Abstract
Doxorubicin (Dox) is one of the most effective chemotherapeutic agents; however, it causes dose-dependent cardiotoxicity. Evaluation of left ventricular function relies on measurements based on M-mode echocardiography. A new technique based on quantification of myocardial motion and deformation, strain echocardiography, has been showed promising profile for early detection of cardiac dysfunction. Different therapy strategies, such as flavonoid plant extracts and stem cells, have been investigated to improve heart function in toxic cardiomyopathy. This work aimed to assess early cardiac function improvement after treatments with either flavonoid extract from Camellia sinensis or mesenchymal stem cells in Dox cardiotoxicity using strain echocardiography. Twenty Wistar rats were randomly assigned to four groups. They received water (control, Dox, Dox + stem cells) or 100 mg/kg C. sinensis extract (Dox + C. sinensis) via gavage, daily, for four weeks. Animals also received saline (control) or 5 mg/kg doxorubicin (Dox, Dox + C. sinensis, Dox + stem cells) via intraperitoneal injection, weekly, for four weeks. Stem cells were injected (3 × 106 cells) through tail vein prior the beginning of the experiment (Dox + stem cells). Animals were evaluated by hematological, electrocardiography, echocardiography, and histopathological examinations. Dox cardiotoxicity was only diagnosed with strain echocardiography, detecting a decrease in ventricular function. C. sinensis extract did not prevent ventricular dysfunction induced by Dox. However, strain echocardiography examination revealed that Dox cardiotoxicity was significantly suppressed in rats treated with stem cells. In conclusion, strain echocardiography was able to detect precocity signs of heart failure and stem cell therapy showed cardioprotection effect against Dox cardiotoxicity.
Keywords: Echocardiography, Cardiotoxicity, Stem cell therapy, Flavonoid
Introduction
One of the most effective chemotherapeutic agents used for treatment of hematological and solid tumors is doxorubicin (Dox) [1]. However, Dox causes a dose-dependent cardiotoxicity that may lead to irreversible heart failure by different pathogenic mechanisms which have not been completely elucidated [2]. A progressive reduction of left ventricular function, observed as decrease in fractional shortening and ejection fraction measured by M-mode, is observed in a significant proportion of patients during the course of Dox therapy, and life-threatening congestive heart failure is observed [3–5]. Production of reactive oxygen species, calcium imbalance, mitochondrial damage, and apoptosis are considered in Dox cardiotoxicity condition [2,6–8]. Regarding such potential causes for cardiotoxicity it is possible to infer that flavonoids which have been studied and showed free radical scavenging properties [9,10] and mesenchymal stem cell (MSC) transplantation replacing injuried cells and due to its paracrine effects and multipotent properties to produce cells with some cardiomyocytes characteristics [11,12] may have beneficial effects toward heart function improvement.
Echocardiography has a leading role in the routine assessment and diagnosis of cardiac diseases and it is a safe study method due to its non-ionizing characteristic [13,14]. Left ventricular ejection fraction is the most commonly used measure to assess left ventricular systolic function, is well established, with strong prognostic and therapeutic implications, nevertheless it may not always be satisfactory in all cases [14]. Other measures of myocardial contractility can provide valuable additive information, as longitudinal function using tissue Doppler data or speckle tracking algorithms [14]. Tissue Doppler imaging is useful in quantifying regional myocardial function, but has a significant limitation of Doppler angle dependency. The recently developed strain echocardiography, based on speckle tracking imaging technology, enables the evaluation of myocardial function independent of ultrasound beam direction [15]. Strain echocardiography has provided quantification of regional myocardial systolic function objectively and is less influenced by tethering effects and cardiac translational artifact than Doppler tissue imaging [14]. Along with echocardiography, other examinations as electrocardiography (ECG), radiography, computed tomography, magnetic resonance imaging, measurement of serum cardiac biomarkers including C-reactive protein (CRP), N-terminal pro-B-type natriuretic peptide (nt-proBNP), troponin T (TnT), creatine kinase MB (CK-MB), and lactate dehydrogenase (LDH) are important diagnostic tools in the evaluation of cardiac function [16–18]. Some reports have already shown differences between M-mode and strain parameters in Dox-cardiotoxicity models [19,20]. However, strain echocardiography was not compared with different cardiac assays at the same time and it was not used to assess therapeutical benefits in diseased subjects. This work was aimed to evaluate the ability of strain echocardiography to detect early ventricular dysfunction and cardiac improvement after MSC and flavonoid extract from Camellia sinensis treatments in a Dox-cardiotoxicity model.
Materials and Methods
Animals
Twenty male Wistar rats weighing 180–200 g were evaluated. They were weighted weekly and were housed in 12 h dark-light manner with food and water available ad libitum. All experimental protocols were in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Federal University of Minas Gerais, Brazil (CETEA, protocol number 176/2010).
Experimental groups
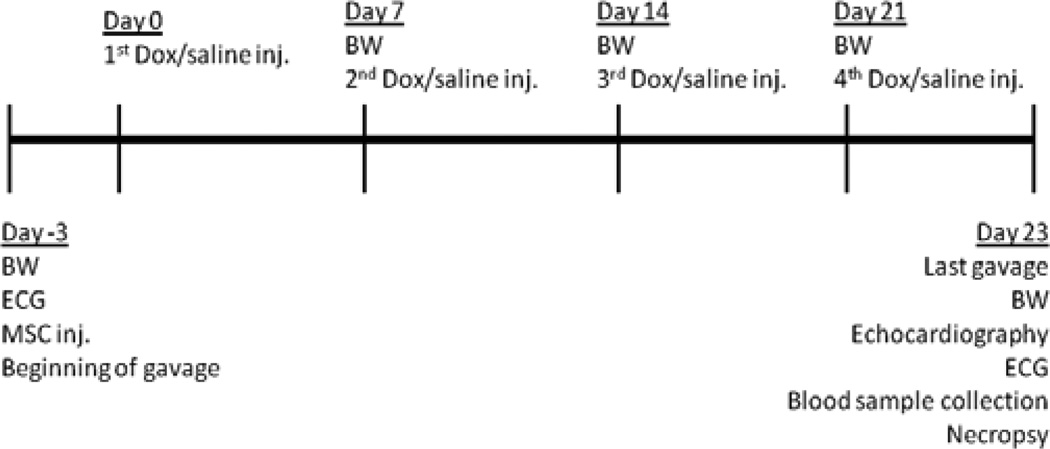
Animals were randomly categorized into four groups: control (distilled water orally (P.O.) daily; saline intraperitoneal (I.P.) weekly); Dox (distilled water P.O. daily; 5 mg/kg Dox I.P. weekly); Dox + C. sinensis (100 mg/kg C. sinensis extract P.O. daily; 5 mg/kg Dox I.P. weekly); Dox + MSC (distilled water P.O. daily; 5 mg/kg Dox IP weekly; MSC via intravenous injection). Distilled water and plant extract (1 ml) were given through gavage procedure, which was first performed three days prior, the beginning of the experiment (first Dox injection). The C. sinensis extract used contains polyphenols > 80%, catechins > 80%, epigallocatechin > 45%, and caffeine < 1% detected by high performance liquid chromatography analysis as informed by the manufacture. The MSCs were isolated from adipose tissue of Lewis LEW-Tg (EGFP) F455.5/Rrrc rats, which were obtained from the Rat Resource and Research Center (Missouri, USA). Adipose tissue derived MSCs were isolated as previously described, with minor modifications [21]. Briefly, inguinal adipose tissue was collected from 6-week-old rats, washed with phosphate-buffered saline (PBS) and digested with 0.15% collagenase II for 1 hour. Collagenase activity was inhibited by the addition of fetal bovine serum (FBS) and the digested tissue was centrifuged at 330 g for 10 min. Pellet was suspended in basal media and plated in T75 tissue flasks. Basal media was composed of Dulbecco’s Modified Eagle’s medium – high glucose supplemented with 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin and 250 ng/ml, amphotericin B. Cell cultures were kept in a humidified atmosphere with 5% CO2 at 37°C. The mesenchymal population was isolated based on its ability to adhere on the culture plate. At 80–90% confluence, cells were detached using 0.25% trypsin-EDTA and replated in other flasks at 1:3 ratios. Cells were cultured up to the fourth passage and, then, were categorized by flow cytometry as expressing the surface molecules CD54, CD73, CD90, and lacking for CD45. Cells (3 × 106) were washed and re-suspended in saline (200 µl) and injected through tail vein 72 h prior the beginning of the experiment. At the end of the experiment, animals were anaesthetized and sacrificed by overdose of isoflurane 48 h after the last fourth Dox injection. A time line for the experimental procedures is shown in Figure 1.
Figure 1.
Time line of the experimental procedures. BW: body weight, ECG: electrocardiography, MSC: mesenchymal stem cell, inj.: injection, Dox: doxorubicin.
Electrocardiography
Computer ECG tracings were 10 min long. Heart rate, heart rhythm, and the measurement of waves and intervals were evaluated. ECG was recorded in 50 mm / s and 2N, animals were anaesthetized using 2.5% isoflurane for induction and were placed in supine position. Traces recorded prior the beginning of the experiment were compared to those recorded at the end.
Echocardiography
Echocardiography images were obtained in bidimensional (2-D), M-mode, Doppler, and strain (speckle-tracking) examinations. Rats were anaesthetized using 2.5% isoflurane for induction and were placed in supine position. Examinations were performed in the last day of the study by the same blind examinator following the recommendations of the American Society of Echocardiography.
Conventional echocardiographic measurements were obtained from gray scale M-mode images, at the midpapillary level in the parasternal short axis view. Conventional measurements of the left ventricle included: end-diastolic diameter, end-systolic diameter, anterior and posterior wall thicknesses, ejection fraction, and fractional shortening.
Echocardiographic speckle-tracking based strain measures of myocardial deformation were obtained from 2D gray scale echocardiography images acquired from the parasternal long- and short-axis views. Strain, strain rate, velocity and displacement were quantified in the longitudinal and radial axes. In accordance with myocardial fiber orientation at varying levels of the left ventricle wall, longitudinal strain is most representative of myocardial shortening at the level of the endocardium, whereas radial strain is at the level of the mesocardium. All images were acquired at an average frame rate of 220 frames per second and at an average depth of 11 mm. Strain analyses were conducted using a speckle-tracking algorithm provided by VisualSonics (VevoStrain, VisualSonics, Toronto, Canada). In brief, suitable B-mode loops were selected from digitally acquired echocardiography images based on adequate visualization of the endocardial border and absence of image artifacts. Three consecutive cardiac cycles were selected for analysis based on image quality. Semi automated tracing of the endocardial and epicardial borders were performed and verified over all three cardiac cycles and, then, corrected as needed to achieve good quality tracking throughout each cine loop. Thereafter tracked images were processed in a frame-by-frame manner for strain measurements. Each long- and short-axis view of the left ventricle myocardium was divided into six standard anatomic segments for regional speckle-tracking based strain analysis throughout the cardiac cycle. For global strain values, velocity, displacement, strain and strain rate measurements were averaged across all six segments.
Clinical pathology
Blood samples were collected at the end of the study by cardiocentesis in tubes with EDTA for determination of hematological and serum biochemical parameters. Hemogram was performed with an electronic cell counter except the white cell differential counting which was done by the microscopic identification of 100 cells in blood smear stained with May-Grunwald Giemsa. Plasma was harvested after centrifugation at 3000 rpm for 10 min, for determination of the serum biochemical parameters CK, CK-MB, LDH, and aspartate aminotransferase (AST) using kinetics method in spectrophotometer.
Pathological analysis
Necropsy was conducted and hearts were weighted and heart weight/body weight ratio was obtained to infer cardiac enlargement. Hearts were sectioned along the longitudinal axes and half of them were fixed in 10% neutral buffered formalin, paraffin embedded, and stained with hematoxylin and eosin for further histopathological analysis on light microscopy.
Assessment of mesenchymal stem cell engraftment in rat cardiac tissues
The other half of each heart was used for detection of the enhanced green fluorescent protein (eGFP) gene using polymerase chain reaction (PCR). eGFP gene detection reaction involved genomic DNA isolation using DNAzol® reagent and following manufacturer’s instructions. A triplex reaction was performed containing the following primers: LWS 455 5F: AACCTCCCAGTGCTTTGAACGCTA, LWS 455 5R: GGTGCAAGCCTCAACTTCTTTGT and U3r-4: ATCAGGGAAGTAGCCTTGTGTGTG. Wild type Lewis rats show amplicons with approximately 400 bp whilst for eGFP positive Lewis rats are 100 bp. Samples of heterozygous or containing genetic material from animals of different genotypes contain amplicons of both sizes.
Statistical analysis
All variables were submitted to normality and homoscedasticity analyses and, then, it was performed analysis of variance (ANOVA). Parametric variables and those with normal distribution after logarithmic transformation (serum biochemical variables) were studied by Student-Newman-Keuls (SNK) post test. Significance was considered for 5% (p<0.05). Analyses were done in R (2.11 version) software program.
Results
Clinical parameters and pathological analysis
Six of the 20 animals died through the experimental period: Dox group (n=2), Dox + C. sinensis group (n=2) and Dox + MSC group (n=2). Early deaths were detected in Dox, Dox + C. sinensis, and Dox + MSC groups on days 19, 20 and 22 after the first Dox injection, respectively. Although six animals died, the remaining sample comprised by 14 animals fitted all basic principles for performing a robust statistical analysis. All animals that received Dox injection showed weight loss compared to control, mainly Dox and Dox + C. sinensis groups (p<0.05). At necropsy, hearts were weighted and the heart weight / body weight ratio was assessed and it was significantly higher for Dox and Dox + C. sinensis groups compared to others. Results are summarized in Table 1.
Table 1.
Body weight, heart weight/body weight ratio, hematological, and serum biochemical parameters [mean (standard deviation)] obtained from Wistar rats by the end of the experimental period.
| Groups | ||||
|---|---|---|---|---|
| Parametersa | Control | Doxorubicin | Doxorubicin + stem cells |
Doxorubicin + C. sinensis |
| Body weight (g) | 264.8(31.1) | 133.3(22.0)* | 203.0(54.8)*† | 117.3(28.7)* |
| Hw / Bw ratio (×103) | 3.9(0.4) | 4.7(0.1)* | 3.5(0.6) | 5.3(0.8)* |
| RBC (×106/µl) | 6.1(0.6) | 6.9(2.4) | 5.9(0.3) | 5.7(0.1) |
| WBC (×103/µl) | 6.3(3.4) | 7.2(5.5) | 5.8(3.6) | 8.7(3.3) |
| CK (U/l) | 1565(2291) | 1079(439) | 1103(300) | 949(648) |
| CK-MB (U/l) | 752(675) | 619(90) | 536(466) | 528(530) |
| LDH (U/l) | 755(650) | 625(465) | 814(207) | 448(357) |
| Urea (mg/dl) | 54(9) | 40(15) | 22(18) | 39(3) |
| Creatinine (mg/dl) | 0.5(0.04) | 1.7(2.2) | 0.7(0.5) | 0.5(0.06) |
Hw / Bw ratio: heart weight/body weight ratio; RBC: red blood cell count; WBC: white blood cell count; CK: creatine kinase; CK-MB: creatine kinase isoenzyme; LDH: lactate dehydrogenase;
p < 0.05 vs. Control;
p < 0.05 vs. Doxorubicin
Electrocardiography
ECGs revealed no arrhythmias. The major finding was an increase and enlargement in T waves whose amplitude was either equal to or higher than R waves. Such condition was detected on groups Dox + C. sinensis (n=2) and Dox (n=1).
Echocardiography
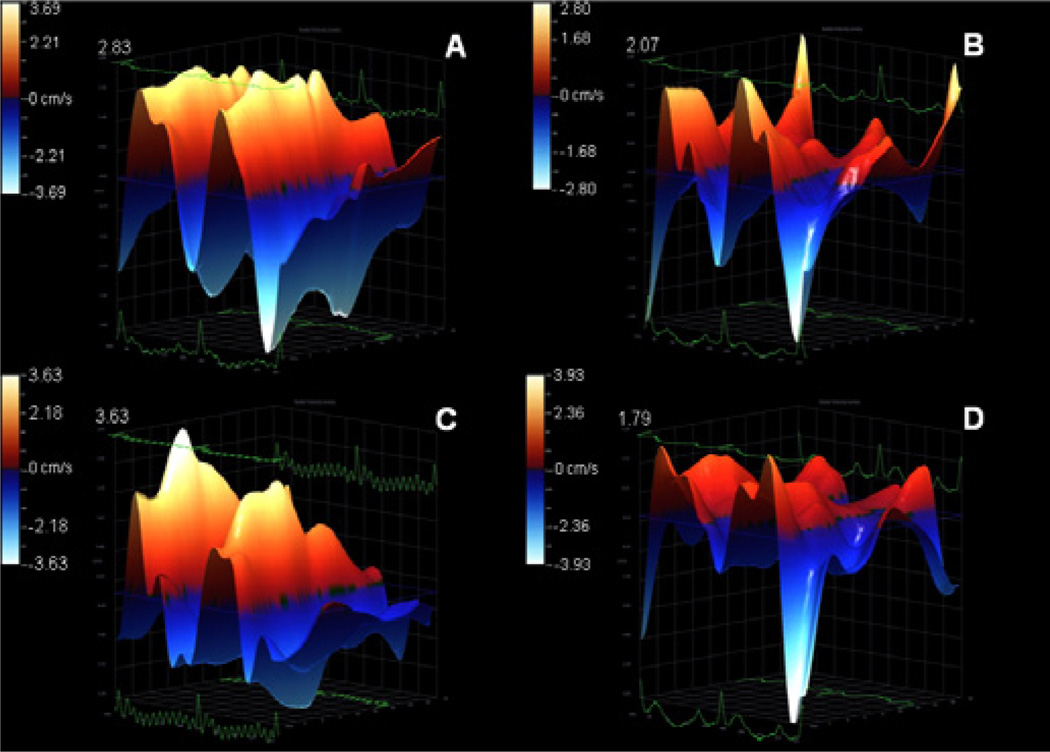
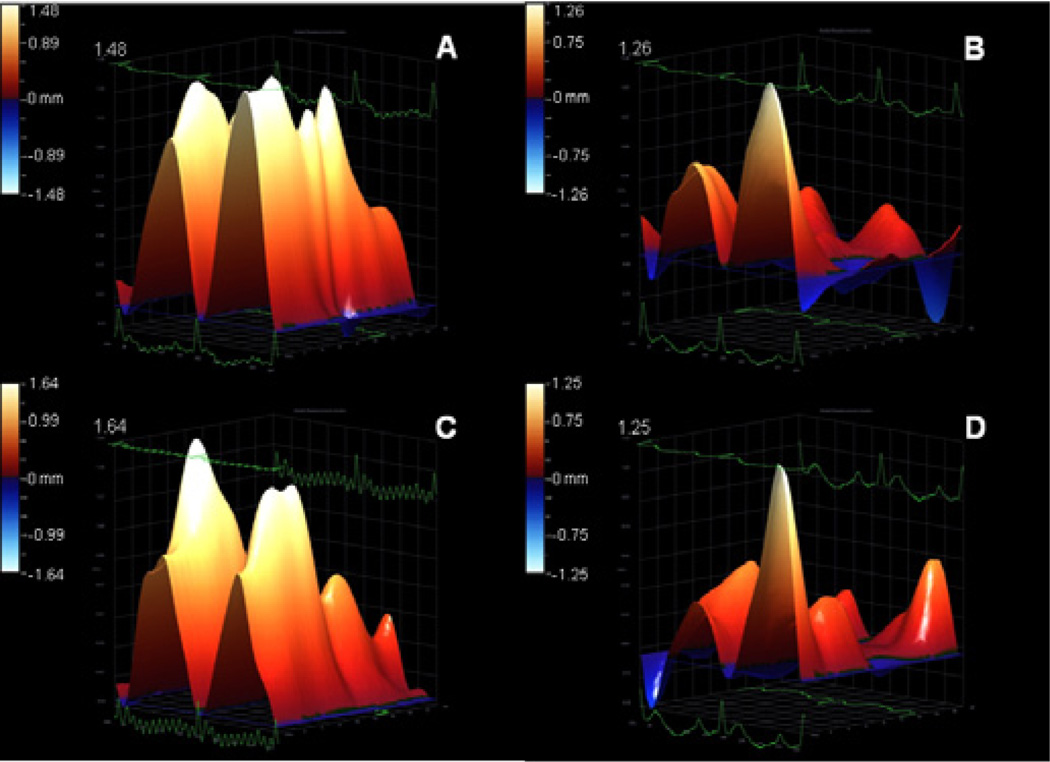
Echocardiography examination showed left ventricular dysfunction in Dox group, compared to control (p<0.05), indicating that cardiotoxicity was effectively induced in the sample studied. Injection of MSC significantly promoted left ventricular function with respect to untreated Dox group. Table 2 summarizes the data. Radial velocity aand radial displacement parameters were significantly decreased in Dox and Dox + C. sinensis groups. On the other hand, animals from Dox + MSC group showed similar measurements to the control group. Global measurements of myocardial velocity (Figure 2) and displacement (Figure 3) were also decreased in Dox and Dox + C.sinensis groups, compared to others.
Table 2.
Strain and M-mode echocardiography parameters for ventricular function [mean (standard deviation)] obtained from Wistar rats by the end of the experimental period.
| Groups | ||||
|---|---|---|---|---|
| Parameters | Control | Doxorubicin | Doxorubicin + stem cells |
Doxorubicin + C. sinensis |
| Radial Strain | ||||
| Velocity (cm/s) | 1.95(0.4) | 1.02(0.4)* | 1.94(0.6)† | 1.08(0.2)* |
| Displacement (mm) | 0.90(0.2) | 0.49(0.2)* | 0.80(0.2)† | 0.51(0.2)* |
| Strain (%) | 21.7(2.8) | 13.0(7.3) | 33.3(25.4) | 11.0(4.2) |
| Strain rate (1/s) | 5.7(0.5) | 3.9(0.5) | 5.9(2.3) | 3.7(0.3) |
| M-mode | ||||
| Ejection fraction (%) | 70.7(3.9) | 60.8(13.0) | 73.6(1.9) | 63.6(4.1) |
| Fractional shortening (%) | 41.6(2.7) | 33.4(9.2) | 43.6(1.6) | 35.2(3.5) |
p < 0.05 vs. Control;
p < 0.05 vs. Doxorubicin
Figure 2.
Illustrative 3D parametric displays of global left ventricle radial velocity with the Vevo strain software in one Wistar rat of each experimental group: control (A), doxorubicin (B), doxorubicin + stem cell (C), doxorubicin + Camellia sinensis (D). The upper value of each graph was highlighted to better visualization.
Figure 3.
Illustrative 3D parametric displays of global left ventricle radial displacement with the Vevo strain software in one Wistar rat of each experimental group: control (A), doxorubicin (B), doxorubicin + stem cell (C), doxorubicin + Camellia sinensis (D) The upper value of each graph was highlighted to better visualization.
Mesenchymal stem cell engraftment
Cell engraftment was evaluated in host cardiac tissue by PCR analysis after 33 days of MSC intravenously injection. No GFP-positive MSCs were detected on rat heart tissues by PCR analysis. The lack of diagnosis may be attributed to the fact that only a sample of the tissue was used to DNA extraction (samples were required for histopathological analysis) and that MSCs had been injected intravenously rather than in situ.
Discussion
Regarding the high incidence of cancer in the population worldwide and the importance of doxorubicin cardiotoxicity, this research shows that the side effects of chemotherapy on the heart may be early detected by strain echocardiography. Such approach is of great importance for allowing premature medical intervention in order to prevent secondary damages. Moreover, stem cell therapy using MSCs shows a promising improvement on left ventricular function against Dox-cardiotoxicity which is detected in advance with strain echocardiography.
Even though no alteration was observed on conventional echocardiography measurements, ECG examination and histopathology in the Dox group, the occurrence of death, weight loss, and the values recorded on strain analysis indicate clinical intoxication if compared with the control group. Death, weight loss, vomiting and diarrhea are commonly reported side effects of Dox administration, in accordance with others using the same animal model [22,23]. Animals from the Dox + C. sinensis group had the most significant body weight loss probably due to a combination of gastrointestinal toxicoses in addition to the use of green tea extract. This combination increments both the metabolic activity and the diuresis [24], improving the weight loss regardless of the fact that neither water intake nor urinary debt were measured in this study. Besides death occurrence and body weight loss, no alterations were detected either at clinical pathology or at histopathological analysis, indicating that the Dox-related heart disease was not severe in this sample. Similar to our results in a previous report [22], rats that received Dox showed body weight loss, decreased core body temperature, and atrial and ventricular arrhythmias; but no evidence of focal degeneration, inflammation or fibrosis, which are indicative of cardiomyopathy, had been detected on histopathological examinations either. Additionally, body weight loss seems to be the reason for the high cardiac enlargement index founded on Dox and Dox + C. sinensis groups. It is known that high heart-weight/bodyweight ratio may be considered as an index for cardiac enlargement [25]. Regarding the sample studied, and taking into consideration that cardiac enlargement was not observed in either echocardiography or histopathological analysis, such high ratio mainly reflects the body weight loss. Furthermore, serum biochemical parameters, such as CK, CK-MB and LDH are considered in the assessment and monitoring of cardiac toxicity [22]. Our results, which showed normal serum biochemical profile, may be explained by the short exposure to the drug, which was not long enough to promote alterations in these values.
Major ECG abnormalities detected due to Dox-cardiotoxicity are atrial and ventricular arrhythmias [3,5,22]. Cardiac arrhythmias were not diagnosed in the sample studied, which may be explained by the severity of the cardiac damage or by the 10-minute length evaluation. As cardiac arrhythmias have a transitory occurrence, the 10-minute recording may not have been enough for the diagnosis, since in a similar Dox cardiomyopathy model it was detected atrioventricular block and ventricular and atrial extrasystoles in animals evaluated with telemetry [22]. The only ECG abnormalities detected were tall-peaked T waves, which are generally related to electrolyte imbalance, mainly hyperkalemia [26]. Even though potassium serum measurement had not been performed, it is possible to infer from poor score condition that rats were in electrolyte imbalance. Like in the present study, such ECG findings were also reported by other researchers studying the same heart disease model [22].
Echocardiographic findings on Dox-induced cardiotoxicity indicate left ventricular dysfunction, mainly decreasing values of ejection fraction and fractional shortening [3,5,20,22]. However, such alterations are frequently detected only when the patient had already developed heart failure. Therefore, early-time point diagnosis is needed, mainly at subclinical stage of the disease, requiring a more precise and accurate evaluation of the ventricular function [2,27]. Strain echocardiography enables the study of myocardial function independently of the ultrasound beam direction [15], providing quantification of regional myocardial systolic function with higher efficiency than Doppler tissue imaging [14], and with higher specificity than conventional measures [19,20,28]. In the present data, both the ejection fraction and the fractional shortening remain normal, although changes in such parameters are, indeed, reported for Dox-induced cardiomyopathy in experimental studies [20,29,30]. These studies, however, were performed with either higher doses of Dox, or intravenous injections or patients remained in treatment for a longer time, leading to a more severe cardiac damage and, as a consequence, lower ejection fraction and fractional shortening values. Furthermore, case report studies describe that patients under Dox chemotherapy are, indeed, kept in treatment for longer periods of time [4,5]. Therefore, it is possible to infer whether the animals had been kept in treatment for a longer time or had been using a higher dose of Dox, so that the changes in M-mode dependent variables could have been detected. The lack of diagnosis by conventional echocardiographic measures may be due to limitations of the technique itself, for in different cardiac pathologies the myocardial wall deformation becomes distorted, misleading the evaluation either with M-mode or 2D [28]. On the other hand, ultrasound deformation imaging obtained by speckle tracking, i.e. strain echocardiography, provides more-sensitive marker of early myocardial dysfunction compared with standard echocardiography [14,15,28], supporting our findings. Besides the Dox-cardiotoxicity, in the present research strain echocardiography also detected an improvement on left ventricular function of Dox treated animals which received MSC through tail vein, prior the beginning of the experiment. However, the flavonoid extract from C. sinensis had no protective effect in the heart, although studies have demonstrated that antioxidants from natural sources preserve heart from toxic damage [9,10,31,32]. Thereafter it is possible to infer that a higher dose of the extract may be required; or that epigallocatechin - the major component of the extract - presents limited potential to disrupt Dox toxicity; or even that other pathological mechanisms, instead of the oxidative stress hypothesis, are involved, as highlighted by the First International Workshop on Anthracycline Cardiotoxicity [2]. On the other hand, the MSC effectively preserved myocardial function and, in reality, global radial strain parameters were similar than those recorded in the control group. Recently it was reported that high doses of MSCs (up to 6 × 106 cells to a single mice) did not lead to any toxic effect or death [33]. So, it is possible to infer that the amount of MSC injected in the animals is a safe dose and the death occurrence in the MSCs treated group is due to toxicity of the doxorubicin, instead of the cell therapy. The fact that GFP positive cells were not detected in cardiac tissue 33 days after injection suggests that engraftment of MSC was either absent or very low. Therefore it is very likely that the MSC protective effects were instead associated with the release of mediators which influence the survival, differentiation, and proliferations of host tissue cells, via paracrine effects [11,12]. Among these mediators it has been reported the expression of VEGF, iNOS, and connexin-43, a GAP junction protein [34,35]. Although the lack of detection of MSC in heart tissue, our group and others as well have already been demonstrating that such cells are able to migrate to sites of tissue injury and inflammation [36,37], even after intravenous injection when a significant number of cells are mostly trapped in the microvasculature of the lung due to their size and the adhesion potential [37]. It was also seen that systemic delivery of manipulated MSC indeed provides a feasible therapeutic approach, as demonstrated by the analysis of the migration behavior of MSC in photogenic transgenic rats that express fluorescent and/or luminescent proteins [38], thus supporting the methodology applied to this work. Similar to this present research, other researchers working with different disease models also failed to detect the presence of the stem cells in the host organs, although the beneficial effects due to cell therapy were also observed [39,40].
In conclusion, both the Dox-induced cardiotoxicity and the ventricular improvement after stem cell therapy were only detected in advance with strain echocardiography. Although the results of this work are promising, more studies are necessary to determine how early and precise the strain echocardiography can detect ventricular function alterations in this setting.
Acknowledgements
National Institutes of Health (NIH Grant 1R03TW008709) and “Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for financial support; “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES) for the scholarship awarded to M. S. Oliveira; Prof. Robson A. S. Santos (ICB – UFMG) for providing the echocardiography facilities; Prof. Angela Maria Quintão Lana and Danilo Gonçalves Bastos (EV – UFMG) for their assistance with statistical analyses; Tayio International Inc. (MN - USA – Scott J. Smith) and REP & SAGA (SP – Brasil – Fernando Santana) for their donation of the green tea extract, Sunphenon DCF.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Butany J, Ahn E, Luk A. Drug-related cardiac pathology. J Clin Pathol. 2009;62:1074–1084. doi: 10.1136/jcp.2008.058255. [DOI] [PubMed] [Google Scholar]
- 2.Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, et al. Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol. 2008;26:3777–3784. doi: 10.1200/JCO.2007.14.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alves de Souza RC, Camacho AA. Neurohormonal, hemodynamic, and electrocardiographic evaluations of healthy dogs receiving long-term administration of doxorubicin. Am J Vet Res. 2006;67:1319–1325. doi: 10.2460/ajvr.67.8.1319. [DOI] [PubMed] [Google Scholar]
- 4.Testore F, Milanese S, Ceste M, de Concilis E, Parello G, et al. Cardioprotective effect of dexrazoxane in patients with breast cancer treated with anthracyclines in adjuvant setting: a 10-year single institution experience. Am J Cardiovasc Drugs. 2008;8:257–263. doi: 10.2165/00129784-200808040-00005. [DOI] [PubMed] [Google Scholar]
- 5.Aapro M, Bernard-Marty C, Brain EG, Batist G, Erdkamp F, et al. Anthracycline cardiotoxicity in the elderly cancer patient: a SIOG expert position paper. Ann Oncol. 2011;22:257–267. doi: 10.1093/annonc/mdq609. [DOI] [PubMed] [Google Scholar]
- 6.Yao CX, Li WY, Zhang SF, Zhang SF, Zhang HF, et al. Effects of Doxorubicin and Fenofibrate on the activities of NADH oxidase and citrate synthase in mice. Basic Clin Pharmacol Toxicol. 2011;109:452–456. doi: 10.1111/j.1742-7843.2011.00748.x. [DOI] [PubMed] [Google Scholar]
- 7.Tan X, Wang DB, Lu X, Wei H, Zhu R, et al. Doxorubicin induces apoptosis in H9c2 cardiomyocytes: role of overexpressed eukaryotic translation initiation factor 5A. Biol Pharm Bull. 2010;33:1666–1672. doi: 10.1248/bpb.33.1666. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Kang YM, Tian C, Zeng Y, Jia LX, et al. Overexpression of Nrdp1 in the heart exacerbates doxorubicin-induced cardiac dysfunction in mice. PLoS One. 2011;6:e21104. doi: 10.1371/journal.pone.0021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bast A, Haenen GR, Bruynzeel AM, Van der Vijgh WJ. Protection by flavonoids against anthracycline cardiotoxicity: from chemistry to clinical trials. Cardiovasc Toxicol. 2007;7:154–159. doi: 10.1007/s12012-007-0018-0. [DOI] [PubMed] [Google Scholar]
- 10.Soucek P, Kondrova E, Hermanek J, Stopka P, Boumendjel A, et al. New model system for testing effects of flavonoids on doxorubicin-related formation of hydroxyl radicals. Anticancer Drugs. 2011;22:176–184. doi: 10.1097/cad.0b013e328341a17b. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Liu W, Li W, Gao C. Autologous bone marrow mesenchymal cell transplantation improves left ventricular function in a rabbit model of dilated cardiomyopathy. Exp Mol Pathol. 2010;88:311–315. doi: 10.1016/j.yexmp.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Triantafyllou KA, Karabinos E, Kalkandi H, Kranidis AI, Babalis D. Clinical implications of the echocardiographic assessment of left ventricular long axis function. Clin Res Cardiol. 2009;98:521–532. doi: 10.1007/s00392-009-0046-9. [DOI] [PubMed] [Google Scholar]
- 14.Sahlen A, Winter R. How should we measure global and regional left ventricular systolic function? J Echocardiogr. 2011;9:41–50. doi: 10.1007/s12574-011-0085-x. [DOI] [PubMed] [Google Scholar]
- 15.Motoki H, Nakatani S, Abe H, Kanzaki H, Kitakaze M. Heterogeneous contraction of the left ventricle demonstrated by 2-dimensional strain imaging. J Echocardiogr. 2010;8:33–39. doi: 10.1007/s12574-009-0027-z. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien PJ. Cardiac troponin is the most effective translational safety biomarker for myocardial injury in cardiotoxicity. Toxicology. 2008;245:206–218. doi: 10.1016/j.tox.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Salcedo EE, Moloo J, Quaife R, Wolfel E. Imaging heart failure in 2010. Curr Cardiovasc Imaging Reports. 2010;3:303–316. [Google Scholar]
- 18.Goda A, Nakao S, Tsujino T, Yuba M, Otsuka M, et al. Determinants of plasma brain natriuretic peptide levels in untreated hypertensive patients. J Echocardiogr. 2011;9:103–108. doi: 10.1007/s12574-011-0086-9. [DOI] [PubMed] [Google Scholar]
- 19.Piegari E, Di Salvo G, Castaldi B, Vitelli MR, Rodolico G, et al. Myocardial strain analysis in a doxorubicin-induced cardiomyopathy model. Ultrasound Med Biol. 2008;34:370–378. doi: 10.1016/j.ultrasmedbio.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Migrino RQ, Aggarwal D, Konorev E, Brahmbhatt T, Bright M, et al. Early detection of doxorubicin cardiomyopathy using two-dimensional strain echocardiography. Ultrasound Med Biol. 2008;34:208–214. doi: 10.1016/j.ultrasmedbio.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hazari MS, Haykal-Coates N, Winsett DW, Costa DL, Farraj AK. Continuous electrocardiogram reveals differences in the short-term cardiotoxic response of Wistar-Kyoto and spontaneously hypertensive rats to doxorubicin. Toxicol Sci. 2009;110:224–234. doi: 10.1093/toxsci/kfp092. [DOI] [PubMed] [Google Scholar]
- 23.Kenk M, Thackeray JT, Thorn SL, Dhami K, Chow BJ, et al. Alterations of pre- and postsynaptic noradrenergic signaling in a rat model of adriamycin-induced cardiotoxicity. J Nucl Cardiol. 2010;17:254–263. doi: 10.1007/s12350-009-9190-x. [DOI] [PubMed] [Google Scholar]
- 24.Basu A, Sanchez K, Leyva MJ, Wu M, Betts NM, et al. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J Am Coll Nutr. 2010;29:31–40. doi: 10.1080/07315724.2010.10719814. [DOI] [PubMed] [Google Scholar]
- 25.Penney D, Benjamin M, Dunham E. Effect of carbon monoxide on cardiac weight as compared with altitude effects. J Appl Physiol. 1974;37:80–84. doi: 10.1152/jappl.1974.37.1.80. [DOI] [PubMed] [Google Scholar]
- 26.Enselberg CD, Simmons HG, Mintz AA. The effects of potassium upon the heart, with special reference to the possibility of treatment of toxic arrhythmias due to digitalis. Am Heart J. 1950;39:713–728. doi: 10.1016/0002-8703(50)90131-1. [DOI] [PubMed] [Google Scholar]
- 27.Tassan-Mangina S, Codorean D, Metivier M, Costa B, Himberlin C, et al. Tissue Doppler imaging and conventional echocardiography after anthracycline treatment in adults: early and late alterations of left ventricular function during a prospective study. Eur J Echocardiogr. 2006;7:141–146. doi: 10.1016/j.euje.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Bauer M, Cheng S, Jain M, Ngoy S, Theodoropoulos C, et al. Echocardiographic speckle-tracking based strain imaging for rapid cardiovascular phenotyping in mice. Circ Res. 2011;108:908–916. doi: 10.1161/CIRCRESAHA.110.239574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandran K, Aggarwal D, Migrino RQ, Joseph J, McAllister D, et al. Doxorubicin inactivates myocardial cytochrome c oxidase in rats: cardioprotection by Mito-Q. Biophys J. 2009;96:1388–1398. doi: 10.1016/j.bpj.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiang P, Deng HY, Li K, Huang GY, Chen Y, et al. Dexrazoxane protects against doxorubicin-induced cardiomyopathy: upregulation of Akt and Erk phosphorylation in a rat model. Canc Chemother Pharmacol. 2009;63:343–349. doi: 10.1007/s00280-008-0744-4. [DOI] [PubMed] [Google Scholar]
- 31.Wattanapitayakul SK, Chularojmontri L, Herunsalee A, Charuchongkolwongse S, Niumsakul S, et al. Screening of antioxidants from medicinal plants for cardioprotective effect against doxorubicin toxicity. Basic Clin Pharmacol Toxicol. 2005;96:80–87. doi: 10.1111/j.1742-7843.2005.pto960112.x. [DOI] [PubMed] [Google Scholar]
- 32.Anjos Ferreira AL, Russell RM, Rocha N, Placido Ladeira MS, Favero Salvadori DM, et al. Effect of lycopene on doxorubicin-induced cardiotoxicity: an echocardiographic, histological and morphometrical assessment. Basic Clin Pharmacol Toxicol. 2007;101:16–24. doi: 10.1111/j.1742-7843.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 33.Sacerdote P, Niada S, Franchi S, Arrigoni E, Rossi A, et al. Systemic administration of human Adipose-derived Stem Cells (hASCs) reverts nociceptive hypersensitivity in an experimental model of neuropathy. Stem Cells Dev. 2012 doi: 10.1089/scd.2012.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hahn JY, Cho HJ, Kang HJ, Kim TS, Kim MH, et al. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51:933–943. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 35.Schlosser S, Dennler C, Schweizer R, Eberli D, Stein JV, et al. Paracrine effects of mesenchymal stem cells enhance vascular regeneration in ischemic murine skin. Microvasc Res. 2012;83:267–275. doi: 10.1016/j.mvr.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Satake K, Lou J, Lenke LG. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine. 2004;29:1971–1979. doi: 10.1097/01.brs.0000138273.02820.0a. [DOI] [PubMed] [Google Scholar]
- 37.Assis AC, Carvalho JL, Jacoby BA, Ferreira RL, Castanheira P, et al. Time-dependent migration of systemically delivered bone marrow mesenchymal stem cells to the infarcted heart. Cell Transplant. 2010;19:219–230. doi: 10.3727/096368909X479677. [DOI] [PubMed] [Google Scholar]
- 38.Hara M, Murakami T, Kobayashi E. In vivo bioimaging using photogenic rats: fate of injected bone marrow-derived mesenchymal stromal cells. J Autoimmun. 2008;30:163–171. doi: 10.1016/j.jaut.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Cargnoni A, Di Marcello M, Campagnol M, Nassuato C, Albertini A, et al. Amniotic membrane patching promotes ischemic rat heart repair. Cell Transplant. 2009;18:1147–1159. doi: 10.3727/096368909X12483162196764. [DOI] [PubMed] [Google Scholar]
- 40.Sant’Anna LB, Cargnoni A, Ressel L, Vanosi G, Parolini O. Amniotic membrane application reduces liver fibrosis in a bile duct ligation rat model. Cell Transplant. 2011;20:441–453. doi: 10.3727/096368910X522252. [DOI] [PubMed] [Google Scholar]