Abstract
Rationale
Aortic aneurysm and dissection (AAD) are major diseases of the adult aorta caused by progressive medial degeneration of the aortic wall. Although the overproduction of destructive factors promotes tissue damage and disease progression, the role of protective pathways is unknown.
Objective
In this study, we examined the role of AKT2 in protecting the aorta from developing AAD.
Methods and Results
AKT2 and phospho-AKT levels were significantly downregulated in human thoracic AAD tissues, especially within the degenerative medial layer. Akt2-deficient mice showed abnormal elastic fibers and reduced medial thickness in the aortic wall. When challenged with angiotensin II (AngII), these mice developed aortic aneurysm, dissection, and rupture with features similar to those in humans, in both thoracic and abdominal segments. Aortas from Akt2-deficient mice displayed profound tissue destruction, apoptotic cell death, and inflammatory cell infiltration that were not observed in aortas from wild-type mice. Additionally, AngII-infused Akt2-deficient mice showed significantly elevated expression of matrix metalloproteinase (MMP)-9 and reduced expression of tissue inhibitor of metalloproteinase (TIMP)-1. In cultured human aortic vascular smooth muscle cells, AKT2 inhibited the expression of MMP-9 and stimulated the expression of TIMP-1 by preventing the binding of transcription factor forkhead box protein O1 (FOXO1) to the MMP-9 and TIMP-1 promoters.
Conclusions
Impaired AKT2 signaling may contribute to increased susceptibility to the development of AAD. Our findings provide evidence of a mechanism that underlies the protective effects of AKT2 on the aortic wall and that may serve as a therapeutic target in the prevention of AAD.
Keywords: Aortic aneurysm and dissection, AKT, FOXO1, MMP-9, TIMP-1
INTRODUCTION
Aortic aneurysm and dissection (AAD) are major diseases of the adult aorta.1 Despite significant improvements in the diagnosis and surgical repair of AAD, the rate of morbidity and mortality from aortic rupture remains high, particularly when the disease involves the thoracic aorta.1 Understanding the pathogenesis of AAD is necessary to develop a better treatment for these conditions.
The key histopathologic feature of aortic aneurysm is progressive degeneration of the medial layer of the aortic wall, which is characterized by the loss of smooth muscle cells (SMCs) and the destruction of extracellular matrix (ECM).2, 3 These events lead to progressive dilatation of the vascular wall and AAD formation, ultimately resulting in lethal aortic rupture. Pharmacologic prevention is ineffective, largely because the molecular mechanisms responsible for the vascular degeneration underlying AAD formation are poorly understood.
The activation of stress signaling pathways and the subsequent production of tissue-destructive factors, such as matrix metalloproteinases (MMPs), promote aortic aneurysm formation and progression. However, there may be native protective systems that counterbalance destructive factors to maintain a healthy vascular wall. When these protective mechanisms are impaired, the uninhibited tissue-destructive factors may drive excessive aortic destruction and lead to AAD formation. Although destructive factors that function in the pathogenesis of AAD have been well documented, protective mechanisms remain to be identified.
The multifunctional AKT signaling pathway, which is activated by insulin, growth factors, and cytokines, is implicated in a diverse range of cellular functions including cell metabolism, survival, proliferation, and migration.4 AKT has profound effects on the cardiovascular system.5 In the heart, the acute activation of AKT promotes cardiomyocyte survival and function,6–8 whereas chronic AKT activation can cause cardiac hypertrophy and maladaptive remodeling.9, 10 In the vascular wall, the AKT pathway regulates endothelial nitric oxide synthase and vascular tone.11 Inflammation12 and metabolic stress13 can impair AKT signaling, resulting in endothelial dysfunction. AKT signaling also promotes vascular SMC (VSMC) proliferation14–16 and migration.5, 17 Although proliferative vascular remodeling can be harmful in neointimal hyperplasia and restenosis,18 it may be beneficial in vascular repair and regeneration in AAD.
In this study, we observed significantly decreased AKT2 expression and AKT phosphorylation/activation in human AAD tissues. We therefore investigated whether AKT2 plays a protective role against AAD formation in the aortic wall in mice and examined the direct effects of AKT2 on MMP-9 and tissue inhibitor of metalloproteinase (TIMP)-1 expression in cultured aortic VSMCs. Our findings suggest that AKT2 has a protective role in the aorta and that AKT2 impairment may contribute to the development of AAD.
METHODS
An expanded Methods section is available in the Online Data Supplement.
Human tissue studies
All protocols were approvedby the Institutional Review Board of Baylor College of Medicine. Informed consent was obtained from all enrolled patients. For this study, we used aortic tissues from 28 patients with descending thoracic AAD, including 12 with thoracic aortic aneurysm (TAA) without dissection (age, 66.8±4.7 years) and 16 with chronic thoracic aortic dissection (TAD) (age, 63.6±5.1 years). Aortic tissues from 12 age-matched organ donors were used as controls (Online Table I). AKT levels and activation in these tissues were examined by Western blot and immunohistochemical analysis.
Animal studies
All animal experiments were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine in accordance with the guidelines of the National Institutes of Health. Wild-type (WT; C57BL/6J) and Akt2−/− (B6.Cg-Akt2tm1.1Mbb/J with C57BL/6J background) mice from The Jackson Laboratory (Bar Harbor, ME) were used. Eight-week-old male mice were infused with either saline or 1,000 ng/min/kg angiotensin II (AngII) for 4 weeks. AAD incidence, aortic destruction, inflammatory cell infiltration, and MMP-9 and TIMP-1 expression were compared between Akt2−/− and WT mice.
Statistical analysis
All quantitative data are presented as the mean ± standard deviation. Data were analyzed with SPSS software, version 11.0 (SPSS Inc., Chicago, IL). Normality of the data was examined by using the Kolmogorov-Smirnov test. Independent t tests were used to compare normally distributed values, and the Mann-Whitney test was used to compare data without a normal distribution. Multiple groups were compared by one-way analysis of variance or by the Kruskal-Wallis test, as appropriate. For all statistical analyses, 2-tailed probability values were used.
RESULTS
AKT2 and Active AKT levels are significantly reduced in the medial layer of human AAD tissue
We first examined AKT and active AKT levels in aortic tissues from patients with descending TAA and chronic TAD. Western blot analysis of AKT protein in the protein extracts of the whole aortic wall (ie, intima, media, and adventitia in TAA; media and adventitia in TAD) (Figure 1A) showed that total AKT protein levels were significantly lower in TAA tissues (but not in TAD tissues) than in control tissues. Furthermore, whereas AKT1 protein levels were similar among groups, AKT2 protein levels were significantly lower in both TAA and TAD tissues than in control tissues. Importantly, phospho-AKT levels and phospho-AKT/total AKT ratios were significantly lower in both TAA and TAD tissues than in control tissues, indicating the reduced activation of AKT in human AAD.
Figure 1.
Decreased phospho-AKT levels in the aortic wall of human aortic aneurysm and dissection (AAD) tissues. (A) Phospho-AKT, total-AKT, AKT1, and AKT2 proteins in human control aortas, thoracic aortic aneurysm (TAA) tissues, and thoracic aortic dissection (TAD) tissues were detected by Western blot by using anti phospho-AKT (Ser 473) and anti-AKT antibodies. Representative blots and quantification of the mean intensities of phospho-AKT, total AKT, AKT1, and AKT2 bands (normalized with those of β-actin) show decreased levels of AKT2 and phospho-AKT, as well as decreased ratios of phospho-AKT/total AKT in aortas from TAA and TAD patients. (B) Phospho-AKT (Ser 473) and total AKT proteins in control, TAA, and TAD tissues were detected by immunostaining. Representative images are shown. For each sample, the positive-staining areas for phospho-AKT and total AKT were measured in five randomly selected microscopic fields (magnification, 400×) and normalized with the total number of cells/nuclei in the counted area. (C) The mean normalized staining signals for phospho-AKT, total AKT, and the ratio of phospho-AKT to total AKT are shown.
Immunostaining analysis of AKT protein in the diseased aortic wall showed a pattern dependent on pathologic changes (Figure 1B). Total AKT protein levels were high in the hyperplastic intima and adventitia, particularly in inflammatory cells and proliferating cells. In the media, although total AKT was abundantly expressed in preserved areas and in hyperplastic areas (ie, areas with increased cell density), it was expressed at substantially lower levels in the cells of degenerative areas (ie, areas with tissue destruction and markedly decreased cell density). Importantly, regardless of total AKT protein levels, phospho-AKT levels and phospho-AKT/total AKT ratios were significantly lower in the media of diseased aortas than in the media of control aortas (Figure 1C). Together, these observations indicate that AKT2 expression is reduced in human AAD tissues, particularly in the degenerative medial layer, and that AKT signaling in these tissues may have been impaired.
Akt2-deficient mice develop AAD when challenged with AngII
To further determine whether impaired AKT2 expression contributes to the development of AAD, we examined AAD formation in Akt2−/− and WT mice. In the absence of exogenous stress, no Akt2−/− mice developed spontaneous AAD (Figure 2A and Figure 2B). When mice were challenged with AngII, which has been shown to induce aortic aneurysms in ApoE−/− mice,19 systolic blood pressure increased similarly in Akt2−/− mice and WT mice (Online Figure IA). In the thoracic and/or abdominal aorta of 70% of AngII-infused Akt2−/− mice, we observed aortic aneurysms (Figure 2A; aortic diameter > 1.5× of the mean aortic diameter of saline-infused Akt2−/− mice), dissections (Figure 2C), or rupture (Figure 2D), whereas no AngII-infused WT mice developed aortic aneurysms (aortic diameter > 1.5× of the mean aortic diameter of saline-infused WT mice) or dissections (P<0.001) (Figure 2B). Furthermore, compared with AngII-infused WT mice, AngII-infused Akt2−/− mice showed significant increases in the diameter of most aortic segments (Figure 2E). When we characterized aortic gross pathology using the classification of Daugherty and colleagues,19 we found that only 25% of AngII-infused WT mice developed aortic lesions (none of which were more severe than Type II), whereas 91% of AngII-infused Akt2−/− mice developed aortic lesions (P<0.001) (52% of which were more severe than Type II; Figure 2F). Furthermore, in AngII-infused Akt2−/− mice, vessel dilatation (aortic diameter > 1.25× of the mean aortic diameter of saline-infused Akt2−/− mice) was observed most frequently in the suprarenal abdominal segment (74%), followed by the ascending (65%), descending thoracic (61%), arch (39%), and infrarenal (39%) aortic segments, 4 weeks after AngII infusion (Online Figure IB). Aneurysms in AngII-infused Akt2−/− mice were most frequently observed in the ascending (39%) and suprarenal abdominal (35%) aortic regions, followed by the descending thoracic (17%), arch (13%), and infrarenal (9%) aortic segments (Online Figure IC). Fatal rupture occurred in 13% of AngII-infused Akt2−/− mice, exclusively in the ascending aortic segment and typically within 1 week after the initiation of AngII infusion (Online Figure ID). Together, our results show that AngII infusion led to severe aortic disease in the setting of Akt2 deficiency, suggesting that AKT2 has protective role against the formation of AAD.
Figure 2.
Development of aortic aneurysms and dissections in Akt2−/− mice infused with AngII. WT and Akt2−/− mice were infused with saline or angiotensin II (AngII) for 4 weeks. (A) Representative excised aortas show that Akt2−/− mice challenged with AngII developed aneurysms involving the thoracic and abdominal aorta. (B) The incidence of all aortic aneurysms and dissections in Akt−/− and WT mice (P<0.001). (C) Histologically confirmed aortic dissection, with a characteristic true lumen (TL) and false lumen (FL), in AngII-infused Akt2−/− mice. (D) In an AngII-infused Akt2−/− mouse that suddenly died, median sternotomy (left panel) revealed that blood surrounded the heart and great vessels, which was caused by the rupture of an ascending aortic aneurysm (arrow, right panel). (E) Comparison of aortic diameter among saline or AngII-infused WT and Akt2−/− mice. (F) The types of aortic lesions, according to a modified classification system based on that of Daugherty and colleagues,19 are shown.
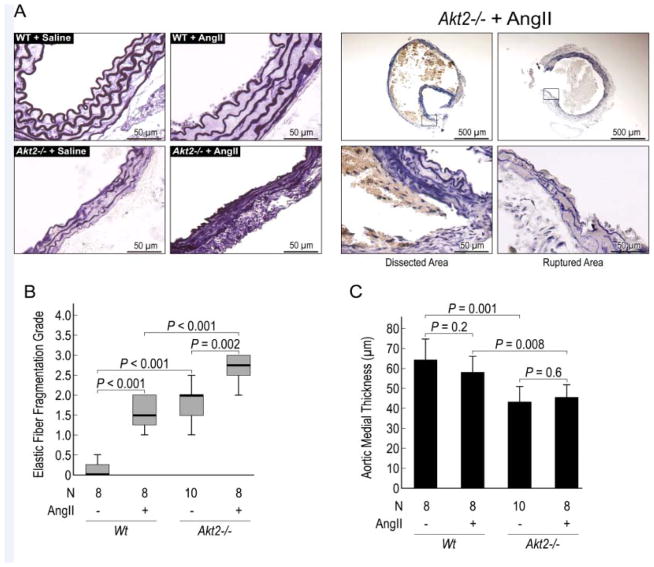
Elastic fiber destruction is significantly increased in AngII-challenged Akt2−/− mice
To characterize the histologic changes in the aortas of Akt2−/− mice, we analyzed elastic lamellar architecture using Verhoeff elastic staining. Compared with aortas from saline-infused WT mice, aortas from saline-infused Akt2−/− mice displayed notable abnormalities in aortic architecture, with thinner and straighter elastic fibers (Figure 3A), increased elastic fiber fragmentation (Figure 3B), and significantly reduced medial thickness (Figure 3C). When infused with AngII, Akt2−/− mice exhibited severe elastic fiber fragmentation (Figure 3B). These findings suggest that AKT2 may prevent AAD formation and progression by promoting and maintaining normal aortic structure and inhibiting elastin destruction.
Figure 3.
Significantly increased elastic fiber destruction and reduced medial thickness in angiotensin II (AngII)-infused Akt2−/− mice. (A) Representative histologic sections of the aorta (Verhoeff elastic staining) demonstrating abnormalities in elastic lamellar architecture in saline-infused Akt2−/− mice and marked elastin destruction in AngII-infused Akt2−/− mice. Comparisons of (B) elastic fiber fragmentation scores (Grade 0 = none; Grade 1 = minimal; Grade 2 = moderate; Grade3 = severe) and (C) medial thickness among groups are shown.
Apoptosis is significantly increased in AngII-challenged Akt2−/− mice
Because AKT plays an important role in cell survival, we analyzed apoptosis in the aortas of Akt2−/− and WT mice using the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay. As shown in Figure 4A, TUNEL-positive cells were observed in AngII-infused WT mice but not in saline-infused WT mice. The number of TUNEL-positive cells was markedly increased in the aortic wall of saline-infused Akt2−/− mice but was even higher when Akt2−/− mice were challenged with AngII, particularly in the lesion regions. Additionally, cleaved caspase 3 (Figure 4B) and apoptosis-inducing factor (Figure 4C) were detected in the aortas of AngII-infused Akt2−/− mice, suggesting the potential involvement of both caspase-dependent and caspase-independent apoptotic pathways.
Figure 4.
Significantly increased apoptosis and inflammatory cell infiltration in angiotensin II (AngII)-infused Akt2−/− mice. (A) Representative terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-stained images and comparison of the number of TUNEL-positive cells normalized to the total number of cells are shown. The number of TUNEL-positive cells was significantly increased in the lesion areas of AngII-infused Akt2−/− mice, particularly in dissected areas. Cleaved caspase 3 (B) and apoptosis inducing factor (AIF) (C) were detected the aortas of AngII-infused Akt2−/− mice. (D) Representative images of immunofluorescence staining and quantification (mean positive staining area normalized with evaluated aortic area) of CD68-positive macrophages in the aortic wall show a significant increase in macrophages in AngII-infused Akt2−/− mice.
Inflammatory cell infiltration is significantly increased in AngII-challenged Akt2−/− mice
We also examined the infiltration of the aorta by inflammatory cells, which have been shown to promote ECM degradation and cell injury. In the absence of AngII infusion, the infiltration of macrophages (marked by the presence of CD68) (Figure 4D) and CD3-positive T cells (Online Figure II) was minimal in the aortas of saline-infused WT mice and Akt2−/− mice. In the presence of AngII, the infiltration of these cells was observed within the aortic wall of WT mice and Akt2−/− mice. However, AngII-challenged Akt2−/− mice had significantly more inflammatory cell infiltration that was even more pronounced in areas of tissue destruction and dissection (Figure 4D). Most of the macrophages in the aortic tissue of AngII-challenged Akt2−/− mice were observed in the adventitia. Macrophages were also detected in the media-adventitia boundary area, and a few macrophages were identified in the media of the aortic wall.
MMP-9 expression is increased and TIMP-1 expression is decreased in AngII-challenged Akt2−/− mice
To further investigate the potential mechanisms for increased elastin destruction in Akt2−/− mice, we examined the expression of MMP-9 and MMP-2, which are key proteinases in ECM protein destruction. We found that the levels of MMP-9 protein (Figure 5A) and mRNA (Online Figure IIIA) and MMP-2 protein (Online Figure IIIB) were significantly increased in the aortic media and adventitia of AngII-challenged Akt2−/− mice, particularly in lesion segments. In these mice, MMP-9 was expressed in CD68-positive macrophages (Figure 5B) and in SM22α-positive SMCs (Figure 5C; Online Figure IIIC). In the aortic media, SMCs appeared to produce more MMP-9 than did macrophages (Figure 5B and 5C), suggesting that MMP-9 from SMCs may play a critical role in medial destruction and aortic dysfunction.
Figure 5.
Significantly increased MMP-9 expression and MMP-9/TIMP-1 ratio in angiotensin II (AngII)-infused Akt2−/− mice. (A) Representative images of immunofluorescence staining and quantification of the mean positive staining area normalized with the evaluated aortic area show increased MMP-9 expression in aortas from AngII-infused Akt2−/− mice. Double staining for MMP-9 and CD68 or MMP-9 and SM22α shows increased MMP-9 expression in (B) CD68-positive macrophages and in (C) SM22α-positive SMCs in the aortas of AngII-infused Akt2−/− mice. (D) Representative images of immunofluorescence staining and quantification of TIMP-1 expression (the mean positive-staining area normalized with the evaluated area) shows lower TIMP-1 expression in aortas from AngII-infused Akt2−/− mice than in AngII-infused WT mice. (E) Double staining for MMP-9 and TIMP-1 in an aorta from an AngII-infused Akt2−/−mouse. Comparison shows increased MMP-9/TIMP-1 ratios in aortas from AngII-infused Akt2−/− mice. (F) Representative zymography images show increased MMP activity in aortas from AngII-infused Akt2−/− mice.
Additionally, TIMP-1 protein (Figure 5D) and mRNA (Online Figure IIID) levels were increased in AngII-infused WT mice, saline-infused Akt2−/− mice, and AngII-infused Akt2−/− mice. Double immunostaining showed the colocalization of TIMP-1 and MMP-9 (Figure 5E) in the aortic wall, indicating that increased TIMP-1 expression may represent a protective response to increased MMP expression and aortic injury. However, the induction of TIMP-1 was lower (Figure 5D) and the MMP-9/TIMP-1 ratio was significantly higher (Figure 5E) in AngII-infused Akt2−/− mice than in AngII-infused WT mice, indicating an insufficient counterbalance of TIMP-1 with MMP-9 in AngII-infused Akt2−/−mice.
Consistent with these findings, the results of in situ gelatin zymography showed increased MMP activity in the aortic media of AngII-infused Akt2−/− mice (Figure 5F), suggesting that MMP-9 and MMP-2 production by SMCs may play an important role in medial destruction.
AKT2 inhibits MMP-9 expression and enhances TIMP-1 expression in cultured aortic VSMCs
To further investigate the mechanism of the AKT2-mediated regulation of MMP-9 and TIMP-1, we examined the effects of AKT2 on MMP-9 and TIMP-1 protein and mRNA expression in human aortic VSMCs. Whereas the overexpression of WT-AKT2 reduced MMP-9 and increased TIMP-1 protein levels, the shRNA-mediated knockdown of AKT2 increased MMP-9 and decreased TIMP-1 protein levels (Figure 6A). Furthermore, qRT-PCR results showed that WT-AKT2 significantly reduced MMP-9 and increased TIMP-1 mRNA levels, whereas AKT2 shRNA increased MMP-9 and decreased TIMP-1 mRNA levels (Figure 6B). Our data suggest that AKT2 inhibits MMP-9 expression and enhances TIMP-1 expression at the mRNA level.
Figure 6.
Effects of AKT2 on TIMP-1 and MMP-9 expression. (A) Western blot analysis of TIMP-1 and MMP-9 protein levels in aortic vascular smooth muscle cells (VSMCs) that were transfected with wild-type AKT2 (WT-AKT2), or AKT2 shRNA. Representative blots from 3 independent experiments are shown. Quantification of the mean intensities of TIMP-1 and MMP-9 bands (normalized with those of β-actin) are shown. (B) TIMP-1 and MMP-9 mRNA levels were examined by reverse transcription and quantitative real-time PCR in VSMCs transfected with WT-AKT2 or AKT2 shRNA. TIMP-1 and MMP-9 mRNA expression was normalized with that of β-actin and is expressed as the percentage of the control. Data represent the mean ± SD (N=3).
Forkhead Box Protein O1 (FOXO1) positively regulates MMP-9 and negatively regulates TIMP-1 expression in human aortic VSMCs
To further investigate the potential mechanism for the AKT2-mediated regulation of MMP-9 and TIMP-1 expression, we examined whether the transcription factor FOXO1, a major downstream target of AKT that can be phosphorylated and inactivated by AKT, regulates the expression of MMP-9 and TIMP-1. WT-FOXO1 but not DN-FOXO1 (dominant-negative FOXO1) or FOXO1 siRNA increased MMP-9 protein levels (Figure 7A), and WT-FOXO1 and CA-FOXO1 (constitutively active FOXO1) but not DN-FOXO1 increased MMP-9 mRNA levels (Figure 7B), indicating that FOXO1 positively regulates MMP-9 expression.
Figure 7.
The effects of FOXO1 on MMP-9 and TIMP-1 expression. Aortic vascular smooth muscle cells (VSMCs) were transfected with plasmids expressing wild-type (WT)-FOXO1, constitutively active (CA)-FOXO1, dominant negative (DN)-FOXO1, or FOXO1 siRNA, and (A) MMP-9 and TIMP-1 protein expression was examined by Western blot. Representative blots from 3 independent experiments are shown. Quantification of the mean intensities of TIMP-1 and MMP-9 bands (normalized with those of β-actin) are shown. (B) MMP-9 and TIMP-1 mRNA levels were examined by reverse transcription and quantitative real-time PCR and were normalized with levels of β-actin mRNA. Levels of mRNA are expressed as the percentage of the control. Data represent the mean ± SD (N=3). (C) Diagram showing the location of FOXO binding sites in the human MMP-9 promoter. (D) ChIP analysis showing that FOXO-1 bound to FOXO binding site 2 of the MMP-9 promoter in aortic VSMCs transfected with WT-FOXO1 and CA-FOXO1 but not DN-FOXO1. FOXO1-DNA complexes were cross-linked by formaldehyde and immunoprecipitated with anti-FOXO1 antibody. Sites in the MMP-9 promoter bound by FOXO1 were detected by PCR and normalized with input DNA. Representative blots from 2 independent experiments are shown. (E) Diagram showing the location of FOXO and GATA1 binding sites in the human TIMP-1 promoter. (F) ChIP analysis showing that FOXO-1 bound to the TIMP-1 promoter in aortic VSMCs transfected with WT-FOXO1 and CA-FOXO1 but not DN-FOXO1. FOXO1-DNA complexes were cross-linked and immunoprecipitated with anti-FOXO1 antibody and anti-GATA1 antibody. Sites in the TIMP-1 promoter bound by FOXO1 and GATA1 were detected by PCR and normalized with input DNA. Representative blots from 2 independent experiments are shown.
In contrast to the positive regulation of MMP-9 expression by FOXO1, we found that TIMP-1 expression is negatively regulated by FOXO1. WT-FOXO1 decreased TIMP-1 protein levels, whereas DN-FOXO1 or FOXO1 siRNA increased TIMP-1 protein levels (Figure 7A). Additionally, WT-FOXO1 and CA-FOXO1 but not DN-FOXO1 decreased TIMP-1 mRNA levels (Figure 7B).
FOXO1 binds to the promoters of MMP-9 and TIMP-1 in human aortic VSMCs
Promoter sequence analysis of the 5′-flanking region of the human MMP-9 gene showed the presence of 3 potential binding sites for FOXO transcription factors (Figure 7C). By using the ChIP assay, we showed that WT-FOXO1 and CA-FOXO1 but not DN-FOXO1 bind to FOXO binding site 2 of MMP-9 (Figure 7D). FOXO1 also bound to sites 1 and 3 (data not shown). Our data suggest that the binding of FOXO1 to FOXO binding sites on the MMP-9 promoter maybe critical for FOXO1-mediated stimulation of MMP-9 gene expression.
Promoter sequence analysis of the 5′-flanking region of the human TIMP-1 gene showed the presence of 5 potential binding sites for FOXO transcription factors. Interestingly, one of the FOXO binding sites we identified and subsequently tested in the TIMP-1 promoter is in close proximity to a binding site for GATA1 (Figure 7E). GATA1 is a transcription factor that is phosphorylated by AKT and mediates AKT-induced TIMP-1 expression.20 We performed ChIP analysis to examine the binding of FOXO1 and GATA1 to the TIMP-1 promoter and found that WT-FOXO1 and CA-FOXO1 increased FOXO1 binding while decreasing GATA1 binding to the TIMP-1 promoter, whereas DN-FOXO1 decreased FOXO1 binding while increasing GATA1 binding to the TIMP-1 promoter (Figure 7F). The inverse relationship between FOXO1 and GATA1 binding to the TIMP-1 promoter suggests that FOXO1 binding to this site may prevent GATA1 from binding to the TIMP-1 promoter, resulting in the suppression of TIMP-1 transcription.
AKT2 regulates MMP-9 and TIMP-1 by inhibiting FOXO1
We examined whether AKT2 regulates MMP-9 expression by regulating FOXO1-mediated MMP-9 and TIMP-1 expression. We found that WT-AKT2 prevented the FOXO1-induced stimulation of MMP-9 protein and mRNA expression, whereas AKT2 shRNA amplified the FOXO1-induced stimulation of MMP-9 protein and mRNA expression (Figure 8A and 8B). ChIP analysis showed that WT-AKT2 reduced the binding of FOXO1 to the MMP-9 promoter, whereas AKT2 shRNA increased the binding of FOXO1 to the MMP-9 promoter (Figure 8C). Furthermore, WT-AKT2 prevented WT-FOXO1 from binding to the MMP-9 promoter, whereasWT-AKT2 failed to prevent CA-FOXO1 from binding to the MMP-9 promoter, indicating that the inhibition of FOXO1 by AKT2 is FOXO1-phosphorylation dependent.
Figure 8.
Inhibition of FOXO1-mediated MMP-9 expression by AKT2. Vascular smooth muscle cells were transfected with wild-type (WT)-FOXO1 in the presence of WT-AKT2 and AKT2 shRNA. (A) MMP-9 and TIMP-1 protein levels were examined by Western blot. Representative blots from 3 independent experiments are shown. Quantification of the mean intensities of TIMP-1 and MMP-9 bands (normalized with those of β-actin) are shown. (B) MMP-9 and TIMP-1 mRNA levels were examined by reverse transcription and quantitative real-time PCR and normalized with those of β-actin mRNA. The relative levels of mRNA are expressed as the percentage of the control. Data represent the mean ± SD (N=3). (C) FOXO1 binding to the MMP-9 promoter was examined by ChIP analysis. (D) FOXO1 and GATA1 binding to the TIMP-1 promoter was examined by ChIP analysis. Representative blots from 2 independent experiments are shown. (E) Immunostaining showed increased FOXO1 nuclear translocation in SMCs transfected with Akt2 siRNA or treated with Wortmannin. Immunostaining showed increased FOXO1 expression and nuclear localization in (F) aortas of AngII-infused Akt2−/− mice and (G) degenerative aortic media in human thoracic aortic aneurysm (TAA) tissue.
Additionally, WT-AKT2 prevented the FOXO1-induced inhibition of TIMP-1 protein and mRNA expression, whereas AKT2 shRNA amplified the FOXO1-induced inhibition of TIMP-1 protein and mRNA expression (Figure 8A and 8B). WT-AKT2 also reduced the binding of FOXO1 to the TIMP-1 promoter, whereas AKT2 shRNA increased the binding of FOXO1 to the TIMP-1 promoter (Figure 8D). In contrast, WT-AKT2 induced GATA1 binding to the TIMP-1 promoter, whereas AKT2 shRNA reduced GATA1 binding to the TIMP-1 promoter (Figure 8D). The inability of WT-AKT2 to prevent CA-FOXO1 from binding to the TIMP-1 promoter indicates that the inhibition of FOXO1 by AKT2 is FOXO1-phosphorylation dependent.
We also showed that AKT2 inhibits FOXO1 action by preventing its nuclear translocation (Figure 8E). Silencing AKT2 expression with AKT2 siRNA or inhibiting AKT activation with Wortmannin promoted the nuclear translocation of FOXO1 (Figure 8E). Furthermore, consistent with our in vitro observations, high levels of FOXO1 protein were observed in the nucleus of aortic SMCs in AngII-infused Akt2−/− mice (Figure 8F), suggesting the activation of FOXO1 in these cells. Moreover, a significant amount FOXO1 protein was also observed in aortic media in human TAA tissue (Figure 8G).
Together, these data suggest that AKT2 may inhibit MMP-9 expression by preventing FOXO1-mediated MMP-9 transcription and that AKT2 may stimulate TIMP-1 expression by inhibiting FOXO1 and stimulating GATA1-regulated TIMP-1 transcription (Online Figure IV).
DISCUSSION
In this study, we have identified a novel molecular mechanism for AKT2 in protecting against the formation of AAD. Following our initial observation that AKT2 protein and AKT activation were reduced in human thoracic AAD, we then showed that Akt2 deficiency in mice markedly increased susceptibility to AngII-induced AAD formation. Furthermore, we found that AKT2 suppressed MMP-9 expression and stimulated TIMP-1 expression by inhibiting FOXO1 in cultured human aortic VSMCs. Our results suggest that impaired AKT2 signaling in the aorta may increase susceptibility to AAD.
Although Akt2−/− mice did not develop spontaneous AAD in the absence of exogenous stress, the aortas of these mice showed significant abnormalities. These results suggest that AKT2 may play a critical role in the response to hemodynamic stress. Furthermore, when challenged with AngII, 70% of Akt2−/− mice but not WT mice developed marked aortic dilatation with aneurysm formation, dissection, and rupture, with features similar to those seen in human aortic disease. Thus, we speculate that AKT2 activation may protect the aortic wall against aneurysm formation in response to stress. Our results support the notion that, in addition to the well-known overactivation of destructive forces, there are native protective mechanisms in place that counterbalance destructive forces to maintain vascular wall integrity.
Several mechanisms may account for AKT2’s protective effects. One possible mechanism through which AKT2 may protect against the formation of aortic aneurysms is by promoting SMC survival. AKT has well-established roles in promoting cell survival6, 21 by directly inhibiting apoptosis signal-regulating kinase 1,22 p53,22 and BAD23—all key molecules in the apoptosis pathway. SMC apoptosis has been reported in human aortic aneurysms,2, 24 and it may play a critical role in SMC depletion and aortic damage during AAD formation. It is noteworthy that we observed a significant increase in the number of TUNEL-positive cells in the lesion regions of AngII-infused Akt2−/− mice, with more in dissected areas and fewer in areas with significant SMC loss. A high rate of TUNEL-positive cells was also observed in human AAD tissues, even in those with significant SMC loss. In human abdominal aortic aneurysm with significant aortic SMC cell loss, the reported rate of apoptosis ranges from 10%24 to 30%.2 Interestingly, in our study, mice with a significant number of TUNEL-positive cells were alive at the end of the experiment (ie, 28 days after initiating AngII infusion; all ruptures occurred within 10 days of AngII infusion), indicating that profound tissue repair and fibrotic remodeling occurred in the aortic wall that strengthened the aortic wall structure and prevented rupture. Although it is unclear how rapidly TUNEL-positive cells in our study died, our evidence suggests that there was enough time for aortic repair and fibrotic remodeling to take place before rupture.
In mice challenged with AngII, we observed inflammatory cell infiltration in the aortic wall, which was significantly higher in the aortas of Akt2−/− mice than in WT mice, especially in dissected aortas. In AngII-infused Akt−/− mice, most macrophages were observed in the adventitial layer. The complicated role of AKT in inflammation is not completely understood; although AKT has been shown to promote inflammation,25 several studies have also shown that AKT activation can inhibit inflammation.26–31 Given that AngII is known to induce inflammation,32 the significant inflammatory cell infiltration observed in AngII-infused Akt2−/− mice may result from the insufficient inhibition of AngII-induced inflammation by AKT2. Another possibility is that the increased inflammatory cell infiltration in these mice represents a response to SMC apoptosis and aortic injury. The significant production of MMP-9 in macrophages suggests that these cells may contribute to aortic destruction.32–34 Thus, when AngII-induced inflammation and aortic injury are not sufficiently counterbalanced, this may lead to inflammatory cell infiltration, which causes further damage to the aortic wall.
MMPs are key destructive factors in the development of AAD.35, 36 MMP activity can be inhibited by TIMPs, and thus signaling pathways that regulate the balance between MMP and TIMP activities are critical in controlling tissue destruction. AKT has been shown to stimulate TIMP-1 expression in erythroid cells.20 However, the AKT pathway has been shown to have complex effects on MMP regulation. Although AKT has been shown to promote MMP production in tumor invasion and metastasis,37–39 recent studies have shown that the AKT pathway inhibits MMP expression. It has been suggested that PI3Kγ, a molecule upstream of AKT, inhibits the expression and activity of several MMPs induced by biomechanical stress.40 Additionally, the inhibition of the AKT pathway with rapamycin drastically enhanced the LPS-induced upregulation of MMP-9 in macrophages,41 suggesting that the AKT pathway may negatively regulate MMP-9 expression. Here, we showed that AKT2 inhibited MMP-9 expression and stimulated TIMP-1 expression in cultured VSMCs. In AngII-infused Akt2−/− mice, MMP-9 expression was significantly elevated in aortic SMCs. Additionally, the induction of TIMP-1 in response to increased MMPs was significantly lower and the MMP-9/TIMP-1 ratio was significantly higher in AngII-infused Akt2−/− mice than in WT mice. Thus, our findings suggest that the AKT2-induced inhibition of MMP-9 and stimulation of TIMP-1 in VSMCs may contribute to the protective effects of AKT2 on the aorta.
In our study, we also found that AKT2 stimulated TIMP-1 and inhibited MMP-9 expression through the inhibition of FOXO1. We showed that FOXO1 upregulated MMP-9 and downregulated TIMP-1 expression, which was reversed by the overexpression of AKT2. Consistent with these observations, a recent study showed that FOXO3 induces the expression of MMP-9 and MMP-13 in cancer cells and promotes tumor metastasis.42 FOXO3a has also been shown to increase MMP-3 expression and activity and decrease TIMP-1 expression in the vascular wall.43 In several regions of the TIMP-1 promoter, we identified FOXO1 binding sites in close proximity to binding sites of GATA1. Given our finding that FOXO1can bind to the TIMP-1 promoter and prevent the DNA binding of GATA1, it is conceivable that FOXO1 binding to the TIMP-1 promoter may prevent GATA1 DNA binding and suppress TIMP-1 transcription. AKT has previously been shown to induce TIMP-1 expression by directly phosphorylating and activating GATA1.20 Thus, in addition to activating GATA1, AKT2 may also promote TIMP-1 expression by preventing FOXO-1 from inhibiting the DNA binding of GATA-1 to the TIMP-1 promoter. Together, our finding that AKT2 regulates MMP-9 and TIMP-1 expression by inhibiting FOXO1 represents a novel mechanism for the control of MMP and TIMP production (Online Figure IV).
Other mechanisms may underlie the protective effects of AKT2. For example, AKT has been shown to inhibit RhoA, a downstream target of the AngII-signaling pathway.29 AKT also can inhibit TGF-β signaling,44, 45 a critical player in aortic destruction and aneurysm formation. Additionally, AKT plays a significant role in VSMC proliferation,46 migration,46 differentiation,47–48 ECM protein production, and aortic repair (our unpublished observations). AKT2 also plays critical roles in glucose49, 50 and lipid51, 52 homeostasis. As a result, even though we used young mice (8 weeks old) to avoid profound metabolic disturbance, the metabolic stress from Akt2 deficiency may also partially contribute to aortic destruction in these mice. Further studies are needed to explore this possibility. Finally, AKT may play an important role in aortic wall development; impaired formation of aortic ECM could cause developmental abnormalities that predispose Akt2-deficient mice to AAD formation after the infusion of AngII.
The AKT kinase family comprises three highly homologous isoforms that have specific functions. AKT1 promotes cell survival,53 and AKT2 regulates glucose49, 50 and lipid51, 52 homeostasis. AKT3 has recently been shown to reduce lipoprotein uptake in macrophages, preventing foam cell formation that leads to atherosclerosis development.54 However, evidence suggests that AKT isoforms share many of these functions. For example, AKT2 also plays a critical role in promoting cell survival.55–57 We have also observed the regulation of MMP-9 and TIMP-1 by AKT1 (data not shown), although AKT1 showed stronger inhibition of MMP-9, and AKT2 showed stronger induction of TIMP-1. Thus, AKT isoforms may act cooperatively to balance TIMPs and MMPs.
We observed significantly reduced levels of active AKT and AKT2 in human TAA and TAD tissues than in control aortas. Interestingly, AKT expression within the diseased aorta exhibited marked heterogeneity; although AKT expression was downregulated in degenerative areas, it was abundantly expressed in preserved areas and in hyperplastic areas. This pattern of variation in AKT expression is consistent with the heterogeneic nature of aortic aneurysm pathology; different areas of the aortic wall exhibit different stages of disease progression, even within a single aneurysm. Importantly, regardless of overall AKT expression, phospho-AKT levels were significantly lower in the aortic media of patients with AAD than in those of age-matched controls, indicating impaired AKT signaling in diseased aortas. The low levels of active AKT observed in diseased aortas may have resulted from reduced cell numbers or from decreased AKT expression and impaired AKT activation in the remaining cells.
In this study, our age-matched control subjects had a mean age of 61 years; many of these subjects had hypertension, dyslipidemia, and diabetes mellitus. Considerable variation in active AKT levels was observed among control tissues. Interestingly, high levels of active AKT were often observed in aortic tissues from control subjects with diabetes mellitus. Similar to our patients, control subjects with diabetes mellitus were presumably exposed to inflammatory and metabolic stress generated from their comorbid condition. The activation of AKT in the aorta of these individuals may provide protection against such biologic insults and counterbalance the stress/destructive factors and pathways.
Compromised AKT signaling has been reported in the thoracic aorta of patients with Marfan syndrome.58 Given the importance of the AKT signaling pathway in protecting the aorta, the downregulation of this pathway may be partially responsible for the formation and progression of thoracic AAD in Marfan syndrome patients. Further studies are necessary to elucidate the causes of AKT downregulation in the aorta. It is well established that inflammation12 and metabolic stress13 can impair AKT signaling. The aorta, especially in aged individuals with atherosclerosis, is constantly exposed to inflammatory factors and metabolic stress, which may affect the medial layer of the aorta and impair AKT signaling.
CONCLUSION
Our findings suggest that AKT2 may have a protective role against AAD in humans. Although these findings require further validation, the possibility remains that the preservation of AKT2 signaling may be an effective strategy for preventing the formation of AAD.
Novelty and Significance.
What Is Known?
Open and endovascular surgical repair of aortic aneurysm and dissection (AAD) are accompanied by high peri-operative morbidity and mortality, andmedical therapy to prevent the formation and progression of AAD is lacking.
Aortic aneurysm and dissection are caused by the progressive degeneration of the aortic media, characterized by the depletion of smooth muscle cells and the destruction of extracellular matrix.
Although the overproduction of destructive factors promotes tissue damage and AAD progression, the role of pathways that protect against the development of AAD is unknown.
What New Information Does This Article Contribute?
Levels of AKT2 protein and phospho-AKT are significantly downregulated in human thoracic AAD tissues.
A causal relationship between Akt2 deficiency and AAD is supported by our findings that the aortas of Akt2-deficient mice challenged with angiotensin II (but not the aortas of wild-type mice challenged with angiotensin II) developed aneurysm, dissection, and rupture and showed evidence of profound tissue destruction and apoptotic cell death.
The mechanism of AKT2 protection against AAD involves the inhibition of MMP-9 and the stimulation of TIMP-1 through the inhibition of the transcription factor forkhead box protein O1 (FOXO1).
Aortic aneurysm and dissection are characterized by degeneration of the aortic media. The role of the pathways that protect against AAD is unknown., We examined the role of AKT2 in AAD formation. We found that protein levels of AKT2 and phospho-AKT were significantly reduced in human thoracic AAD tissues, especially within the degenerative medial layer. Our studies in Akt2-deficient mice showed that these mice have abnormal elastic fibers and reduced medial thickness in the aortic wall. When challenged with AngII, Akt2-deficient mice developed aortic aneurysm, dissection, and rupture. Aortas from AngII-infused Akt2-deficient mice displayed profound tissue destruction, apoptotic cell death, and inflammatory cell infiltration, changes that were not observed in aortas from AngII-infused wild-type mice. Additional findings in Akt2-deficient mice and human aortic VSMCs indicated that the protective mechanism of AKT2 involves the regulation of MMP-9 and TIMP-1 expression. The results of these studies showing that impaired AKT2 signaling may contribute to AAD formation may lead to the development of a new therapeutic target for the treatment of AAD.
Acknowledgments
We gratefully acknowledge Sue Zhang of the Texas Heart Institute at St. Luke’s Episcopal Hospital, for critical discussion of the manuscript; Darren Woodside and Deenadayalan Bakthavatsalam of the Texas Heart Institute at St. Luke’s Episcopal Hospital, for their expert advice regarding use of the confocal microscope; Scott A. Weldon of Baylor College of Medicine, for assistance with illustrations; and Nicole Stancel of the Texas Heart Institute at St. Luke’s Episcopal Hospital, for providing editorial support.
SOURCES OF FUNDING
This study was supported by NIH R01HL085341 (to S.A.L.) and NIH K08 HL080085 (to S.A.L.). The Thoracic Aortic Disease Tissue Bank at Baylor College of Medicine was supported in part through the Tissue Banking Core of the Specialized Center of Clinically Oriented Research in Thoracic Aortic Aneurysms and Dissections (NIH P50 HL083794).
Non-standard Abbreviations
- AAD
aortic aneurysm and dissection
- SMC
smooth muscle cell
- ECM
extracellular matrix
- MMP
matrix metalloproteinase
- VSMC
vascular smooth muscle cell
- TIMP
tissue inhibitor of metalloproteinase
- TAA
thoracic aortic aneurysm
- TAD
thoracic aortic dissection
- WT
wild type
- AngII
angiotensin II
- TUNEL
terminal deoxynucleotidyltransferase dUTP nick end labeling
- DN
dominant negative
- CA
constitutively active
- FOXO1
forkhead box protein O1
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
Footnotes
DISCLOSURES
None.
References
- 1.Kochanek KD, Kirmeyer SE, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2009. Pediatrics. 2012;129:338–348. doi: 10.1542/peds.2011-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, Thompson RW. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997;150:993–1007. [PMC free article] [PubMed] [Google Scholar]
- 3.Schlatmann TJ, Becker AE. Pathogenesis of dissecting aneurysm of aorta. Comparative histopathologic study of significance of medial changes. Am J Cardiol. 1977;39:21–26. doi: 10.1016/s0002-9149(77)80005-2. [DOI] [PubMed] [Google Scholar]
- 4.Manning BD, Cantley LC. Akt/pkb signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiojima I, Walsh K. Role of akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 6.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 8.Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MV, Napoli C, Sadoshima J, Croce CM, Ross J., Jr Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated akt in the heart. J Biol Chem. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 10.Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, Ogawa W, del Monte F, Gwathmey JK, Grazette L, Hemmings BA, Kass DA, Champion HC, Rosenzweig A. Pi3k rescues the detrimental effects of chronic akt activation in the heart during ischemia/reperfusion injury. J Clin Invest. 2005;115:2128–2138. doi: 10.1172/JCI23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo Z, Fujio Y, Kureishi Y, Rudic RD, Daumerie G, Fulton D, Sessa WC, Walsh K. Acute modulation of endothelial akt/pkb activity alters nitric oxide-dependent vasomotor activity in vivo. J Clin Invest. 2000;106:493–499. doi: 10.1172/JCI9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen YH, Zhang L, Gan Y, Wang X, Wang J, Lemaire SA, Coselli JS, Wang XL. Up-regulation of pten (phosphatase and tensin homolog deleted on chromosome ten) mediates p38 mapk stress signal-induced inhibition of insulin signaling: A cross-talk between stress signaling and insulin signaling in resistin-treated human endothelial cells. J Biol Chem. 2006;281:7727–7736. doi: 10.1074/jbc.M511105200. [DOI] [PubMed] [Google Scholar]
- 13.Wang XL, Zhang L, Youker K, Zhang MX, Wang J, LeMaire SA, Coselli JS, Shen YH. Free fatty acids inhibit insulin signaling-stimulated endothelial nitric oxide synthase activation through upregulating pten or inhibiting akt kinase. Diabetes. 2006;55:2301–2310. doi: 10.2337/db05-1574. [DOI] [PubMed] [Google Scholar]
- 14.Shigematsu K, Koyama H, Olson NE, Cho A, Reidy MA. Phosphatidylinositol 3-kinase signaling is important for smooth muscle cell replication after arterial injury. Arterioscler Thromb Vasc Biol. 2000;20:2373–2378. doi: 10.1161/01.atv.20.11.2373. [DOI] [PubMed] [Google Scholar]
- 15.Auge N, Garcia V, Maupas-Schwalm F, Levade T, Salvayre R, Negre-Salvayre A. Oxidized ldl-induced smooth muscle cell proliferation involves the egf receptor/pi-3 kinase/akt and the sphingolipid signaling pathways. Arterioscler Thromb Vasc Biol. 2002;22:1990–1995. doi: 10.1161/01.atv.0000043453.21629.3b. [DOI] [PubMed] [Google Scholar]
- 16.Stabile E, Zhou YF, Saji M, Castagna M, Shou M, Kinnaird TD, Baffour R, Ringel MD, Epstein SE, Fuchs S. Akt controls vascular smooth muscle cell proliferation in vitro and in vivo by delaying g1/s exit. Circ Res. 2003;93:1059–1065. doi: 10.1161/01.RES.0000105086.31909.1B. [DOI] [PubMed] [Google Scholar]
- 17.Yao JS, Chen Y, Zhai W, Xu K, Young WL, Yang GY. Minocycline exerts multiple inhibitory effects on vascular endothelial growth factor-induced smooth muscle cell migration: The role of erk1/2, pi3k, and matrix metalloproteinases. Circ Res. 2004;95:364–371. doi: 10.1161/01.RES.0000138581.04174.2f. [DOI] [PubMed] [Google Scholar]
- 18.Calabro P, Samudio I, Willerson JT, Yeh ET. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004;110:3335–3340. doi: 10.1161/01.CIR.0000147825.97879.E7. [DOI] [PubMed] [Google Scholar]
- 19.Daugherty A, Manning MW, Cassis LA. Antagonism of at2 receptors augments angiotensin ii-induced abdominal aortic aneurysms and atherosclerosis. Br J Pharmacol. 2001;134:865–870. doi: 10.1038/sj.bjp.0704331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadri Z, Maouche-Chretien L, Rooke HM, Orkin SH, Romeo PH, Mayeux P, Leboulch P, Chretien S. Phosphatidylinositol 3-kinase/akt induced by erythropoietin renders the erythroid differentiation factor gata-1 competent for timp-1 gene transactivation. Mol Cell Biol. 2005;25:7412–7422. doi: 10.1128/MCB.25.17.7412-7422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amaravadi R, Thompson CB. The survival kinases akt and pim as potential pharmacological targets. J Clin Invest. 2005;115:2618–2624. doi: 10.1172/JCI26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of bad couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 24.Henderson EL, Geng YJ, Sukhova GK, Whittemore AD, Knox J, Libby P. Death of smooth muscle cells and expression of mediators of apoptosis by t lymphocytes in human abdominal aortic aneurysms. Circulation. 1999;99:96–104. doi: 10.1161/01.cir.99.1.96. [DOI] [PubMed] [Google Scholar]
- 25.Calamito M, Juntilla MM, Thomas M, Northrup DL, Rathmell J, Birnbaum MJ, Koretzky G, Allman D. Akt1 and akt2 promote peripheral b-cell maturation and survival. Blood. 2010;115:4043–4050. doi: 10.1182/blood-2009-09-241638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guha M, Mackman N. The phosphatidylinositol 3-kinase-akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 27.Schabbauer G, Tencati M, Pedersen B, Pawlinski R, Mackman N. Pi3k-akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler Thromb Vasc Biol. 2004;24:1963–1969. doi: 10.1161/01.ATV.0000143096.15099.ce. [DOI] [PubMed] [Google Scholar]
- 28.Yin W, Signore AP, Iwai M, Cao G, Gao Y, Johnnides MJ, Hickey RW, Chen J. Preconditioning suppresses inflammation in neonatal hypoxic ischemia via akt activation. Stroke. 2007;38:1017–1024. doi: 10.1161/01.STR.0000258102.18836.ca. [DOI] [PubMed] [Google Scholar]
- 29.Zhang WJ, Wei H, Hagen T, Frei B. Alpha-lipoic acid attenuates lps-induced inflammatory responses by activating the phosphoinositide 3-kinase/akt signaling pathway. Proc Natl Acad Sci U S A. 2007;104:4077–4082. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kidd LB, Schabbauer GA, Luyendyk JP, Holscher TD, Tilley RE, Tencati M, Mackman N. Insulin activation of the phosphatidylinositol 3-kinase/protein kinase b (akt) pathway reduces lipopolysaccharide-induced inflammation in mice. J Pharmacol Exp Ther. 2008;326:348–353. doi: 10.1124/jpet.108.138891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carpintero R, Brandt KJ, Gruaz L, Molnarfi N, Lalive PH, Burger D. Glatiramer acetate triggers pi3kdelta/akt and mek/erk pathways to induce il-1 receptor antagonist in human monocytes. Proc Natl Acad Sci U S A. 2010;107:17692–17697. doi: 10.1073/pnas.1009443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial il-6/mcp1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, Sun C, Zhang Y, Liu J, Ennis TL, Knispel R, Xiong W, Thompson RW, Baxter BT, Shi GP. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117:3359–3368. doi: 10.1172/JCI31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong W, MacTaggart J, Knispel R, Worth J, Persidsky Y, Baxter BT. Blocking tnf-alpha attenuates aneurysm formation in a murine model. J Immunol. 2009;183:2741–2746. doi: 10.4049/jimmunol.0803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segura AM, Luna RE, Horiba K, Stetler-Stevenson WG, McAllister HA, Jr, Willerson JT, Ferrans VJ. Immunohistochemistry of matrix metalloproteinases and their inhibitors in thoracic aortic aneurysms and aortic valves of patients with marfan’s syndrome. Circulation. 1998;98:II331–337. discussion II337–338. [PubMed] [Google Scholar]
- 36.Zhang X, Shen YH, LeMaire SA. Thoracic aortic dissection: Are matrix metalloproteinases involved? Vascular. 2009;17:147–157. doi: 10.2310/6670.2008.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng JC, Chou CH, Kuo ML, Hsieh CY. Radiation-enhanced hepatocellular carcinoma cell invasion with mmp-9 expression through pi3k/akt/nf-kappab signal transduction pathway. Oncogene. 2006;25:7009–7018. doi: 10.1038/sj.onc.1209706. [DOI] [PubMed] [Google Scholar]
- 38.Zhang D, Bar-Eli M, Meloche S, Brodt P. Dual regulation of mmp-2 expression by the type 1 insulin-like growth factor receptor: The phosphatidylinositol 3-kinase/akt and raf/erk pathways transmit opposing signals. J Biol Chem. 2004;279:19683–19690. doi: 10.1074/jbc.M313145200. [DOI] [PubMed] [Google Scholar]
- 39.Zhang D, Brodt P. Type 1 insulin-like growth factor regulates mt1-mmp synthesis and tumor invasion via pi 3-kinase/akt signaling. Oncogene. 2003;22:974–982. doi: 10.1038/sj.onc.1206197. [DOI] [PubMed] [Google Scholar]
- 40.Guo D, Kassiri Z, Basu R, Chow FL, Kandalam V, Damilano F, Liang W, Izumo S, Hirsch E, Penninger JM, Backx PH, Oudit GY. Loss of pi3k{gamma} enhances camp-dependent mmp remodeling of the myocardial n-cadherin adhesion complexes and extracellular matrix in response to early biomechanical stress. Circ Res. doi: 10.1161/CIRCRESAHA.110.229054. [DOI] [PubMed] [Google Scholar]
- 41.Mendes Sdos S, Candi A, Vansteenbrugge M, Pignon MR, Bult H, Boudjeltia KZ, Munaut C, Raes M. Microarray analyses of the effects of nf-kappab or pi3k pathway inhibitors on the lps-induced gene expression profile in raw264.7 cells: Synergistic effects of rapamycin on lps-induced mmp9-overexpression. Cell Signal. 2009;21:1109–1122. doi: 10.1016/j.cellsig.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 42.Storz P, Doppler H, Copland JA, Simpson KJ, Toker A. Foxo3a promotes tumor cell invasion through the induction of matrix metalloproteinases. Mol Cell Biol. 2009;29:4906–4917. doi: 10.1128/MCB.00077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee HY, You HJ, Won JY, Youn SW, Cho HJ, Park KW, Park WY, Seo JS, Park YB, Walsh K, Oh BH, Kim HS. Forkhead factor, foxo3a, induces apoptosis of endothelial cells through activation of matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2008;28:302–308. doi: 10.1161/ATVBAHA.107.150664. [DOI] [PubMed] [Google Scholar]
- 44.Conery AR, Cao Y, Thompson EA, Townsend CM, Jr, Ko TC, Luo K. Akt interacts directly with smad3 to regulate the sensitivity to tgf-beta induced apoptosis. Nat Cell Biol. 2004;6:366–372. doi: 10.1038/ncb1117. [DOI] [PubMed] [Google Scholar]
- 45.Remy I, Montmarquette A, Michnick SW. Pkb/akt modulates tgf-beta signalling through a direct interaction with smad3. Nat Cell Biol. 2004;6:358–365. doi: 10.1038/ncb1113. [DOI] [PubMed] [Google Scholar]
- 46.Poon M, Marx SO, Gallo R, Badimon JJ, Taubman MB, Marks AR. Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest. 1996;98:2277–2283. doi: 10.1172/JCI119038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin KA, Merenick BL, Ding M, Fetalvero KM, Rzucidlo EM, Kozul CD, Brown DJ, Chiu HY, Shyu M, Drapeau BL, Wagner RJ, Powell RJ. Rapamycin promotes vascular smooth muscle cell differentiation through insulin receptor substrate-1/phosphatidylinositol 3-kinase/akt2 feedback signaling. J Biol Chem. 2007;282:36112–36120. doi: 10.1074/jbc.M703914200. [DOI] [PubMed] [Google Scholar]
- 48.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase balpha is critical for ischemic and vegf-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase akt2 (pkb beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 50.Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish JD, Coleman KG. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking akt2/pkb beta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leavens KF, Easton RM, Shulman GI, Previs SF, Birnbaum MJ. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 2009;10:405–418. doi: 10.1016/j.cmet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wan M, Leavens KF, Saleh D, Easton RM, Guertin DA, Peterson TR, Kaestner KH, Sabatini DM, Birnbaum MJ. Postprandial hepatic lipid metabolism requires signaling through akt2 independent of the transcription factors foxa2, foxo1, and srebp1c. Cell Metab. 2011;14:516–527. doi: 10.1016/j.cmet.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding L, Biswas S, Morton RE, Smith JD, Hay N, Byzova TV, Febbraio M, Podrez EA. Akt3 deficiency in macrophages promotes foam cell formation and atherosclerosis in mice. Cell Metab. 2012;15:861–872. doi: 10.1016/j.cmet.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bacus SS, Altomare DA, Lyass L, Chin DM, Farrell MP, Gurova K, Gudkov A, Testa JR. Akt2 is frequently upregulated in her-2/neu-positive breast cancers and may contribute to tumor aggressiveness by enhancing cell survival. Oncogene. 2002;21:3532–3540. doi: 10.1038/sj.onc.1205438. [DOI] [PubMed] [Google Scholar]
- 56.Fujio Y, Mitsuuchi Y, Testa JR, Walsh K. Activation of akt2 inhibits anoikis and apoptosis induced by myogenic differentiation. Cell Death Differ. 2001;8:1207–1212. doi: 10.1038/sj.cdd.4400919. [DOI] [PubMed] [Google Scholar]
- 57.Kim MA, Kim HJ, Jee HJ, Kim AJ, Bae YS, Bae SS, Yun J. Akt2, but not akt1, is required for cell survival by inhibiting activation of jnk and p38 after uv irradiation. Oncogene. 2009;28:1241–1247. doi: 10.1038/onc.2008.487. [DOI] [PubMed] [Google Scholar]
- 58.Chung AW, Au Yeung K, Cortes SF, Sandor GG, Judge DP, Dietz HC, van Breemen C. Endothelial dysfunction and compromised enos/akt signaling in the thoracic aorta during the progression of marfan –syndrome. Br J Pharmacol. 2007;150:1075–1083. doi: 10.1038/sj.bjp.0707181. [DOI] [PMC free article] [PubMed] [Google Scholar]