Abstract
The prefrontal cortex has a crucial role in cognitive control, executive function and sensory processing. Functional imaging, neurophysiological and animal studies provide evidence for a functional connectivity between the dorsolateral prefrontal cortex (DFLPC) and the primary motor cortex (M1) during free choice but not instructed choice selection tasks. In this study, twin coil, neuronavigated transcranial magnetic stimulation (TMS) was used to examine the precise timing of the functional interaction between human left DLPFC and ipsilateral M1 during the execution of a free/specified choice-selection task involving the digits of the right hand. In a thumb muscle that was not involved in the task, a conditioning pulse to the left DLPFC enhanced the excitability of the ipsilateral M1 during free-selection more than specified selection 100 ms after presentation of the cue; the opposite effect was seen at 75 ms. However, the difference between free and externally specified conditions disappeared when a task specific muscle was investigated. In this case, the influence from DLPFC was dominated by task involvement rather than mode of selection, suggesting that other processes related to movement execution were also operating. Finally, we show that the effects were spatially specific since they were absent when an adjacent area of DLPFC was stimulated. These results reveal temporally and spatially selective interactions between BA46 and M1 that are both task and muscle specific.
Keywords: Cortical Connectivity, Dorsolateral Prefrontal Cortex, Primary Motor Cortex, Transcranial magnetic stimulation, Choice-Reaction-Task
Introduction
The prefrontal cortex (PFC) is highly developed in primates (Miller & Cohen, 2001) and plays important roles in cognitive control, executive function, working-memory and top-down modulation of sensory processing (Miller, 2000; Miller & Cohen, 2001). Within the PFC, the dorsolateral prefrontal cortex (DLPFC) has a central integrative function for motor control and behaviour. In particular, Brodmann’s area 46 (BA46) has diverse neuronal connections to several different motor regions such as the premotor cortices, supplementary motor area, cerebellum and basal ganglia (Alexander, DeLong, & Strick, 1986; Bates & Goldman-Rakic, 1993; Lu, Preston, & Strick, 1994; Miller & Cohen, 2001). Animal studies involving monkeys indicate that the lateral PFC in particular plays a crucial and superordinate role in motor selection decisions for adapting to two behavioural rules (Hoshi, Shima, & Tanji, 2000). In humans, imaging studies have shown that activation of the DLPFC (especially BA46) is prominent during action selection, particularly in tasks in which subjects are required to freely select their movement (Deiber, Ibanez, Sadato, & Hallett, 1996; Deiber et al., 1991; Frith, Friston, Liddle, & Frackowiak, 1991; Hadland, Rushworth, Passingham, Jahanshahi, & Rothwell, 2001; Hoshi et al., 2000; Jueptner et al., 1997; Rowe, Stephan, Friston, Frackowiak, & Passingham, 2005; Spatt & Goldenberg, 1993). For example, one early study in which regional cerebral blood flow (rCBF) was measured with positron emission tomography (PET), showed increased activation of the DLPFC when subjects made free selection responses relative to when they were specified (Frith et al., 1991). Another PET study showed that free selection conditions activated various cortical areas, including different motor cortical fields, but that there was an exclusive increase of rCBF in the PFC compared to the activation pattern following cued conditions. The authors concluded that the internal selection process for self selection of movements involves a distributed network located mainly in the frontal lobe (Deiber et al., 1996). Later functional MRI (fMRI) studies confirmed these ideas and showed that the coupling between DLPFC and M1 is greater for freely selected choices compared with external instructed choices (Rowe et al., 2005). Finally, work using transcranial magnetic stimulation (TMS) has revealed a distinct inhibitory network involving two frontal brain regions, the lateral prefrontal cortex and the dorsal premotor cortex, and the interconnected M1 during response preparation of selected and unselected effectors (Duque, Labruna, Verset, Olivier, & Ivry, 2012; Duque, Lew, Mazzocchio, Olivier, & Ivry, 2010). This work suggests that during freely selected movements, specific interactions exist between the DLPFC (especially BA46) and M1. However, little is known regarding the exact timing and the excitatory and inhibitory nature of this DLPFC-M1 interaction during action selection tasks. Therefore, the present experiments were designed to probe the details of a specific interaction between DLPFC and M1, using twin coil TMS. In this design, one coil is used to stimulate M1 in order to probe the excitability of corticospinal output to hand muscles involved in the task; the other is used to stimulate BA46 6 to 12 ms beforehand. There are no direct anatomical connections between DLPFC and M1 (Miller & Cohen, 2001), but TMS connectivity studies indicate a coupling between PFC and M1 at subsecond timescales. For example, one TMS study investigated the connections between M1 and frontal/medial cortices at rest and showed an inhibitory influence of premotor stimulation on the M1 at short interstimulus intervals (4 – 6 ms) (Civardi, Cantello, Asselman, & Rothwell, 2001). Some of the positions of the conditioning coil used in those experiments (6 cm anterior to the hot spot) could be considered as overlapping with the area defined as DLPFC (Fitzgerald, Maller, Hoy, Thomson, & Daskalakis, 2009; Rusjan et al., 2010). However, the translation from this pioneering work to cognitive neuroscience is not simple, as no neuronavigation was used and connectivity was examined at rest rather than during the execution of a task as in the present study. As noted by others, connectivity between brain areas is often quite different in different behavioural states (Rothwell, 2011).
By varying the time of stimulation after a cue which signalled either a free selection or specified finger movement, we assessed whether the interactions between BA46 and M1 occurred at particular intervals during task preparation and if this was specific to free selection. In addition, since electromyography (EMG) activity evoked by M1 stimulation can be recorded in separate hand muscles we also ask whether the influence of BA46 is specific to muscles involved in the task. Finally, we used neuronavigation to position the site of DLPFC stimulation. Therefore, we could investigate whether the interaction was spatially specific to BA46 by applying the conditioning stimulus to the rostral part of the superior frontal gyrus (BA9), which is also considered part of the DLPFC (Petrides & Pandya, 1999).
We tested the hypothesis that the excitability of the functional connection between a given region of DLPFC, namely BA46, and the ipsilateral motor cortex is modulated during a choice-reaction task. In our model, modulation would depend on the modality of the task, the timing of the cue presentation, the selection/non-selection of an effector and the localisation of the PFC stimulation.
Methods
Subjects
Seventeen subjects (10 females, mean age = 30.2 ± 7.0) participated in one or more of the experiments of this study. 10 subjects (8 females) participated in experiment 1, 7 subjects (3 females) participated in experiment 2 and experiment 3 and 4 were conducted each with 8 subjects (4 females). For all experiments, subjects had individual T1-weighted MRI scans. All subjects were right-handed according to the Edinburgh handedness inventory (Oldfield, 1971), and had normal or corrected-to-normal vision. There was no history of neurological or mental illness, alcohol or drug abuse, metallic cerebral implants, and no subject was taking any neuroactive medication. The study protocol, which is in accordance with the Declaration of Helsinki, was approved by the Ethics Committee of University College London.
Behavioural Task
Subjects performed an instructed free selection/external specified selection task similar to that described in previous publications (Rowe et al., 2005). Subjects sat in front of a standard computer screen which was approximately 80 cm in front of them. In brief, a white arrow was presented every 5 seconds in the middle of a black screen. In the externally specified condition, the arrow could occur at four different orientations (9, 11, 1 and 3 o’clock) each of which specified a button press of a different finger (respectively: index finger, middle finger, ring finger, small finger). A fifth arrow with an orientation at 12 o’clock indicated that this was a free selection trial in which subjects had to select at will any finger press. To avoid perseveration, in the free selection trials subjects were instructed not to repeatedly use the same finger, but to make a random choice on each occasion (Rowe et al., 2005). Subjects performed one practice block with 30 trials before the experiment started. In Experiment 1, 960 trials were applied in 4 blocks (240 trials/block, 120 free selection and 120 specified selection). In Experiment 2, 3 and 4, 480 trials were applied in 4 blocks (120 trials/block, 60 free selection and 60 specified selection). After each block, a pause of approximately 7 minutes was given. Each 24 trials were fully randomized; therefore neither the subject nor the experimenter could predict the trial order.
Transcranial magnetic stimulation
We recorded surface electromyography (EMG) from the right abductor pollicis brevis muscle (APB, experiment 1 and 3) and the right first-dorsal-interosseous muscle (FDI, experiment 1, 2 and 4) via Ag/AgCl electrodes in a belly-tendon montage. Raw-signals were amplified (Digitimer 360, Digitimer Ltd., Welwyn Garden City, Herts, UK), band-pass filtered (10 Hz - 3 kHz) and digitalized using a 1401 data acquisition interface (Cambridge Electronic Design Ltd., Cambridge UK) controlled by Signal Software (Cambridge Electronic design). To investigate the BA46-M1 connectivity within the left hemisphere, two figure-of-eight coils (7 cm outer diameter for the primary motor cortex (M1), 5 cm outer diameter for the BA46 region) connected to two single-pulse monophasic stimulators (Magstim Co., Whitland, Dyfeld, UK) were used. With this experimental design, the influence of DLPFC on M1 could be quantified by measuring the extent to which DLPFC stimulation changed the excitability of the ipsilateral M1 outputs. In contrast to most other TMS connectivity studies, we investigated the PFC (in our study BA 46)-M1 connection within the same hemisphere. This was achievable through the use of a small custom-made figure-of-eight coil, and in the selection of an area which was located at a sufficient distance to M1 to allow a reliable placement of two figure-of-eight coils on the same hemisphere. This setup reduced the bias derived from interhemispheric measures and allowed us to focus on the dominant hemisphere. The intensity of the conditioning pulse (BA46) was set at 105% of resting motor threshold (RMT) and the intensity of the test pulse (M1) was set to evoke a 1mV-motor-evoked-potential (MEP) at rest with the large TMS coil. The decision of setting the intensity of the conditioning pulse at 105% RMT was based on the findings that a suprathreshold conditioning pulse can elicit functional interactions between the frontal lobe and M1 (Koch et al., 2006; O’Shea, Sebastian, Boorman, Johansen-Berg, & Rushworth, 2007) and on the observation that higher stimulation intensities used over this area were less well tolerated by our subjects. RMT was defined as the lowest intensity that produced an MEP of >50 μV in 5 out of 10 trials in the relaxed target muscle with the small TMS-coil placed over the left M1. Left M1 was defined functionally as the position where single-pulse TMS induced consistently the largest MEPs in both reference muscles (figure 1).
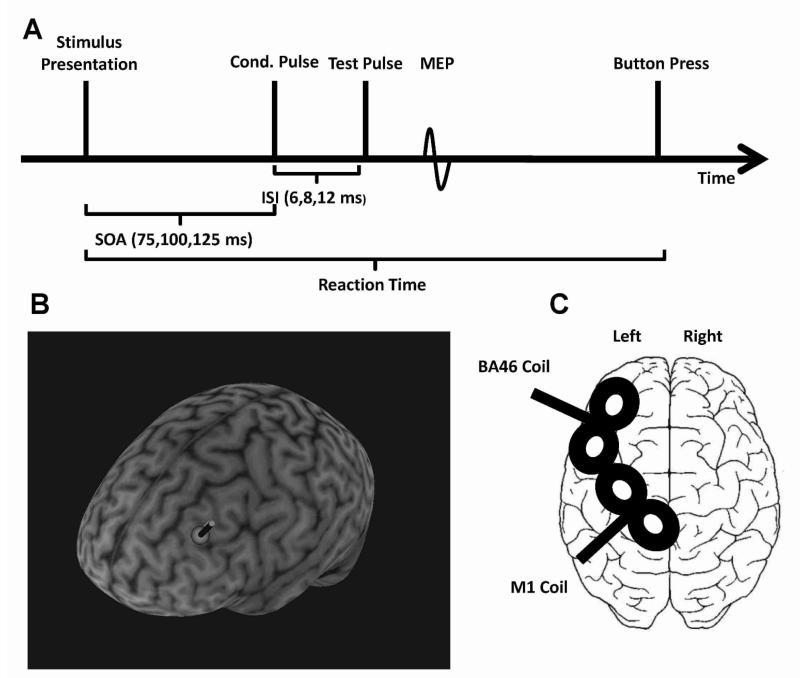
Figure 1.
A) Time course of the BA46-M1 experiment. The conditioning pulse was applied 75, 100, or 125 ms after the cue appeared on the screen. The test-pulse followed this conditioning pulse with a latency of 6, 8, or 12 ms in experiment 1. SOA: stimulus-onset asynchrony; ISI: interstimulus-interval. B) Stimulation site and coil placement of the BA46-coil (3D reconstructed brain images of one representative subject). C) Schematic presentation of the coil placements over the left hemisphere.
Individual anatomical T1 MRI-scans and Brainsight-Neuronavigation (Rogue Research, Canada) was used to determine the exact location of the left BA46 site (Talairach coordinates (x; y; z): - 40; 28; 30) previously linked to the specification of freely selected actions (Rowe et al., 2005). This position was visually inspected and corrected when necessary by A.H to ensure a target position on the grey matter. Talairach coordinates (Talairach & Tournoux, 1998) were transformed into native space using the brain atlas function Brainsight-Neuronavigation (Rogue Research, Canada).
Experimental design
During all experiments, subjects were placed in front of a screen and wore a tight-fitting EEG cap with the marked TMS-coil positions.
Experiment 1: Influence of BA46 stimulation on the excitability of the ipsilateral M1 measured in an unselected muscle
This experiment tested the effect of stimulation of BA46 on the excitability of corticospinal output from M1 to a muscle that was not involved in any of the 4 possible finger movements (APB) and to an involved muscle (FDI). In the first experiment, we used an unselected muscle as primary outcome measure for two reasons: first, we wanted to avoid any possible effect of movement preparation on corticospinal excitability of M1, which is expected in task related muscles. Second, the findings of Rowe and colleagues from their fMRI study (2005) indicated that DLPFC was exerting a non-somatotopic effect on M1 suggesting that it would be apparent in all muscles of the involved hand. However, to test our hypothesis of this unspecific connectivity, we analyzed the data of the FDI as a secondary outcome measure and we compared the results from both muscles in this experiment. Three different stimulus-onset asynchronies (SOA) between the appearance of an arrow on the visual display and the conditioning TMS pulse were examined (SOA; 75, 100, 125 ms) at 3 different inter-stimulus intervals between stimulation of BA46 and M1 (ISI; 6, 8, 12 ms). These SOAs and ISIs were based on those used to investigate the connectivity of premotor/frontal brain regions and the M1 within and between hemispheres (Buch, Mars, Boorman, & Rushworth, 2010; Koch et al., 2006; Mars et al., 2009; Neubert, Mars, Buch, Olivier, & Rushworth, 2010; O’Shea et al., 2007).
Experiment 2: Specific and muscle-dependent BA46-M1 connectivity
Experiment 2 tested the effect of BA46 stimulation on corticospinal excitability to selected and unselected muscles at different stimulus-onset asynchronies (SOA; 75, 100, 125 ms) and a single ISI (12 ms) which was identified as optimal from experiment 1. A single ISI was chosen so that we could record a sufficient amount of trials for each finger response to allow a comparison between selected and unselected muscles during free and specified trial types. In these trials, reaction times (RTs) and EMG data from movements with an index finger press (FDI selected) were contrasted with data from movements in which the correct finger press was middle, ring or small finger (FDI not selected).
Experiments 3 and 4: Anatomic specificity of the BA46-M1 connectivity
Anatomical specificity was tested in two additional control experiments. Experiments 3 and 4 were similar to experiments 1 and 2 (uninvolved and involved muscles respectively) except that the conditioning coil was placed over BA9 rather than BA46 (x, y, z: -9; 50; 21, BA9 region).
Presentation of visual stimuli and synchronization with TMS was implemented by MATLAB 2008b (The MathWorks, Natick, MA) and the Cogent toolbox developed by LON, FIL and ICN at UCL (http://www.vislab.ucl.ac.uk/cogent.php).
Assessing randomness of free choices
Though subjects were instructed to choose a response randomly within free selection it is possible that certain patterns would emerge (Jahanshahi, Dirnberger, Fuller, & Frith, 2000; Robertson, Hazlewood, & Rawson, 1996). We compared the level of randomness within free and specified trials. To this end, we calculated the entropy conveyed by trials (Harrison, Duggins, & Friston, 2006). Trial-by-trial entropy (H) was calculated as
where × (1 of the 16 possible combinations between finger selected on trial t and trial t-1) is a discrete random variable and f (x) is the value of its probability distribution at x. Entropy was estimated separately for the free selection and specified trial types within experiment 2 (this experiment had a valuable amount of homogenous trials to allow such a post-hoc analyses) and compared across subjects with a paired t-test.
Data analysis/Statistical analyses
To correct for small differences in coil placement and possible alterations in baseline MEPs and SOAs between blocks, MEP sizes were normalized within each block and analysis was performed across blocks. Reaction times (RT) were defined as the onset of the cue until the button press and analyzed as absolute values. Trials with incorrect responses pre-contraction in the target muscle (EMG amplitude in 100 ms before the TMS pulse > 2.5 × EMG amplitude 800 – 1000 ms before the TMS pulse) or RTs less than 80 ms were excluded from further analyses. RTs were analysed as absolute values to allow the assessment of single-pulse and paired-pulse TMS on RTs.
For statistical analyses, SPSS 20 for Windows was used. Level of significance was set at α = 0.05. Shapiro-Wilk-Tests confirmed normal distribution for the data (p > 0.05). Electrophysiological data (MEP-Amplitude) and behavioural data (RT-Duration) were analysed with Repeated-Measures-ANOVAs (RM-ANOVA) in a within subject-design. If appropriate (significant interactions in the RM-ANOVA), Student’s t-tests (paired-sample or one-sample, two-tailed) were performed to determine changes between different conditions and in comparison to the baseline. In the linear models, sphericity was tested with Mauchly’s test and if necessary (Mauchly’s test < 0.05), the Greenhouse-Geisser correction was used.
Results
Assessing randomness of free choices
The following post-hoc analysis was conducted on all subjects in experiment 2. A paired t-test showed no significant difference (t(6) = 1.451, p = 0.197) in entropy between the conditions (free: 1.74 ± 0.07 bits, specified: 1.78 ± 0.05 bits). This indicates that the degree of randomness of finger selection was similar across free and specified trial types. Additionally, in the free-selection condition finger one was chosen in 26.0 % (± 9.2 %), finger two in 27.1 % (± 3.1 %), finger three in 29.5 % (± 9.1 %) and finger four in 18.0 % (± 4.8 %). RM-ANOVA with the factor “Finger” showed no significant difference in the distribution of fingers used within the free trial types (F(1.3, 7.7) = 3.141, p = 0.112).
Correct and incorrect trials
In experiment 1 4.6% of the trials in the free selection condition and 6.4 % of the trials in the externally cued condition were incorrect. Experiment 2 had 2.9% incorrect free and 5.3 % incorrect specified trials. The control experiments 3/4 had 5.4%/5.2% incorrect free selected trials and 6.9%/6.7% incorrect specified selected trials. All incorrect trials were excluded from the analysis.
Experiment 1: BA46-M1 connectivity in an unselected muscle (APB, non-specific connectivity)
Behavioural data
One subject had to be excluded from the analysis because she did not complete all blocks. The three-way RM-ANOVA (RT absolute values) with the factors “condition” (free-selection vs. specified-selection), “SOA” (75, 100, 125 ms) and “TMS” (single-pulse (test-pulse only), 6, 8, 12ms) revealed a significant main effect of “condition” (F(1, 8) = 22.539, p = 0.01), indicating, as expected, faster RTs in the specified-selection trials. Furthermore, analyses revealed a significant main effect of “SOA” (F(2, 16) = 9.789, p = 0.01), but no further main effects or interactions (all F < 1.849, p > 0.110).
Electrophysiological data
The aim of experiment 1 was to investigate whether the influence of BA46 on corticospinal excitability of M1 (assessed at ISIs of 6, 8, 12 ms) changed at different times after the appearance of the visual “go” signal (SOAs of 75, 100, 125ms). As detailed below the results indicate that during trials with externally specified responses, stimulation of BA46 increases excitability of M1 at a SOA of 75 ms, but did not modulate it at SOAs of 100 and 125 ms. However, in freely selected trials, stimulation of BA 46 at a SOA of 100 ms facilitates M1 excitability. Averaged data suggested that these effects occurred at all three ISIs, but additional analyses show that the main effect is at an ISI of 12 ms.
To compare the MEPs recorded from both muscles, we used a four-way ANOVA with the factors “muscle” (APB vs. FDI), “condition” (free-selection vs. specified-selection), “SOA” (75, 100, 125 ms) and “ISI” (6, 8, 12 ms). This analysis revealed a trend for a “muscle x condition x SOA” interaction (F(2, 16) = 2.824, p = 0.089) and a trend for a “condition x ISI” interaction (F(2, 16) = 2.931, p = 0.082), but not further main effects or interactions (all F < 2.544, p > 0.111).
For the APB muscle (unselected muscle, non-specific connectivity), we performed a three-way RM-ANOVA with factors “condition” (free vs. specified), “SOA” (75, 100, 125 ms) and “ISI” (6, 8, 12 ms). This revealed a significant “condition x SOA” interaction (F(2, 16) = 6.674, p = 0.008), a trend for an interaction “condition x ISI” (F(2, 16) = 2.773, p = 0.092), but no further main effects or interactions ( all F < 1.386, p > 0.279). To enhance the power of this analysis by reducing the input to the ANOVA, ISIs were merged together as one factor “mean-ISI”. As expected by the results of the first ANOVA, this analysis revealed a significant “condition x SOA” interaction (F(2, 16) = 6.163, p = 0.010), but no further main effects of interactions ( all F < 0.835 p > 0.453). These “mean-ISI” values were used for contrasting the “condition x SOA” interaction.
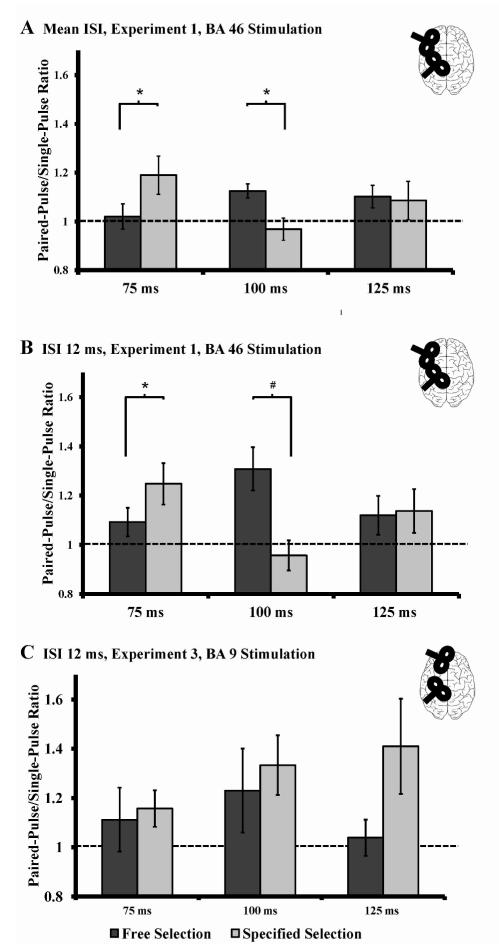
Paired-sample t-tests showed a significantly higher paired-pulse/single-pulse ratio for free-selection (1.12 ± 0.09) compared to specified-selection (0.97 ± 0.15) at a SOA of 100 ms (t(8) =3.138, p = 0.014), and a lower paired-pulse/single-pulse ratio for free-selection (1.02 ± 0.16) compared to specified-selection (1.19 ± 0.25) at a SOA of 75 ms (t(8) = 2.312, p = 0.050) (figure 2 A). One-sample t-tests of the ratios against baseline (test value = 1.00, (Neubert, Mars, Buch, Olivier, & Rushworth, 2010)) showed that the MEPs were significantly facilitated at a SOA of 100 ms in the free selection condition (t(8) = 4.063, p = 0.004) and that MEPs showed a trend towards facilitation at a SOA of 125 ms (t(8) = 2.067, p = 0.072). In the specified condition, MEPs showed a trend towards a facilitation at a SOA of 75 ms (t(8) = 2.283, p = 0.052) (figure 2 A).
Figure 2.
Timing of the unspecific functional connectivity (data recorded from the right APB, experiment 1 (cross-interaction) and experiment 3). A) BA46 stimulation: Paired-pulse/single-pulse ratio averaged for all ISIs (6, 8, 12 ms) at different SOAs (75, 100, 125 ms). At SOA 75 ms, the functional BA46-M1 connectivity is enhanced for trials with an external specified action and at SOA 100 ms the functional BA46-M1 connectivity is enhanced for free-selection trials. This indicates different timings of stimulus processing in the visual and frontal lobe. B) BA46 stimulation: Paired-pulse/single-pulse ratio for one ISI (12 ms) at different SOAs (75, 100, 125 ms). This shows that the main effect is driven by an ISI of 12 ms and for that reason all further experiment have been conducted using an ISI of 12 ms. C) BA9 stimulation: Paired-pulse/single-pulse ratio for one ISI (12 ms) at different SOAs (75, 100, 125 ms). The cross-interaction at the SOAs of 75 ms and 100 ms disappeared and analyses could not detect an effect of BA 9 stimulation on M1 excitability. The visual difference at a SOA of 125 ms is due to an outlier and not statistically significant. *p ≤ 0.05; # p < 0.08 (trend level). Error bars are expressed as standard error of the mean.
These results could not be confirmed in the FDI muscle. The three-way-ANOVA with factors “condition” (free vs. specified), “SOA” (75, 100, 125 ms) and “ISI” (6, 8, 12 ms) did not show, apart from a trend for an interaction “condition x ISI” (F(2, 16) = 2.921, p = 0.083) any main effects or interactions (all F < 0.913, p > 0.423).
The interaction between PMd/PMv/SMA and the ipsilateral and contralateral M1 was found to be within 10 ms at rest and during the performance of various behavioural tasks (Baumer et al., 2006; Baumer et al., 2009; Buch, Mars, Boorman, & Rushworth, 2010; Davare, Lemon, & Olivier, 2008; Koch et al., 2006; Mochizuki, Huang, & Rothwell, 2004). Therefore, we can assume that the interaction, which is very likely to be polysynaptic, between BA46 and ipsilateral M1 should be in the range of ISIs longer than 10 ms. For that reason we hypothesised that our observed effect would be greatest at an ISI of 12 ms and although we had no effect of the factor “ISI” in the initial ANOVA, we repeated our analyses with this ISI (12 ms) to confirm our initial findings which were calculated with the factor “mean-ISI”.
To compare both muscles, we used a three-way ANOVA with the factors “muscle” (APB vs. FDI), “condition” (free-selection vs. specified-selection) and “SOA” (75, 100, 125 ms). This analysis revealed a trend for a significant “muscle x condition x SOA” interaction (F(2, 16) = 2.951, p = 0.083), but no further main effects or interactions (all F < 0.633, p > 0.545).
For the APB muscle, a RM-ANOVA with a RM-ANOVA with the factors “conditions” and “SOA” revealed a significant “condition x SOA” interaction (F(2, 16) = 7.327, p = 0.006), but no main effects of (all F < 1.328, p > 0.283). Paired-sample t-tests showed a significantly higher paired-pulse/single-pulse ratio for free-selection (1.31 ± 0.26) compared to specified-selection (0.96 ± 0.18) at a SOA of 100 ms (t(8) = 3.692, p = 0.006), and a trend for a lower paired-pulse/single-pulse ratio for free-selection (1.09 ± 0.17) compared to specified-selection (1.25 ± 0.25) at a SOA of 75 ms (t(8) = 2.036, p = 0.076) (figure 2 B). One-sample t-tests of the ratios against baseline (test value = 1.00) showed that the MEPs were significantly facilitated at a SOA of 100 ms in the free selection condition (t(8) = 3.500, p = 0.008) and that MEPs were facilitated in the specified selection condition at a SOA of 75 ms (t(8) = 2.932 p = 0.019) (figure 2 B).
For the FDI muscle, a RM-ANOVA with the factors “conditions” and “SOA” revealed no “condition x SOA” interaction (F(2, 16) = 0.169, p = 0.846) and no main effects (all F < 0.258, all p > 0.627).
Baseline cortical excitability
To examine possible changes in baseline cortical excitability we performed a statistical comparison of the single-pulse TMS trials. RM-ANOVA for the APB with the factors “condition” and “SOA” revealed no main effects < 3.125, all p > 0.112), but a significant “condition x SOA” interaction (F(2, 16) = 4.369, p = 0.030). Post-hoc paired t-tests indicate that the baseline MEP amplitudes were smaller in the free-selection condition (0.42 ± 0.30 mV) compared to the specified selection condition (0.52 ± 0.38 mV) at a SOA of 100 ms (t(8) = 3.332 p = 0.011). At a SOA of 75 ms (free: 0.47 ± 0.40 mV; specified: 0.43 ± 0.32 mV) and a SOA of 125 ms (free: 0.45 ± 0.34 mV; specified: 0.45 ± 0.35 mV) post-hoc t-tests showed no differences of baseline MEPs.
For the FDI, RM-ANOVA revealed no main effects for “condition” (F(1, 8) = 0.733, p = 0.417) or “SOA” (F(2, 16) = 0.343, p = 0.715) and no “condition x SOA” interaction F(2, 16) = 1.002, p = 0.389). There were no differences in baseline MEPs at a SOA of 75 ms (free: 1.41 ± 1.07 mV; specified: 1.35 ± 1.03 mV), a SOA of 100 ms (free: 1.42 ± 1.10 mV; specified: 1.47 ± 1.19 mV) or a SOA of 125 ms (free: 1.43 ± 1.14 mV; specified: 1.35 ± 0.97 mV).
In summary, these results indicate an interaction between BA46 and M1 at SOAs of 75 ms and 100 ms which is dependent on task modality. However, we cannot determine the precise ISI of this interaction. On the basis of our literature-based hypothesis that a longer ISI most likely underlies this interaction, and the additional analyses focussing on an ISI of 12 ms, we decided to use only one ISI, namely 12 ms, for the following experiments. This allowed us to accumulate more trials for the involved and non-involved muscles.
Experiment 2: BA46-M1 connectivity in a selected muscle (FDI, muscle-specific connectivity)
Behavioural data
This experiment, conducted in 7 subjects (3 females) was similar to experiment 1 except that in this case we examined corticospinal excitability to a muscle involved in the task (FDI: index finger press). A four-way RM-ANOVA (RT absolute values) with the factors “condition” (free vs. specified), “SOA” (75, 100, 125 ms), “Selection” (selected vs. not Selected) and “TMS” (single pulse vs. 12 ms) revealed an expected significant main effect of condition (F(1, 6) = 10.881, p = 0.016), a significant “condition x selection” interaction (F(1, 6) = 12.010, p = 0.013), a trend for a significant “condition x SOA” interaction (F(3, 12) = 3.157, p = 0.079), but no further main effects or interactions (all F < 2.408 p > 0.173). RTs for this experiment and further contrasts are presented in table 2. In general, as in experiment 1, RTs were faster in the specified condition than the freely selected condition.
Table 2.
Reaction times for experiment 2 and 4.
| Specified Selection | Free Selection | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Pulse | SOA | Mean [ms] |
SD | Pulse | SOA | Mean [ms] |
SD | P | |
| Experiment 2 | |||||||||
| Selected | Testpulse | 75 | 615.9 | 124.4 | Testpulse | 75 | 731.2 | 126.0 | 0.043 |
| PP 12 ms | 75 | 642.6 | 117.9 | PP 12 ms | 75 | 747.6 | 187.7 | 0.098 | |
| Testpulse | 100 | 763.0 | 250.6 | Testpulse | 100 | 784.6 | 206.5 | 0.388 | |
| PP 12 ms | 100 | 647.8 | 119.0 | PP 12 ms | 100 | 747.5 | 187.7 | 0.031 | |
| Testpulse | 125 | 633.6 | 95.5 | Testpulse | 125 | 809.0 | 151.1 | 0.001 | |
| PP 12 ms | 125 | 641.0 | 108.0 | PP 12 ms | 125 | 774.0 | 194.0 | 0.026 | |
| Not Selected | Testpulse | 75 | 656.9 | 124.8 | Testpulse | 75 | 691.9 | 184.4 | 0.307 |
| PP 12 ms | 75 | 663.7 | 124.0 | PP 12 ms | 75 | 694.4 | 170.4 | 0.304 | |
| Testpulse | 100 | 687.8 | 158.0 | Testpulse | 100 | 650.4 | 327.0 | 0.011 | |
| PP 12 ms | 100 | 679.3 | 132.5 | PP 12 ms | 100 | 753.6 | 188.0 | 0.723 | |
| Testpulse | 125 | 689.5 | 131.2 | Testpulse | 125 | 713.5 | 153.6 | 0.216 | |
| PP 12 ms | 125 | 682.5 | 118.9 | PP 12 ms | 125 | 762.1 | 157.9 | 0.004 | |
| Experiment 4 | |||||||||
| Selected | Testpulse | 75 | 661.6 | 110.9 | Testpulse | 75 | 682.5 | 99.0 | 0.609 |
| PP 12 ms | 75 | 648.7 | 72.6 | PP 12 ms | 75 | 703.5 | 97.5 | 0.117 | |
| Testpulse | 100 | 665.7 | 90.0 | Testpulse | 100 | 668.8 | 131.0 | 0.901 | |
| PP 12 ms | 100 | 672.6 | 79.0 | PP 12 ms | 100 | 727.7 | 99.4 | 0.018 | |
| Testpulse | 125 | 691.0 | 104.1 | Testpulse | 125 | 758.0 | 102.8 | 0.226 | |
| PP 12 ms | 125 | 664.4 | 76.3 | PP 12 ms | 125 | 758.4 | 94.3 | 0.019 | |
| Not Selected | Testpulse | 75 | 647.3 | 84.1 | Testpulse | 75 | 682.4 | 93.7 | 0.237 |
| PP 12 ms | 75 | 617.0 | 60.4 | PP 12 ms | 75 | 663.3 | 111.9 | 0.111 | |
| Testpulse | 100 | 661.1 | 87.7 | Testpulse | 100 | 684.6 | 124.7 | 0.278 | |
| PP 12 ms | 100 | 655.1 | 94.6 | PP 12 ms | 100 | 714.7 | 108.7 | 0.037 | |
| Testpulse | 125 | 668.3 | 62.4 | Testpulse | 125 | 702.8 | 135.1 | 0.502 | |
| PP 12 ms | 125 | 656.4 | 74.3 | PP 12 ms | 125 | 688.5 | 95.4 | 0.108 | |
PP: paired-pulse; SD: standard deviation; SOA: stimulus-onset asynchrony; bold: p < 0.05 (comparison specified-selection vs. free selection, paired t tests, two-tailed)
Electrophysiological data
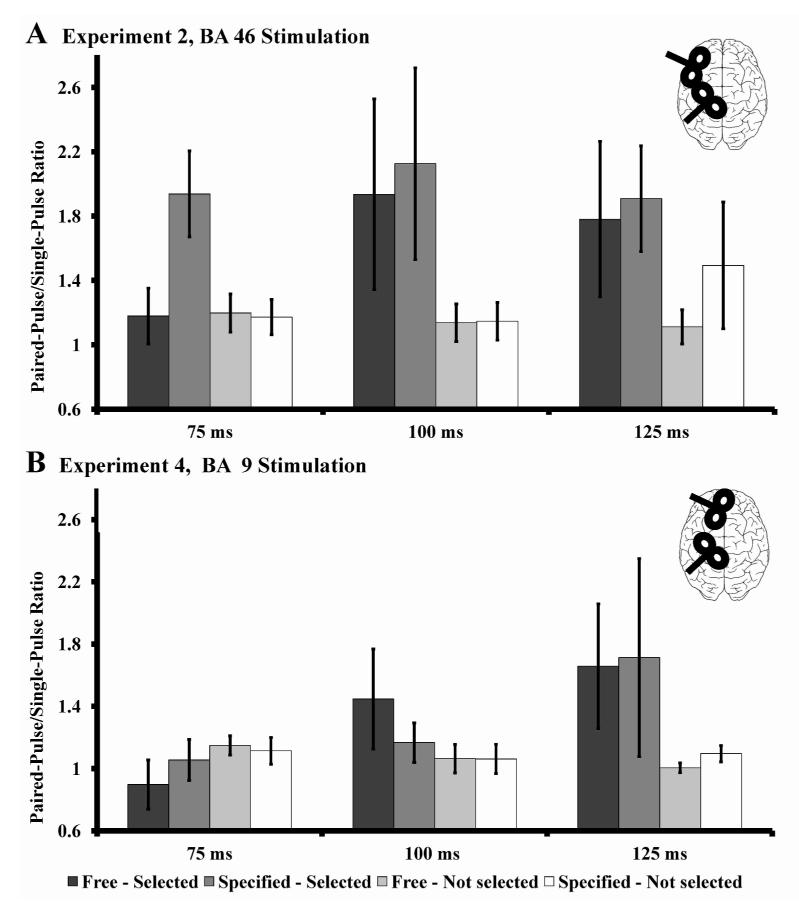
We separated out trials into those in which the movement was an index finger press (FDI involved) and movements of any of the other three fingers (FDI not involved). In contrast to experiment 1, there was no difference between freely selected and externally instructed movements (figure 3 A). The main result was that BA46-M1 connectivity was facilitated in trials in which an index finger press was to be made but there was no effect in trials where a different finger was moved. This was confirmed using a RM-ANOVA with the factors “condition” (free vs. specified), “SOA” (75, 100, 125 ms) and “Selection” (selected vs. not selected). This revealed a significant main effect of “Selection” (F(1, 6) = 22.516, p = 0.003), but no further main effects or interactions (all F < 3.30, p > 0.120) (figure 3).
Figure 3.
Timing of the specific functional connectivity with regard to muscle-involvement (data recorded from the right FDI, experiment 2 and 4). A) BA 46 stimulation: The difference between free and externally specified conditions disappeared (compare to figure 2, experiment 1) when a task specific muscle was investigated (significant main effect of “selection”). Different relay stations of BA46, like premotor cortices, might influence the BA46-M1 connectivity when task-specific, selected muscles are investigated. B) BA 46 stimulation: Analyses did not reveal an effect of BA 9 stimulation and the initially described effect (A) disappeared. The visual difference at a SOA of 125 ms is due to an outlier and not statistically significant. The data of 75 ms and 100 ms shows clearly that BA 46 stimulation has an impact on selected movements and the stimulation of BA 9 is not able to replicate this finding. Error bars are expressed as standard error of the mean.
Baseline cortical excitability
RM-ANOVA with the factors “condition”, “selection” and “SOA” revealed a significant “condition x selection” interaction (F(1, 6) = 6.312, p = 0.046), but no main effects (all F < 1.720, p > 0.238) and no further interactions (all F < 0.992, p > 0.399). Post-hoc t-tests showed a higher MEP baseline for not-selected trials compared to selected trials at a SOA of 125 ms (t(6) = 2.472, p = 0.048; selected: 0.71 ± 0.51 mV, not-selected: 1.00 ± 0.58 mV) during the execution of an instructed task. In the free-selection task the baseline MEPs at a SOA of 75 ms were larger in selected trials (1.24 ± 0.86 mV) compared to not-selected trials (0.79 ± 0.52 mV) (t(6) = 2.484, p = 0.048). Comparing both conditions, MEPs in a selected muscle were marginally larger in freely selected trials at a SOA of 125 ms compared to specified trials (t(6) = 2.402, p = 0.053; free: 1.00 ± 0.78 mV; specified: 0.71 ± 0.51 mV). In an unselected muscle, MEPs were marginally smaller in freely selected trials at a SOA of 75 ms (t(6) = -2.297, p = 0.053; free: 0.80 ± 0.52 mV; specified: 1.00 ± 0.70 mV). No other contrasts showed significant results (all T < 1.521, p > 0.179).
Experiment 3: Control experiment for the non-specific BA46-M1 connectivity (anatomical specificity): BA9-M1 connectivity in an unselected muscle (APB, non-specific connectivity)
Behavioural data
8 subjects (4 females) participated in this experiment. One subject did not have enough valid recordings and was excluded. A three-way RM-ANOVA (RT absolute values) with the factors “condition” (free-selection vs. specified-selection), “SOA” (75, 100, 125 ms) and “TMS” (single-pulse (test-pulse only), 12ms) revealed a significant main effect of “SOA” (F(2, 12) = 5.761, p = 0.018), a trend for “condition” (F(1, 6) = 5.192, p = 0.063), a trend for “TMS” (F(1, 6) = 5.623, p = 0.055), but no interactions (F < 1.781, p > 0.211) (table 3).
Table 3.
Reaction times for experiment 3.
| Specified Selection | Free Selection | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Pulse | SOA | Mean [ms] |
SD | Pulse | SOA | Mean [ms] |
SD | P |
| Testpulse | 75 | 645.3 | 82.1 | Testpulse | 75 | 661.5 | 89.5 | 0.042 |
| PP 12 ms | 75 | 620.2 | 63.5 | PP 12 ms | 75 | 658.9 | 97.5 | 0.004 |
| Testpulse | 100 | 647.6 | 66.1 | Testpulse | 100 | 663.1 | 109.6 | 0.003 |
| PP 12 ms | 100 | 641.5 | 80.4 | PP 12 ms | 100 | 667.9 | 106.3 | 0.001 |
| Testpulse | 125 | 644.2 | 65.9 | Testpulse | 125 | 705.9 | 111.9 | 0.118 |
| PP 12 ms | 125 | 652.0 | 69.3 | PP 12 ms | 125 | 693.0 | 84.9 | 0.014 |
PP: paired-pulse; SD: standard deviation; SOA: stimulus-onset asynchrony; bold: p < 0.05 (comparison specified-selection vs. free selection, paired t tests, two-tailed)
Electrophysiological data
RM – ANOVA with the factors “condition” (free vs. specified) and “SOA” (75, 100, 125ms) did not reveal any main effect or interactions (all F < 2.136, p > 0.194) showing that the observed connectivity (experiment 1) is critically dependent on BA46 and not on a general frontal-lobe activation (figure 2 C).
Experiment 4: Control experiment for the muscle-specific BA46-M1 connectivity (anatomical specificity): BA9-M1 connectivity in a selected muscle (FDI, muscle-specific connectivity)
Behavioural data
8 subjects (4 females) participated in this experiment. Two subjects did not have enough valid recordings and so excluded. A four-way RM-ANOVA (RT absolute values) with the factors “condition” (free vs. specified), “SOA” (75, 100, 125 ms), “Selection” (selected vs. not Selected) and “TMS” (single pulse vs. 12 ms) revealed a trend for a main effect of condition (F(1, 5) = 5.047, p = 0.075), a significant effect of SOA (F(2, 10) = 5.236, p = 0.028), but no further main effects (all F < 1.858, p > 0.232). Apart from a “condition x TMS” interaction (F(1, 5) = 7.739, p = 0.042), no other interactions could be detected (all F < 2.044, p > 0.213) (table 2).
Electrophysiological data
RM-ANOVA with the factors “condition” (free vs. specified), SOA” (75, 100, 125 ms) and selection (selected vs. not selected) did not reveal any main effect or interactions (all F < 1.387, p > 0.292). In accordance with the findings of experiment 3, the muscle specific connectivity found in experiment 2, is dependent on the stimulation of BA46 (figure 3).
Discussion
The present results reveal temporally and spatially selective interactions between BA46 and M1 that are both task and muscle specific. The latency of the effects was short and occurred with stimulation of BA46 only 6, 8 or 12 ms prior to M1. Although additional analyses suggested that the main effect occurred at the longest ISI of 12 ms, the data are in line with the idea that BA46 has an intimate influence on motor cortical excitability. However, whether later effects also occur is unknown as we did not investigate longer ISIs. Since there are no direct connections between BA46 and M1, likely candidates might involve a relay in dorsal premotor cortex (PMd) or other secondary cortical motor areas (Lu et al., 1994; Luppino, Matelli, Camarda, & Rizzolatti, 1993; Miller, 2000; Strick, 1985). An anatomically direct pathway between premotor and primary motor cortex can be activated at ISIs of 4 to 6 ms (Civardi et al., 2001; Godschalk, Mitz, van Duin, & van der Burg, 1995). Subcortical pathways through the basal ganglia might also contribute (Alexander et al., 1986; Miller & Cohen, 2001; Neubert et al., 2010) although this is perhaps more likely at the longer intervals given the correspondingly longer pathways and multiple relays that would be involved.
Stimulation of BA46 has a bidirectional and timing-specific effect on motor cortical excitability during the execution of a choice-selection task
Experiment 1 showed that during a free selection task, stimulation of BA46 facilitated M1 excitability. This effect was maximal what 100 ms after the instruction cue, occurred in a muscle controlling a digit (thumb) that was not involved in the task itself (finger pressing), and was not seen if the movement was instructed rather than freely selected. At the earlier SOA (75 ms), stimulation of BA46 facilitated M1 to a greater extent during instructed movement than during free selection in this non-involved muscle. This facilitation was greater than at baseline. The first implication of these findings is that visual information about the instruction signal rapidly reaches prefrontal areas. This signal is processed within the PFC and, dependent on the timing of the stimulus presentation and the modality of the stimulus, the connectivity to the motor system is modulated. When this action-signal indicates that subjects must freely choose their next finger movement, it increases the excitability of facilitatory interactions between BA46 and muscle representations in M1 whereas this connectivity is significantly inhibited if the cue specifies the required movement. On the other hand, the early timing of the facilitatory interaction at a SOA of 75 ms following a cue for an instructed movement may indicate that this information is evaluated more quickly than free choice. Since it was facilitatory it could contribute to the shorter reaction times to externally instructed compared to freely selected movements. In summary, the excitability of the BA46-M1 interaction varied with the mode of selection and the time point of the task.
One previous fMRI study using a related task design found greater activation of dorsal prefrontal cortex (especially BA46) and of M1 in the free selection condition, whereas both conditions resulted in activation of the prefrontal lobe. Furthermore, there was significantly greater coupling between left BA46 and M1 in the free selection of the task (Rowe et al., 2005). The results of our experiments provide additional evidence about the task-related timing of BA46-M1 interactions, but further studies focussing on disrupting possible cortical relay areas (e.g. with repetitive TMS protocols) are needed to clarify the precise functional pathway of this connection. We suggest that a facilitatory influence of BA46 on M1 excitability at a SOA of 100 ms may contribute to the increased functional coupling between these two cortical areas observed during free-selection tasks in fMRI studies (Rowe et al., 2005). Furthermore, our findings indicate the BA46-M1 connection can be facilitated when PFC is processing external instructed movements at earlier timings, a finding which has not been presented before.
The role of the DLPFC in free-selection tasks has been well established by fMRI, PET and TMS studies (Deiber et al., 1996; Deiber et al., 1991; Frith et al., 1991; Hadland et al., 2001; Jueptner et al., 1997) and is reinforced by our findings. During the free-selection process, we propose that the DLPFC sends a facilitatory and specific output to ipsilateral M1. However, it should be noted that although the DLPFC is associated with action selection, it may not be involved in action execution (Bunge, Hazeltine, Scanlon, Rosen, & Gabrieli, 2002). This role in selection but not generation of a specific movement may explain why we could observe an influence of DLPFC on corticospinal outputs to a muscle that was not involved in the task itself.
At a SOA of 100 ms, we observed higher single-pulse MEP amplitudes in cued conditions and a facilitation of paired-pulse MEP amplitudes for free conditions. Studies using paired-pulse paradigms applied to the primary motor cortex (e.g short-interval intracortical inhibition or intracortical facilitation) indicate that the inhibitory or facilitatory effect is related to the sizes of test MEPs (Chen, 2004). Therefore, a direct intra-area effect within the left M1 could be one possible additional explanation on the observed excitability shift.
The impact of the ipsilateral DLPFC is reduced in muscles involved in the choice-reaction task
Unlike experiment 1, the modality of movement selection (experiment 2) had no effect on the excitability of muscles involved in the task. M1 output to these muscles was influenced by whether or not the muscle was used in the upcoming movement. Thus, a muscle involved in index finger flexion was facilitated from BA46 whenever subjects had to press the response button with their index finger, but was unaffected when a different finger was used. This occurred whether the movement was chosen freely or specified by the instruction cue. Therefore, involvement of a muscle in the task had a stronger influence on BA46-M1 connectivity than the free and specified conditions. Indeed, the magnitude of the effect was much larger in task related muscles than in those that were never used. In addition, the effects when the muscle was not selected were the same in the specified and free conditions and did not change with time, unlike the effects we saw on the non-involved muscle in experiment 1. It is possible that the input from BA46 to M1 interacts with other inputs that either excites or suppresses task relevant muscles. These other inputs may mask the smaller effects observed in uninvolved muscles within experiment 1. This is unlikely to occur within M1 itself since facilitation from BA46 is expressed relative to the ongoing level of excitability in M1. Given that the anatomical BA46-M1 connection is necessarily indirect, the observed interaction may well occur at an intermediate stage(s) of the pathway.
One possibility is that the effects during the specified trials are relayed via PMd. Duque et al. (2012) recently showed that stimulation of the contralateral dorsal premotor cortex during the presentation of a preparatory cue in a choice-reaction task facilitated motor cortical output to an involved (effector) muscle but had no effect in a non-selected muscle (Duque et al., 2012). In contrast, stimulation of the contralateral lateral prefrontal region reduced inhibition in both selected and not-selected effectors suggesting that the lateral PFC is responsible for general and abstract aspects of motor control (Duque et al., 2012). Note, however, that our results are based on ipsilateral BA46-M1 connectivity, whereas the effect observed by Duque et al. (2012) represents an interhemispheric connectivity.
Other data confirm that the intermediate relay stations from BA46, such as premotor areas, influence activation in muscles involved in the task, and at similar timings to BA46 (Miller & Cohen, 2001). For example, Koch and colleagues (2006) showed that PMd modulates activation in muscle groups involved in a task while having no effect on muscles that are uninvolved. In choice reaction tasks, like that in the present experiments, it facilitated muscles when they were selected in the task but suppressed them when they were not selected. Despite some differences in experimental paradigms, it could be that similar effects occur even in freely chosen movements, with facilitation of the chosen muscle and suppression of any potential candidate muscles. Indeed, input from BA46 during free selection trials could act as an appropriate trigger for such behaviour, which may dominate the influence of BA46 on M1 that are described in experiment 1.
In this hypothetical framework, we can assume that the results of experiment 1 reflect a relatively “pure” influence of BA46 on M1 which we observe as changes in excitability of uninvolved muscles during free selection, whereas the results of experiment 2 might represent a cumulative effect of different inhibitory and facilitatory inputs to M1.
The effect of the DLPFC on motor-cortical excitability is anatomically specific and dependent on the stimulation of BA46
It is important to note that within the frontal lobe, different subregions have unique functions in cognitive control, as well as interconnections that fulfil their biological function (Miller, 2000). The DLFPC is occupied by the interconnected cytoarchitectonic areas BA9 and BA46 (Petrides & Pandya, 1999) and the findings of our study indicate that during a selection task with a motor response (finger press), the functional connectivity from one part of the DLPFC, namely BA46, to M1 is of particular importance. In our additional experiments, we found no connectivity between BA9 and M1 using the experimental configuration which showed a prominent effect after stimulating BA 46. It should be noted that determination of exact anatomical borders of BA9 and BA46 is difficult (Petrides & Pandya, 1999) and that other SOAs, ISIs or another task-design might be necessary to probe the BA9-M1 connection. However, our findings underlie the importance of subdividing the DLPFC according to function.
Conclusions
The present results suggest that there is anatomically specific functional connectivity between left BA46 and left M1 during free and specified selection of a movement. In selected muscles, the input of the DLPFC has only limited impact on the M1 excitability, as other more powerful inputs from various areas of the motor-network may modulate M1 excitability. A direct functional connection between DLPFC and M1, as suggested by imaging studies, seems to have a minor role in this complex network and is only unmasked in uninvolved muscles. Our results provide further evidence for a functional specialisation within the DLPFC and reveal that connectivity changes at specific time intervals during a choice-reaction task.
Table 1.
Reaction times for experiment 1.
| Specified Selection | Free Selection | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Pulse | SOA | Mean [ms] |
SD | Pulse | SOA | Mean [ms] |
SD | P |
| Testpulse | 75 | 620.9 | 35.1 | Testpulse | 75 | 714.6 | 54.4 | 0.0004 |
| PP 6 ms | 75 | 636.3 | 55.5 | PP 6 ms | 75 | 697.0 | 64.5 | 0.023 |
| PP 8 ms | 75 | 655.0 | 56.0 | PP 8 ms | 75 | 714.6 | 61.0 | 0.020 |
| PP 12 ms | 75 | 635.4 | 55.0 | PP 12 ms | 75 | 712.7 | 53.3 | 0.007 |
| Testpulse | 100 | 651.5 | 51.2 | Testpulse | 100 | 718.1 | 57.6 | 0.002 |
| PP 6 ms | 100 | 660.1 | 60.1 | PP 6 ms | 100 | 731.9 | 57.0 | 0.002 |
| PP 8 ms | 100 | 661.2 | 59.0 | PP 8 ms | 100 | 724.8 | 60.5 | 0.006 |
| PP 12 ms | 100 | 650.0 | 59.0 | PP 12 ms | 100 | 736.1 | 58.4 | 0.002 |
| Testpulse | 125 | 676.3 | 56.6 | Testpulse | 125 | 726.6 | 85.9 | 0.032 |
| PP 6 ms | 125 | 647.7 | 68.4 | PP 6 ms | 125 | 723.6 | 50.8 | 0.002 |
| PP 8 ms | 125 | 654.0 | 59.0 | PP 8 ms | 125 | 729.0 | 63.7 | 0.003 |
| PP 12 ms | 125 | 660.8 | 58.5 | PP 12 ms | 125 | 737.0 | 73.9 | 0.009 |
PP: paired-pulse; SD: standard deviation; SOA: stimulus-onset asynchrony; bold: p < 0.05 (comparison specified-selection vs. free selection, paired t tests, two-tailed)
Acknowledgements
Grants Alkomiet Hasan is supported by the Deutsche Forschungsgemeinschaft (DFG grant HA 6091/1-1) and by the Research Program of the Faculty of Medicine Goettingen (Georg-August-University Goettingen). Sven Bestmann is supported by the Biotechnology and Biological Sciences Research Council (BBSRC) and European Research Council (ERC; ActSelectContext 260424)
Footnotes
Conflict of interest The authors have no potential conflict of interests related to the subject of this report.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Bates JF, Goldman-Rakic PS. Prefrontal connections of medial motor areas in the rhesus monkey. J Comp Neurol. 1993;336(2):211–228. doi: 10.1002/cne.903360205. [DOI] [PubMed] [Google Scholar]
- Baumer T, Bock F, Koch G, Lange R, Rothwell JC, Siebner HR, et al. Magnetic stimulation of human premotor or motor cortex produces interhemispheric facilitation through distinct pathways. J Physiol. 2006;572(Pt 3):857–868. doi: 10.1113/jphysiol.2006.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumer T, Schippling S, Kroeger J, Zittel S, Koch G, Thomalla G, et al. Inhibitory and facilitatory connectivity from ventral premotor to primary motor cortex in healthy humans at rest--a bifocal TMS study. Clin Neurophysiol. 2009;120(9):1724–1731. doi: 10.1016/j.clinph.2009.07.035. [DOI] [PubMed] [Google Scholar]
- Buch ER, Mars RB, Boorman ED, Rushworth MF. A network centered on ventral premotor cortex exerts both facilitatory and inhibitory control over primary motor cortex during action reprogramming. J Neurosci. 2010;30(4):1395–1401. doi: 10.1523/JNEUROSCI.4882-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage. 2002;17(3):1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154(1):1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14(6):1444–1453. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- Davare M, Lemon R, Olivier E. Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. J Physiol. 2008;586(Pt 11):2735–2742. doi: 10.1113/jphysiol.2008.152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiber MP, Ibanez V, Sadato N, Hallett M. Cerebral structures participating in motor preparation in humans: a positron emission tomography study. J Neurophysiol. 1996;75(1):233–247. doi: 10.1152/jn.1996.75.1.233. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res. 1991;84(2):393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- Duque J, Labruna L, Verset S, Olivier E, Ivry RB. Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. J Neurosci. 2012;32(3):806–816. doi: 10.1523/JNEUROSCI.4299-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Lew D, Mazzocchio R, Olivier E, Ivry RB. Evidence for two concurrent inhibitory mechanisms during response preparation. J Neurosci. 2010;30(10):3793–3802. doi: 10.1523/JNEUROSCI.5722-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Maller JJ, Hoy KE, Thomson R, Daskalakis ZJ. Exploring the optimal site for the localization of dorsolateral prefrontal cortex in brain stimulation experiments. Brain Stimul. 2009;2(4):234–237. doi: 10.1016/j.brs.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston K, Liddle PF, Frackowiak RS. Willed action and the prefrontal cortex in man: a study with PET. Proc Biol Sci. 1991;244(1311):241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- Godschalk M, Mitz AR, van Duin B, van der Burg H. Somatotopy of monkey premotor cortex examined with microstimulation. Neurosci Res. 1995;23(3):269–279. doi: 10.1016/0168-0102(95)00950-7. [DOI] [PubMed] [Google Scholar]
- Hadland KA, Rushworth MF, Passingham RE, Jahanshahi M, Rothwell JC. Interference with performance of a response selection task that has no working memory component: an rTMS comparison of the dorsolateral prefrontal and medial frontal cortex. J Cogn Neurosci. 2001;13(8):1097–1108. doi: 10.1162/089892901753294392. [DOI] [PubMed] [Google Scholar]
- Harrison LM, Duggins A, Friston KJ. Encoding uncertainty in the hippocampus. Neural Netw. 2006;19(5):535–546. doi: 10.1016/j.neunet.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Shima K, Tanji J. Neuronal activity in the primate prefrontal cortex in the process of motor selection based on two behavioral rules. J Neurophysiol. 2000;83(4):2355–2373. doi: 10.1152/jn.2000.83.4.2355. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Dirnberger G, Fuller R, Frith CD. The role of the dorsolateral prefrontal cortex in random number generation: a study with positron emission tomography. Neuroimage. 2000;12(6):713–725. doi: 10.1006/nimg.2000.0647. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. I. Frontal cortex and attention to action. J Neurophysiol. 1997;77(3):1313–1324. doi: 10.1152/jn.1997.77.3.1313. [DOI] [PubMed] [Google Scholar]
- Koch G, Franca M, Del Olmo MF, Cheeran B, Milton R, Alvarez Sauco M, et al. Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J Neurosci. 2006;26(28):7452–7459. doi: 10.1523/JNEUROSCI.1158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MT, Preston JB, Strick PL. Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J Comp Neurol. 1994;341(3):375–392. doi: 10.1002/cne.903410308. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol. 1993;338(1):114–140. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1(1):59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Huang YZ, Rothwell JC. Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J Physiol. 2004;561(Pt 1):331–338. doi: 10.1113/jphysiol.2004.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Buch ER, Olivier E, Rushworth MF. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc Natl Acad Sci U S A. 2010;107(30):13240–13245. doi: 10.1073/pnas.1000674107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea J, Sebastian C, Boorman ED, Johansen-Berg H, Rushworth MF. Functional specificity of human premotor-motor cortical interactions during action selection. Eur J Neurosci. 2007;26(7):2085–2095. doi: 10.1111/j.1460-9568.2007.05795.x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11(3):1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Robertson C, Hazlewood R, Rawson MD. The effects of Parkinson’s disease on the capacity to generate information randomly. Neuropsychologia. 1996;34(11):1069–1078. doi: 10.1016/0028-3932(96)00031-0. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Using transcranial magnetic stimulation methods to probe connectivity between motor areas of the brain. Hum Mov Sci. 2011;30(5):906–915. doi: 10.1016/j.humov.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Stephan KE, Friston K, Frackowiak RS, Passingham RE. The prefrontal cortex shows context-specific changes in effective connectivity to motor or visual cortex during the selection of action or colour. Cereb Cortex. 2005;15(1):85–95. doi: 10.1093/cercor/bhh111. [DOI] [PubMed] [Google Scholar]
- Rusjan PM, Barr MS, Farzan F, Arenovich T, Maller JJ, Fitzgerald PB, et al. Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Hum Brain Mapp. 2010;31(11):1643–1652. doi: 10.1002/hbm.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatt J, Goldenberg G. Components of random generation by normal subjects and patients with dysexecutive syndrome. Brain Cogn. 1993;23(2):231–242. doi: 10.1006/brcg.1993.1057. [DOI] [PubMed] [Google Scholar]
- Strick PL. How do the basal ganglia and cerebellum gain access to the cortical motor areas? Behav Brain Res. 1985;18(2):107–123. doi: 10.1016/0166-4328(85)90067-1. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Coplanar sterotaxic atlas of the human brain. Thieme Medical Publishers; New York: 1998. [Google Scholar]