Abstract
Purpose
Biomarkers of ionising radiation exposure are useful in a variety of scenarios, such as medical diagnostic imaging, occupational exposures, and spaceflight. This study investigates to what extent microRNA (miRNA) expression signatures in mouse peripheral blood can be used as biomarkers for exposures to radiation with low and high linear energy transfers.
Materials and methods
Mice were irradiated with doses of 0.5, 1.5, or 5.0 Gy γ-rays (dose rate of 0.0136 Gy/s) or with doses of 0.1 or 0.5 Gy 56Fe ions (dose rate of 0.00208 Gy/s). Total RNA was isolated from whole blood at 6 h or 24 h after irradiation. Three animals per irradiation condition were used. Differentially expressed miRNA were determined by means of quantitative real-time polymerase chain reaction.
Results
miRNA expression signatures were radiation type-specific and dose- and time-dependent. The differentially expressed miRNA were expressed in either one condition (71%) or multiple conditions (29%). Classifiers based on the differentially expressed miRNA predicted radiation type or dose with accuracies between 75% and 100%. Gene-ontology analyses show that miRNA induced by irradiation are involved in the control of several biological processes, such as mRNA transcription regulation, nucleic-acid metabolism, and development.
Conclusion
miRNA signatures induced by ionising radiation in mouse blood are radiation type- and radiation dose-specific. These findings underline the complexity of the radiation response and the importance of miRNA in it.
Keywords: microRNA, biodosimetry, whole body irradiation, high-LET radiation, class prediction, gene ontology analysis
Introduction
Biomarkers of ionising radiation exposure are useful in a variety of scenarios, such as medical diagnostic imaging, occupational exposures, and spaceflights. In contrast with physical dosimeters, radiation biomarkers have the potential to provide information on both radiation dose and radiation-induced changes of biological functions. In general, two types of biomarkers have been developed. The first type is based on the detection of radiation-induced chromosomal damage, such as chromosomal translocations and micronuclei formation. Such biomarkers have been successfully used in retrospective radiation biodosimetry and have become established biomarkers for radiation exposure (Durante et al. 2003, Rodrigues et al. 2005, George et al. 2010). The second group of radiation biomarkers shows radiation-induced functional changes in cells, tissues, and organisms. Recently, high-throughput methodologies have been used to estimate the extent of radiation-induced functional changes. Comparisons of gene expression between irradiated and control samples effectively discriminate irradiated from non-irradiated samples, as well as samples exposed to low doses of radiation from samples exposed to high doses. Additionally, functional analyses of the differentially expressed genes show that radiation induces changes in many cellular processes (Fachin et al. 2007, Amundson 2008, Paul and Amundson 2008, Roy et al. 2009, Brengues et al. 2010). These studies demonstrate the feasibility of using gene expression to estimate both radiation dose and the physiological effects of ionising radiation.
The main goal of our study is to show to what degree microRNA (miRNA) can be used as biomarkers for exposure to ionising radiation. miRNA are a very important class of non-protein-coding genes. They are predicted to regulate the expression of more than 50% of the human protein-coding genes by means of mRNA destabilisation and translational repression (Shkumatava et al. 2009). miRNA have been shown to play important roles in cell signalling pathways, physiological processes, and human pathologies (Mehler and Mattick 2007, Carthew and Sontheimer 2009, Croce 2009, Fineberg et al. 2009, Umbach and Cullen 2009). Previous studies have shown that radiation with low linear energy transfer (LET) can induce changes in miRNA expression profiles in normal human fibroblasts (Maes et al. 2008, Simone et al. 2009) and immortalised cell lines (Ishii and Saito 2006, Marsit et al. 2006, Josson et al. 2008, Chaudhry 2009, Shin et al. 2009).
In the present study, we validated miRNA as biomarkers for exposure to low-LET γ-rays and high-LET 56Fe ions. 56Fe and other heavy ions are components of galactic cosmic rays which pose a health threat to astronauts, particularly on missions beyond low earth orbit. Our investigation is an in vivo proof-of-principle study, in which we exposed C57BL/6 mice to various doses of γ-ray or 56Fe-ion total body irradiation and measured miRNA expression levels in the blood of the irradiated and control mice. Due to its easy accessibility and the relative non-invasiveness of phlebotomy, blood is an excellent tissue for biomarker tests.
We identified 31 differentially expressed miRNA in the various irradiation conditions used in our study, including four dose-dependent ones, the expression levels of which gradually increase with increasing radiation dose. Based on this set of radiation-responsive miRNA, we developed miRNA-based statistical classifiers that can determine the type of radiation and the radiation dose of samples with unknown irradiation status. The analysis of miRNA target genes shows that they participate in biological processes such as transcription regulation, nucleic-acid binding and metabolism, and developmental processes.
Materials and methods
Animals and irradiation
All animal husbandry and experimental procedures were conducted in accordance with applicable federal and state guidelines and approved by the Animal Care and Use Committees of Columbia University Medical Center and Brookhaven National Laboratory. Forty-two male C57BL/6 mice obtained from Taconic (Hudson, NY, USA) were used in this study. Animals were kept in holding cages in groups of three, on a regular 12 h/12 h L/D cycle with ad libitum access to food and water. Animals were 12–15 weeks of age when they were exposed to ionising radiation. Three animals were used per condition. Twenty-four mice were exposed to doses of 0 (control), 0.5, 1.5, or 5 Gy 137Cs γ-rays at a dose rate of 0.0136 Gy/s at the Center for Radiological Research at Columbia University Medical Center. Eighteen animals were irradiated with doses of 0 (control), 0.1, or 0.5 Gy 1 GeV/n 56Fe ions at a dose rate of 0.00208 Gy/s at the National Aeronautics and Space Administration (NASA) Space Radiation Laboratory at Brookhaven National Laboratory. Extra care was taken to avoid stressing the animals during the experiments in order to minimise stress-induced changes in transcription and to ensure that the measured miRNA expression changes were indeed caused by irradiation. For this reason, we irradiated the mice in relatively large cages that did not unnecessarily restrain the animals’ movements. The mice used at Columbia University were irradiated three at a time in specially designed cylindrical containers with a diameter of 30 cm and a height of 10 cm, whereas the animals used at Brookhaven National Laboratory were individually irradiated in boxes sized 11 × 8 × 6 cm (w/l/h). Animals that did not receive the maximum radiation dose were kept in their cages in the radiation facility for the same time as the animals exposed to the highest dose in order to standardise treatment conditions (~6 min for γ irradiation and ~4 min for 56Fe irradiation).
Blood collection and RNA isolation
Six or 24 h after irradiation, 250 µl blood was collected from the mice using the submandibular method. The blood was collected directly in tubes containing lysis solution, a procedure which preserves the in vivo miRNA expression signatures. Three samples per condition were collected, resulting in a sample size of n = 3 biological repeats per irradiation dose and time point. Each mouse was bled only once, and different animals were used for each irradiation condition (radiation type, time point, and dose). Total RNA was purified using the mirVana™ PARIS™ kit according to the manufacturer’s instructions (Applied Biosystems/Ambion, Austin, TX, USA). Neither blood nor RNA from different animals was pooled, and all samples were processed and analysed separately. RNA concentration was measured on a Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and RNA integrity was determined using the Agilent 2100 Bioanalyzer microelectrophoretic system (Agilent Technologies, Santa Clara, CA, USA).
Determination of miRNA expression signatures by means of quantitative polymerase chain reaction
In order to determine miRNA expression in the blood samples, 384-well low-density TaqMan® rodent miRNA expression plates for quantitative real-time polymerase chain reaction (PCR) were used according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA, USA). This methodology has been shown to provide data with greater precision than the data obtained from microarrays, which are based on solid-surface hybridisations between oligonucleotide probes and target molecules, and this technology is routinely used to validate data obtained from microarray studies (Pradervand et al. 2010, Yauk et al. 2010). Also, it has been shown that the determination of relative miRNA expression by means of quantitative PCR is highly correlated to expression results obtained from RNA sequencing-based techniques (Pradervand et al. 2010).
Data processing for determination of differentially expressed miRNA
The quantitative real-time PCR data was imported into RQ Manager v. 1.2 (Applied Biosystems) to determine threshold-cycle (CT) values. The data was then exported to Excel (Microsoft Corporation, Redmond, WA, USA) for preprocessing, which included the removal of the non-relevant rat miRNA and of the miRNA that did not have detectable expression (CT = 40) in both irradiated and control samples. The preprocessed data was then imported into RealTime StatMiner™ v. 3.1 software (Integromics™, Madrid, Spain) for statistical analysis. The statistical analysis included three steps: (1) Normalisation of the expression data, (2) calculation of the p-values of the differentially expressed miRNA, and (3) calculation of the false discovery rate (FDR) for the results from step 2. The median value of each quantitative-PCR plate was used for normalisation in step 1 because all three endogenous controls supplied with a plate proved to be radiation-sensitive (Hunter et al. 2008). In step 2, a moderated t-test (Bioconductor limma package; Smyth 2004) was used to determine the p-values for the miRNA expression levels in the different irradiation conditions, with the respective calibrator control samples serving as the reference group. In step 3, the FDR was adjusted according to the method of Benjamini and Hochberg (Benjamini and Hochberg 1995). miRNA with FDR-adjusted p-values of less than 0.07 were included in the set of differentially expressed miRNA. Additionally, we monitored the statistical power, using the methodology of Lee and Whitmore (Lee and Whitmore 2002), in order to determine the sensitivity of our analyses. This methodology assumes that the differential expression values follow an approximate multivariate normal distribution, based on independent log-intensity values derived from multiple repeated observations and the central limit theorem. Our analysis is based on the R package ‘sizepower’ by Qiu, Lee, and Whitmore (2010), which requires as input parameters the mean number of false positives, the anticipated number of undifferentially expressed genes, the mean difference in log-expression between treatment and control conditions as postulated under the alternative hypothesis, the anticipated standard deviation of the difference in log-expression between treatment and control conditions, and the group sample size.
Standard errors of the mean (SEM) for fold changes shown in Tables I and II contain the variability of both control and irradiated samples and were calculated according to the following formula:
with SDΔCT depicting the standard deviation (SD) among the normalised CT (ΔCT) values of the samples belonging to a group.
Table I.
MicroRNA differentially expressed in whole mouse blood after exposure to ionizing radiation in vivo. Mice were irradiated with γ-rays (doses of 0, 0.5, 1.5, or 5.0 Gy) or 56Fe particles (doses of 0, 0.1, or 0.5 Gy), 250 µl blood was collected directly in lysis solution, and total RNA was purified 6 and 24 h after irradiation. miRNA expression levels were determined by 384-well low-density TaqMan® real-time PCR miRNA expression assays. The expression data was preprocessed and normalised. Fold changes in expression level, relative to non-irradiated control samples, with a false discovery rate of < 0.07 were considered to be statistically significant.
| Irradiation condition | miRNA ID | Only expressed in one condition?a |
Fold change ± SEM |
p-value | FDR |
|---|---|---|---|---|---|
| γ 6 h 0.5 Gy | miR-511 | R | 197.0 ± 1.18 | 5.2E-08 | 9.4E-06 |
| miR-598 | R | 20.0 ± 2.89 | 1.6E-06 | 1.4E-04 | |
| γ 6 h 1.5 Gy | miR-135a | 4.0 ± 1.26 | 6.3E-04 | 0.0182 | |
| miR-147 | R | 80.6 ± 3.30 | 3.1E-07 | 2.7E-05 | |
| miR-150 | −2.2 ± 1.17 | 0.0028 | 0.0676 | ||
| miR-200b | C | −686.4 ± 28.72 | 2.1E-07 | 2.7E-05 | |
| miR-547 | C | −18.8 ± 4.14 | 9.9E-06 | 5.6E-04 | |
| miR-680 | R | 182.8 ± 1.59 | 1.3E-05 | 5.6E-04 | |
| miR-685 | R | 170.0 ± 12.34 | 3.6E-05 | 0.0029 | |
| miR-708 | C | −43.0 ± 5.35 | 2.2E-04 | 0.0075 | |
| γ 6 h 5.0 Gy | miR-10a | 1.8 ± 1.08 | 0.0027 | 0.0529 | |
| miR-135a | 19.9 ± 1.15 | 8.0E-07 | 4.6E-05 | ||
| miR-135b | 8.1 ± 1.54 | 0.0010 | 0.0267 | ||
| miR-139-5p | 3.8 ± 1.23 | 4.0E-04 | 0.0140 | ||
| miR-147 | R | 215.0 ± 3.24 | 0.0011 | 0.0267 | |
| miR-223 | 3.6 ± 1.09 | 4.1E-05 | 0.0018 | ||
| miR-450a-5p | 2.2 ± 1.12 | 0.0012 | 0.0267 | ||
| miR-680 | R | 2530.0 ± 1.35 | 4.9E-08 | 4.3E-06 | |
| miR-685 | R | 2430.0 ± 12.55 | 5.9E-09 | 1.0E-06 | |
| γ 24 h 1.5 Gyb | miR-335-3p | 4.2 ± 1.25 | 2.6E-04 | 0.0229 | |
| miR-667 | C | −91.8 ± 6.05 | 4.5E-09 | 8.0E-07 | |
| γ 24 h 5.0 Gy | miR-125a-3p | 3.6 ± 1.35 | 0.0020 | 0.0327 | |
| miR-146a | −3.9 ± 1.33 | 0.0011 | 0.0221 | ||
| miR-150 | −19.7 ± 1.39 | 1.5E-05 | 5.3E-04 | ||
| miR-151-3p | 2.5 ± 1.21 | 0.0031 | 0.0453 | ||
| miR-211 | C | −377.2 ± 5.97 | 3.0E-07 | 1.3E-05 | |
| miR-335-3p | 3.7 ± 1.27 | 7.5E-04 | 0.0166 | ||
| miR-337-3p | C | −30.0 ± 3.67 | 1.4E-04 | 0.0042 | |
| miR-339-3p | 3.1 ± 1.24 | 0.0013 | 0.0235 | ||
| miR-342-3p | −6.5 ± 1.39 | 3.3E-04 | 0.0083 | ||
| miR-501-3p | R | 12300.0 ± 1.18 | 3.3E-10 | 5.9E-08 | |
| miR-511 | R | 107.0 ± 6.45 | 1.1E-07 | 9.6E-06 | |
| miR-667 | C | −100.1 ± 4.22 | 2.4E-07 | 1.3E-05 | |
| 56Fe 6 h 0.1 Gy | miR-125a-3p | C | −117.1 ± 3.75 | 1.5E-08 | 2.7E-06 |
| miR-350 | −1.7 ± 1.06 | 3.7E-04 | 0.0169 | ||
| miR-379 | R | 680.3 ± 1.61 | 8.1E-06 | 5.0E-04 | |
| miR-383 | C | −21.5 ± 1.24 | 6.9E-06 | 5.0E-04 | |
| 56Fe 6 h 0.5 Gy | miR-383 | C | −20.2 ± 1.25 | 4.4E-06 | 2.7E-04 |
| miR-501-3p | R | 336.9 ± 16.75 | 1.7E-10 | 3.1E-08 | |
| miR-680 | R | 107.6 ± 7.62 | 5.2E-07 | 4.7E-05 | |
| 56Fe 24 h 0.1 Gy | miR-409-3p | R | 64.1 ± 6.47 | 1.0E-08 | 9.8E-07 |
| miR-685 | R | 292.8 ± 10.76 | 7.0E-10 | 1.3E-07 | |
| 56Fe 24 h 0.5 Gy | miR-150 | −2.5 ± 1.17 | 8.6E-04 | 0.0326 | |
| miR-211 | −2.2 ± 1.10 | 2.5E-04 | 0.0232 | ||
| miR-342-3p | −2.2 ± 1.12 | 4.4E-04 | 0.0278 | ||
| miR-494 | −2.4 ± 1.17 | 8.1E-04 | 0.0326 | ||
| miR-879 | R | 35.3 ± 6.15 | 1.8E-08 | 3.4E-06 |
ID, identification; SEM, standard error of the mean; FDR, false discovery rate.
MicroRNA that are only expressed in the sham-irradiated controls (C) or after exposure to ionising radiation (R).
No miRNA were differentially expressed after 0.5 Gy γ irradiation at the 24 h time point.
Table II.
Dose-dependent microRNA. Dose-dependent miRNA show dose-dependent changes in expression levels, i.e., they show monotonic increases in expression levels with increasing radiation dose. Only results for miRNA whose expression levels are statistically significantly different from control expression levels as well as significantly different between doses of 1.5 Gy and 5.0 Gy are reported.
| miRNA ID | Irradiation condition | Fold change ± SEM |
|---|---|---|
| miR-135a | γ 6 h 1.5 Gy | 4.0 ± 1.26 |
| 5.0 Gy | 19.9 ± 1.15 | |
| miR-147 | γ 6 h 1.5 Gy | 80.6 ± 3.30 |
| 5.0 Gy | 215.0 ± 3.24 | |
| miR-680 | γ 6 h 1.5 Gy | 182.8 ± 1.59 |
| 5.0 Gy | 2530.0 ± 1.35 | |
| miR-685 | γ 6 h 1.5 Gy | 170.0 ± 12.34 |
| 5.0 Gy | 2430.0 ± 12.55 |
ID, identification; SEM, standard error of the mean.
Multidimensional scaling analysis of miRNA signatures
Multidimensional scaling (MDS) was performed in BRB-Array Tools v. 3.8.0 (Simon et al. 2007). A Euclidean distance metric was used to compute a distance matrix, and the first three principal components of miRNA expression were used as the axes for the MDS plot.
Development of miRNA-based radiation class predictors
BRB-Array Tools (Simon et al. 2007) was also used to perform class prediction using the ΔCT values of the differentially expressed miRNA. The nearest-centroid method was used as the prediction method. The centroid of each class is a vector containing the averages of the ΔCT values of the miRNA belonging to the class. The distance of the miRNA expression profile of a sample to each of the centroids is determined, and the sample is assigned to the class with the shortest distance. The robustness of the predictor was assessed by the leave-one-out cross-validated misclassification error rate with 10,000 random permutations, which produced error p-values of less than 0.02 for all predictors.
Gene ontology analysis of miRNA target genes
miRNA target genes were determined using TargetScanMouse v. 5.1 (Grimson et al. 2007). Only phylogenetically conserved targets with a context score ≤ −0.3, corresponding to a log2 gene expression ratio of ≤ −0.3, as determined by multivariate linear regression fitting of gene expression microarray data (Grimson et al. 2007), were used for gene-ontology (GO) analysis.
The predicted miRNA target genes were uploaded to the PANTHER GO database v. 6.0 (Thomas et al. 2003), which classifies genes according to GO terms, using published scientific experimental evidence. Gene set enrichment analysis was performed, with the mouse AB1700 gene list (Applied Biosystems) serving as the reference whole-genome list. A binomial test with Bonferroni correction for multiple testing was used for enrichment analysis for biological processes or molecular functions.
Results
miRNA expression signatures induced by radiation
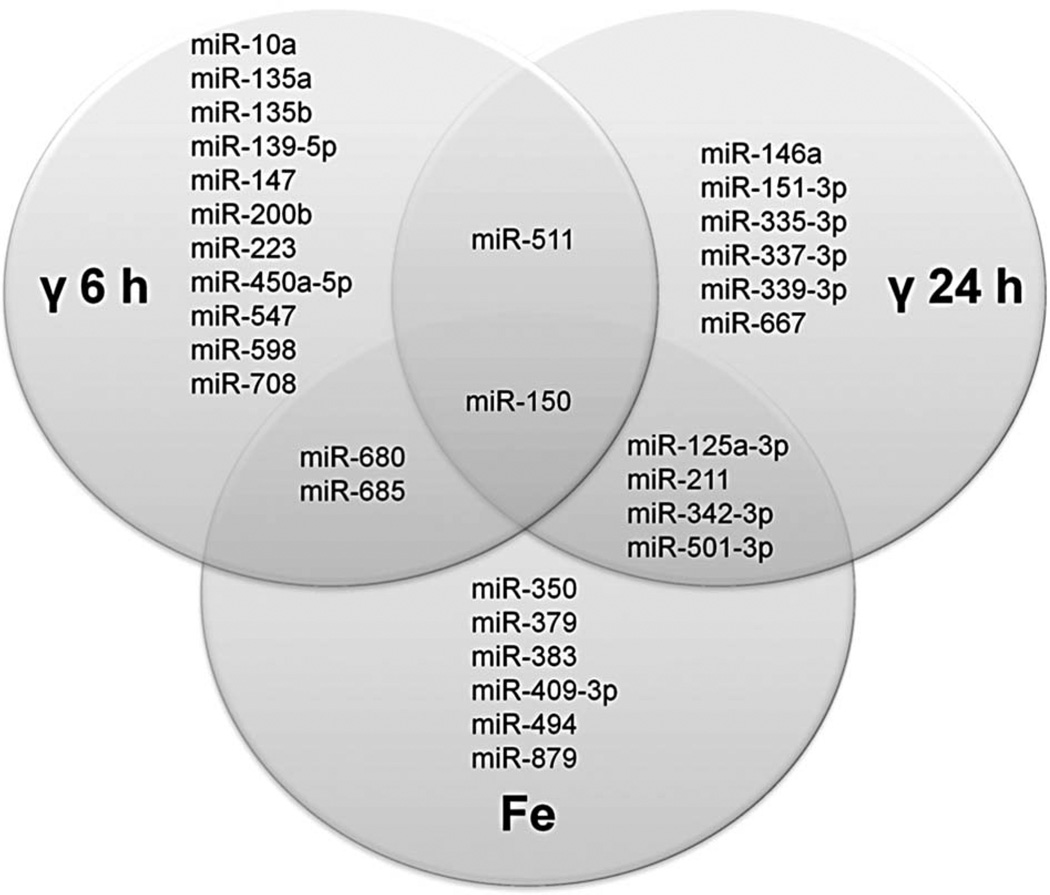
Mice were irradiated with γ-rays or 56Fe particles, 250 µl blood was collected, and total RNA was purified 6 and 24 h after irradiation. The γ irradiation regimen consisted of doses of 0 (control), 0.5, 1.5, and 5.0 Gy, and doses of 0 (control), 0.1, and 0.5 Gy were used for the iron-irradiated animals. miRNA expression levels were determined by 384-well low-density rodent miRNA TaqMan® quantitative real-time PCR expression assays. The filtered and normalised expression data was subjected to thorough statistical analysis, in which we controlled the p-values and the FDR of the differentially expressed miRNA and additionally monitored the statistical power of the analysis. A total of 98 mouse miRNA (out of 160 per sample that were, on average, amplified above background level) were differentially expressed with p-values of less than 0.05. The differentially expressed miRNA were subjected to an additional FDR analysis in order to account for the multiple comparison problem (Benjamini and Hochberg 1995). The FDR was set to 0.07, which means that only 7% of the miRNA declared as differentially expressed are expected to be false positives. After applying this restrictive adjustment, we identified 31 miRNA to be differentially expressed (FDR < 0.07, average p-value of 0.00008) (Table I). The differentially expressed miRNA were expressed in either one condition (71%) or multiple conditions (29%) (Figure 1). Interestingly, we found that the expression values of some differentially expressed miRNA monotonically increase with increasing radiation dose. These ‘dose-dependent’ miRNA, their fold changes, and SEM are depicted in Table II.
Figure 1.
Venn diagram showing miRNA differentially expressed in the indicated irradiation conditions. Areas of overlap among different circles depict miRNA differentially expressed in multiple conditions. For simplicity, miRNA expression at different doses of irradiation is not distinguished in the diagram, and miRNA that are differentially expressed at any dose corresponding to the respective radiation type and time point are shown. Both time points are combined for miRNA differentially expressed upon 56Fe irradiation.
The results from the power analysis show that the power to detect 2-fold differences between the compared experimental conditions is relatively low (average power of 0.37), but for differences of 3-fold and over, the statistical power increases considerably and is, on average, 0.83 for 3-fold differences, 0.96 for 4-fold differences, and 0.99 for 5-fold differences. Correspondingly, 23 out of the 31 detected differentially expressed miRNA exhibit a radiation-induced up- or downregulation of 3-fold or more. Since the effect of miRNA on gene downregulation depends on their expression levels (Bartel 2009), the miRNA exhibiting high fold changes are likely to have larger effects on gene expression than the ones that differed less between the irradiated and control samples. Therefore, the lack of detection power for low fold changes, although not to be underestimated, most probably does not significantly impact the results of the gene ontology analysis reported here.
Multidimensional scaling analysis of miRNA signatures
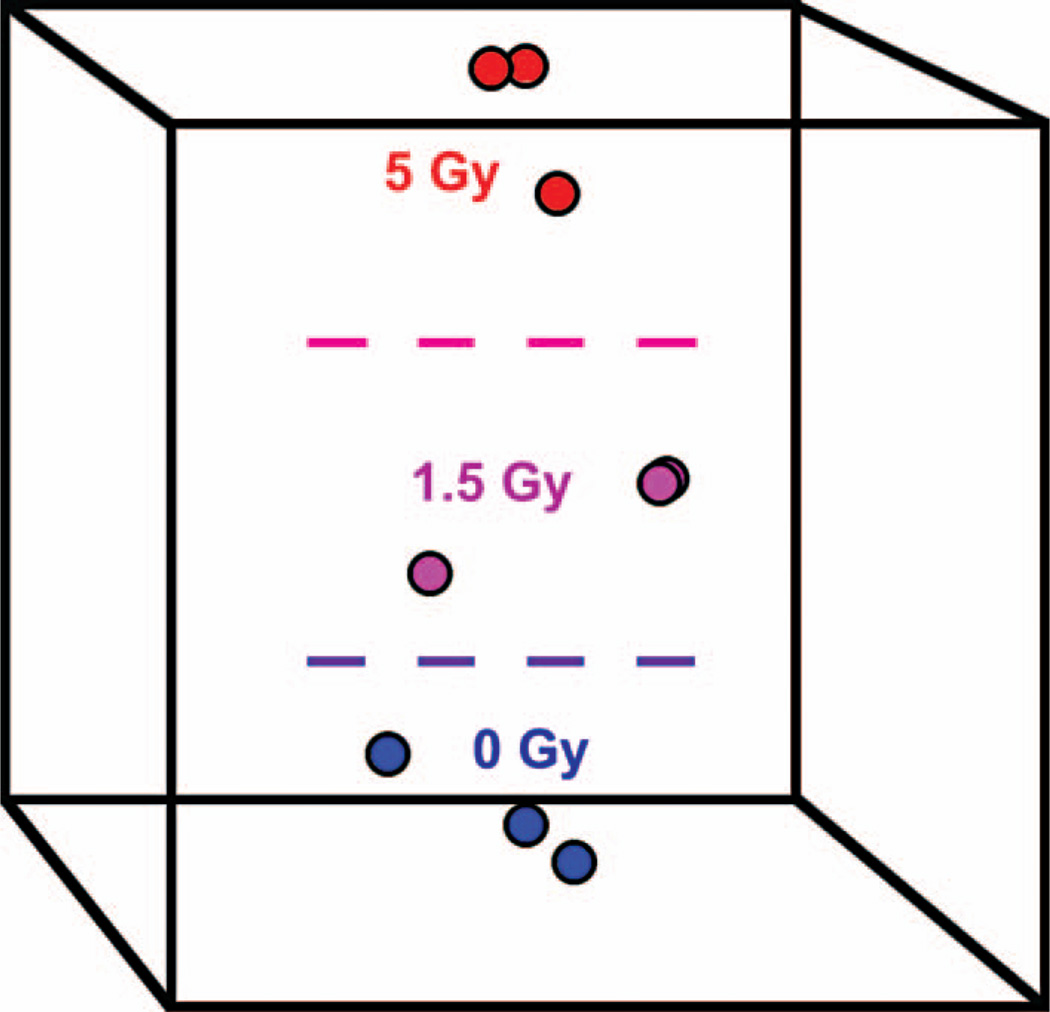
In order to visualise to what degree miRNA signatures can reveal the global differences between the different irradiation conditions, we used MDS for graphical representation. MDS was applied separately to each irradiation condition (radiation type, time point, and dose), and only miRNA that were differentially expressed were used to build up an MDS space. Figure 2 shows an MDS plot for the nine samples that were obtained 24 h after γ irradiation. As can be seen from the plot, MDS shows good separation between the samples, which confirms that the observed differences in miRNA expression are significant.
Figure 2.
Clustering of samples by means of multidimensional scaling (MDS). The nine γ ray-treated samples collected at 24 h after irradiation were projected onto a space spanned by three orthogonal axes (represented by the box) that optimally preserves the information about the differences in miRNA expression levels among the three irradiation conditions. A Euclidian distance metric was used to construct the MDS axes. The distance between any two points (samples) reflects their overall similarity with respect to the expression levels of the miRNA included in the classifier. As can be seen from the plot, MDS is able to separate the samples according to radiation dose.
miRNA signature-based class prediction
The differentially expressed miRNA were used to build up classifiers in order to test to what degree miRNA signature-based class prediction is able to indicate the irradiation status of mouse blood samples. The leave-one-out cross validation method was used to test the robustness of the classifiers. In this method, all samples – except one – are used to build the classifier, and the ability of the classifier to correctly predict the class membership of the left-out sample is computed. This process is continued until each sample has been left out once, and the parameters that characterise the performance of the classifier are based on the overall ability of the classifier to correctly assign the samples to the classes they belong to.
Three parameters were calculated to gauge classifier performance: Accuracy, sensitivity, and specificity. Accuracy is the percentage of samples correctly assigned to the class they belong to. Sensitivity is the probability for a sample belonging to a class to be correctly predicted as belonging to that class. Specificity is the probability for a sample not belonging to a class to be correctly predicted as not belonging to that class.
Two different types of classifiers were created. Their performances are shown in Table III. The first type of classifier was designed to classify samples according to radiation type (γ, iron, or control), irrespective of time point after irradiation and radiation dose. Seven miRNA were included in this predictor. This predictor correctly classified 75% of the unknown samples, with specificities and sensitivities ranging from 0.667–0.917.
Table III.
Accuracy, sensitivity, and specificity of the class-prediction classifiers for predicting the irradiation type or dose, based on miRNA expression signatures.
| Irradiation condition | Accuracy | Sensitivity | Specificity |
|---|---|---|---|
| γ | 75% | 0.667 | 0.833 |
| 56Fe | 0.75 | 0.875 | |
| Control | 0.833 | 0.917 | |
| γ 0.0 Gy | 88% | 1 | 0.889 |
| 0.5 Gy | 0.833 | 0.944 | |
| 1.5 Gy | 0.667 | 1 | |
| 5.0 Gy | 1 | 1 | |
| 56Fe 0.0 Gy | 100% | 1 | 1 |
| 0.1 Gy | 1 | 1 | |
| 0.5 Gy | 1 | 1 |
The second type of classifier was designed to classify samples according to the received radiation doses (γ: 0.5, 1.5 and 5 Gy; 56Fe: 0.1 and 0.5 Gy) irrespective of time point after radiation exposure. This type of predictor is used for a sample for which it is known in advance to what type of radiation (γ or 56Fe) it was exposed. The γ predictor contained 24 miRNA and correctly classified 88% of the unknown samples, with specificities and sensitivities ranging from 0.667–1.00. The iron predictor contained 13 miRNA and correctly classified 100% of the samples, with all specificities and sensitivities equal to 1.00. These results show that, in principle, blood miRNA expression profiles derived from C57BL/6 mice can be used to predict the radiation conditions that the animals were exposed to. It would be informative to conduct additional studies to further investigate the suitability of miRNA as radiation classifiers.
miRNA targets and gene ontology analysis
Changes in miRNA expression induce changes in the expression of a large number of target genes. Therefore, miRNA expression changes potentially reveal important information not only about the irradiation conditions as shown above, but also about the biological changes in the irradiated cells and organisms. We attempted to determine the physiological significance of radiation-induced miRNA expression changes by: (1) Determining the genes that are targeted by the differentially expressed miRNA, and (2) reconstructing the processes and functions that the target genes are involved in.
Because blood-cell composition depends significantly on the dose and time after irradiation, it is difficult to distinguish which miRNA expression levels change as a result of functional changes in the irradiated cells and which change simply because the cell subsets before and after irradiation are different. For doses ≥1 Gy X-rays, lymphocyte counts change considerably over time, reaching very low numbers 24 h after irradiation, in contrast with other blood cells, such as neutrophils (Dainiak 2002, Ossetrova et al. 2010). For this reason, we performed GO analysis of the targets of only those differentially expressed miRNA which had no detectable expression in the control samples, but whose expression was switched on by radiation. Using this approach we ensure that the results of the GO analysis reflect indeed a cellular response to ionising radiation, rather than merely radiation-induced differences in the composition of blood cells.
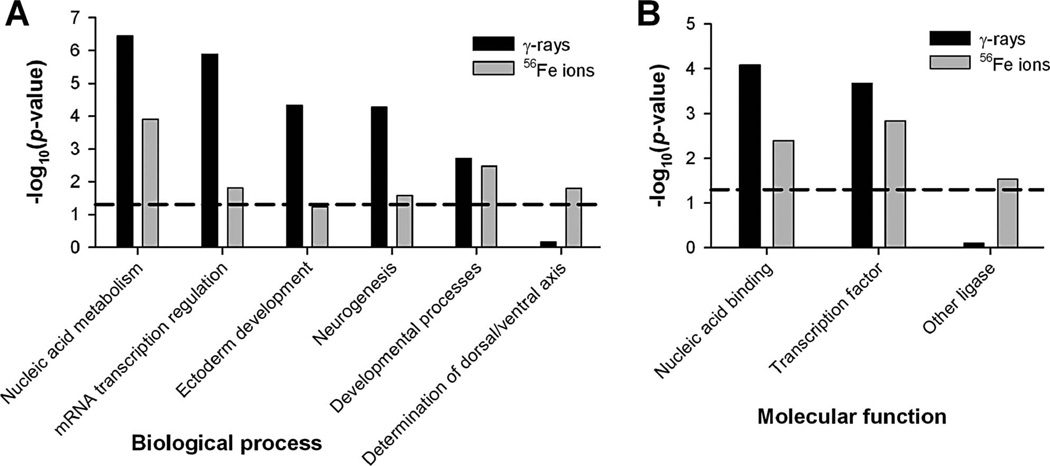
miRNA target genes were determined using TargetScanMouse v. 5.1 (Grimson et al. 2007, Friedman et al. 2009). Only high-probability target genes with context scores of ≤ −70.3 and at least one conserved miRNA target site, as defined by phylogenetic-tree analysis, were selected. Using these criteria, only conserved target genes that were downregulated by at least 23% by the respective miRNA were included in the analysis. GO analysis for biological process and molecular function was performed using the PANTHER GO database v. 6.0 (Thomas et al. 2003). All results are based on the Bonferroni correction for multiple testing. The results show that after irradiation, miRNA control of biological processes associated with nucleic-acid processing, especially transcription, is increased (Figure 3A). Developmental processes are also under the control of radiation-induced miRNA, which is not unexpected considering the important roles of miRNA in development (Olsen and Ambros 1999, Kloosterman and Plasterk 2006). Equally important is the enrichment of miRNA target genes in molecular functions involving transcription and DNA/RNA binding (Figure 3B), emphasising the important regulatory function of miRNA (Mattick 2004, Bartel 2009). Overall, these results demonstrate that radiation induces changes in the processes and functions controlled by miRNA. This indicates that miRNA has the potential to be used as an indicator of functional cellular changes.
Figure 3.
Biological processes and molecular functions with increased miRNA control after irradiation. The GO analysis is based on genes targeted only by miRNA that were switched on by irradiation. The target genes were determined using TargetScanMouse v. 5. (Grimson et al. 2007) and then uploaded to the PANTHER GO database v. 6.0 (Thomas et al. 2003) to determine the biological processes (A) and molecular functions (B) in which the target genes are overrepresented. The mouse AB1700 gene list (Applied Biosystems) was used as the reference genome. Processes and functions above the horizontal dashed line exhibit significant enrichment at a Bonferroni-corrected p-value of < 0.05.
Discussion
In this paper we analyse miRNA expression in vivo in the blood of mice irradiated with γ-rays (as an example of low-LET radiation) or 56Fe ions (as an example of high-LET radiation). Our main finding is that ionising radiation induces changes in the miRNA signature of whole blood and that these changes depend on the irradiation parameters.
Overall we found that 31 miRNA (19% of all detected miRNA) were differentially expressed after irradiation. Noticeably, the differentially expressed miRNA and their numbers differ among the various irradiation conditions. More miRNA are generally differentially expressed at high radiation doses, compared to low doses. However, for the low doses (0.5 Gy and 1.5 Gy γ-rays and 0.1 Gy 56Fe ions) the numbers of miRNA whose expression levels changed upon radiation exposure were lower at 24 h after irradiation compared to the earlier time point. This decrease may be explained by the repair of radiation damage. For the high doses (5.0 Gy γ-rays and 0.5 Gy 56Fe ions) the numbers of differentially expressed miRNA were approximately the same for both time points.
The differentially expressed miRNA fall into two categories: (1) miRNA expressed in one condition only, and (2) miRNA expressed in multiple conditions. Out of the differentially expressed miRNA, 71% are specific to a particular radiation condition. The rest is expressed in two conditions (26%) or three conditions (3%). These findings underline the complexity of the radiation response and the importance of miRNA in this response. A comparison of miRNA signatures induced by equitoxic doses of two different types of radiation shows that miRNA profiles are radiation type-specific. For example, 1.5 Gy γ and 0.5 Gy 56Fe irradiation, the estimated relative biological effectiveness of which varies from 2–4 depending on the endpoint investigated (Peng et al. 2009), induce different miRNA expression signatures. These results are in agreement with previous studies that showed differences between gene expression signatures induced by low- and high-LET irradiation in cells and tissues (Ding et al. 2005, Zhang et al. 2006, Matsumoto et al. 2008). In some cases the expression of a small number of miRNA appears inconsistent, i.e., they are differentially expressed at radiation types, time points, and/or doses that are quite different from each other. Four miRNA belong to this category. These are miR-125a-3p (differential expression at γ 24 h 5.0 Gy and at 56Fe 6 h 0.1 Gy, but not at 0.5 Gy 56F), miR-501-3p (differential expression at γ 24 h 5.0 Gy and at 56Fe 6 h 0.5 Gy, but not at 56Fe 24 h), miR-511 (differential expression at γ 6 h 0.5 Gy and at γ 24 h 5.0 Gy, but not at γ 1.5 Gy), and miR-685 (differential expression at γ 6 h 5.0 Gy and at 56Fe 24 h 0.1 Gy, but not at 56Fe 0.5 Gy). The detected differential expression of these miRNA in disparate irradiation conditions might be explained by the fact that some miRNA may belong to multiple ‘response networks’ that are activated by different cellular stimuli. Moreover, the limited power of our analysis to detect differentially expressed miRNA with low fold changes may have prevented the detection of these miRNA in additional irradiation conditions.
We attempted to cross-validate our results with other studies. We did not find any other published work describing radiation-induced miRNA expression changes in mouse blood for comparison. A comparison of our findings with radiation-induced miRNA expression profiles in human cell lines and primary cells shows very little overlap, probably because of differences in species, model system, cell type, and irradiation conditions (Maes et al. 2008, Cha et al. 2009a, 2009b).
Biomarkers determined by high-throughput assays are frequently used to build classifiers that can predict the treatment status of unknown samples. Gene expression signatures of mouse and human peripheral blood cells were successfully used to build radiation class-prediction classifiers (Meadows et al. 2008, Paul and Amundson 2008, Meadows et al. 2010). Dressman et al. (2007) showed that such classifiers perform well in distinguishing between irradiated and non-irradiated human samples (accuracy of 90%, sensitivity of 85%, and specificity of 94%). Importantly, the authors also showed that radiation-induced gene expression signatures derived from mouse blood can predict the irradiation status of human samples with very good accuracy (Dressman et al. 2007).
Since miRNA expression signatures contain information about the regulation of hundreds to thousands of target genes, it is very reasonable to use them for the development of radiation classifiers. Based on the detected differences in miRNA expression signatures, we developed such classifiers and showed that miRNA can be used, in principle, as predictors of radiation exposure. Our classifiers performed with variable accuracy: They classified the samples reasonably well according to radiation type (accuracy of 75%) and were very exact for dose estimation (accuracy of 88% for γ-rays and of 100% for 56Fe ions). An advantage of our classifiers is that they encompass various scenarios that require predictions about received radiation exposures. It is possible to run two predictors in sequence. For example, if neither the radiation type nor the dose of exposure is known, the classifier predicting radiation type can be applied first, and, after the radiation type has been determined, a γ- or 56Fe-specific classifier can be used to estimate the dose of exposure. A further advantage is that the classifiers do not require a pre-exposure control sample because: (1) All samples that were used to develop the classifiers were derived from separate, independent organisms, and (2) the nearest-centroid classifiers that we developed are able to assign a radiation type and a radiation dose to a sample with unknown radiation status based on the absolute normalised expression values (ΔCT values) of the miRNA contained in the classifier. It has to be noted, however, that the miRNA used for class prediction are not necessarily radiation-specific; differential expression of miRNA as reported in this study might also be induced by other cellular stresses, such as inflammation, reactive oxygen species, and cytotoxic chemicals (Simone et al. 2009).
We also analysed the functions of the genes targeted by the differentially expressed miRNA in order to obtain information on the physiological changes in mouse blood induced by γ or 56Fe irradiation. The numbers of the target genes of the differentially expressed miRNA per irradiation condition are considerable. For example, 6 h after γ irradiation with 1.5 Gy, 515 genes (conserved targets only) are targeted by the differentially expressed miRNA, using our context-score cut-off criterion. As a result, the effects of the radiation-responsive miRNA on cellular processes could be quite substantial. The GO analysis results show that irradiation changes miRNA control of specific biological processes, such as nucleic acid metabolism, transcription regulation, and development (Figure 3).
Conclusion
In recent years, it has become increasingly evident that miRNA signatures describe cell and tissue status very precisely. Several studies have shown that miRNA expression profiles have a greater classificatory power to characterise some tumour types and other diseases than gene expression signatures (Negrini et al. 2009, Small et al. 2010). The results of our study are in line with these findings by demonstrating that mouse blood miRNA signatures change in response to radiation. While the results of our study cannot be directly applied to human biodosimetry, they do demonstrate the feasibility of using blood miRNA as in vivo biomarkers that can distinguish between low- and high-LET radiation, as well as between different doses of ionising radiation. Other studies, aimed to show to what degree ionising radiation induces changes in miRNA expression in the blood of human radiotherapy patients, are in progress in our laboratory.
Acknowledgements
We are grateful to the staff at the NASA Space Radiation Laboratory and at the Medical and Biology Departments of Brookhaven National Laboratory. In particular, we wish to thank Drs Adam Rusek, Michael Sivertz, and I-Hung Chiang for their help with dosimetry and irradiation of animals, and Dr Peter Guida for his organisational support. Additionally, we acknowledge the assistance of MaryAnn Petry and the staff of the Brookhaven Animal Facility. This study was supported in part by NASA, grant number NNX07AT41G, and by the Center for High-Throughput Minimally-Invasive Radiation Biodosimetry, National Institute of Allergy and Infectious Diseases, grant number U19 AI067773.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Amundson SA. Functional genomics in radiation biology: A gateway to cellular systems-level studies. Radiation and Environmental Biophysics. 2008;47:25–31. doi: 10.1007/s00411-007-0140-1. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B. 1995;57:289–300. [Google Scholar]
- Brengues M, Paap B, Bittner M, Amundson S, Seligmann B, Korn R, Lenigk R, Zenhausern F. Biodosimetry on small blood volume using gene expression assay. Health Physics. 2010;98:179–185. doi: 10.1097/01.HP.0000346706.44253.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha HJ, Seong KM, Bae S, Jung JH, Kim CS, Yang KH, Jin YW, An S. Identification of specific microRNAs responding to low and high dose gamma-irradiation in the human lymphoblast line IM9. Oncology Reports. 2009a;22:863–868. [PubMed] [Google Scholar]
- Cha HJ, Shin S, Yoo H, Lee EM, Bae S, Yang KH, Lee SJ, Park IC, Jin YW, An S. Identification of ionizing radiation-responsive microRNAs in the IM9 human B lymphoblastic cell line. International Journal of Oncology. 2009b;34:1661–1668. doi: 10.3892/ijo_00000297. [DOI] [PubMed] [Google Scholar]
- Chaudhry MA. Real-time PCR analysis of micro-RNA expression in ionizing radiation-treated cells. Cancer Biotherapy and Radiopharmaceuticals. 2009;24:49–56. doi: 10.1089/cbr.2008.0513. [DOI] [PubMed] [Google Scholar]
- Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nature Reviews Genetics. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainiak N. Hematologic consequences of exposure to ionizing radiation. Experimental Hematology. 2002;30:513–528. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- Ding LH, Shingyoji M, Chen F, Chatterjee A, Kasai KE, Chen DJ. Gene expression changes in normal human skin fibroblasts induced by HZE-particle radiation. Radiation Research. 2005;164:523–526. doi: 10.1667/rr3350.1. [DOI] [PubMed] [Google Scholar]
- Dressman HK, Muramoto GG, Chao NJ, Meadows S, Marshall D, Ginsburg GS, Nevins JR, Chute JP. Gene expression signatures that predict radiation exposure in mice and humans. PLoS Medicine. 2007;4:e106. doi: 10.1371/journal.pmed.0040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante M, Snigiryova G, Akaeva E, Bogomazova A, Druzhinin S, Fedorenko B, Greco O, Novitskaya N, Rubanovich A, Shevchenko V, et al. Chromosome aberration dosimetry in cosmonauts after single or multiple space flights. Cytogenetic and Genome Research. 2003;103:40–46. doi: 10.1159/000076288. [DOI] [PubMed] [Google Scholar]
- Fachin AL, Mello SS, Sandrin-Garcia P, Junta CM, Donadi EA, Passos GA, Sakamoto-Hojo ET. Gene expression profiles in human lymphocytes irradiated in vitro with low doses of gamma rays. Radiation Research. 2007;168:650–665. doi: 10.1667/RR0487.1. [DOI] [PubMed] [Google Scholar]
- Fineberg SK, Kosik KS, Davidson BL. MicroRNAs potentiate neural development. Neuron. 2009;64:303–309. doi: 10.1016/j.neuron.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Research. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George K, Chappell LJ, Cucinotta FA. Persistence of space radiation induced cytogenetic damage in the blood lymphocytes of astronauts. Mutation Research. 2010;701:75–79. doi: 10.1016/j.mrgentox.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Molecular Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, Xiao T, Schafer J, Lee ML, Schmittgen TD, et al. Detection of microRNA expression in human peripheral blood micro-vesicles. PLoS One. 2008;3:e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H, Saito T. Radiation-induced response of micro RNA expression in murine embryonic stem cells. Medicinal Chemistry. 2006;2:555–563. doi: 10.2174/1573406410602060555. [DOI] [PubMed] [Google Scholar]
- Josson S, Sung SY, Lao K, Chung LW, Johnstone PA. Radiation modulation of microRNA in prostate cancer cell lines. Prostate. 2008;68:1599–1606. doi: 10.1002/pros.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Developmental Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Lee ML, Whitmore GA. Power and sample size for DNA microarray studies. Statistics in Medicine. 2002;21:3543–3570. doi: 10.1002/sim.1335. [DOI] [PubMed] [Google Scholar]
- Maes OC, An J, Sarojini H, Wu H, Wang E. Changes in microRNA expression patterns in human fibroblasts after low-LET radiation. Journal of Cellular Biochemistry. 2008;105:824–834. doi: 10.1002/jcb.21878. [DOI] [PubMed] [Google Scholar]
- Marsit CJ, Eddy K, Kelsey KT. MicroRNA responses to cellular stress. Cancer Research. 2006;66:10843–10848. doi: 10.1158/0008-5472.CAN-06-1894. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Iwakawa M, Furusawa Y, Ishikawa K, Aoki M, Imadome K, Matsumoto I, Tsujii H, Ando K, Imai T. Gene expression analysis in human malignant melanoma cell lines exposed to carbon beams. International Journal of Radiation Biology. 2008;84:299–314. doi: 10.1080/09553000801953334. [DOI] [PubMed] [Google Scholar]
- Mattick JS. RNA regulation: A new genetics? Nature Reviews Genetics. 2004;5:316–323. doi: 10.1038/nrg1321. [DOI] [PubMed] [Google Scholar]
- Meadows SK, Dressman HK, Muramoto GG, Himburg H, Salter A, Wei Z, Ginsburg GS, Chao NJ, Nevins JR, Chute JP. Gene expression signatures of radiation response are specific, durable and accurate in mice and humans. PLoS One. 2008;3:e1912. doi: 10.1371/journal.pone.0001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows SK, Dressman HK, Daher P, Himburg H, Russell JL, Doan P, Chao NJ, Lucas J, Nevins JR, Chute JP. Diagnosis of partial body radiation exposure in mice using peripheral blood gene expression profiles. PLoS One. 2010;5:e11535. doi: 10.1371/journal.pone.0011535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler MF, Mattick JS. Noncoding RNAs and RNA editing in brain development, functional diversification, and neurological disease. Physiological Reviews. 2007;87:799–823. doi: 10.1152/physrev.00036.2006. [DOI] [PubMed] [Google Scholar]
- Negrini M, Nicoloso MS, Calin GA. MicroRNAs and cancer – new paradigms in molecular oncology. Current Opinion in Cell Biology. 2009;21:470–479. doi: 10.1016/j.ceb.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Olsen PH, Ambros V. The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Developmental Biology. 1999;216:671–680. doi: 10.1006/dbio.1999.9523. [DOI] [PubMed] [Google Scholar]
- Ossetrova NI, Sandgren DJ, Gallego S, Blakely WF. Combined approach of hematological biomarkers and plasma protein SAA for improvement of radiation dose assessment triage in biodosimetry applications. Health Physics. 2010;98:204–208. doi: 10.1097/HP.0b013e3181abaabf. [DOI] [PubMed] [Google Scholar]
- Paul S, Amundson SA. Development of gene expression signatures for practical radiation biodosimetry. International Journal of Radiation Oncology Biology Physics. 2008;71:1236–1244. doi: 10.1016/j.ijrobp.2008.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Brown N, Finnon R, Warner CL, Liu X, Genik PC, Callan MA, Ray FA, Borak TB, Badie C, et al. Radiation leukemogenesis in mice: loss of PU.1 on chromosome 2 in CBA and C57BL/6 mice after irradiation with 1 GeV/nucleon 56Fe ions, X rays or gamma rays. Part I. Experimental observations. Radiation Research. 2009;171:474–483. doi: 10.1667/RR1547.1. [DOI] [PubMed] [Google Scholar]
- Pradervand S, Weber J, Lemoine F, Consales F, Paillusson A, Dupasquier M, Thomas J, Richter H, Kaessmann H, Beaudoing E, et al. Concordance among digital gene expression, microarrays, and qPCR when measuring differential expression of microRNAs. Biotechniques. 2010;48:219–222. doi: 10.2144/000113367. [DOI] [PubMed] [Google Scholar]
- Qiu W, Lee MLT, Whitmore GA. Sample size and power calculation in microarray studies using the sizepower package. 2010 [Google Scholar]
- Rodrigues AS, Oliveira NG, Gil OM, Leonard A, Rueff J. Use of cytogenetic indicators in radiobiology. Radiation Protection Dosimetry. 2005;115:455–460. doi: 10.1093/rpd/nci072. [DOI] [PubMed] [Google Scholar]
- Roy L, Gruel G, Vaurijoux A. Cell response to ionising radiation analysed by gene expression patterns. Annali dell Istituto Superiore di Sanita. 2009;45:272–277. [PubMed] [Google Scholar]
- Shin S, Cha HJ, Lee EM, Lee SJ, Seo SK, Jin HO, Park IC, Jin YW, An S. Alteration of miRNA profiles by ionizing radiation in A549 human non-small cell lung cancer cells. International Journal of Oncology. 2009;35:81–86. [PubMed] [Google Scholar]
- Shkumatava A, Stark A, Sive H, Bartel DP. Coherent but overlapping expression of microRNAs and their targets during vertebrate development. Genes & Development. 2009;23:466–481. doi: 10.1101/gad.1745709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Lam A, Li MC, Ngan M, Menenzes S, Zhao Y. Analysis of gene expression data using BRB-Array Tools. Cancer Informatics. 2007;3:11–17. [PMC free article] [PubMed] [Google Scholar]
- Simone NL, Soule BP, Ly D, Saleh AD, Savage JE, Degraff W, Cook J, Harris CC, Gius D, Mitchell JB. Ionizing radiation-induced oxidative stress alters miRNA expression. PLoS One. 2009;4:e6377. doi: 10.1371/journal.pone.0006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small EM, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121:1022–1032. doi: 10.1161/CIRCULATIONAHA.109.889048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Statistical Applications in Genetics and Molecular Biology. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, Diemer K, Muruganujan A, Narechania A. PANTHER: A library of protein families and subfamilies indexed by function. Genome Research. 2003;13:2129–2141. doi: 10.1101/gr.772403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach JL, Cullen BR. The role of RNAi and microRNAs in animal virus replication and antiviral immunity. Genes & Development. 2009;23:1151–1164. doi: 10.1101/gad.1793309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauk CL, Rowan-Carroll A, Stead JD, Williams A. Cross-platform analysis of global microRNA expression technologies. BMC Genomics. 2010;11:330. doi: 10.1186/1471-2164-11-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Burns FJ, Chen H, Chen S, Wu F. Alterations in gene expression in rat skin exposed to 56Fe ions and dietary vitamin A acetate. Radiation Research. 2006;165:570–581. doi: 10.1667/RR3556.1. [DOI] [PubMed] [Google Scholar]