantitative studies, in which HSC numbers in each of the tissues of the conceptus was determined 68, suggest that the aorta (the only tissue shown to autonomously generate HSCs) cannot produce all of the HSCs that eventually are found in the foetal liver and the adult bone marrow (tissues that harbour but do not generate HSCs) (Figure 2). In this regard, the midgestation mouse placenta contains an abundance of HSCs 16, 17, supporting the notion that this highly vascularized tissue generates HSCs from hemogenic endothelium and/or that it provides a unique supportive growth niche for expansion of aorta-derived HSCs. Similarly, the human placenta might also autonomously generate HSCs and/or promote their expansion to large numbers before they migrate to the bone marrow. Recent studies showing the importance of mechanical stimuli provided by circulation in AGM HSC development 69 suggests that mechanical stimuli as well as hypoxia 70 could be important factors in placental and HSC development.
The placenta in hematopoietic development
Hematopoiesis in the mouse and human conceptus progresses in wave-like stages (reviewed in 13, 25). The three hemogenic/hematopoietic tissues, the yolk sac (YS), the AGM region (and its precursor tissue the para-aortic splanchnopleura) and the chorio-allantoic placenta are active at different but overlapping stages of development. They produce varying repertoires and quantities of hematopoietic cells and support them in distinct microenvironments. It is curious that eutherian mammals use the placenta as a hemogenic and hematopoietic tissue, whereas other mammals and nonmammalian vertebrates develop a fully functional hematopoietic system in the absence of a placenta. However, in these other vertebrate embryos, it is possible that hematopoietic cells emerging in the allantois are amplified elsewhere. In marsupials, the allantois is mainly avascular and does not fuse with the chorion. Many variations in placental structure are seen in mammals. It is discoid in mouse and man, and in species like the pig, horse and whale it is diffuse and distributed over most of the uterus inner surface 4. Interestingly, the evolution of the placenta as a complex organ has occurred multiple times, as found in the fish genus Poeciliopsis 71. Hence, is the more fully developed placenta irrelevant as a hematopoietic tissue or does it play a special role in blood development in mammals?
One notion is that the large size of the mammalian foetus and the extended length of the gestational period might require a greater quantity of hematopoietic progenitors and HSCs for foetal growth, and this can only be accomplished in an extra hematopoietic tissue such as the placenta. Indeed, the placenta is large as compared to the size of the other hematopoietic tissues of the embryo, and thus, it represents a significant space for producing, amplifiying and/or harbouring hematopoietic cells.
Another idea concerning the functional relevance of the placenta comes from studies of Runx1 haploinsufficient mouse embryos; in these mice, which have half a dose of the Runx1 transcription factor protein, the AGM region produces fewer HSCs 43, 72. However, Runx1 haploinsufficiency does not have negative effects on HSCs in the extraembryonic tissues (yolk sac and placenta) or fetal liver, and instead results in a surprising increase in HSC numbers in the placenta 43. Hence, the placenta appears to be more resistant to genetic/physiologic changes, implicating it as a highly robust hematopoietic tissue in nonhomeostatic conditions.
Additionally, the provision of growth factors such as interleukin 3 (IL-3) by the maternal part of the placenta can stimulate the hematopoiesis 73. IL-3 is a potent HSC survival and proliferation factor during embryonic development 43 and the IL-3 gene is a known direct downstream transcriptional target of Runx1. Interestingly, human placenta MSCs produce SCF (stem cell factor), Flt3 ligand, IL-6 and M-CSF (macrophage-colony stimulating factor), among other hematopoietic factors 23. Thus, both maternal- and foetal-derived factors may contribute to the growth of the placenta hematopoietic niche and the support of hematopoietic cells.
Overall, the human placenta plays an important and multifaceted role in the development and growth of the foetus. Its structural complexity and its immense vascular network containing large quantities of circulating blood cells and progenitors, make it a difficult tissue to understand, particularly concerning its precise role in the development of the mammalian hematopoietic system. The ongoing challenge is to determine whether the placenta just contains reserve cohorts of hematopoietic progenitor and stem cells, or whether these cohorts of cells are required for adult hematopoiesis and migrate to the bone marrow at the end of fetal development before the time of delivery.
Concluding remarks
The discovery of HSCs in the human placenta throughout development and their localization to the highly vascular compartment of this tissue opens new lines of inquiry with potential medical implications (Box 1). As a new source of HSCs, the term human placenta as yet yields only relatively small numbers. The present cell extraction procedures that include dissection, enzymatic digestion with three enzymes (collagenase, dispase and pancreatin) and mechanical dispersion yield about 10% of the total number of HSCs found in a unit of umbilical cord blood 22, 74. Although placenta HSCs can be stored much like umbilical cord blood HSCs, harvest efficiencies together with the cost- and labor-effectiveness must improve before any potential clinical applications can be considered. In this regard, a more easily isolatable source of HSCs with potential for regenerative medicine has recently been discovered, the amniotic fluid 75.
Box 1. Outstanding Questions.
Does the placenta have the ability to generate hematopoietic stem cells? The placenta contains hematopoietic stem cells 22, but it is as yet undetermined whether the placenta can intrinsically produce these potent, therapeutically important stem cells 18.
Does the placenta contain hemangioblasts and/or hemogenic endothelial cells? Results from studies of the mouse chorion and allantois have shown that these tissues possess intrinsic hematopoietic potential 62, 63. In the absence of circulation, the mouse placenta intrinsically generates multipotent progenitors 18; however, it is as yet unknown whether the vasculature of the labyrinth and/or the mesenchymal cells within the villi are hemogenic.
How many hematopoietic stem cells does a human placenta contain? If placenta-derived cells are to be contemplated for hematopoietic transplantation purposes, they must be at least as abundant as those available in the umbilical cord blood. Improvements in extraction procedures should optimize both quantitative yields and viability of these cells.
How robust are placenta HSCs in transplantation scenarios? Xenotransplants of human placenta cells reveal the presence of multipotent, long-term engrafting HSCs. However, it is uncertain whether placental HSCs are qualitatively as potent (in proliferation, differentiation and self-renewal) as those from umbilical cord blood and adult bone marrow.
Does the placenta contain a unique hematopoietic inductive/supportive microenvironment? The placenta is an extraembryonic and transient tissue; it is uncertain whether unique placental cell types and genetic programs regulate the development of hematopoietic cells differently than in the other embryonic and adult hematopoietic tissues.
Importantly, knowledge from developmental studies demonstrating the hemogenic nature of the early-stage chorio-allantoic placenta 18, 62, 63 offers a new and exciting challenge in the field. Insights into the signals that drive hematopoietic cell generation from mesodermal precursors, endothelial cells and the development of placenta labyrinth and villi could provide new strategies for the production of hematopoietic cells from the numerous vascular endothelial cells of the foetal part of this tissue. A recent study with early stage human embryos has identified a marker ACE (angiotensin converting enzyme, CD143) recognized by the BB9 antibody, on a subset of human mesoderm that establishes a population of hemogenic endothelial cells within the fetus 76. The ACE-expressing cells possess hematopoietic potential in long term cultures and in SCID mouse in vivo reconstitution assays. Moreover, ACE-expressing cells isolated from human ES cells differentiated into embryoid bodies are hemangioblastic-like angiohematopoietic cells. It will be interesting to examine the human placenta for BB9 expression and if such cells are found to examine their hemogenic potential. Characterization of the molecular program of ACE-expressing cells should yield information on the genes involved in HSC specification, amplification and maintenance.
Of future interest is the prospect of using this knowledge of the genetic program to induce the differentiation of early populations of hemogenic cells to HSC fate, particularly if large numbers of such cells can be isolated from the placenta. Alternatively, with the advancement of the technology of reprogramming somatic cells to pluripotent stem cells, it may be possible, with our complete knowledge of the pivotal factors involved in HSC generation to obtain other patient-specific cells that can be reprogrammed into therapeutically potent HSCs 77. In this regard, the human placenta, allantoic and chorionic foetal membranes 36 provide an abundant source of developmentally young somatic cells such as MSCs, that may be stored, differentiated and/or reprogrammed for regenerative medicine.
Acknowledgments
The authors thank lab members and particularly K. Bollerot, S. Mendes, E. Haak, M. Crisan, F. Cerisoli, I. Lauw, P. Kaimakis, R. Jorna, P. Imanirad, R. van der Linden, E. Steegers and T. Cupedo who contributed to these studies. We also thank Mihaela Crisan for critical reading of the manuscript. Support was provided by the Landsteiner Society for Blood Research (0614), NIH R37 (DK51077), Dutch BSIK Stem Cells in Development and Disease (03038) and NWO VIDI (917.76.345).
Glossary
- Placenta
extraembryonic tissue derived from the chorion and allantois of the early stage mammalian conceptus.
- Allantois
posterior outgrowth of the early stage embryo with hematopoietic potential. It gives rise to the vessels in the umbilical cord of mammals. In birds and reptiles, it is involved in oxygen exchange and is a reservoir of nitrogenous waste.
- Chorion
membrane separating the fetus and mother that is formed by the extraembryonic mesoderm and trophoblast.
- Endosteal and endothelial niches
specific anatomical locations/microenvironments within the adult bone marrow that produce factors or provide important cell-cell interactions for the maintenance, self-renewal and/or differentiation of hematopoietic progenitors and stem cells. The endosteal niche containing osteoblasts juxtaposed to the bone. The endothelial niche is the vasculature within the bone marrow.
- Hematopoietic stem cells
the rare cells existing within the bone marrow cavities of the adult that contribute to the life-long production of all blood cells of the hematopoietic system. These cells are long-lived, self renewing and possess potential to produce all hematopoietic cell lineages.
- Hematopoietic progenitor cells
intermediate cells within the adult hematopoietic cell differentiation hierarchy. Having restricted differentiation potential, they expand and differentiate to one or a few hematopoietic lineages. They are generally short-lived and do not self-renew.
- Hemangioblastic cords
In the early stage human placenta, mesodermal/mesenchymal cells that are the precursors of vascular endothelial and hematopoietic cells.
- Labyrinth
region of the placenta in which the trophoblast and its associated fetal blood vasculature undergoes extensive branching of the villi into a densely packed structure.
- Pericytes
a relatively undifferentiated cell type that surrounds blood vessels. It possesses multilineage differentiation potential and is thought to be the precursor of mesenchymal stem/stromal cells (MSC).
- Syncytiotrophoblasts
the outer most cell type of the fetal placenta. They invade the uterine wall and provide the large surface area for exchange of oxygen, nutrients and waste between the fetus and mother.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Longo LD, Reynolds LP. Some historical aspects of understanding placental development, structure and function. Int J Dev Biol. 2010;54:237–255. doi: 10.1387/ijdb.082774ll. [DOI] [PubMed] [Google Scholar]
- 2.Pijnenborg R, Vercruysse L. Erasmus Darwin’s enlightened views on placental function. Placenta. 2007;28:775–778. doi: 10.1016/j.placenta.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Gude NM, et al. Growth and function of the normal human placenta. Thromb Res. 2004;114:397–407. doi: 10.1016/j.thromres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 4.Rossant J, Cross JC. Lineage Specification and Differentiation: Extraembryonic Lineages. In: Rossant J, Tam PL, editors. Mouse development: patterning, morphogenesis and organogenesis. Academic Press; 2001. pp. 155–174. [Google Scholar]
- 5.Cross JC, et al. Genes, development and evolution of the placenta. Placenta. 2003;24:123–130. doi: 10.1053/plac.2002.0887. [DOI] [PubMed] [Google Scholar]
- 6.Rawn SM, Cross JC. The evolution, regulation, and function of placenta-specific genes. Annu Rev Cell Dev Biol. 2008;24:159–181. doi: 10.1146/annurev.cellbio.24.110707.175418. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Porrero JA, et al. Potential intraembryonic hemogenic sites at pre-liver stages in the mouse. Anat Embryol (Berl) 1995;192:425–435. doi: 10.1007/BF00240375. [DOI] [PubMed] [Google Scholar]
- 8.Georgiades P, et al. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- 9.Mikkola HK, et al. Placenta as a site for hematopoietic stem cell development. Exp Hematol. 2005;33:1048–1054. doi: 10.1016/j.exphem.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Demir R, et al. Fetal vasculogenesis and angiogenesis in human placental villi. Acta Anat (Basel) 1989;136:190–203. doi: 10.1159/000146886. [DOI] [PubMed] [Google Scholar]
- 11.Caprioli A, et al. Blood-borne seeding by hematopoietic and endothelial precursors from the allantois. Proc Natl Acad Sci U S A. 1998;95:1641–1646. doi: 10.1073/pnas.95.4.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez-Silva M, et al. Mouse placenta is a major hematopoietic organ. Development (Cambridge, England) 2003;130:5437–5444. doi: 10.1242/dev.00755. [DOI] [PubMed] [Google Scholar]
- 13.Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ottersbach K, Dzierzak E. The placenta as a haematopoietic organ. Int J Dev Biol. 2010;54 doi: 10.1387/ijdb.093057ko. [DOI] [PubMed] [Google Scholar]
- 15.Gekas C, et al. Isolation and visualization of mouse placental hematopoietic stem cells. Current protocols in stem cell biology. 2008;Chapter 2(Unit 2A 8 1-2A 8 14) doi: 10.1002/9780470151808.sc02a08s6. [DOI] [PubMed] [Google Scholar]
- 16.Gekas C, et al. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell. 2005;8:377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Rhodes KE, et al. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2:252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Challier JC, et al. Immunocytological evidence for hematopoiesis in the early human placenta. Placenta. 2005;26:282–288. doi: 10.1016/j.placenta.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Barcena A, et al. The human placenta is a hematopoietic organ during the embryonic and fetal periods of development. Dev Biol. 2009;327:24–33. doi: 10.1016/j.ydbio.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Barcena A, et al. A new role for the human placenta as a hematopoietic site throughout gestation. Reprod Sci. 2009;16:178–187. doi: 10.1177/1933719108327621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robin C, et al. Human placenta is a potent hematopoietic niche containing hematopoietic stem and progenitor cells throughout development. Cell Stem Cell. 2009;5:385–395. doi: 10.1016/j.stem.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, et al. Human placenta-derived mesenchymal progenitor cells support culture expansion of long-term culture-initiating cells from cord blood CD34+ cells. Exp Hematol. 2004;32:657–664. doi: 10.1016/j.exphem.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Tavian M, et al. The human embryo, but not its yolk sac, generates lympho-myeloid stem cells: mapping multipotent hematopoietic cell fate in intraembryonic mesoderm. Immunity. 2001;15:487–495. doi: 10.1016/s1074-7613(01)00193-5. [DOI] [PubMed] [Google Scholar]
- 25.Tavian M, Peault B. Embryonic development of the human hematopoietic system. Int J Dev Biol. 2005;49:243–250. doi: 10.1387/ijdb.041957mt. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia M, et al. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med. 1998;4:1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, et al. SCID-repopulating cell activity of human cord blood-derived CD34- cells assured by intra-bone marrow injection. Blood. 2003;101:2924–2931. doi: 10.1182/blood-2002-09-2782. [DOI] [PubMed] [Google Scholar]
- 28.Yaniv I, et al. The tale of early hematopoietic cell seeding in the bone marrow niche. Stem Cells Dev. 2006;15:4–16. doi: 10.1089/scd.2006.15.4. [DOI] [PubMed] [Google Scholar]
- 29.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 30.Lo Celso C, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendes SC, et al. Mesenchymal progenitor cells localize within hematopoietic sites throughout ontogeny. Development (Cambridge, England) 2005;132:1127–1136. doi: 10.1242/dev.01615. [DOI] [PubMed] [Google Scholar]
- 32.Moore KA, et al. In vitro maintenance of highly purified, transplantable hematopoietic stem cells. Blood. 1997;89:4337–4347. [PubMed] [Google Scholar]
- 33.Oostendorp RA, et al. Stromal cell lines from mouse aorta-gonads-mesonephros subregions are potent supporters of hematopoietic stem cell activity. Blood. 2002;99:1183–1189. doi: 10.1182/blood.v99.4.1183. [DOI] [PubMed] [Google Scholar]
- 34.Durand C, et al. Mesenchymal lineage potentials of aorta-gonad-mesonephros stromal clones. Haematologica. 2006;91:1172–1179. [PubMed] [Google Scholar]
- 35.Zipori D. Mesenchymal stem cells: harnessing cell plasticity to tissue and organ repair. Blood Cells Mol Dis. 2004;33:211–215. doi: 10.1016/j.bcmd.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Parolini O, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26:300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 37.Crisan M, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Fukuchi Y, et al. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- 39.Igura K, et al. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy. 2004;6:543–553. doi: 10.1080/14653240410005366-1. [DOI] [PubMed] [Google Scholar]
- 40.Li CD, et al. Mesenchymal stem cells derived from human placenta suppress allogeneic umbilical cord blood lymphocyte proliferation. Cell research. 2005;15:539–547. doi: 10.1038/sj.cr.7290323. [DOI] [PubMed] [Google Scholar]
- 41.Miao Z, et al. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006;30:681–687. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Portmann-Lanz CB, et al. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol. 2006;194:664–673. doi: 10.1016/j.ajog.2006.01.101. [DOI] [PubMed] [Google Scholar]
- 43.Robin C, et al. An unexpected role for IL-3 in the embryonic development of hematopoietic stem cells. Dev Cell. 2006;11:171–180. doi: 10.1016/j.devcel.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Wulf GG, et al. Mesengenic progenitor cells derived from human placenta. Tissue Eng. 2004;10:1136–1147. doi: 10.1089/ten.2004.10.1136. [DOI] [PubMed] [Google Scholar]
- 45.Yen BL, et al. Isolation of multipotent cells from human term placenta. Stem Cells. 2005;23:3–9. doi: 10.1634/stemcells.2004-0098. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, et al. Mesenchymal progenitor cells derived from chorionic villi of human placenta for cartilage tissue engineering. Biochem Biophys Res Commun. 2006;340:944–952. doi: 10.1016/j.bbrc.2005.12.091. [DOI] [PubMed] [Google Scholar]
- 47.Kim SJ, et al. Human placenta-derived feeders support prolonged undifferentiated propagation of a human embryonic stem cell line, SNUhES3: comparison with human bone marrow-derived feeders. Stem Cells Dev. 2007;16:421–428. doi: 10.1089/scd.2006.0098. [DOI] [PubMed] [Google Scholar]
- 48.Miyamoto K, et al. Human placenta feeder layers support undifferentiated growth of primate embryonic stem cells. Stem Cells. 2004;22:433–440. doi: 10.1634/stemcells.22-4-433. [DOI] [PubMed] [Google Scholar]
- 49.Anson-Cartwright L, et al. The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nature genetics. 2000;25:311–314. doi: 10.1038/77076. [DOI] [PubMed] [Google Scholar]
- 50.Lebestky T, et al. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science (New York, N Y) 2000;288:146–149. doi: 10.1126/science.288.5463.146. [DOI] [PubMed] [Google Scholar]
- 51.Basyuk E, et al. Murine Gcm1 gene is expressed in a subset of placental trophoblast cells. Dev Dyn. 1999;214:303–311. doi: 10.1002/(SICI)1097-0177(199904)214:4<303::AID-AJA3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Behringer RR. Esx1 is an X-chromosome-imprinted regulator of placental development and fetal growth. Nature genetics. 1998;20:309–311. doi: 10.1038/3129. [DOI] [PubMed] [Google Scholar]
- 53.Murray P. The development in vitro of the blood of the early chick embryo. Proc Roy Soc London. 1932;11:497–521. [Google Scholar]
- 54.Sabin F. Studies on the origin of blood vessels and of red blood corpuscles as seen in the living blastoderm of chicks during the second day of incubation. Carnegie Inst Wash Pub # 272, Contrib Embryol. 1920;9:214. [Google Scholar]
- 55.Chen MJ, et al. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zovein AC, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eilken HM, et al. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 58.Lancrin C, et al. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boisset JC, et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 60.Caprioli A, et al. Hemangioblast commitment in the avian allantois: cellular and molecular aspects. Dev Biol. 2001;238:64–78. doi: 10.1006/dbio.2001.0362. [DOI] [PubMed] [Google Scholar]
- 61.Downs KM, et al. Vascularization in the murine allantois occurs by vasculogenesis without accompanying erythropoiesis. Development (Cambridge, England) 1998;125:4507–4520. doi: 10.1242/dev.125.22.4507. [DOI] [PubMed] [Google Scholar]
- 62.Corbel C, et al. Hematopoietic potential of the pre-fusion allantois. Dev Biol. 2007;301:478–488. doi: 10.1016/j.ydbio.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 63.Zeigler BM, et al. The allantois and chorion, when isolated before circulation or chorio-allantoic fusion, have hematopoietic potential. Development (Cambridge, England) 2006;133:4183–4192. doi: 10.1242/dev.02596. [DOI] [PubMed] [Google Scholar]
- 64.North TE, et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- 65.de Bruijn MF, et al. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. 2002;16:673–683. doi: 10.1016/s1074-7613(02)00313-8. [DOI] [PubMed] [Google Scholar]
- 66.Koushik SV, et al. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. Faseb J. 2001;15:1209–1211. doi: 10.1096/fj.00-0696fje. [DOI] [PubMed] [Google Scholar]
- 67.Lux CT, et al. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood. 2008;111:3435–3438. doi: 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumaravelu P, et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development (Cambridge, England) 2002;129:4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- 69.North TE, et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dunwoodie SL. The role of hypoxia in development of the Mammalian embryo. Dev Cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 71.Reznick DN, et al. Independent origins and rapid evolution of the placenta in the fish genus Poeciliopsis. Science (New York, N Y) 2002;298:1018–1020. doi: 10.1126/science.1076018. [DOI] [PubMed] [Google Scholar]
- 72.Cai Z, et al. Haploinsufficiency of AML1 affects the temporal and spatial generation of hematopoietic stem cells in the mouse embryo. Immunity. 2000;13:423–431. doi: 10.1016/s1074-7613(00)00042-x. [DOI] [PubMed] [Google Scholar]
- 73.Chamley LW, et al. Is interleukin-3 important in antiphospholipid antibody-mediated pregnancy failure? Fertil Steril. 2001;76:700–706. doi: 10.1016/s0015-0282(01)01984-7. [DOI] [PubMed] [Google Scholar]
- 74.Bhatia M, et al. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci U S A. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ditadi A, et al. Human and murine amniotic fluid c-Kit+Lin- cells display hematopoietic activity. Blood. 2009;113:3953–3960. doi: 10.1182/blood-2008-10-182105. [DOI] [PubMed] [Google Scholar]
- 76.Zambidis ET, et al. Emergence of human angiohematopoietic cells in normal development and from cultured embryonic stem cells. Annals of the New York Academy of Sciences. 2007;1106:223–232. doi: 10.1196/annals.1392.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Graf T, Enver T. Forcing cells to change lineages. Nature. 2009;462:587–594. doi: 10.1038/nature08533. [DOI] [PubMed] [Google Scholar]


