Abstract
Clonal responses of Mycobacterium tuberculosis-specific CD4+ or CD8+ T effector cells producing antituberculosis cytokine IFN-γ in the context of immune protection against tuberculosis remain poorly characterized in humans. Utilizing decade-long TCR expertise, we previously developed a useful method to isolate clonotypic TCR sequences from Ag-specific IFN-γ–producing T cells and to specifically measure clonotypic TCR frequencies in the T cell pool. In this study, we investigated TCR Vβ repertoires/CDR3 usage, clonal expansion or dominance, and pulmonary trafficking or accumulation for purified protein deritative (PPD)-specific T effector cells producing IFN-γ during bacillus Calmette-Guérin (BCG) vaccination and subsequent M. tuberculosis challenge of macaques. We found that while PPD-specific CD4+ and CD8+ T effector clones employed diverse TCR Vβ repertoires, 30–33% of IFN-γ+CD4+ T cell clones from three M. tuberculosis-infected macaques expressed TCR bearing a conserved residue leucine in CDR3. Many Ag-specific IFN-γ+ CD4+ and few CD8+ T effector cells emerged as dominant clones during mycobacterial infections and underwent major recall expansion after pulmonary M. tuberculosis infection of BCG-vaccinated macaques. PPD-specific T cell clones readily trafficked to the airway or lung after BCG vaccination or M. tuberculosis infection, and some of them continuously accumulated in lungs during M. tuberculosis infection even after they became undetectable in the circulation. Importantly, remarkable recall expansion and pulmonary accumulation of T effector cells coincided with BCG-induced protection against tuberculosis. Thus, rapid clonal expansion and pulmonary accumulation of Ag-specific T effector cells appear to be one of the immune mechanisms underlying immunity against tuberculosis.
Tuberculosis (TB) remains one of the major causes of global mortality, and it has become increasingly prevalent and deadly as a result of the HIV/AIDS pandemic and the emergence of extensively drug resistant strains of Mycobacterium tuberculosis (1). Precise protective elements in immunity against human TB are poorly characterized, although HIV-mediated CD4+ T cell deficiency clearly increases the susceptibility to TB (2–4). Elucidating precise immune elements for controlling human TB is therefore of central importance for ultimately developing a better vaccine and immunotherapeutic against TB and for reducing TB epidemics. It is widely accepted that CD4+ T cells play an important role in the ability of humans and experimental animals to resist active M. tuberculosis infection (5–8). In this regard, Th1 cytokine IFN-γ has been shown to be crucial for immune protection against TB in mice (9, 10). Our recent study has also demonstrated that vaccine-elicited CD8+ T cells play a critical role in immunity against active TB (11). However, clonal responses and potential lung-trafficking of M. tuberculosis-specific CD4+ or CD8+ T effector cells producing anti-TB cytokine IFN-γ in the context of anti-TB immunity remain poorly characterized in humans (12–14). Studies of Ag-specific T cells for TCR repertoires/CDR3 usage, clonal expansion, and pulmonary trafficking in immunity against TB should help to elucidate immune mechanisms for protective T cell immune responses.
We have recently used our decade-long TCR expertise and developed a useful method to isolate TCR VDJ clonotypic sequences from a limited number of purified protein deritative (PPD)-specific IFN-γ–producing T cells and to specifically measure clonotypic TCR frequencies in the T cell pool using the PCR-based real-time quantitation (15). The real-time quantitation technique using clonotypic primers specifically quantitates a target clonotypic TCR clone at a detection limit of 10–5 Ag-specific T cells, and it discriminates other clones that differ by two or more bases in DJ regions (15). Employing this useful method, we investigated TCR Vβ repertoires/CDR3 usage, clonal expansion or dominance, and pulmonary trafficking or accumulation for PPD-specific T effector cells producing anti-TB cytokine IFN-γ during bacillus Calmette-Guérin (BCG) vaccination and subsequent M. tuberculosis challenge of macaques. We found that while PPD-specific T effector clones employed diverse TCR Vβ repertoires, 30–33% of IFN-γ+ CD4+ T cell clones from three M. tuberculosis-infected macaques expressed TCR bearing a conserved residue leucine in CDR3 despite different Vβ usage. Many CD4+ and few CD8+ T effector clones emerged as dominant clones during BCG vaccination and underwent major recall expansion and trafficking to the airway after M. tuberculosis infection. Such major recall expansion and rapid pulmonary accumulation coincided with BCG-induced anti-TB immunity.
Materials and Methods
Macaque animals
A total of eight juvenile Indian rhesus macaques (2 y old) were used for evaluating clonal immune responses of M. tuberculosis-specific CD4+ and CD8+ T cells. Studies using all the animals were documented in animal protocols and approved by the Institutional Animal Care and Use Committee.
BCG vaccination and M. tuberculosis challenge
Two groups of juvenile Indian rhesus macaques (four animals per group) were vaccinated i.v. or intradermally (below) with 106 CFUs BCG (Pasteur strain) and saline control, respectively. One and one-half months later each of them was challenged with 400 CFUs M. tuberculosis H37Rv strain by aerosol as previously described (16). Four control naive animals became moribund and had to be euthanized due to the development of fatal tuberculosis within 1.5 mo after the infection, whereas the BCG-vaccinated macaques survived fatal tuberculosis during a 2.5-mo follow-up (16). The surviving macaques with transient low levels of bacillus but no evidence of active tuberculosis were then treated daily for 3 mo with anti-TB drugs; that is, isoniazid (5 mg/kg) and pyrazinamide (15 mg/kg) mixing with yogurt as previously described (17).
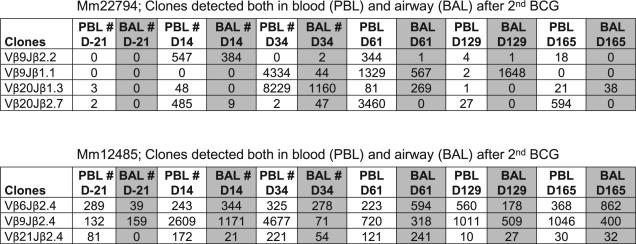
To optimally investigate Ag-specific T effector clones for pulmonary trafficking, two additional rhesus macaques received the first i.v. BCG vaccination and, 4 mo later, the second BCG vaccination. Ag-specific clonal responses were extensively followed for 6 mo. Because BCG organisms were not detected in bronchoalveolar lavage (BAL) fluid without increases in neutrophils during the secondary BCG infection (Fig. 6), detection of Ag-specific clonotypic TCR clones in both blood and BAL fluid implicated the trafficking of T cells to the airway.
FIGURE 6.
PPD-specific IFN-γ–producing CD4+ T cell clones readily trafficked to the airway as well after the second i.v. BCG vaccination. Shown are the frequencies of clonotypic TCR clones detected both in PBLs and BAL fluid after the second BCG vaccination of macaques. The first BCG vaccination was done 4 mo earlier. The primary BCG infection is usually resolved within 2 mo after the first i.v. BCG inoculation. No BCG mycobacteria were detected in BAL samples after the second BCG vaccination. # indicates that no BCG bacteria were isolated from the blood and BAL fluid collected from denoted times (#) after the second BCG vaccination. BCG CFUs were measured using 7H10 agar plates (16). There were no or few neutrophils seen over time in BAL fluid.
To examine if intradermal BCG vaccination was similar to i.v. BCG administration in inducing accumulation of T effector cells in the airway, four rhesus macaques were intradermally vaccinated with BCG and assessed for the occurrence of PPD-specific IFN-γ+ T cells in BAL fluid (Fig. 7). The intradermal BCG vaccination appeared to be relevant somewhat to i.v. BCG administration, as T effector cell recall expansion and anti-TB immunity were also seen in adult rhesus macaques that received intradermal BCG vaccination (18), a standard vaccination route.
FIGURE 7.
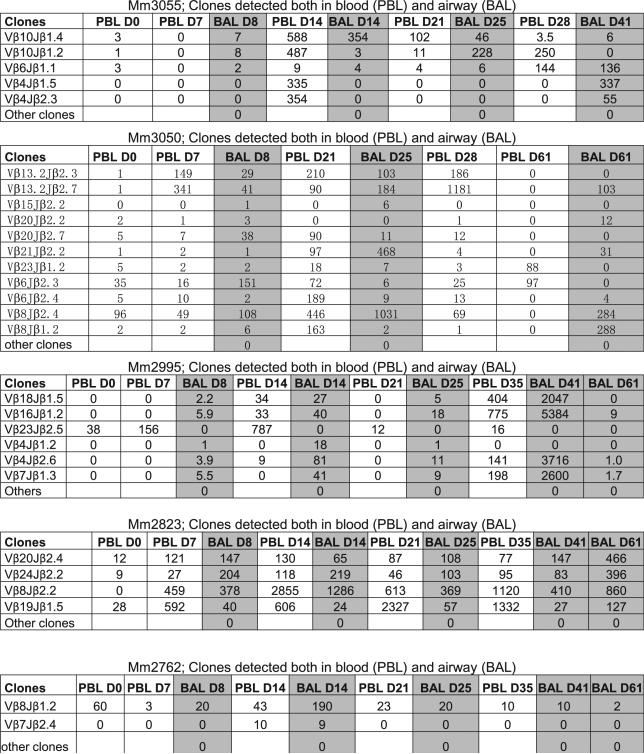
Accumulation of T effector cells in the airway was also induced by intradermal BCG vaccination. Shown are ICS data indicating mean frequencies of PPD-specific IFN-γ+ T cells in BAL cells from four rhesus macaques vaccinated intradermally with BCG (18).
BAL
BAL was done essentially the same as previously described (11, 19, 20). Clear BAL fluid without blood contamination was used for evaluation of clonal T cell responses.
Sampling approaches
Two parallel approaches were employed to quantitate Ag-specific T cell clones in infection. To isolate clonotypic TCR and design clonotypic primers for real time quantitation, PBLs from M. bovis BCG-infected macaques were isolated from blood and used to generate Ag-specific T cells by means of peptide stimulation and intracellular IFN-γ staining. The Ag-specific IFN-γ– producing T cells were purified by flow sorting; TCR VDJ DNA was isolated by megaplex PCR and sequenced for designing clonotypic primers.
In parallel, 10 × 106 PBL or 3 × 106 BAL cells were collected and frozen in a liquid nitrogen tank over time after BCG vaccination and M. tuberculosis infection. The frozen cells were then used for RNA isolation and cDNA synthesis. cDNA and clonotypic primers were used to quantitate T cell clones during BCG vaccination and M. tuberculosis infection.
ELISPOT measuring PPD-specific IFN-γ–producing cells
PPD-specific IFN-γ+ cells in PBMCs and BAL fluid were detected as we previously described (11, 18, 21, 22).
Intracellular cytokine staining and purification of PPD-specific IFN-γ+ CD4+ and CD8+ T cells by flow cytometry sorting
Intracellular cytokine staining (ICS) for IFN-γ+ cells in PBMCs was done as previously described (11, 15, 18, 22). Stained cells were fixed in 2% formaldehyde. IFN-γ+ CD4+CD3+ T cells and IFN-γ+ CD8+CD3+ T cells were then purified and enumerated by flow cytometry sorting, essentially as we previously described (15, 23). The formaldehyde-fixed PPD-specific CD4 T cells were subjected to TCRVDJ DNA isolation using the megaplex PCR.
Isolation of clonotypic TCR VDJ DNA from formaldehyde-fixed PPD-specific CD4+ or CD8+ T cells
To isolate and amplify TCR β VDJ DNA from formaldehyde-fixed cells, three-round nested PCRs (megaplex PCRs) were employed using DNA prepared from the sorted cells as well as three sets of 24 forward Vβ family-specific primers and three sets of 13 reverse Jβ primers as shown in Table 1 and Fig. 1 in Du et al. (15). The first and second round of nested megaplex PCRs were performed in a single PCR tube using external, middle sets of mixed 24 forward Vβ family-specific primers and mixed 13 reverse Jβ primers, respectively, whereas the third megaplex PCR amplified and displayed clonotypic Vβ-D-Jβ DNA at the level of individual Vβ-Jβ recombinations for direct sequencing (15). The method allowed isolation clonotypic TCR DNA in fidelity from 20 to 2000 sorted cells (15). The extremely low frequencies of specific Teffector cells in three naive controls that died at an early stage precluded the clonotypic TCR sequence isolation and subsequent quantitation of TCR clones measured over time by real-time PCR.
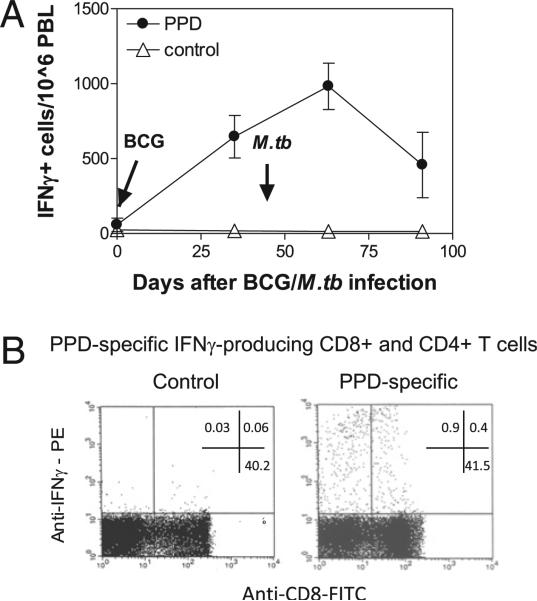
FIGURE 1.
PPD-specific T effector cells producing IFN-γ upon stimulation were detectable after BCG vaccination and M. tuberculosis infection. Shown are ELISPOT data (A) indicating IFN-γ+ cellular responses in PBMCs after BCG vaccination and M. tuberculosis infection of juvenile Indian rhesus macaques (A) and representative flow histogram data (B) displaying PPD-specific IFN-γ+ CD8+ and CD8– (CD4+) T cells measured by intracellular cytokine staining after stimulation with PPD or medium control. Data were gated on CD3, with percentages indicated in quadruples. Both IFN-γ+ CD8+ and CD4+ T cell populations were purified by flow cytometry sorting (15). ELSPOT data are mean values with SEM from four macaques.
Direct sequencing of TCR VDJ DNA
The individual clonotypic Vβ-D-Jβ DNA recovered from a gel extraction kit from Qiagen (Valencia, CA) was directly sequenced using the third-round PCR primers, as previously described (15). If the direct sequencing showed multiple templates, the DNA was then inserted into the plasmid SP65 (Promega, Madison, WI) for cloning and sequencing, as previously described (23).
Isolation of RNA from frozen cells and cDNA synthesis
Total RNA was isolated from PBLs using the TRIzol isolation method (15). The RNA pellet was resuspended in RNase-free deionized water and then used immediately for synthesis of cDNA using the protocol provided in the cDNA synthesis kit from Clontech Laboratories (Palo Alto, CA).
Real-time quantitative PCR for quantitating Ag-specific T cell clones
To quantitate clonotypic T cell clones in a T cell pool, individual clonotypic TCR transcripts were measured for their frequencies in total TCR β transcripts (Cβ) (15). This was done essentially the same as previously described (15). Forward Vβ family-specific primers and TaqMan probes for real-time quantitative PCRs were designed at the region close to the 3′ end of Vβ regions; Jβ clonotypic reverse primers were designed based on the unique sequences in D-J regions [please see Table 1b, 1c in Du et al. (15)]. The real-time quantitative PCR was performed using PE Applied Biosystems 7700 single reporter Sequence Detection Systems 1.6.3 (Applied Biosystems, Foster City, CA) as we previously described (15). All data were analyzed using the GeneAmp 7700 SDS software (Applied Biosystems).
To standardize expression levels of Vβ and Cβ transcripts, CT Vβ and CT Cβ were verified by CT values for a housekeeping gene, β-actin (CT β-actin) (15). The expression levels of individual clonotypic TCR transcripts among a total of TCR β transcripts within the sample were then calculated using the formula, 2–[(CT Vβ – CT β-actin) – (CT Cβ – CT β-actin)], as we and others previously described (15, 24–26). The calculation gave rise to the ratio of clonotypic Vβ-bearing transcripts versus Cβ transcripts (15). The data were then expressed as a relative expression of Ag-specific IFN-γ–producing T cell clones, (Vβ transcripts/Cβ transcripts) × 105 (15). Such an expression of data was based on the consideration that the expression levels of clonotypic VβDJβ-bearing transcripts were always less than those of Cβ transcripts, and that the detection limit for Ag-specific T cell clones in our assay system was 10–5 cells (15).
Statistical analysis
Statistical analysis was done using a Student t test, as previously described (16).
Results
PPD-specific CD4+ and CD8+ T effector clones employed diverse TCR Vβ repertoires; 30–33% of IFN-γ+ CD4+ T cell clones from three M. tuberculosis-infected macaques expressed TCR bearing a conserved residue leucine in CDR3
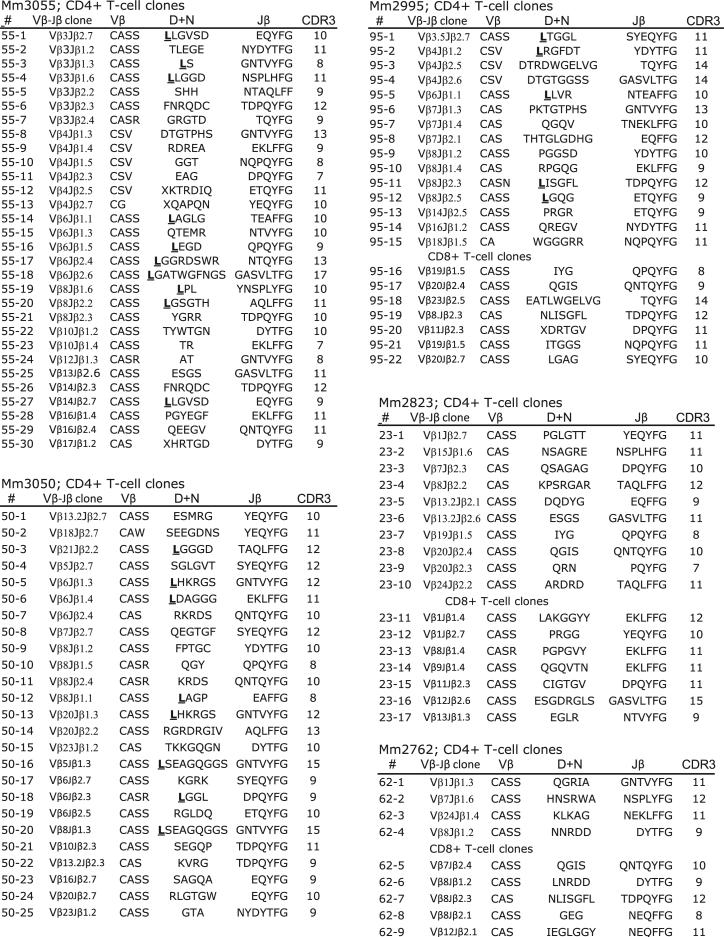
TCR Vβ repertoire and clonotypic sequences of M. tuberculosis-specific T effector cells producing anti-TB cytokine IFN-γ remain incompletely known (12–14). Because IFN-γ is one of the major T effector cytokines contributing to anti-TB immunity (9, 10), we sought to determine the breadth and clonotypes of Ag-specific IFN-γ+ T effector cells using our validated method for TCR isolation and extensive TCR Vβ repertoire/CDR3 analyses (15, 23, 27–29). PPD-specific IFN-γ+ cells in PBLs were detectable after BCG vaccination and M. tuberculosis infection (Fig. 1A). CD4+ and CD8+ T cells in PBLs were reproducibly detected by ICS at week 5 after pulmonary M. tuberculosis infection of macaques (Fig. 1B) (18, 22). These M. tuberculosis-specific T effector cells were therefore isolated by flow cytometry sorting as we described previously (15, 23) and were assessed for TCR Vβ families and clonotypic CDR3 sequences by taking advantage of the recombined VDJ DNA derived from the isolated cells (15). Notably, M. tuberculosis PPD-specific IFN-γ+ CD4+ and CD8+ T effector cells in M. tuberculosis-infected macaques employed multiple TCR Vβ families during primary M. tuberculosis infection of naive or BCG-vaccinated macaques (Fig. 2). These Ag-specific T effector cells in each macaque often expressed selected TCR comprised of 4–11 Vβ family genes and diverse CDR3 sequences/lengths (Fig. 2). However, some Vβ families were predominantly used for recognition of MHC/peptide complexes despite the fact that their CDR3 sequences and lengths were not identical (Fig. 2). Interestingly, 30–33% of IFN-γ+CD4+ T cell clones from three infected macaques (Mm3055, Mm2995, and Mm3050) expressed TCRs bearing a conserved residue leucine in the N-D junctional region (Fig. 2). Although many clones sharing the leucine residue in CDR3 were not dominant clones, the data suggested a selection by potentially conserved MHC class II/peptide complexes. Thus, while PPD-specific T effector clones employed diverse TCR Vβ repertoires during M. tuberculosis infection, some IFN-γ+CD4+ T cell clones could express TCRs bearing a conserved residue leucine in CDR3.
FIGURE 2.
PPD-specific CD4+ and CD8+ T effector clones employed diverse TCR Vβ repertoires; 30–33% of IFN-γ+CD4+ T cell clones from three M. tuberculosis-infected macaques expressed TCR bearing a conserved residue leucine in CDR3. Shown are TCR sequences in V-D-J junctional regions for CD4+ and CD8+ T cell clones. Clone numbers (#) from TCR isolation, clone ID (Vβ-Jβ usage), amino acid sequences of Vβ (3′ end), D+N and Jβ, and CDR3 lengths are indicated. CDR3 lengths were defined as previously described (23, 27, 29). Note a conserved residue leucine that is bold and underlined in the TCR N+D region of the clones isolated from macaques 3055, 2995, and 3050. To maximize the detection of M. tuberculosis-specific IFN-γ+ T effector cells using the ICS method, PBLs collected at weeks 5 or 7 after M. tuberculosis infection were stimulated with PPD, stained for IFN-γ and surface CD3, CD4, and CD8, and subjected to isolation of IFN-γ+ CD4+ or CD8+ T effector cells by flow cytometry sorting as we previously described (15, 23). Thirty to 55% of the clones were isolated from PBLs obtained at both weeks 5 and 7 after M. tuberculosis infection.
Many Ag-specific IFN-γ+ CD4+ and few CD8+ T effector cells emerged as dominant clones during mycobacterial infections and underwent major recall expansion after pulmonary M. tuberculosis infection of BCG-vaccinated macaques
Once we isolated clonotypic TCR sequences of PPD-specific IFN-γ+ CD4+ and CD8+ T effector cells, we sought to determine frequencies of these individual T effector clones over time after pulmonary M. tuberculosis infection. We used PCR-based real-time quantitation to measure the frequencies of these clones in cDNA derived from PBLs collected and frozen after BCG vaccination and subsequent M. tuberculosis infection. The real-time quantitation of each T cell clone was done using a 5′-Vβ–specific primer and a 3′-clonotypic primer corresponding to the unique sequence in the D+N and J (start) region as we previously described (15).
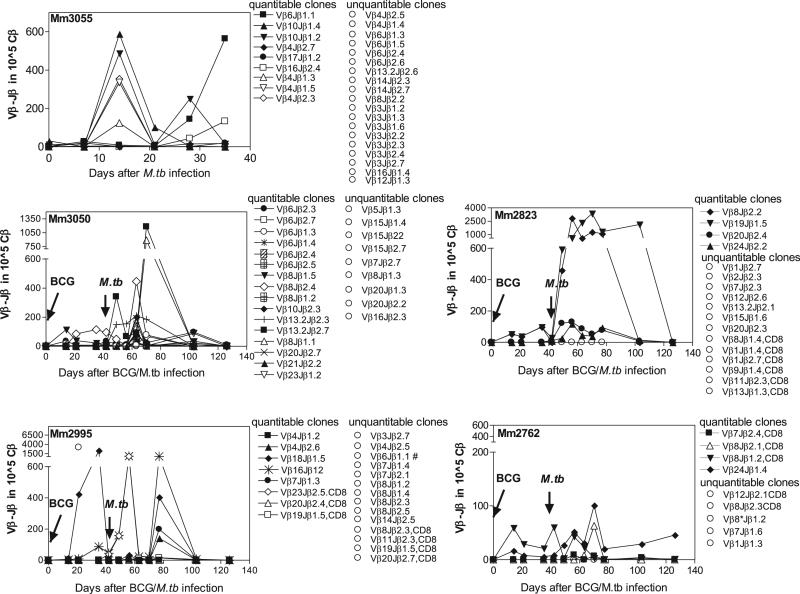
A number of TCR transcripts of CD4+ T effector cells were readily quantitatable or measurable 2–3 wk after i.v. BCG infection or pulmonary M. tuberculosis infection (Fig. 3). The occurrence of these measurable clones indicated a clonal expansion of the dominant T effector cell clones. However, many T cell clones were at low frequency and unquantitatable due to the detection limit for the PCR-based real-time quantitation (Fig. 3) (15). Interestingly, a number of T cell clones measurable after BCG infection were detected at higher frequencies in PBLs weeks after pulmonary M. tuberculosis infection (Fig. 3), whereas most clones from BCG-vaccinated macaques without M. tuberculosis infection became unquantitatable or at very low frequencies at 5–6 wk after BCG vaccination (15). These contrasting data suggested that BCG-primed clones had undergone recall expansion after M. tuberculosis infection of the BCG-vaccinated macaques. Some clones undergoing recall expansion could increase up to 1000–3000 copies per 105 total TCR (Cβ) transcripts (Fig. 3). Notably, these increased clones were quite dynamic in the blood circulation over time after the infection (Fig. 3). Most clones became unquantitatable 2.5 mo after M. tuberculosis challenge (Fig. 3), presumably due to BCG-induced control of acute M. tuberculosis infection.
FIGURE 3.
Many Ag-specific IFN-γ+ CD4+ and few CD8+ T effector cells emerged as dominant clones during mycobacterial infections and underwent major recall expansion after pulmonary M. tuberculosis infection of BCG-vaccinated macaques. Shown are the frequencies for individual clonotypic TCR clones in the blood T cell pool after i.v. BCG vaccination (four macaques) and pulmonary M. tuberculosis infection (five macaques). Data are expressed as clonotypic TCR copy numbers in 105 total TCR β (Cβ) transcripts. The time for BCG vaccination or M. tuberculosis infection is pointed to by an arrow in each panel. CD8+ T effector clones were indicated; otherwise, they were CD4+ T effector clones. Note that the quantitatable and unquantitatable TCR clones are grouped, respectively, for each macaque. The real-time quantitation method confers a detection limit for TCR transcripts corresponding to 10–5 Ag-specific T cells (15). Most clones became unquantitatable around the time point of anti-TB chemotherapy (denoted by drug), which might be attributed to the vaccine-induced immune control of acute M. tuberculosis infection. Note that Vβ23Jβ2.5 (◇) and Vβ16Jβ1.2 (*) clones from macaque 2995 exhibited similar frequencies early after M. tuberculosis infection, whereas Vβ16Jβ1.1 clone (marked by #) became unquantitatable after M. tuberculosis infection. The low-level recall clonal expansion for macaque 2762 appeared to correlate with extremely low bacterial burden after M. tuberculosis infection (16).
Six clonotypic TCR sequences representing CD8+ T cell clones were also quantitatable, but only two of them were at a “sub-dominant” or “dominant” level in the blood after i.v. BCG infection or pulmonary M. tuberculosis infection (Fig. 3). The abundance of CD4+ clones and the paucity of CD8+ clones appeared to be consistent with the general idea that CD4+ T cells constitute greater numbers of IFN-γ–producing cells than do CD8+ T cells. We could not rule out the possibility that the CD8+ paucity was also attributed to the fact that PPD used for ICS stimulation was less efficient than optimal 9- to 12-mer peptides to stimulate CD8+ T cells that could be sorted by flow for clonotypic TCR sequence selection and subsequent frequency quantitation.
Thus, many Ag-specific CD4+ T effector clones but few CD8+ T clones emerged as dominant clones in mycobacterial infections, and they underwent major recall expansion after pulmonary M. tuberculosis infection of BCG-vaccinated macaques.
PPD-specific T cell clones readily trafficked to the airway after BCG vaccination or M. tuberculosis infection, and some of them continuously accumulated in lungs during M. tuberculosis infection even after they became undetectable in the circulation
Rapid accumulation of Ag-specific T effector cells in lungs may be critical for mounting anti-TB immunity after infection. Despite the fact that many T cells infiltrated into the lungs in forming granulomas during M. tuberculosis infection, it remains poorly characterized whether certain numbers of Ag-specific CD4+ or CD8+ T effector clones traffick to the pulmonary compartment after clonal expansion in lymphoid tissues. To address this, we first demonstrated that PPD-specific IFN-γ+ T cells were detectable in the airway (BAL fluid) after pulmonary M. tuberculosis infection (Fig. 4). We then made use of the quantitatable (dominant) clonotypic TCR clones identified in the circulation as described above to determine whether they were measurable as well in the airway. Interestingly, at 8 d after M. tuberculosis infection, many dominant CD4+ T effector clones expressing clonotypic TCR in PBLs were also detected at high frequencies in the airway, with two CD8+ T clones having been detected at low levels (Fig. 5). The accumulation of these dominant PPD-specific IFN-γ+ T cell clones in the airway was sustained at days 14, 25, 41, and 61 after pulmonary M. tuberculosis infection (Fig. 5). Some CD4+ clones could accumulate at the frequency of up to 1000–5000 per 105 total TCR (Cβ) transcripts in the airway at days 14 and 41 despite some fluctuation for these clones (Fig. 5). Notably, at late time points some clones continuously accumulated in the airway despite the fact that they had already become undetectable for weeks in the blood (Vβ4Jβ1.5; Vβ4Jβ2.3 for 3055; Vβ8Jβ1.2 and Vβ21Jβ2.2 for 3050; Fig. 5).
FIGURE 4.
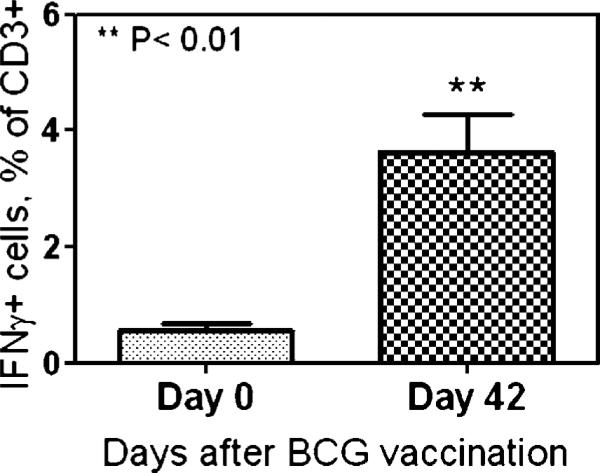
PPD-specific IFN-γ–producing cells were detectable in the airway in M. tuberculosis-infected macaques. Shown are ELSPOT data for PPD-specific IFN-γ+ cell responses in BAL fluid collected from three M. tuberculosis-infected macaques at day 41 after the infection, as well as from three uninfected healthy macaques (control). Data were subtracted from values of medium alone and expressed as IFN-γ+ cells in 106 BAL cells. From Student t test analysis, p < 0.05 between the groups.
FIGURE 5.
PPD-specific CD4+ and CD8+ T cell clones readily trafficked to the airway after M. tuberculosis infection, and some of them continuously accumulated in lungs even after they became undetectable in the circulation. Shown are the frequencies (clonotypic TCR copy numbers in 105 total TCR β (Cβ) transcripts) of clonotypic TCR clones detected both in blood (PBLs) and airway (BAL) at different days (D) after M. tuberculosis infection of macaques. Data for BAL are highlighted in light black. Only those dominant clones quantitatable in PBLs were subjected to real-time quantitation in BAL samples due to the limited numbers of BAL cells.
To further investigate the airway trafficking of Ag-specific T effector clones, rhesus macaques immunized previously with BCG 4 mo earlier were vaccinated again with BCG and assessed for PPD-specific clonotypic T cell clones that had trafficked to the airway. While BCG organisms were not detected in BAL fluid, with no or few neutrophils found over time after the second BCG (Fig. 6; please see legend for the # symbol), we consistently found that PPD-specific clonotypic TCR clones identified in the circulation were also measurable in BAL cells over time after the second BCG vaccination (Fig. 6). In examining whether intradermal BCG vaccination was similar to i.v. BCG administration in inducing accumulation of T effector cells in the airway, we found that accumulation of T effector cells in the airway was also induced by intradermal BCG vaccination (Fig. 7). The data suggested that both routes were comparable in this setting.
These results therefore suggested that PPD-specific IFN-γ+ T cell clones could traffick to the airway after BCG vaccination or M. tuberculosis infection, and that some clones could continuously accumulate in the airway during M. tuberculosis infection even after they were no longer detectable in the peripheral blood.
Remarkable recall expansion and rapid pulmonary accumulation of T effector cells coincided with BCG vaccine-induced protection against fatal M. tuberculosis infection
Given the possibility that IFN-γ+ CD4+ and CD8+ T effector cells in lungs contribute to anti-TB immunity, we sought to determine whether major recall expansion and pulmonary accumulation of PPD-specific T effector clones after M. tuberculosis infection of BCG-vaccinated macaques coincided with protection against TB (16). While naive juvenile Indian rhesus controls died of fatal M. tuberculosis infection, all BCG-vaccinated macaques survived with much lower M. tuberculosis burdens (16). Interestingly, the BCG vaccine-induced anti-TB immunity coincided with the major recall expansion of PPD-specific CD4+ and CD8+ T effector cells in the airway after M. tuberculosis challenge (Fig. 3). Importantly, the detectable protection was also associated with the high-frequency accumulation of those dominant T cell clones in the airway (Fig. 5). Comparative analysis of the PPD-specific IFN-γ+ T effector cells between BCG-vaccinated macaques and naive controls also indicated that rapid major expansion of BCG-primed T effector cells was associated with protection against fatal TB (Fig. 8). It is also noteworthy that T effector cell recall expansion and anti-TB immunity were similarly seen in adult rhesus macaques that received intradermal BCG vaccination (18), a standard BCG vaccination route. Thus, clonal recall expansion and pulmonary accumulation of T effector clones appeared to contribute to BCG-induced anti-TB immunity.
FIGURE 8.
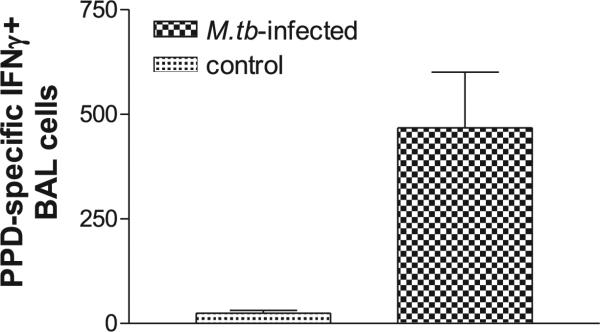
Rapid major expansion of BCG-primed T effector cells after M. tuberculosis infection was associated with protection against fatal TB in juvenile Indian rhesus macaques. Shown are mean comparative ELI-SPOT data from BCG-vaccinated and naive control groups at 3 wk after M. tuberculosis infection. All vaccinated macaques survived with very low-level M. tuberculosis burden, whereas all naive controls died (16).
Discussion
To our knowledge, the present study represents the first extensive experiment dissecting TCR repertoires/CDR3, clonal dominance/recall expansion, and pulmonary trafficking/accumulation for Ag-specific CD4+ and CD8+ T effector clones producing anti-TB cytokine IFN-γ during BCG vaccination and M. tuberculosis infection of nonhuman primates. A recent human study reported a marked CD8+ T cell clonal expansion in the blood of children with TB, although Ag specificity, expansion versus time course, and pulmonary responses for these CD8+ T cells were not known (13). Another human study identified highly focused responses of T cells in lungs in the latent M. tuberculosis infection (14). However, it would be interesting to see whether such responses are also apparent in primary M. tuberculosis infection or TB, and how these highly focused T cell responses are interrelated with the naturally presented epitopes and the TCR repertoires in the circulation or lymphoid tissues. Thus, our study extends human studies to provide further information regarding clonal immune responses of Mycobacterium-specific T effector cells in M. tuberculosis infection, particularly demonstrating that recall clonal expansion and rapid pulmonary accumulation of Ag-specific T effector cells closely correlate with vaccine-induced immunity against TB.
PPD-specific CD4+ and CD8+ T cells exhibited broad TCR repertoires involving multiple Vβ families and heterogeneous CDR3 sequences and lengths during M. tuberculosis infection. This might be due to the fact that PPD protein contains a number of epitopes that can be presented by different MHC class I and II alleles. In fact, we previously showed that even the Mamu-A*01–restricted CD8+ T cell population recognizing an immunodominant epitope indeed employed remarkably broad TCR repertoire during acute and chronic macaque SIV infection (23, 28).
Interestingly, 30–33% of PPD-specific CD4+ T cell clones in three M. tuberculosis-infected macaques expressed TCR bearing a selected residue leucine at the CDR3 N-D junctional region despite the fact that they employed several different Vβ families. These results suggest that these CD4+ T effector clones might share some TCR structural features required for recognition of a conserved peptide of PPD, and such a hypothetical peptide might serve as an immunodominant epitope selecting for the leucine-bearing TCRs expressed by the CD4+ T effector cells. Further studies are needed to confirm TCR binding to a defined peptide/MHC complex.
Clonal expansion of PPD-specific CD4+ and CD8+ T effector clones could occur as early as 2–3 wk after i.v. BCG vaccination or pulmonary M. tuberculosis infection. Some of them sustained clonal expansion as dominant clones, whereas others were no longer quantitatable at later time points. The dynamic clonal representation in the circulation appears to be driven by cytokine environments, which are presumably different between acute and chronic infections. In fact, our earlier studies demonstrated that a number of epitope-specific T cell clones identified in acute SIV infection can lose their dominance in the circulation in chronic infection (23, 28).
Recall expansion of dominant CD4+ T cell clones after M. tuberculosis infection of BCG-vaccinated macaques was remarkable, as clonotypic TCR transcript numbers of a CD4+ T cell clone could increase to 3000 copies among 105 total TCR (Cβ) transcripts. The recall expansion of these T effector clones was likely stimulated by same epitope peptides derived similarly from BCG-and M. tuberculosis-infected macrophages or dendritic cells. More importantly, the recall expansion of these dominant clones correlated with BCG vaccine-induced immunity against fatal M. tuberculosis infection in the juvenile Indian rhesus macaques. Such findings suggest that these T effector clones contribute to the immune protection. This notion is indeed supported by our recent observation indicating that vaccine-elicited CD4+ and CD8+ T cells play a role in antimycobacterial immunity (18, 30).
Interestingly, animal Mm2762 had a log less clonal expansion than did the other immunized animals, but it also had the lowest bacterial burden. It is possible that low levels of clonal expansion may reflect susceptibility to M. tuberculosis, as it did in the unimmunized animals, but it can also reflect superior control of mycobacterial infection.
To our knowledge, our results provided the first clonotypic TCR evidence suggesting that some immunodominant T cell clones could traffick to lungs and continuously accumulate in the airway during M. tuberculosis infection. This presumption was implicated since the dominant clonal TCR sequences were identified and quantitated both in the circulation and airway. The observation was consistent with the recent human study demonstrating that Ag-specific T cells were detectable in lung biopsies in humans with latent M. tuberculosis infection (14). Although the human study was not able to link all expanded TCR Vβ families to the recognition of naturally processed and presented M. tuberculosis epitopes (14), our results clearly demonstrated that many Ag-specific CD4+ but few CD8+ T effector cells producing anti-TB cytokine IFN-γ could remarkably expand as dominant clones in both circulation and airway during M. tuberculosis infection. It is noteworthy that some dominant T effector clones could continuously accumulate in the airway even after these clones were no longer detectable in the blood circulation. The airway trafficking of Ag-specific T effector clones was also supported by the extensive follow-up study in the BCG-vaccinated macaques. Because there was no evidence of lung infection or damage in the BCG-vaccinated macaques (Fig. 6), Ag-specific T cell clones that accumulated in the airway during the 6-mo period might be recruited through transendothelial trafficking or migration rather than due to the damage of lung tissues. This notion is indeed consistent with our previous observation that phosphoantigen-specific IFN-γ+ Vγ2Vδ2 T effector cells can readily traffic to the airway after their peripheral activation even in the absence of lung infection or injury (19). The i.v. and intradermal BCG vaccinations appear to be somewhat relevant since both can elicit accumulation of T effector cells in the airway and induce anti-TB immunity (16, 18). It is important to note that although IFN-γ controls murine TB, human studies have not found a correlation between blood T effectors producing this cytokine and anti-TB immunity. Our data suggest that pulmonary recruitment of Ag-specific T effector cells may be the immune correlate of protection. Collectively, our findings suggest that Ag-specific CD4+ and CD8+ T cells can efficiently traffick to lungs from local or remote lymphoid tissues during BCG vaccination and M. tuberculosis infection. The pulmonary trafficking and accumulation of these dominant Ag-specific T effector clones producing anti-TB cytokine IFN-γ may contribute to the resistance to active TB.
Acknowledgments
We thank other members at the Chen Laboratory for technical assistance.
This work was supported in part by National Institutes of Health Grants R01HL64560 (to Z.W.C.) and R01RR13601 (to Z.W.C.).
Abbreviations used in this paper
- BAL
bronchoalveolar lavage
- BCG
Mycobacterium bovis bacillus Calmette-Guérin
- D
days
- ICS
intracellular cytokine staining
- PPD
purified protein deritative
- TB
tuberculosis
Footnotes
G.D. and C.Y.C. contributed equally to this work and share the first authorship.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Wells CD, Cegielski JP, Nelson LJ, Laserson KF, Holtz TH, Finlay A, Castro KG, Weyer K. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J. Infect. Dis. 2007;196(Suppl. 1):S86–S107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 2.Jones BE, Young SM, Antoniskis D, Davidson PT, Kramer F, Barnes PF. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am. Rev. Respir. Dis. 1993;148:1292–1297. doi: 10.1164/ajrccm/148.5.1292. [DOI] [PubMed] [Google Scholar]
- 3.Mukadi Y, Perriëns JH, St Louis ME, Brown C, Prignot J, Willame JC, Pouthier F, Kaboto M, Ryder RW, Portaels F, et al. Spectrum of immunodeficiency in HIV-1-infected patients with pulmonary tuberculosis in Zaire. Lancet. 1993;342:143–146. doi: 10.1016/0140-6736(93)91346-n. [DOI] [PubMed] [Google Scholar]
- 4.Theuer CP, Hopewell PC, Elias D, Schecter GF, Rutherford GW, Chaisson RE. Human immunodeficiency virus infection in tuberculosis patients. J. Infect. Dis. 1990;162:8–12. doi: 10.1093/infdis/162.1.8. [DOI] [PubMed] [Google Scholar]
- 5.Boom WH, Canaday DH, Fulton SA, Gehring AJ, Rojas RE, Torres M. Human immunity to M. tuberculosis: T cell subsets and antigen processing. Tuberculosis (Edinb.) 2003;83:98–106. doi: 10.1016/s1472-9792(02)00054-9. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann SH. How can immunology contribute to the control of tuberculosis? Nat. Rev. Immunol. 2001;1:20–30. doi: 10.1038/35095558. [DOI] [PubMed] [Google Scholar]
- 7.Shen Y, Zhou D, Chalifoux L, Shen L, Simon M, Zeng X, Lai X, Li Y, Sehgal P, Letvin NL, Chen ZW. Induction of an AIDS virus-related tuberculosis-like disease in macaques: a model of simian immunodeficiency virus-Mycobacterium coinfection. Infect. Immun. 2002;70:869–877. doi: 10.1128/IAI.70.2.869-877.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orme I. Adaptive immunity to mycobacteria. Curr. Opin. Microbiol. 2004;7:58–61. doi: 10.1016/j.mib.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol. Rev. 2008;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao S, Huang D, Chen CY, Halliday L, Zeng G, Wang RC, Chen ZW. Differentiation, distribution and γδ T cell-driven regulation of IL-22-producing T cells in tuberculosis. PLoS Pathog. 2010;6:e1000789. doi: 10.1371/journal.ppat.1000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gambón-Deza F, Pacheco Carracedo M, Cerdá Mota T, Montes Santiago J. Lymphocyte populations during tuberculosis infection: Vβ repertoires. Infect. Immun. 1995;63:1235–1240. doi: 10.1128/iai.63.4.1235-1240.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobsen M, Detjen AK, Mueller H, Gutschmidt A, Leitner S, Wahn U, Magdorf K, Kaufmann SH. Clonal expansion of CD8+ effector T cells in childhood tuberculosis. J. Immunol. 2007;179:1331–1339. doi: 10.4049/jimmunol.179.2.1331. [DOI] [PubMed] [Google Scholar]
- 14.Tully G, Kortsik C, Höhn H, Zehbe I, Hitzler WE, Neukirch C, Freitag K, Kayser K, Maeurer MJ. Highly focused T cell responses in latent human pulmonary Mycobacterium tuberculosis infection. J. Immunol. 2005;174:2174–2184. doi: 10.4049/jimmunol.174.4.2174. [DOI] [PubMed] [Google Scholar]
- 15.Du G, Qiu L, Shen L, Sehgal P, Shen Y, Huang D, Letvin NL, Chen ZW. Combined megaplex TCR isolation and SMART-based real-time quantitation methods for quantitating antigen-specific T cell clones in mycobacterial infection. J. Immunol. Methods. 2006;308:19–35. doi: 10.1016/j.jim.2005.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, Kou Z, Wang Q, Jiang L, Estep J, et al. Adaptive immune response of Vγ2Vδ2+ T cells during mycobacterial infections. Science. 2002;295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen Y, Shen L, Sehgal P, Zhou D, Simon M, Miller M, Enimi EA, Henckler B, Chalifoux L, Sehgal N, et al. Antiretroviral agents restore Mycobacterium-specific T cell immune responses and facilitate controlling a fatal tuberculosis-like disease in macaques coinfected with simian immunodeficiency virus and Mycobacterium bovis BCG. J. Virol. 2001;75:8690–8696. doi: 10.1128/JVI.75.18.8690-8696.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CY, Huang D, Wang RC, Shen L, Zeng G, Yao S, Shen Y, Halliday L, Fortman J, McAllister M, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 2009;5:e1000392. doi: 10.1371/journal.ppat.1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali Z, Shao L, Halliday L, Reichenberg A, Hintz M, Jomaa H, Chen ZW. Prolonged (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate-driven antimicrobial and cytotoxic responses of pulmonary and systemic Vγ2Vδ2 T cells in macaques. J. Immunol. 2007;179:8287–8296. doi: 10.4049/jimmunol.179.12.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang D, Shen Y, Qiu L, Chen CY, Shen L, Estep J, Hunt R, Vasconcelos D, Du G, Aye P, et al. Immune distribution and localization of phosphoantigen-specific Vγ2Vδ2 T cells in lymphoid and nonlymphoid tissues in Mycobacterium tuberculosis infection. Infect. Immun. 2008;76:426–436. doi: 10.1128/IAI.01008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai X, Shen Y, Zhou D, Sehgal P, Shen L, Simon M, Qiu L, Letvin NL, Chen ZW. Immune biology of macaque lymphocyte populations during mycobacterial infection. Clin. Exp. Immunol. 2003;133:182–192. doi: 10.1046/j.1365-2249.2003.02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei H, Wang R, Yuan Z, Chen CY, Huang D, Halliday L, Zhong W, Zeng G, Shen Y, Shen L, et al. DR*W201/P65 tetramer visualization of epitope-specific CD4 T cell during M. tuberculosis infection and its resting memory pool after BCG vaccination. PLoS ONE. 2009;4:e6905. doi: 10.1371/journal.pone.0006905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen ZW, Li Y, Zeng X, Kuroda MJ, Schmitz JE, Shen Y, Lai X, Shen L, Letvin NL. The TCR repertoire of an immunodominant CD8+ T lymphocyte population. J. Immunol. 2001;166:4525–4533. doi: 10.4049/jimmunol.166.7.4525. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher RE, Yeap BY, Bi W, Livak KJ, Beaubier N, Rao S, Bloomfield CD, Appelbaum FR, Tallman MS, Slack JL, Willman CL. Quantitative real-time RT-PCR analysis of PML-RARα mRNA in acute promyelocytic leukemia: assessment of prognostic significance in adult patients from intergroup protocol 0129. Blood. 2003;101:2521–2528. doi: 10.1182/blood-2002-05-1357. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔC(T) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Semighini CP, Marins M, Goldman MH, Goldman GH. Quantitative analysis of the relative transcript levels of ABC transporter Atr genes in Aspergillus nidulans by real-time reverse transcription-PCR assay. Appl. Environ. Microbiol. 2002;68:1351–1357. doi: 10.1128/AEM.68.3.1351-1357.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou D, Shen Y, Chalifoux L, Lee-Parritz D, Simon M, Sehgal PK, Zheng L, Halloran M, Chen ZW. Mycobacterium bovis bacille Calmette-Guérin enhances pathogenicity of simian immunodeficiency virus infection and accelerates progression to AIDS in macaques: a role of persistent T cell activation in AIDS pathogenesis. J. Immunol. 1999;162:2204–2216. [PubMed] [Google Scholar]
- 28.Chen ZW, Shen L, Regan JD, Kou Z, Ghim SH, Letvin NL. The T cell receptor gene usage by simian immunodeficiency virus gag-specific cytotoxic T lymphocytes in rhesus monkeys. J. Immunol. 1996;156:1469–1475. [PubMed] [Google Scholar]
- 29.Chen ZW, Kou ZC, Lekutis C, Shen L, Zhou D, Halloran M, Li J, Sodroski J, Lee-Parritz D, Letvin NL. T cell receptor Vβ repertoire in an acute infection of rhesus monkeys with simian immunodeficiency viruses and a chimeric simian-human immunodeficiency virus. J. Exp. Med. 1995;182:21–31. doi: 10.1084/jem.182.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen Y, Shen L, Sehgal P, Huang D, Qiu L, Du G, Letvin NL, Chen ZW. Clinical latency and reactivation of AIDS-related mycobacterial infections. J. Virol. 2004;78:14023–14032. doi: 10.1128/JVI.78.24.14023-14032.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]