Abstract
OBJECTIVE
The purpose of this study was to estimate pharmacokinetic parameters and to evaluate placental transport of 17-hydroxyprogesterone caproate (17-OHPC) in singleton gestation.
STUDY DESIGN
Sixty-one women who received weekly injections of 17-OHPC underwent 2 pharmacokinetic studies at 20 + 0 to 24 + 6 weeks’ gestation (study 1) and 31 + 0 to 34 + 6 weeks’ gestation (study 2); daily blood samples were obtained between injections. In 18 women, blood samples were obtained over a 28-day period beyond the last injection (extended study). Maternal and/or cord blood were obtained at delivery.
RESULTS
The half-life (median ± SD) of 17-OHPC was 16.2 ± 6 days. Concentrations of 17-OHPC were higher during study 2 than during study 1. Body mass index affected maternal 17-OHPC concentrations. Cord:maternal 17-OHPC concentration ratios averaged 0.2; 17-OHPC was detectible in cord plasma 44 days after the last maternal injection.
CONCLUSION
The apparent half-life of 17-OHPC is long, and pharmacokinetic parameters vary widely between subjects and are affected by maternal body mass index. The drug crosses the placental barrier.
Keywords: cord blood, pharmacokinetics, placenta, preterm birth
Seventeen-hydroxyprogesterone caproate (17-OHPC) reduces preterm birth rates in women with a previous preterm birth1 but has not proved effective in women with multifetal gestation2, 3 or an ultrasonically identified short cervix.4 The American Congress of Obstetricians and Gynecologists in a 2009 Committee Opinion recommended that this therapy be offered to all women with a previous preterm birth5 and that more research be done with the pharmacology of 17-OHPC and other progestin preparations. Despite widespread clinical use, there are no reports that have described pharmacokinetics of 17-OHPC in singleton gestation, the plasma concentrations that are achieved during therapy for preterm birth prevention, and whether the medication is detectible in fetal blood. In this multicenter Obstetrical-Fetal Pharmacology Research Units Network study, we evaluated the pharmacokinetics and placental transport of 17-OHPC in women with singleton gestation who were receiving 17-OHPC because of a previous preterm birth.
Materials and Methods
Study design
We recruited 61 women from 4 centers who were receiving or planned to receive 17-OHPC for the prevention of recurrent preterm birth based on a history of at least 1 previous spontaneous preterm (<37 weeks’ gestation) birth. In keeping with clinical practice recommendations, all women who were receiving 17-OHPC began therapy between 16 0/7 and 20 6/7 weeks’ gestation. Each subject agreed to participate in 2 pharmacokinetic studies lasting 7 days each. The first pharmacokinetic study (PK1) was scheduled to occur between 20 0/7 and 24 6/7 weeks’ gestation after a minimum of 4 weekly injections had been administered. The second pharmacokinetic study (PK2) occurred between 31 0/7 and 34 6/7 weeks’ gestation. This second study evaluated possible gestational age-related changes in the pharmacokinetics of 17-OHPC. Eighteen women who completed PK2 agreed to have blood drawn an additional 7 times during the 28 days after the start of PK2 to determine the terminal disposition rate constant and apparent terminal disposition half-life (extended study). In these women, blood samples were obtained after the last injection on days 1, 2, 3, 4, 5, 6, and 7 as in all other subjects; however, in addition, blood was obtained on day 9, 11, 14, 17, 20, 24, and 28 after the last injection of 17-OHPC. For all subjects, when possible, blood was obtained at delivery from a maternal vein and the umbilical cord (artery or vein).
All subjects received their weekly 17-OHPC injections by the research staff up to the completed week 35 of gestation or delivery. The weekly injection times were scheduled within a 4-hour window of the initial injection time. The 17-OHPC was obtained from a central compounding pharmacy (Eminent Services Corp, Frederick, MD). At each weekly visit the site of injection was noted, and injection sites were alternated between the right and left hip/buttocks areas. A venous blood sample was obtained, and subjects were queried about side-effects, hospitalizations or episodes of preterm labor that required hospitalization or tocolytic treatment. Data that were recorded for each patient included maternal age, parity, self-reported race, body mass index (BMI), and gestational age at enrollment, at each blood sampling, and at delivery. Women who missed ≥1 injections were not included in the pharmacokinetic analyses. No attempt was made to alter or mandate clinical treatment of the subjects. The institutional review boards of each clinical site approved this study. This trial is registered at Clinicaltrials.gov (NCT00409825).
Sample analysis
For all 17-OHPC measurements, blood was collected in 10-mL tubes with ethylenediaminetetraacetic acid and centrifuged within 1 hour of collection at 3500g for 10 minutes. The supernatant plasma was aliquoted into 1-mL polypropylene tubes and frozen at −70°C until analysis of 17-OHPC by high performance liquid chromatography with tandem mass spectrometry as reported previously.6 The standard curve was linear in the range of 1–200 ng/mL. The lower limit of quantitation for 17-OHPC was 1 ng/mL. Inter- and intraassay variability at 10 ng/mL was 7.9 and 5.2%, respectively.
Pharmacokinetic analysis
Pharmacokinetic parameters (Appendix) in each of the 2 pharmacokinetic studies and the extended study were estimated by the standard noncompartmental approach implemented in Win-Nonlin software (version 4.0; Pharsight Corp, Mountain View, CA). Maximum concentration and time to maximum concentration were determined from the observed data. The terminal disposition rate constant (λz) was determined by log-linear regression of terminal linear disposition phase with the data from the extended study. Half-life was estimated by 0.693/ λz. The area under the plasma concentration vs time curve (AUCt1t2) was calculated from time t1 and t2, which are time of consecutive doses (beginning at the end of a dosing interval). The apparent oral clearance (clearance/bioavailability) was estimated as dose/(AUC)t1t2, and the apparent volume of distribution (VD/F) was calculated as dose/λz. AUCt1t2 used the AUC data from PK2 and the terminal disposition rate constant from the second study in 18 subjects from whom samples were collected for up to 28 days after the last injection (extended study).
Statistical analysis
The primary outcome variable was the gestational change in AUC (0–7 days). The sample size estimate was based on the assumptions that gestational change in AUC (0–7 days) of 30% is clinically relevant and that the variance in 17-OHPC concentrations is similar to that reported in nonpregnant women by Onsrud et al7 because there are no published data on singleton gestation. Based on these considerations, a total sample size of 47 women would be sufficient to detect such a difference, assuming a power of 0.8 and alpha of .05. We assumed a 25–30% dropout rate; therefore, the final sample size of 61 recruited subjects would be more than adequate for the evaluation of the outcome of interest. Secondary outcome variables included the pharmacokinetic variables that were described earlier (maximum concentration; time to maximum concentration; the minimum concentration at time zero, just before the next injection (Ctrough); half-life; volume of distribution; apparent clearance) and maternal and cord 17-OHPC concentrations.
GraphPad Prism software (version 4.01; GraphPad Software, Inc, La Jolla, CA) was used for the performance of the statistical tests for significance. Pairwise group comparisons used nonparametric (Wilcoxon signed rank and Mann Whitney U) tests. Population medians in multiple groups were compared with Kruskal-Wallis 1-way analysis of variance with Dunn’s posttest for multiple comparisons. We considered probability values of < .05 to be significant. All pharmacokinetic parameter results are reported as median (interquartile range). Demographic variables are reported as mean ±SD.
Results
Demographics
The characteristics of the study population are summarized in Table 1. These 61 subjects each experienced 1–4 previous preterm births. Treatment with 17-OHPC started on average at 18 weeks’ gestation, and subjects received an average of 6 injections by PK1 and 17 injections by PK2.
TABLE 1.
Characteristics of enrolled patients
| Characteristic | Measure | |
|---|---|---|
| Cases, n | 61 | |
| Parity, na | 2.2 (1–6) | |
| Previous preterm births, na | 1.5 (1–4) | |
| Age, yb | 28 ± 5.9 | |
| Race, n | ||
| African American | 13 | |
| Hispanic | 16 | |
| White | 30 | |
| Other | 2 | |
| Body mass index, kg/m2b | 29 ± 6 | |
| Gestational age, weeks-mean ± SDb | ||
| First injection | 18 ± 1.5 | |
| Delivery | 37 ± 3.6 | |
| Injections given, nb | ||
| Pharmacokinetic 1 | 5.7 ± 0.9 | |
| Pharmacokinetic 2 | 16.5 ± 2.3 | |
Data are given as mean (range);
Data are given as mean ± SD.
Caritis. Pharmacology and placental transport of 17-OHPC. Am J Obstet Gynecol 2012.
Noncompartmental pharmacokinetics
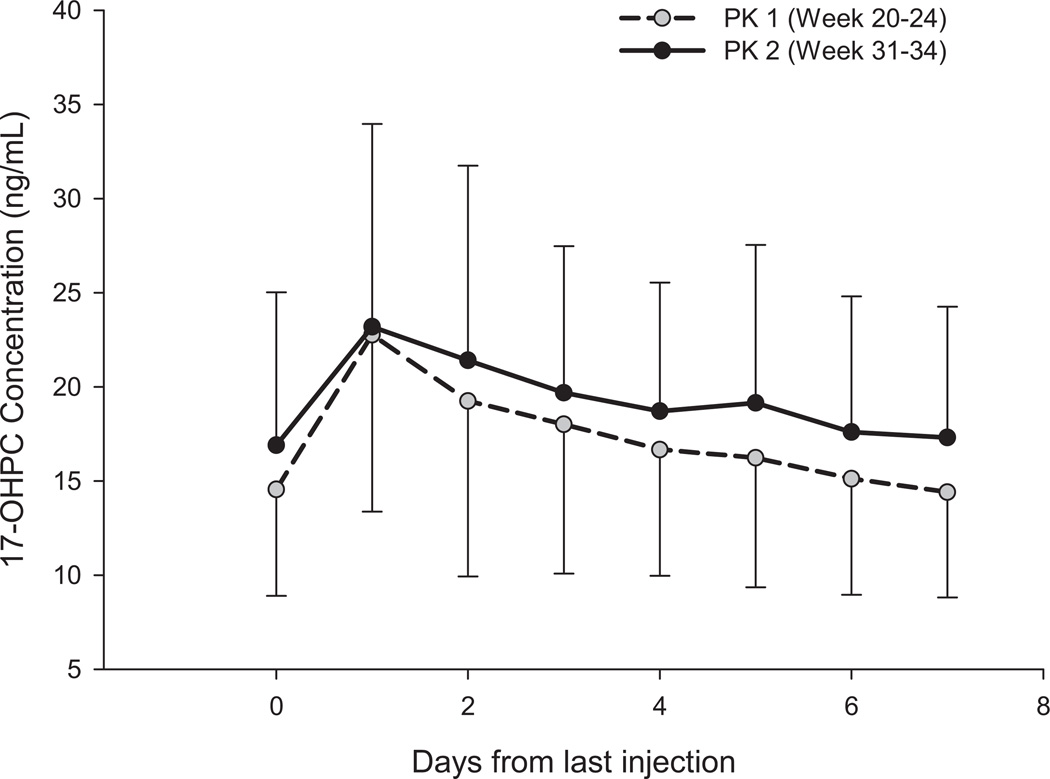
Figure 1 shows the mean (±SD) plasma concentration of 17-OHPC during each of the 2 pharmacokinetic studies. Only women who had received all their scheduled injections and remained undelivered through PK1 (20 6/7–24 6/7 weeks’ gestation) and PK2 (31 0/7–34 6/7 weeks’ gestation) were included in this analysis (n = 47). Ten subjects did not undergo PK2 because of preterm delivery (n = 6 women), study withdrawal (n = 3 women), or hospitalization (n = 1 woman). Overall, the plasma concentrations varied widely and decreased slowly during the 7 days of the pharmacokinetic studies. Trough plasma concentrations were higher (approximately 15%) during PK2 compared with PK1.
FIGURE 1. 17-OHPC concentrations (mean ± SD) during PK1 and PK2.
The open circle with dashed line represents the plasma concentrations during pharmacokinetic 1 study (gestational week 20–24); the closed circle with the solid line represents the pharmacokinetic 2 study (gestational week 31–34) in 47 subjects who underwent both PK studies.
IM, intramuscular; PK, pharmacokinetic; 17-OHPC, 17-hydroxyprogesterone caproate.
Caritis. Pharmacology and placental transport of 17-OHPC. Am J Obstet Gynecol 2012.
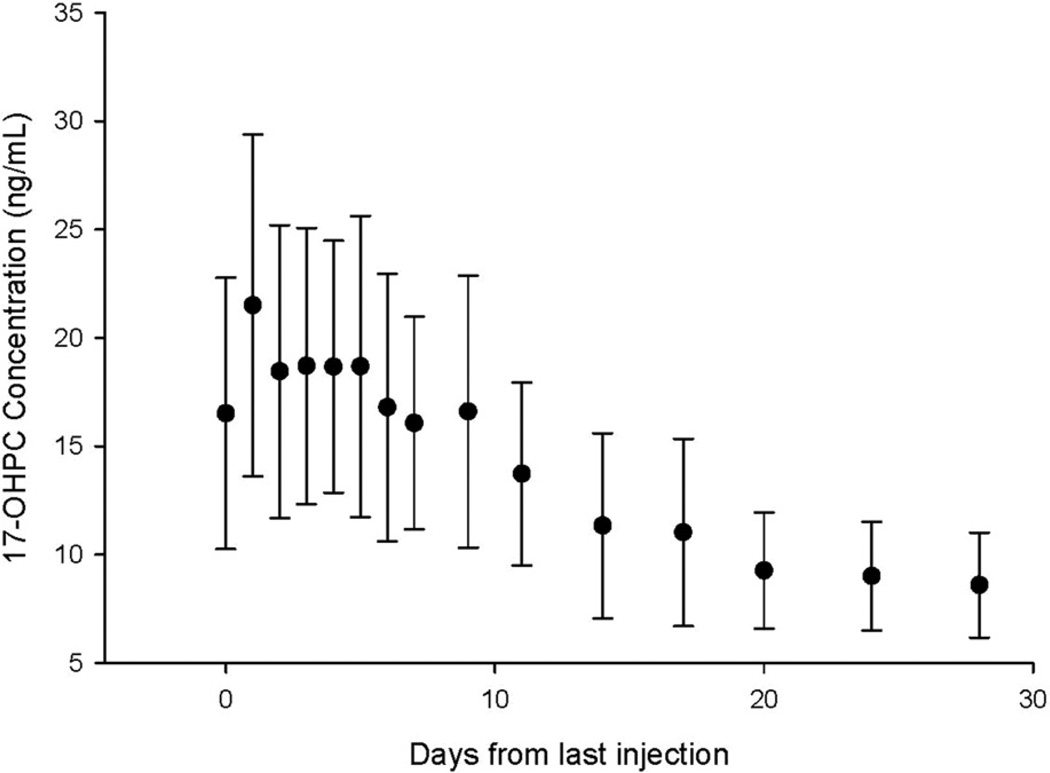
Table 2 compares selected pharmacokinetic parameters (median and interquartile range) for 17-OHPC in the PK1, PK2, and extended studies. The higher plasma concentrations that were seen in PK2 are reflected in higher AUCt1t2, higher Ctrough and maximum concentrations. Figure 2 shows plasma 17-OHPC concentrations in 18 women who underwent the extended pharmacokinetic study. The estimated half-life in these subjects was 18 ± 6 days. The estimation of half-life during drug disappearance is generally more precise because additional drug is not introduced into the body and because drug behavior is monitored for a longer period with increased number of plasma samples. During the extended study, plasma 17-OHPC concentrations disappeared slowly; at 28 days after the injection, the plasma concentrations were still approximately 10 ng/mL, which suggests that 17-OHPC is either slowly released from the castor oil depots or from maternal fat stores.
TABLE 2.
Noncompartmental pharmacokinetic parameters of 17-OHPC during pharmacokinetic studies 1 and 2
| Variable | AUCt1t2 (ng/mL/d) | Ctrough (ng/mL) | CMAX (ng/mL) | TMAX (d) | t1/12 (d) | Vd/F (*103) (L) | Cl/F (*103) (L/d) | |
|---|---|---|---|---|---|---|---|---|
| Pharmacokinetic study | ||||||||
| 1 (n = 47) | 107 (81.1–144.2) | 12.4 (10.0–18.0) | 21.0 (15.8–27.4) | 1.0 (1.0–3.0) | ||||
| 2 (n = 47) | 125 (96.5–165.1) | 14.6 (11.5–21.0) | 24.3 (18.0–31.1) | 1.0 (1.0–3.5) | ||||
| P valuea | .002 | .011 | .007 | .51 | ||||
| Extended pharmacokinetic study 2b (n = 18) | 120 (92.0–168.0) | 14.0 (12.8–19.8) | 23.8 (17.3–29.0) | 1.0 (1.0–4.0) | 16.2 (10.6–21.0) | 56 (25.2–69.6) | 2.1 (1.5–2.7) | |
Data are presented as median pharmacokinetic parameter and interquartile range (IQR; Q1–Q3, where Q1 is the 25th percentile and Q3 is the 75th percentile) that were observed for 47 subjects with singleton pregnancies who had sampling done daily for 7 days.
AUCt1t2, area under the concentration vs time curve; Cl/F, clearance/bioavailability; CMAX, maximum concentration during the dosing interval; Ctrough, the minimum concentration at time zero, just before the next injection; 17-OHPC, 17-hydroxyprogesterone caproate; t1/2, apparent half-life; TMAX, time to maximum concentration; Vd/F, apparent volume of distribution/bioavailability.
Wilcoxon signed rank test–generated probability values (P < .05) indicate significant difference between pharmacokinetic studies 1 and 2;
Extended pharmacokinetic study, median pharmacokinetic parameters and interquartile range that were observed for 18 subjects with singleton pregnancies who had sampling done for 28 days after the last injection.
Caritis. Pharmacology and placental transport of 17-OHPC. Am J Obstet Gynecol 2012.
FIGURE 2. Plasma 17-OHPC concentrations (mean ± SD) in 18 subjects who underwent the PK 2 study after the final injection of 17-OHPC.
Samples were obtained daily during the pharmacokinetic 2 study and 7 times between days 9–28 after the last injection.
PK, pharmacokinetic; 17-OHPC, 17-hydroxyprogesterone caproate.
Caritis. Pharmacology and placental transport of 17-OHPC. Am J Obstet Gynecol 2012.
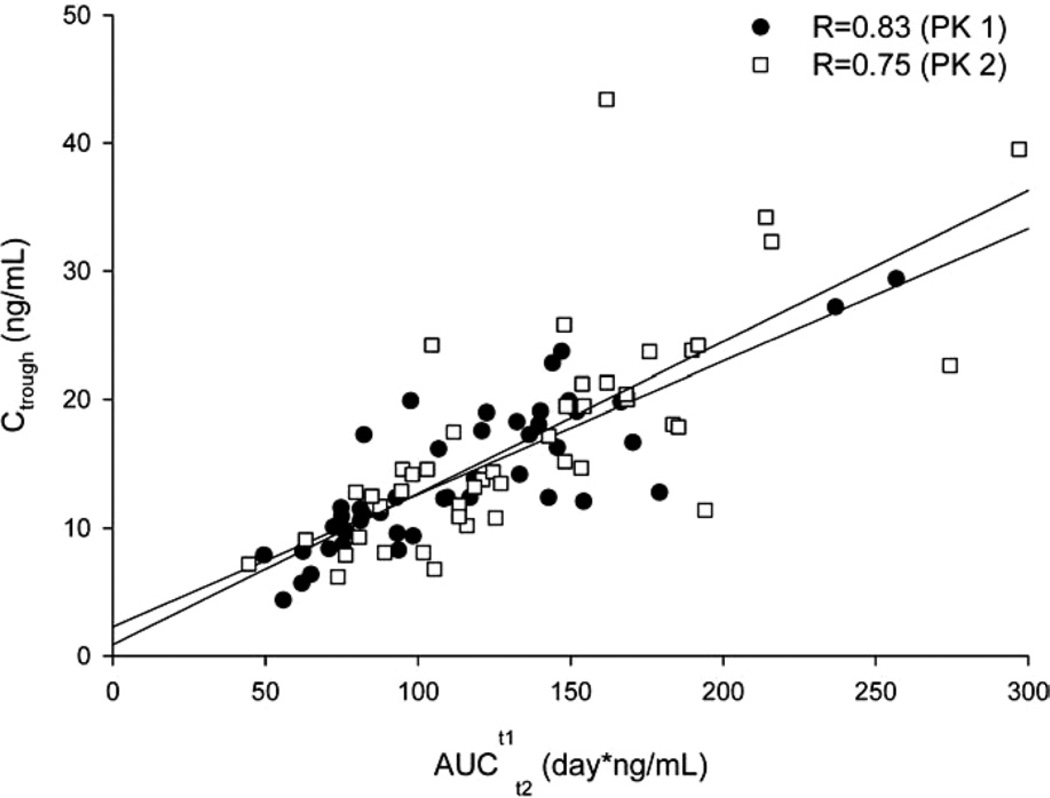
Figure 3 shows the relationship between the concentration of 17-OHPC just before an injection (Ctrough) and AUCt1t2 after the injection. The strong correlation between Ctrough and AUCt1t2 during both PK1(r = 0.0.83; P < .01) and PK2 (r = 0.75; P < .01) suggests that Ctrough could be used as a surrogate measure of drug exposure in singleton gestation after an injection of 17-OHPC.
FIGURE 3. Relationship between the AUC and trough concentrations of 17-OHPC.
Closed circles represent data from the PK 1 study; open circles represent data from the pharmacokinetic 2 study.
AUC, area under the plasma concentration vs time curve; PK, pharmacokinetic; 17-OHPC, 17-hydroxyprogesterone caproate.
Caritis. Pharmacology and placental transport of 17-OHPC. Am J Obstet Gynecol 2012.
Relationship between race, BMI, and parity and 17-OHPC concentrations
We evaluated the relationship between enrollment BMI, race, and parity and plasma 17-OHPC concentrations. For the assessment of the relationship between BMI and plasma 17-OHPC concentrations we included women for whom an enrollment BMI had been recorded and who had received all their scheduled injections of 17-OHPC and remained undelivered at the time of PK1 (n = 53 women) and PK2 (n = 43 women). BMI demonstrated a weak but statistically significant inverse relationship with trough plasma 17-OHPC concentrations and AUC (Table 3) during both pharmacokinetic studies, with heavier women having lower AUCs and lower trough concentrations.
TABLE 3.
Relationship between BMI and 17-OHPC exposures for pharmacokinetic studies 1 and 2
| Pharmacokinetic study |
BMI r | P value | |
|---|---|---|---|
| BMI vs AUCt1t2 | |||
| PK1 | 0.36 | < .01 | |
| PK2 | 0.50 | < .01 | |
| BMI vs Ctrough | |||
| PK1 | 0.29 | < .01 | |
| PK2 | 0.41 | < .01 | |
Correlation coefficient (r) between BMI (independent variable) and area under the concentration (AUC) or the minimum concentration at time zero just before the next injection (Ctrough; dependent variables). The AUC vs time curve (AUCt1t2) was measured in nanograms per milliliter per day; Ctrough was measured in nanograms per milliliter.
BMI, body mass index; 17-OHPC, 17-hydroxyprogesterone caproate.
Caritis. Pharmacology and placental transport of 17-OHPC. Am J Obstet Gynecol 2012.
In assessing the relationship between self-reported race and plasma 17-OHPC concentrations, we included only the 59 women in PK1 and 49 women in PK2 who had received all of their scheduled injections and for whom race was recorded. Race was not related to 17-OHPC exposure (ie, AUC), with similar results in African American and white women in both PK1 (120 ± 58 and 120 ± 43 ng*d/mL, respectively; P = .57) and PK2 (127 ± 50 and 143 ± 54 ng*d/mL, respectively; P = .53).
Parity was not related to plasma trough 17-OHPC concentrations at either PK1 or PK2 (data not shown).
Placental transport
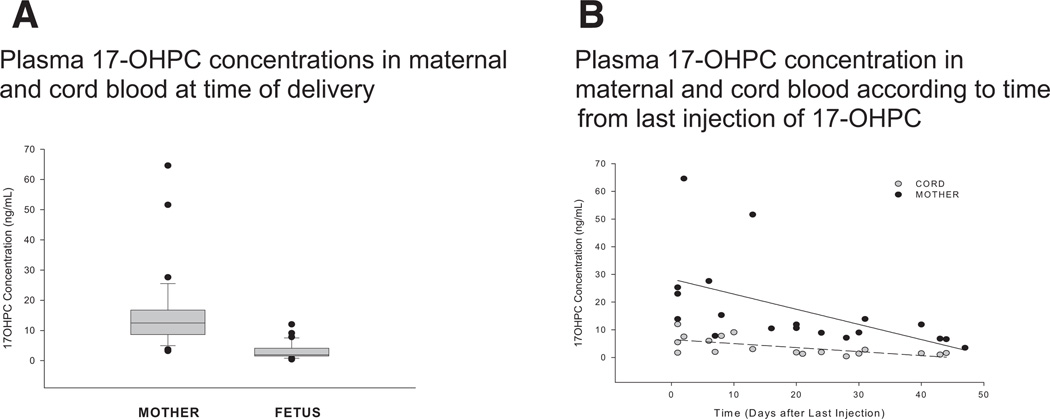
Plasma concentrations of 17-OHPC in maternal and cord blood at the time of delivery were 15.1 ng/mL (range, 3.2–64.6 ng/mL) and 3.2 ng/mL (range, 0.4–12 ng/mL), respectively, and are shown in Figure 4. The time from the last injection until delivery ranged from 1–44 days. The maternal concentrations were higher than the concentration in cord blood in every case. The cord:maternal drug ratio averaged 0.2 (range, 0.06–0.51) and was unaffected by the time from the last injection (slope is not significantly different from zero), although absolute drug concentrations were lower in both compartments as a function of time from last dose.
FIGURE 4. Relationship between maternal and cord blood concentrations of 17-OHPC.
A, Box plot of 17-hydroxyprogesterone caproate concentration in maternal blood at the time of delivery and of cord blood. The horizontal bar is the median value; the error bars represent 25th (lower bar) and 75th (upper bar) percentiles. The closed circles outside the 25th and 75th percentiles represent outliers. B, Relationship between maternal and cord blood concentrations of 17-OHPC. The figure illustrates maternal blood and cord blood concentrations of 17-OHPC according to time from last maternal injection of 17-OHPC.
17-OHPC, 17-hydroxyprogesterone caproate.
Caritis. Pharmacology and placental transport of 17-OHPC. Am J Obstet Gynecol 2012.
Comment
This is the first report of the plasma concentrations and pharmacokinetic analysis of 17-OHPC in pregnant women with a singleton gestation. We have demonstrated that plasma concentrations of 17-OHPC continue to rise with repeated weekly administration. This rise is consistent with the long half-life of 17-OHPC and the time it takes to achieve steady state and is reflected in alterations of descriptive pharmacokinetics, which include increases in Ctrough, maximum concentration, and AUC.
Taken together, the high lipid solubility of 17-OHPC, the negative association between 17-OHPC concentrations and BMI, and the previously reported rapid metabolism of 17-OHPC by human liver microsomes in vitro support the premise that plasma concentrations remain elevated because of the slow and continued release of 17-OHPC from the increasing number of castor oil depots and/or the maternal fat stores that may take up 17-OHPC and release it very slowly or both.8 The slow release of 17-OHPC from these depots would explain the sustained concentrations long after each injection. Clinically this provides considerable latitude in the dosing regimen. An injection that is 2–3 days late will not have an immediate or substantive impact on the plasma concentrations. This knowledge could improve patient convenience and minimize the impact of any noncompliance.
In this study, the plasma concentrations of 17-OHPC varied considerably (coefficient of variation, 40–50%) between subjects, despite the use of an identical dosing regimen. This wide variability may impact effectiveness or safety that is related with 17-OHPC use. We have reported a similar wide variation in plasma concentrations of 17-OHPC in women with twin gestation.9
In the current study, there was an association between the plasma concentration and BMI, but not parity and race. Our observations suggest that obese women may require a higher 17-OHPC dose to achieve plasma concentrations similar to those in nonobese women. In twin pregnancies, there was a similar association with BMI; however, neither parity nor fetal number were related to the plasma concentrations of 17-OHPC and African American women demonstrated a significantly greater clearance of 17-OHPC than white women.9 The current study, however, was not powered to evaluate the relationship between race and plasma 17-OHPC concentration. It is also possible that genetic polymorphism in the CYP3A enzyme that is responsible for the metabolism of 17-OHPC might also contribute to the observed variability in plasma concentrations; genotyping of these enzymes was not performed.
Other covariates may also be related to the plasma concentrations of 17-OHPC in pregnant subjects. We reported recently that endogenous hormones and certain prescription medications may compete with the enzymes that metabolize 17-OHPC.10, 11 Using human liver microsomes, we have found that metabolism of 17-OHPC can be reduced significantly by CYP3A inhibitors. Coadministration of such drugs will lead to large variation in plasma concentrations and may impact the effectiveness and/or any adverse events that may be related to this drug.
Our data provide the first suggestion of therapeutic 17-OPHC concentrations because the dosing regimen used in this pharmacokinetic study was identical to that used by Meis et al1 in the Maternal-Fetal Medicine Unite (MFMU) Network clinical trial to prevent recurrent preterm birth in singleton gestations. Plasma concentrations were not assessed in that trial so that a therapeutic concentration range could not be established directly. The 250-mg weekly dose that was selected in that study was based on previous reports and practice but was not informed by pharmacologic data. The current Food and Drug Administration–approved dosing regimen is likewise 250 mg weekly and is based primarily on data from the MFMU trial.1 This dosing regimen may not be optimal. The large variation in plasma concentration with a similar dosing regimen suggests that efficacy in the MFMU trial of Meis et al may have been impacted because the preterm birth rate was reduced only by 33% by 17-OHPC treatment. Data from the current study do not allow the determination of an optimal dosing regimen, which requires a clinical trial with at-risk women randomly assigned to one of several dosage options (ie, a dose ranging study). The option of the use of a surrogate biomarker as an indicator of 17-OHPC efficacy for the establishment of an optimal dosing regimen is not possible at this time because we do not have a surrogate biomarker for preterm birth nor do we know the mechanism of action by which 17-OHPC reduces preterm birth rates. However, the option of relating plasma trough 17-OHPC concentrations, which is a surrogate marker of AUC (drug exposure parameter) to gestational length, may prove useful in the establishment of an optimal dosing regimen.
The pharmacokinetic parameters that we obtained in singleton gestation were qualitatively similar to those we reported previously in multifetal gestation,9 although quantitative differences were evident (Table 4). The comparisons in Table 4 were performed at 24–28 weeks’ gestation in both studies after an average of 6 injections. Although sample size was small in women with twin gestation, making parameter estimates less precise than with a larger sample size, meaningful comparisons can still be made. Plasma concentrations were approximately 40% higher in singleton gestation.
TABLE 4.
A comparison of pharmacokinetic parameters of 17-OHPC in singleton and twin gestation
| Variable | AUCt1t2 (ng/mL/d) | Ctrough (ng/mL) | CMAX (ng/mL) | TMAX (d) |
| Singleton gestation (n = 47) | 115.2 ± 44.2 (78.8–141.4) | 14.1 ± 5.6 (10–18.1) | 22.6 ± 9.5 (15.8–27.4) | 2.1 ± 1.9 (1.0–3.0) |
| Twin gestation (n = 6) | 86.0 ± 33.5 (61.0–114.0) | 9.7 ± 2.8 (8.0–12.0) | 17 ± 6.7 (12.0–22.0) | 1.2 ± 0.4 (1.0–2.0) |
| P value | .06 | .05 | .12 | .36 |
Data are given as mean pharmacokinetic parameters ± SD (interquartile range; Q1–Q3, where Q1 is the 25th percentile and Q3 is the 75th percentile) that were observed for 47 subjects with singleton gestation and 6 subjects with twin gestation who had sampling done daily for 7 days after an injection and received all scheduled injections. Wilcoxon signed rank test used to compare pharmacokinetic parameters between groups.
AUCt1t2, area under the concentration vs time curve; CMAX, maximum concentration during the dosing interval; Ctrough, the minimum concentration just before the next dose; 17-OHPC, 17-hydroxyprogesterone caproate; TMAX, time to maximum concentration.
Caritis. Pharmacology and placental transport of 17-OHPC. Am J Obstet Gynecol 2012.
This is also the first report of 17-OHPC concentrations in fetal plasma during and after therapy for preterm birth prevention. The drug is detectable in both maternal and fetal blood for at least 44 days after the last injection. The mother serves as a repository for 17-OHPC long after the last maternal injection, as noted earlier, because of the slow release from the castor oil depots or from maternal fat stores. The placenta may also serve as a repository or 17-OHPC or its metabolites, based on placental perfusion studies.12 Seventeen-OHPC is also highly bound to plasma proteins, particularly albumin, which may contribute to the long half-life of the drug in the mother. Thus, there may be prolonged fetal exposure to 17-OHPC long after the last maternal injection. The cord:maternal ratio of 0.2 that was measured at delivery, which was 1–44 days after the last dose, suggests several possibilities: (1) that binding of 17-OHPC is greater in maternal plasma than fetal plasma, (2) that the fetus actively metabolizes the drug, (3) that efflux transporters in the placenta may be involved in actively transporting 17-OHPC from fetus to mother, or (4) a combination of these mechanisms. Indeed, we have demonstrated that the fetus is capable of metabolizing 17-OHPC; Sharma et al13 and Fokina et al14 have also demonstrated the placental metabolism of 17-OHPC. The lack of change in the slope of the fetal-to-maternal concentration ratio with time after last injection would suggest that, if there are any active transport processes involved, they are not saturated at the concentrations that have been observed. Although 17-OHPC is detectable in cord plasma, evidence to date is reassuring in terms of fetal/neonatal safety. The impact of 17-OHPC on the neonate has been evaluated by Northen et al,15 who found no relationship between intra-uterine 17-OHPC exposure and physical findings or developmental parameters that included communication, gross and fine motor skills, problem solving, and personal/social or gender-specific roles at a mean postnatal age of 48 months. In the 1960s and later, 17-OHPC was used more extensively for prevention/treatment of threatened miscarriage. Those studies demonstrated safety of the agent with no evidence of malformations or disordered sexual development.16–21
In summary, we have defined the pharmacokinetics of 17-OHPC in singleton gestation. There is a wide variation in plasma concentrations, despite an identical dosing regimen. This variation may impact overall efficacy. Measurement of trough concentrations of 17-OHPC to guide dose adjustment may prove valuable once the therapeutic concentration range of the drug is identified. Our observations also suggest that the relative safety of the drug for the newborn infants may be related to the lower concentration of the drug in the fetal compartment.
Acknowledgments
Supported by the Obstetric Fetal Pharmacology Research Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development through cooperative agreements U10HD047905, U10HD047892, U10HD047892, and U10HD047892 with additional support by 1 UL1RR031975.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health.
Appendix
Definitions
Apparent half-life: a measure of the time that it takes for the drug concentration to decrease from a given value to one-half of its value (approximately 5–6 half-lives for most of the drug to be out of the body; approximately 5–6 half-lives to reach steady state)
AUC: the area under the blood or plasma concentration vs time curve for a drug
C max: the highest concentration of a drug in blood or plasma during a dosing interval after the administration of a drug
C trough: the point of minimum concentration of a drug that generally occurs immediately before the administration of a drug’s next dose
Clearance: the overall ability of the body to clear the drug (the volume of blood or plasma that is cleared completely of the drug per unit time); calculated as the amount of drug cleared (this equals dose for intravenous administration) divided by the area under the blood or plasma concentration vs time
Pharmacodynamics: the relationship between the pharmacologic response and the drug concentration that includes physiologic or biochemical effects of drugs on the body or on microorganisms or parasites within or on the body
Pharmacokinetics: the time course of a drug in the body that includes absorption, distribution, metabolism, and elimination; what the body does to an administered drug
Steady state concentration: achieved when the rate at which a drug comes into the body equals the rate at which the drug leaves the body (At steady state, the plasma concentration of a drug is constant during continuous intravenous infusion, or the plasma concentration vs time profile during a dosing interval is identical to the plasma concentration vs time profile during the subsequent dosing intervals for a fixed dose and dosing frequency. Steady state plasma concentrations are achieved in approximately 5–6 half-lives.)
T peak: time to maximum concentration or the time at which maximum blood or plasma concentrations are achieved during a dosing interval
Volume of distribution: a hypothetic volume that relates the concentration of the drug in the measured biologic fluid (normally plasma or serum or blood) to the amount of drug in the body; the apparent volume into which the drug has to be distributed at a concentration equal to the concentration that is measured in the biologic fluid (typically expressed in liters or in liters per kilogram), which provides information about the extent to which the drug is distributed outside the vascular system.
Footnotes
The authors report no conflict of interest.
REFERENCES
- 1.Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. Erratum in: N Engl J Med 2003;349;1299. [DOI] [PubMed] [Google Scholar]
- 2.Rouse DJ, Caritis SN, Peaceman AM, et al. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357:454–461. doi: 10.1056/NEJMoa070641. [DOI] [PubMed] [Google Scholar]
- 3.Caritis SN, Rouse DJ, Peaceman AM, et al. Prevention of preterm birth in triplets using 17 alpha-hydroxyprogesterone caproate: a randomized controlled trial. Obstet Gynecol. 2009;113:285–292. doi: 10.1097/AOG.0b013e318193c677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grobman WA. Randomized controlled trial of progesterone treatment for preterm birth prevention in nulliparous women with cervical length less than 30 mm. Am J Obstet Gynecol. 2012;206(suppl):S367. [Google Scholar]
- 5.American College of Obstetricians and Gynecologists. ACOG committee opinion no. 419: use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112:963–965. doi: 10.1097/AOG.0b013e31818b1ff6. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S, Mada SR, Sharma S, et al. Simultaneous determination of 17-alpha hydroxyprogesterone caproate, 17-alpha hydroxyprogesterone and progesterone using high-performance liquid chromatography-mass spectrometric (HPLCMS/MS) method in human plasma. J Pharm Biomed Anal. 2008;48:1174–1180. doi: 10.1016/j.jpba.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onsrud M, Paus E, Huag E, Kjorstak K. Intramuscular administration of hydroxyprogesterone caproate in patients with endometrial carcinoma. Acta Obstet Gynecol Scand. 1985;64:519–523. doi: 10.3109/00016348509156732. [DOI] [PubMed] [Google Scholar]
- 8.Sharma S, Ou J, Strom S, Mattison D, Caritis S, Venkataramanan R. Identification of enzymes involved in the metabolism of 17-hydroxyprogesterone caproate: an effective agent for prevention of preterm birth. Drug Metab Dispos. 2008;36:1896–1902. doi: 10.1124/dmd.108.021444. [DOI] [PubMed] [Google Scholar]
- 9.Caritis S, Sharma S, Venkataramanan R, et al. Pharmacokinetics of 17-hydroxyprogesterone caproate in multifetal gestation. Am J Obstet Gynecol. 2011;205:40.e1–40.e8. doi: 10.1016/j.ajog.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuppett C, Zhao W, Caritis S, Venkataramanan R. Effect of endogenous steroids on 17 alpha-hydroxyprogesterone caproate (17-OHPC) metabolism. Am J Obstet Gynecol. 2011;204(suppl):S29. doi: 10.1016/j.ajog.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y, Cuppett C, Caritis S, Venkataramanan R. Effect of prescription medications on 17-alpha-hydroxyprogesterone caproate (17-OHPC) metabolism. Am J Obstet Gynecol. 2012;206(suppl):S9. doi: 10.1016/j.ajog.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Hemauer SJ, Yan R, Patrikeeva SL, et al. Transplacental transfer and metabolism of 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2008;199:169.e1–169.e5. doi: 10.1016/j.ajog.2007.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma S, Ellis EC, Dorko K, et al. Metabolism of 17 alpha-hydroxyprogesterone caproate, an agent for preventing preterm birth, by fetal hepatocytes. Drug Metab Dispos. 2010;38:723–727. doi: 10.1124/dmd.109.029918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fokina VM, Zharikova OL, Hankins GD, Ahmed MS, Nanovskaya TN. Metabolism of 17-alpha-hydroxyprogesterone caproate by human placental mitochondria. Reprod Sci. 2012;19:290–297. doi: 10.1177/1933719111419248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Northen AT, Norman GS, Anderson K, et al. Follow-up of children exposed in utero to 17-hydroxyprogesterone caproate compared with placebo. Obstet Gynecol. 2007;110:865–872. doi: 10.1097/01.AOG.0000281348.51499.bc. [DOI] [PubMed] [Google Scholar]
- 16.Varma TR, Morsman J. Evaluation of the use of Proluton-Depot (hydroxyprogesterone hexanoate) in early pregnancy. Int J Gynaecol Obstet. 1982;20:137. doi: 10.1016/0020-7292(82)90039-x. [DOI] [PubMed] [Google Scholar]
- 17.Michaelis J, Michaelis H, Gluck E, Keller S. Prospective studies of suspected association between certain drugs administered in early pregnancy and congenital malformations. Teratology. 1983;27:57. doi: 10.1002/tera.1420270109. [DOI] [PubMed] [Google Scholar]
- 18.Resequie LJ, Hick JF, Bruen JA, Noller KL, O’Fallon WM, Kurland LT. Congenital malformations among offspring exposed in utero to progestins, Olmstead County, Minnesota 1936–1974. Fertil Steril. 1985;43:514–519. doi: 10.1016/s0015-0282(16)48490-6. [DOI] [PubMed] [Google Scholar]
- 19.Check JH, Rankin A, Teichman M. The risk of fetal anomalies as a result of progesterone therapy during pregnancy. Fertil Steril. 1986;45:575. doi: 10.1016/s0015-0282(16)49292-7. [DOI] [PubMed] [Google Scholar]
- 20.Katz Z, Lancet M, Skornik J, Chemke J, Mogilner BM, Klinberg M. Teratogenicity of progestogens given during the first trimester of pregnancy. Obstet Gynecol. 1985;65:775–780. [PubMed] [Google Scholar]
- 21.Kester PA. Effects of prenatally administered 17 alpha-hydroxyprogesterone caproate in adolescent males. Arch Sex Behav. 1984;13:441–455. doi: 10.1007/BF01541429. [DOI] [PubMed] [Google Scholar]