Abstract
Abnormalities during brain development are thought to cause psychiatric illness and other neurodevelopmental disorders. However, developmental processes such as neurogenesis continue in restricted brain regions of adults, and disruptions of these processes could contribute to the phenotypes of neurodevelopmental disorders. As previously reported, we show that Disc1 knockdown specifically in adult-born dentate gyrus (DG) neurons results in increased mTOR signaling, hyper-excitability and neuronal structure deficits. Disc1 knockdown also resulted in pronounced cognitive and affective deficits, which could be reversed when the affected DG neurons were inactivated. Importantly, reversing increases in mTOR signaling with an FDA approved inhibitor, both prevented and treated these behavioral deficits, even when associated structural deficits were not reversed. Our findings suggest that a component of the affective and cognitive phenotypes in neurodevelopmental disorders may be caused by disruptions in adult-born neurons. Consequently, treatments directed at this cell population may have a significant impact on these phenotypes.
Neurodevelopmental disorders have long been thought to be caused by irreversible changes during development (Ehninger et al., 2008b). However, developmental processes, such as neuronal progenitor cell proliferation, migration and differentiation, continue throughout life in defined brain cell populations, such as adult-born dentate gyrus (DG) neurons. These neurons are functionally integrated into existing circuitry, and importantly, there is evidence suggesting that they are involved in learning & memory and other behaviors (Arruda-Carvalho et al., 2011; Deng et al., 2009; Mao et al., 2009). Thus, besides affecting prenatal and post-natal development, gene mutations (e.g. in Disc1 or Disrupted-in-schizophrenia 1) responsible for neurodevelopmental disorders could also affect adult-born neurons, and therefore disrupt learning & memory and other behaviors.
The Disc1 gene was originally discovered in a large Scottish family with a high incidence of psychiatric illness, including schizophrenia, bipolar disorder and depression (St Clair et al., 1990). Disc1’s expression pattern, its role in proliferation and cell migration, as well as its interacting proteins all demonstrate an important role for this gene in neuronal development (Austin et al., 2004; Camargo et al., 2007; Ishizuka et al., 2011). Several animal models with Disc1 mutations during development reported altered brain morphology, abnormal plasticity, and cognitive and affective deficits (Clapcote et al., 2007; Hikida et al., 2007; Kvajo et al., 2008; Li et al., 2007).
Our studies of DISC1 function in adult-born DG neurons provide evidence that disruption of processes that modulate adult neurogenesis could play an important role in the cognitive and affective deficits associated with neurodevelopmental disorders. Our results also challenge the widely held view that reversing neuronal structural deficits associated with neurodevelopmental disorders is critical for reversing associated behavioral phenotypes.
Results
Disc1 Knockdown in Adult-Born Neurons Results in Cognitive and Affective Phenotypes
To disrupt DISC1 function specifically in DG newborn neurons in the adult mouse brain, we used a validated oncoretrovirus-approach (Duan et al., 2007; Kim et al., 2009). To infect proliferating neural progenitors in vivo, we stereotaxically injected DISC1 (shRNA-DISC1) or control (shRNA-Cont) retrovirus, which only infect proliferating neural progenitors instead of post-mitotic DG cells, into the hilar region of hippocampus. Previous studies using the same shRNA-DISC1 retrovirus showed specific and effective knocked-down of Disc1 levels, and Disc1 knockdown caused aberrant dendritic morphology and mispositioning of adult-born dentate granule cells (Duan et al., 2007), as well as abnormal axonal targeting (Faulkner et al., 2008).
We confirmed that Disc1 knockdown causes abnormal development of adult-born DG neurons, including mispositioning of these neurons, dendritic structural abnormalities with multiple primary dendrites, and severe axon targeting defects of DISC1 down-regulated adult-born neurons (Figure 1A and Figure S1A–D). Since the retroviruses included a GFP marker, we were able to count the number of DG cells infected with these viruses. Three weeks after retrovirus infection, there were approximately 500 GFP+ neurons in the DG of mice infected with either the shRNA-Cont or shRNA-DISC1 viruses (Figure 1B).
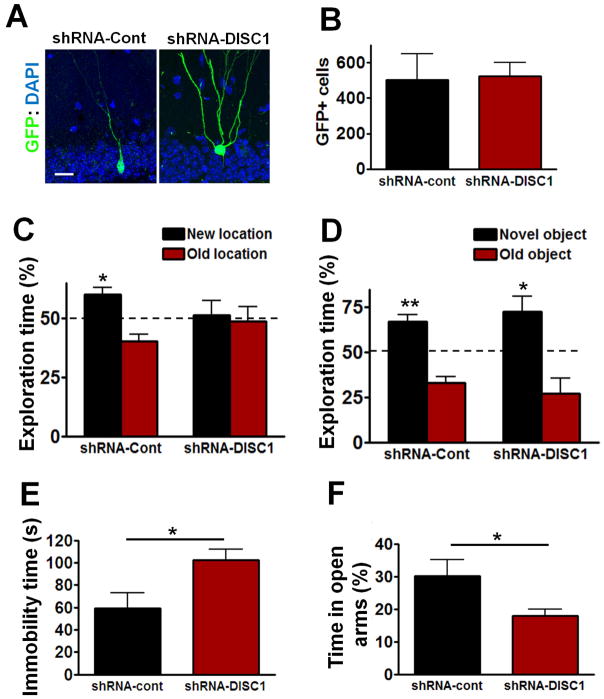
Figure 1. Effects of Disc1 Knockdown on Cognitive and Affective Behaviors.
(A) Representative images of adult-born DG neurons infected with shRNA-Cont or shRNA-DISC1 virus (blue: DAPI; green: GFP, imaged 3 weeks after virus injection). Scale bar: 20 μm. (B) Total number of GFP+ neurons in DG. (C) The percentage of exploration time during the object-place recognition test at 24 h after training (shRNA-Cont n=8, shRNA-DISC1 n=7; *P<0.05, one sample t-test compared to 50%). (D) The percent of exploration time during the novel object recognition test at 24 h after training (shRNA-Cont n=8, shRNA-DISC1 n=7; *P<0.05, **P<0.01, one sample t-test compared to 50%). (E) In the forced swim test, the immobility time of shRNA-DISC1 mice (n=7) was significantly higher than shRNA-Cont mice (n=8; *P<0.05, t-test). (F) In the elevated plus maze test, shRNA-DISC1 mice spent less percentage time in the open arms of the plus maze than shRNA-Cont mice (shRNA-Cont n=7, shRNA-DISC1 n=7; *P<0.05, t-test). All data are shown as means ± s.e.m.
Cognitive deficits are commonly associated with neurodevelopmental disorders (Ehninger et al., 2008b). To test whether Disc1 knockdown in adult-born DG neurons affected cognitive function, mice were tested in the object-place recognition task, an hippocampal dependent test (Oliveira et al., 2010). Disc1 knockdown in adult-born neurons causes profound deficits during the object-place recognition test: while control mice spent significantly more time exploring the object at the new location, the shRNA-DISC1 mice did not (Figure 1C).
Interestingly, the shRNA-DISC1 mice did not show deficits in novel object recognition (NOR), a non-hippocampal-dependent task (Oliveira et al., 2010) that shares many of the same motivational and visual-perceptual demands with the object place task. During the NOR test at 24h after training, both shRNA-Cont mice and shRNA-DISC1 mice spent more time exploring the novel object (Figure 1D). In agreement with the idea that Disc1 knockdown in adult-born neurons causes deficits in hippocampal-dependent learning, shRNA-DISC1 mice showed. deficits in the hidden platform version of the water maze (see Figure S2B).
Changes in adult newborn DG neurons have been associated with affective phenotypes, including anxiety and depression (Santarelli et al., 2003; Snyder et al., 2011). These phenotypes are also common in neurodevelopmental disorders. For example, depression and other affective phenotypes were found to be widespread in a family segregating a chromosomal translocation that disrupted the Disc1 locus (St Clair et al., 1990). The immobility time during the forced-swim test has been used to assess depression-like behaviors in rodents (Mao et al., 2009; Strekalova et al., 2004). During the forced swim test, shRNA-DISC1 mice showed a significant increase in immobility time as compared to shRNA-Cont mice (Figure 1E), suggesting that the disruption of DISC1 function in DG adult-born neurons causes depression-like phenotypes. To determine whether Disc1 knockdown also leads to abnormalities in another affective behavior common in neurodevelopmental disorders (i.e., anxiety) (Rinehart et al., 2002; Tsiouris and Brown, 2004), we tested mice in the elevated plus maze. shRNA-DISC1 mice spent less percent time in the open arms than shRNA-Cont mice (Figure 1F), suggesting that Disc1 knockdown in adult-born DG neurons results in increased anxiety.
Considering the neuroanatomically and developmentally restricted nature of the DISC1 manipulation studied here, it is not surprising that the shRNA-DISC1 mice did not recapitulate all of the phenotypes previously observed in more global Disc1 disruptions in mice (Clapcote et al., 2007; Mao et al., 2009). For example, the shRNA-DISC1 mice did not show deficits in the open field activity test (Figure S4A).
To test if the behavioral deficits of the shRNA-DISC1 mice were caused by the knockdown of Disc1 specifically in the dentate gyrus, control or shRNA-DISC1 virus were injected to CA1, another hippocampal region that is known to play an important role in learning and memory. shRNA-DISC1 virus injection to CA1 did not cause any behavioral changes in all of the tasks tested, including the open field, elevated plus maze, forced swim, and object-place recognition tests (Figure S3A, B, C & D). Additionally, no GFP+ neurons were observed in CA1 at 3 weeks after virus injection (data not shown).
Inactivation of Adult-born Neurons Expressing Disc1 shRNA Reverses Specific Behavioral Phenotypes
To directly test if DISC1 deficient adult-born neurons in DG are the cause of the cognitive and affective phenotypes observed in shRNA-DISC1 mice, we used the allatostatin receptor system (Tan et al., 2006; Zhou et al., 2009) to inactivate these neurons prior to testing. Retroviruses co-expressing the allatostatin receptor as well as control (shRNA-Cont-Alst) or Disc1 shRNA (shRNA-DISC1-Alst) were injected into the dentate gyrus (Figure 2A and B).
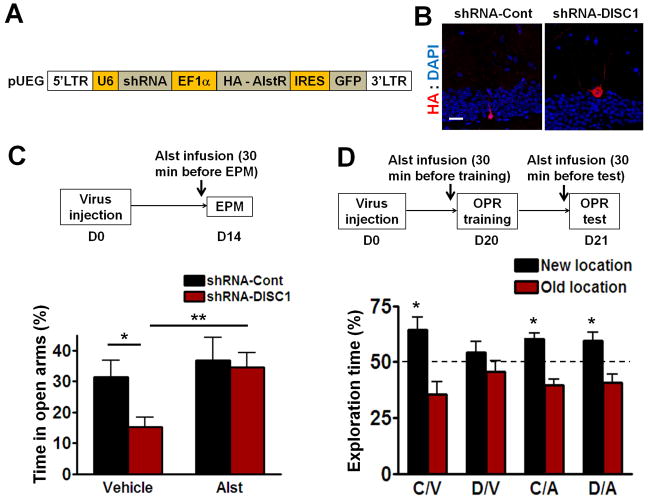
Figure 2. Inactivation of Adult-born Neurons Expressing Disc1 shRNA Reverses Deficits in the Elevated Plus Maze and Object-Place Recognition.
(A) A diagram of the retroviral vector expressing control or Disc1 shRNA and HA tagged allatostatin receptor (AlstR). (B) Representative images of AlstR expression (blue: DAPI; red: HA, imaged 3 weeks after virus injection). Scale bar: 20 μm. (C) In the elevated plus maze test, allatostatin (10 μM) was infused into the dentate gyrus at 30 min before the test. DISC1/veh mice (n=10) spent less percentage time in the open arms of the plus maze than mice in the other three groups (Cont/veh n=11, Cont/Alst n=8, DISC1/Alst n=9; *P<0.05, **P<0.01, planed t-test). (D) In the object-place recognition task, allatostatin was infused into the dentate gyrus at 30 min before both training and testing. With the exception of the DISC1/veh mice (n=10), mice from the other three groups (Cont/veh n=11, Cont/Alst n=6, DISC1/Alst n=9) spent significantly more time exploring the object at the new location (*P<0.05, one sample t-test compared to 50%). All data are shown as means ± s.e.m.
Two weeks after virus injection, mice were tested in the elevated plus-maze test. The DGs of these mice were infused with allatostatin 30 min before testing. Consistent with the results presented above, shRNA-DISC1-Alst mice with vehicle infusion into the DG (DISC1/veh group) spent less time in the open arms as compared to shRNA-Cont-Alst mice treated with vehicle (Cont/veh; Figure 2C, Cont/Veh vs DISC1/Veh, P<0.05). Allatostatin inactivation of adult-born neurons with the shRNA-DISC1-Alst virus significantly increased the time mice spent in the open arms (Figure 2C, DISC1/Alst vs DISC1/Veh, P<0.01).
Approximately three weeks after virus injection, mice were tested in the object-place recognition task. Infected adult-born neurons were inactivated with DG allatostatin infusions 30 min before both training and testing. The shRNA-Cont-Alst mice infused with either vehicle (cont/Veh) or allatostatin (cont/Alst) performed normally: they spent more time exploring the object at the new location (Figure 2D). shRNA-DISC1-Alst mice infused with vehicle (DISC1/Veh) spent similar percentage of time exploring the object at the new location compared to the old location (i.e. showed learning and memory deficits). In contrast, shRNA-DISC1-Alst mice treated with allatostatin (DISC1/Alst) spent significantly more time exploring the object at the new location (Figure 2D). Taken together, these results demonstrate that the adult-born neurons with DISC1 knockdown are responsible for the affective (elevated plus maze) and cognitive (object-place recognition) deficits described here.
Rapamycin Treatment Prevents Morphogenesis, Excitability, and Specific Behavioral Deficits Resulting from Disc1 Knockdown
Rapamycin, an FDA approved compound that inhibits mTOR, has been shown to prevent the morphogenesis deficits caused by Disc1 knockdown in adult-born DG neurons when neuronal morphology was examined 1 day after the last rapamycin treatment (Kim et al., 2009). Our analyses show that rapamycin treatment prevented the increase in the number of primary dendrites of adult-born neurons infected with shRNA-DISC1 [Figure 3B; Two-way ANOVA, (shRNA virus x treatment) interaction: F(1,10)=34.41; Post hoc linear contrast: DISC1/veh vs DISC1/rap, t(10)=8.23, P<0.001] and ameliorated the mispositioning of adult-born DG neurons (Figure 3C). In our study mice were treated with rapamycin (20 mg/kg, i.p.) at 5, 7, 9, 11, and 13 days after retroviral injection, and neuronal morphology was examined 4 weeks after the last rapamycin treatment (Figure 3A). Thus, our results demonstrated that the effects of the rapamycin treatment last at least 4 weeks.
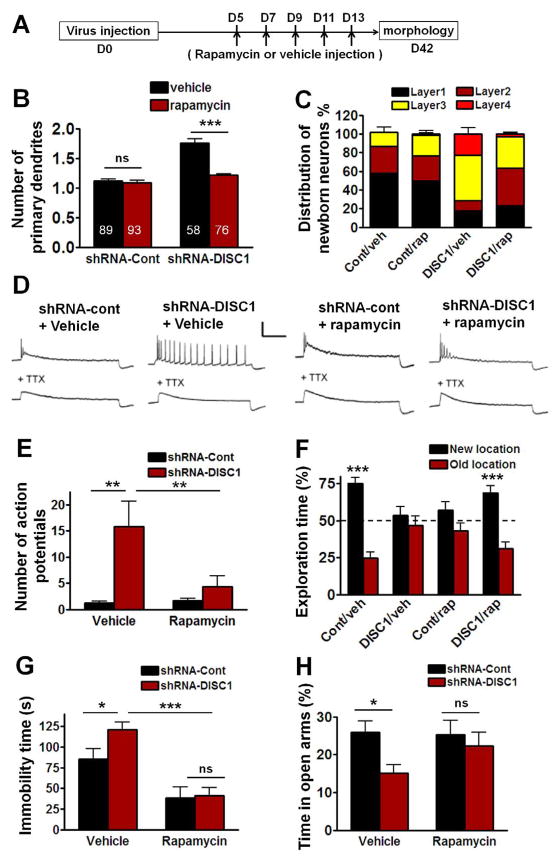
Figure 3. Effects of Rapamycin Treatment on the Morphogenesis and Excitability of adult-born neurons and on Behavioral Deficits Caused by Disc1 Knockdown.
(A) Diagram of rapamycin treatment for morphology examination. (B) Compared to control neurons, the adult-born neurons with Disc1 knockdown have increased number of primary dendrites which is prevented by rapamycin treatment (***P<0.001, Two-way ANOVA). Numbers associated with bar graph indicate the number of neurons examined from at least 3 mice from each group. (C) Positioning of adult-born neurons in DG. Layer 1, layer 2 and layer 3 refer to the inner, the middle, and the outer layers of granule cells in DG, respectively; layer 4 refers to the molecular layer. (D) Sample traces recorded from GFP+ neurons in response to 800 ms 100 pA current injection before and after the addition of TTX (1 μM) at 14 days after virus injection. Scale bar: 50 mV and 200 ms. (E) Quantification of the total number of action potentials with 800ms stimulation (Con/veh, Cont/rap and DISC1/veh: n=5, DISC1/rap n=8; **P<0.01, Two-way ANOVA). (F) The percentage of exploration time during the object-place recognition test at 24h after training. Cont/veh and DISC1/rap mice spent significantly more time exploring the object at the new location (Con/veh n=14, Cont/rap n=12, DISC1/veh n=14, DISC1/rap n=15; ***P<0.001, one sample t-test compared to 50%). (G) In the forced swim test, the immobile time of DISC1/veh mice (n=11) was significantly higher than Cont/veh mice (n=12), and this increased immobility was prevented by rapamycin treatment (DISC1/rap n=9). Two-way ANOVA, *P<0.05, ***P<0.001. (H) DISC1/veh mice spent less percentage time in the open arms of the plus maze than Cont/veh mice (Cont/veh n=16, DISC1/veh n=17; *P<0.05). There was no significant difference between DISC1/rap and Cont/rap (DISC1/rap n=15, Cont/rap n=13, P>0.05, Two-way ANOVA). All data are shown as means ± s.e.m.
To test the effect of rapamycin on the increased excitability reported for adult-born DG neurons with Disc1 knockdown (Duan et al., 2007), mice were treated with rapamycin at 5, 7, 9, 11, and 13 days, and the excitability of GFP+ neurons was recorded at 14 days after retroviral injection (Figure 3D). As before, quantification of the number of action potentials shows that Disc1 knockdown resulted in enhanced excitability. Additionally, our findings showed that rapamycin treatment prevented this enhanced excitability [Figure 3E; Two-way ANOVA, (shRNA virus x treatment) interaction: F(1,19)=5.23; Post hoc linear contrast: Cont/veh vs DISC1/veh, t(19)=3.80, P<0.01; DISC1/veh vs DISC1/rap, t(19)=3.30, P<0.01].
Importantly, the same rapamycin treatment could also prevent the memory deficits in the object-place recognition test. Although shRNA-DISC1 mice treated with vehicle (DISC1/veh group) spent the same percentage of time exploring the object at the new location compared to the old location, the DISC1/rap group and Cont/veh group spent significantly more time exploring the object at the new location (Figure 3F). During training, mice from all groups spent a similar time exploring the two objects (Figure S4B), indicating that the deficits are not caused by changes in exploratory behavior. Remarkably, the Cont/rap group spent a similar percentage of time exploring the object at the new location compared to the old location, demonstrating that the rapamycin treatment that prevented the learning deficits in the shRNA-DISC1 mice caused learning deficits in the control mice.
Rapamycin improved the performance of the shRNA-DISC1 mice in the forced swim test: although the DISC1/veh group showed a significant increase in immobility as compared to Cont/veh group, the immobility time of DISC1/rap group was decreased when compared to that of DISC1/veh group [Figure 3G; Two-way ANOVA, (Treatment x shRNA virus) interaction: F(1,37)=2.134; Post hoc linear contrast: Cont/veh vs DISC1/veh, t(37)=2.37, P<0.05; DISC1/rap vs DISC1/veh, t(37)=4.90, P<0.001]. Interestingly, the Cont/rap group (n=9) also showed decreased immobility compared to the Cont/veh group [Figure 3G; Post hoc linear contrast: Cont/veh vs Cont/rap, t(37)=2.91, P<0.01], a result consistent with previous reports that rapamycin has anti-depressant effects (Cleary et al., 2008).
Rapamycin also improved the performance of the shRNA-DISC1 mice in the elevated plus maze. Although the DISC1/veh group spent significantly less time in the open arms when compared with Cont/veh group [Figure 3H; Two-way ANOVA, overall (Treatment x shRNA virus) interaction: F(1,57)=1.46; Main effect of shRNA virus: F(1,57)=4.68, Post-hoc linear contrast: Cont/veh vs DISC1/veh, t(57)=2.49, P<0.05], the time spent in the open arms by the DISC1/rap, Cont/rap and Cont/veh groups were indistinguishable. In contrast to the object-place recognition test, rapamycin did not seem to prevent the spatial learning deficits in the water maze (Figure S2D).
Late Rapamycin Treatment Rescues Abnormal Signaling and Specific Behavioral Deficits without Reversing Structural Abnormalities
Two-weeks old adult-born DG neurons with Disc1 knockdown show aberrant dendritic morphology, mispositioning, and hyperactivation of mTOR signaling pathway (Kim et al., 2009). We next tested whether treatment of mTOR signaling deficits, specifically started at two weeks post-injection when structural deficits are already present, ameliorate the behavioral phenotypes.
We confirmed that two weeks after virus injection, Disc1 knockdown causes aberrant dendritic morphology and mispositioning of adult-born dentate granule cells (Figure S4E&F). We next determined whether rapamycin treatment (i.p. injection, 20 mg/kg) initiated at 14 days post injection (d.p.i.), and given every other day from day 14 to day 26 (henceforth referred to as Late (L) rapamycin treatment), reversed the dendritic morphogenesis and mispositioning examined on day 28 (Figure 4A). Compared to adult-born neurons in the shRNA-Cont (Cont/veh-L or Cont/rap-L) groups, those in the shRNA-DISC1 (DISC1/veh-L or DISC1/rap-L) groups still showed increased number of primary dendrites [Figure 4B; Two-way ANOVA, (shRNA virus x Treatment) interaction: F(1,15)=1.622; main effect of shRNA virus: F(1,15)=183.2, P<0.001 between shRNA-cont and shRNA-DISC1]. There was no difference between DISC1/veh and DISC1/rap groups in mispositioning scores (Figure 4C), indicating that the late rapamycin treatment did not rescue the morphological abnormalities caused by Disc1 knockdown.
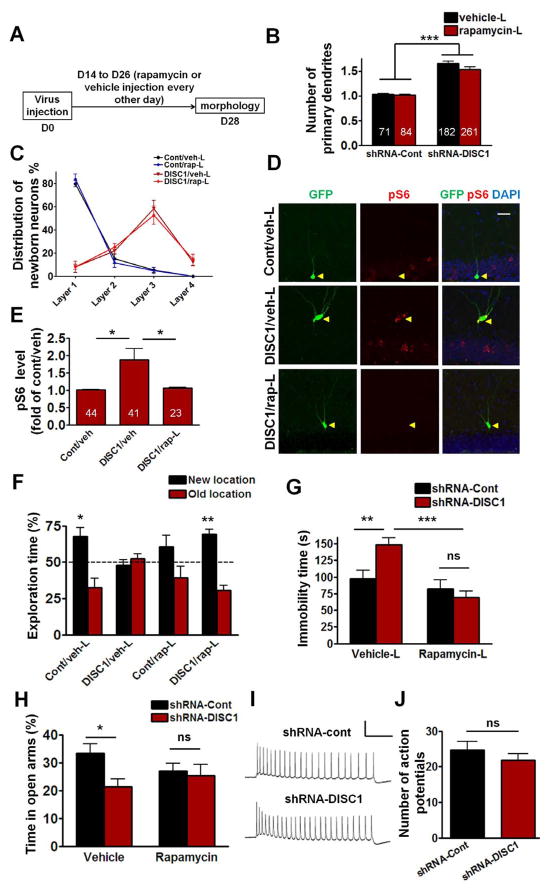
Figure 4. Effects of Late Rapamycin Treatment on the Morphogenesis, Phosphorylated S6 Levels, and Behavioral Deficits Caused by Disc1 Knockdown.
(A) Diagram of the late rapamycin treatment. (B) Compared to control adult-born neurons, the neurons with Disc1 knockdown had significantly higher number of primary dendrites (Two-way ANOVA, ***P<0.001 between shRNA-cont and shRNA-DISC1. Numbers in the bar graph indicate the number of neurons examined in each group from at least four mice). (C) Positioning of adult-born neurons in DG. Layers were defined in Figure 3C. (D) Sample confocal images of immunostainining for GFP, phosphorylated S6 (pS6), and DAPI. Scale bar: 20 μm. (E) Quantifications of pS6 levels in the cytosol of GFP+ neurons. The pS6 fluorescence intensity of individual GFP+ neuron was first normalized to that of the neighboring granule cells in the same image. Numbers in the bar graph indicate the number of neurons examined in each group from at least three mice (*p < 0.05, One-way ANOVA). (F) During the object-place recognition test, Cont/veh mice (n=11; *P<0.05, one sample t-test compared to 50%) and DISC1/rap mice (n=10; ***P<0.001, one sample t-test compared to 50%) significantly spent more time exploring the object at the new location, while the DISC1/veh mice (n=10) and Cont/rap mice (n=10) did not. (G) Effect of late rapamycin treatment on forced swim. The immobility time of DISC1/veh mice (n=13) was significantly higher than Cont/veh mice (n=15; **P<0.01), and this increased immobility was reversed by rapamycin treatment (DISC1/rap n=13; ***P<0.001, Two-way ANOVA). (H) In the elevated plus maze test, compared to Cont/veh mice, DISC1/veh mice spent less percentage time in the open arms of the plus maze (Cont/veh n=14; DISC1/veh n=12; *P<0.05, Two-way ANOVA). There was no significant difference between DISC1/rap and Cont/rap (DISC1/rap n=13, Cont/rap n=12; P>0.05). (I) Sample traces recorded from GFP+ neurons in response to 800 ms 100 pA current injection at 26 days after virus injection. Scale bar: 50 mV and 200 ms. (J) Quantification of the total number of action potentials with 800ms stimulation (shRNA-con n=8, shRNA-DISC1 n=10). All data are shown as means ± s.e.m.
To determine whether the late rapamycin treatment had an impact on mTOR signaling in these neurons, we tested the phosphorylation (i.e., activation) of an mTOR substrate (S6). Importantly, the increase we found in S6 phosphorylation was completely reversed by late rapamycin treatment (Figure 4D&E). This result shows that although late rapamycin treatment failed to rescue the morphological deficits, it rescued the abnormal mTOR signaling of adult-born neurons with a Disc1 knockdown.
We next tested whether the rescue of mTOR signaling with late rapamycin treatment could reverse learning & memory deficits in the object-place test. For this experiment, rapamycin was injected every other day from day 14 to day 22 and also 3h before training in object-place recognition (at 26 d.p.i.). One day after training, the Cont/veh-L and DISC1/rap-L groups, but not DISC1/veh-L or Cont/rap-L groups, spent significantly more time exploring the object at the new location (Figure 4F), demonstrating that rapamycin treatment that causes deficits in controls rescues the learning deficits of shRNA-DISC1 mice. During training, mice from all groups spent similar time exploring the two objects (Figure S4C).
Late rapamycin treatment also has an impact on the affective phenotype of shRNA-DISC1 mice. Mice were injected with rapamycin every other day from day 14 to day 20 after virus injection, as well as 3h before the forced swim test on day 22. The DISC1/veh-L group showed longer immobility time than Cont/veh-L group. In contrast, the performance of the DISC1/rap-L group was indistinguishable from that of the Cont/rap-L or Cont/veh-L groups [Figure 4G; Two-way ANOVA, (Treatment x shRNA virus) interaction: F(1,50)=7.364, P<0.01; Post hoc linear contrast: Cont/veh-L vs DISC1/veh-L, t(50)=3.12, P<0.01; DISC1/rap-L vs DISC1/veh-L, t(50)=4.68, P<0.001], indicating that the depression-like behavior can also be rescued with late rapamycin treatment.
We also tested whether an acute injection of rapamycin 3h before testing (14 days after virus injection) in the elevated plus maze could rescue the anxiety phenotype of shRNA-DISC1 mice. As before, DISC1/veh group spent less percent time in the open arms when compared to the Cont/veh group. However, the performance of DISC1/rap and Cont/rap groups was indistinguishable [Figure 4H; Two-way ANOVA, overall (Treatment x shRNA virus) interaction: F(1,47)=2.46; Main effect of shRNA virus: F(1,47)=4.26, P<0.05, Post hoc linear contrast: Cont/veh vs DISC1/veh, t(47)=2.59, P<0.05]. Additionally, there were no statistically significant differences between the performances of Cont/veh, DISC1/rap and Cont/rap groups (Figure 4H).
We showed that knockdown of Disc1 in adult-born DG neurons results in enhanced excitability measured 14 days after retroviral injection. However, when the excitability of GFP+ neurons was measured at 26 days after retroviral injection (Figure 4I), there was no significant difference between these two groups (Figure 4J, P>0.05, student’s t-test), indicating that by day 26 the shRNA-Cont neurons had similar excitability as shRNA-DISC1 neurons. This result also demonstrates that increased excitability could not be the cause of the behavioral deficits of shRNA-DISC1 mice measured 26 days after retroviral injection.
Discussion
In this study, we found that disruption of DISC1 function only in ≈500 adult-born DG neurons is sufficient to cause several profound behavioral phenotypes, including pronounced learning and memory deficits, as well as clear anxiety and depression-like phenotypes. These results suggest that an important component of the behavioral phenotypes common in neurodevelopmental disorders, such as those caused by Disc1 mutations, could be due to disruptions of developmental-like processes associated with adult-born DG neurons. Our studies demonstrated that the knockdown of Disc1 leads to increases in mTOR signaling in the targeted newborn DG neurons, and indicated that this signaling abnormality is responsible for the cognitive and affective deficits. Indeed, an FDA approved drug that decreases mTOR signaling reversed these behavioral deficits even when associated neuroanatomical abnormalities persisted. These results offer new hope to the many individuals affected by neurodevelopmental disorders, since it demonstrates the feasibility of adult rescue of profound behavioral phenotypes associated with this class of disorders.
Previous results that we confirmed here indicated that knocking down Disc1 in adult newborn DG neurons results in increases in mTOR signaling, enhanced excitability, aberrant dendritic structure, abnormal axonal targeting, and mispositioning of adult-born dentate granule cells. Studies that specifically inactivated the Disc1 knockdown neurons with the allatostatin receptor system allowed us to demonstrate that the behavioral phenotypes described are caused by these neurons, since their inactivation reversed key cognitive and affective phenotypes.
A knockdown of the Disc1 gene specifically and exclusively in adult-born DG neurons resulted in pronounced deficits in two hippocampal-dependent tasks, the object-place recognition task and the spatial version of the Morris water maze. Interestingly, performance in the novel object recognition task, which can be solved with non-hippocampal strategies, appeared to be unaffected by the Disc1 knockdown. This result attests to the hippocampal specificity of the learning & memory deficits. As with the cognitive phenotypes of the shRNA-DISC1 mice, their affective phenotypes were also specific. For example, a dentate gyrus Disc1 knockdown that was not specific to newborn neurons resulted in open field hyperactivity (Mao et al., 2009). In contrast, our newborn neuron specific knockdown of Disc1 in DG did not affect performance in the open field task. The difference between the results of these two studies is likely due to the difference in the neuronal specificities of the tools used.
Here, we show that rapamycin treatment reverses key behavioral deficits of shRNA-DISC1 mice. However, the exact mechanism of rapamycin rescue is still unclear. Remarkably, the rapamycin treatment, that rescued memory deficits in the object-place recognition test in the shRNA-DISC1 mice, caused memory deficits in control mice. mTOR signaling has been shown to play an important role in synaptic protein translation and long-term plasticity (Tang et al., 2002), as well as in long-term memory consolidation and reconsolidation (Stoica et al., 2011). Therefore, it is not surprising that the multi-day rapamycin treatment impairs memory in control mice. Additionally, increased mTOR signaling has been associated with pronounced cognitive and affective deficits in mouse models of neurodevelopmental disorders (Ehninger et al., 2008a). Altogether, our results demonstrate that the increase in mTOR signaling observed in newborn DG neurons with Disc1 knockdown is a key contributor to the behavioral deficits of shRNA-DISC1 mice.
Recently, animal model studies suggested that treating the molecular deficits underlying neurodevelopmental disorders could result in significant amelioration of associated behavioral phenotypes, even when treatments were initiated in adults (Ehninger et al., 2008b). This suggests that the complex behavioral phenotypes associated with neurodevelopmental disorders have developmental (e.g., abnormal circuitry) and adult components (e.g. abnormal signaling and plasticity), and that treatment of one of these two components may be sufficient to result in significant improvements in overall function. Remarkably, our late rapamycin treatment studies show that significant improvement in behavioral deficits can be obtained even when structural and morphological deficits are not reversed.
Our results also demonstrate the importance and specificity of the involvement of adult-born DG neurons in both cognitive and affective behaviors. It is important to note that our shRNA-DISC1 manipulation did not block adult neurogenesis. Instead, it dramatically altered the neuroanatomical and neurophysiological properties of a subset of adult-born DG neurons. In contrast to previous studies that used approaches to block neurogenesis, we show for the first time that altering the signaling, morphology and physiology of even a very small subset of these neurons can have profound effects on multiple cognitive and affective behaviors.
In summary, the studies reported here provide evidence for the novel hypothesis that a key component of the behavioral deficits associated with neurodevelopmental disorders, such as those associated with Disc1 mutations, could be caused by disruption of development-like processes involved in the generation and maturation of newborn DG neurons in adults. Furthermore, we show that a key component of these behavioral deficits can be reversed by adult treatments that target signaling deficits, even when associated structural and morphological deficits are not reversed. Understanding adult mechanisms contributing to neurodevelopmental disorders may lead to the development of treatments for millions of adults affected with this class of disorders.
Experimental Procedures
Mouse Behavior
In the elevated plus maze test, mice were allowed to explore an elevated plus maze for 5 minutes. The elevated plus maze has two open arms and two close arms (with walls of 16.5 cm height), and each arm is 29 cm long and 8 cm wide. The percentage of time mice spent in the open arms was analyzed. During the forced swim test, mice were placed in an 18 cm diameter cylindrical container filled with water (20 cm height) at 25 °C. The behavior of the mice was recorded for 5 min and the total immobility time of each mouse was measured. In the object-place recognition and novel object recognition tasks, mice were trained in the experimental box for 10 min and mice were put back into the same box for the recognition test at 24h after training. For complete details of the recognition test, please see the supplemental experimental procedures.
Immunostaining
Mice were transcardially perfused with 4% PFA (4% paraformaldehyte in 0.1 M phosphate buffer) and after perfusion, brains were sliced coronally (50 μm thick) with vibratome and processed for immunostaining. Primary antibodies, including rabbit anti-GFP (Abcam, 1:500), rabbit anti-pS6 (Cell Signaling, 1:200) and mouse anti-BrdU (Invitrogen, 1:500) were used for immunostaining. Brain slices were incubated with 4′,6-diaminodino-2-phenylindole (DAPI, Invitrogen, 1:2000) for 15 min before mounting onto slides. Immunostaining images were acquired with an Olympus FluoView 1000 Laser Scanning Confocal Microscope (LSCM), and 700 nm femtosecond pulses from Ti:Saphire (Mai Tai, Spectra Physics) were used for two-photon excitation of DAPI.
Electrophysiology
The whole-cell patch-clamp configuration was employed in the current-clamp mode with current pulses (100pA, 800ms) injected. Microelectrodes (4–6 MΩ) were filled with the internal solution containing (in mM): 120 potassium gluconate, 15 KCl, 4 MgCl2, 0.1 EGTA, 10.0 HEPES, 4 ATP (magnesium salt), 0.3 GTP (sodium salt), 7 phosphocreatine (pH 7.4, 300 mOsm). Series and input resistances were monitored, and only those with changes less than 20% during experiments were analyzed. For complete details please see the supplemental experimental procedures.
Statistical Analysis
Results are expressed as mean ± s.e.m. ANOVA analyses or Student’s t-tests were used for statistical comparisons between groups as described in the results or figure legends. P < 0.05 indicates significant difference between groups.
Supplementary Material
Acknowledgments
We thank Denise Cai for help with statistical analysis, Yong-Seok Lee and Justin Shobe for helpful advice, Tawnie Silva, Aida Amin and Katie Cai for technical support. This work was supported by grants from the National Institutes of Mental Health (P50-MH0779720) and the Staglin IMHRO Center for Cognitive Neuroscience at UCLA to A.J.S., National Institute of Health (NS048271, HD069184) and NARSAD Investigator Award to G.L.M., National Institute of Health (NS047344) to H.J.S., China 973 Program (2010CB529604), National Scientific Foundation of China (81271511, 30900432), National Science & Technology Pillar Program (2012BAI01B08), Shanghai Pujiang Program (11PJ1405100), “Eastern Scholar”, “Shu Guang” (10SG14), NARSAD Young Investigator Award to WL, and NARSAD Young Investigator Awards to MZ.
Footnotes
Author contributions
M.Z., W.L. and A.J.S. designed the experiments. M.Z. and S.H. performed the behavioral experiments. M.Z. did immunostaining and data analysis. J.S. did eletrophysiological recording. E.K. carried out the axonal targeting experiment. J.Y.K. provided the virus. H.S. and G.M. helped with paper writing. M.Z. and A.J.S. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Arruda-Carvalho M, Sakaguchi M, Akers KG, Josselyn SA, Frankland PW. Posttraining ablation of adult-generated neurons degrades previously acquired memories. J Neurosci. 2011;31:15113–15127. doi: 10.1523/JNEUROSCI.3432-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin CP, Ky B, Ma L, Morris JA, Shughrue PJ. Expression of Disrupted-In-Schizophrenia-1, a schizophrenia-associated gene, is prominent in the mouse hippocampus throughout brain development. Neuroscience. 2004;124:3–10. doi: 10.1016/j.neuroscience.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Camargo LM, Collura V, Rain JC, Mizuguchi K, Hermjakob H, Kerrien S, Bonnert TP, Whiting PJ, Brandon NJ. Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, Lerch JP, Trimble K, Uchiyama M, Sakuraba Y, Kaneda H, Shiroishi T, Houslay MD, Henkelman RM, Sled JG, Gondo Y, Porteous DJ, Roder JC. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Cleary C, Linde JA, Hiscock KM, Hadas I, Belmaker RH, Agam G, Flaisher-Grinberg S, Einat H. Antidepressive-like effects of rapamycin in animal models: Implications for mTOR inhibition as a new target for treatment of affective disorders. Brain Res Bull. 2008;76:469–473. doi: 10.1016/j.brainresbull.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008a;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Li W, Fox K, Stryker MP, Silva AJ. Reversing neurodevelopmental disorders in adults. Neuron. 2008b;60:950–960. doi: 10.1016/j.neuron.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner RL, Jang MH, Liu XB, Duan X, Sailor KA, Kim JY, Ge S, Jones EG, Ming GL, Song H, Cheng HJ. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc Natl Acad Sci U S A. 2008;105:14157–14162. doi: 10.1073/pnas.0806658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, Wu D, Xue R, Andrade M, Tankou S, Mori S, Gallagher M, Ishizuka K, Pletnikov M, Kida S, Sawa A. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka K, Kamiya A, Oh EC, Kanki H, Seshadri S, Robinson JF, Murdoch H, Dunlop AJ, Kubo KI, Furukori K, Huang B, Zeledon M, Hayashi-Takagi A, Okano H, Nakajima K, Houslay MD, Katsanis N, Sawa A. DISC1-dependent switch from progenitor proliferation to migration in the developing cortex. Nature. 2011 doi: 10.1038/nature09859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Pow-anpongkul N, Kang E, Song H, Ming GL. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron. 2009;63:761–773. doi: 10.1016/j.neuron.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvajo M, McKellar H, Arguello PA, Drew LJ, Moore H, MacDermott AB, Karayiorgou M, Gogos JA. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci U S A. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhou Y, Jentsch JD, Brown RA, Tian X, Ehninger D, Hennah W, Peltonen L, Lonnqvist J, Huttunen MO, Kaprio J, Trachtenberg JT, Silva AJ, Cannon TD. Specific developmental disruption of disrupted-in-schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proc Natl Acad Sci U S A. 2007;104:18280–18285. doi: 10.1073/pnas.0706900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, Biechele T, Petryshen TL, Moon RT, Haggarty SJ, Tsai LH. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AM, Hawk JD, Abel T, Havekes R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem. 2010;17:155–160. doi: 10.1101/lm.1625310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart NJ, Bradshaw JL, Brereton AV, Tonge BJ. A clinical and neurobehavioural review of high-functioning autism and Asperger’s disorder. Aust N Z J Psychiatry. 2002;36:762–770. doi: 10.1046/j.1440-1614.2002.01097.x. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair D, Blackwood D, Muir W, Carothers A, Walker M, Spowart G, Gosden C, Evans HJ. Association within a family of a balanced autosomal translocation with major mental illness. Lancet. 1990;336:13–16. doi: 10.1016/0140-6736(90)91520-k. [DOI] [PubMed] [Google Scholar]
- Stoica L, Zhu PJ, Huang W, Zhou H, Kozma SC, Costa-Mattioli M. Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc Natl Acad Sci U S A. 2011;108:3791–3796. doi: 10.1073/pnas.1014715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- Tan EM, Yamaguchi Y, Horwitz GD, Gosgnach S, Lein ES, Goulding M, Albright TD, Callaway EM. Selective and quickly reversible inactivation of mammalian neurons in vivo using the Drosophila allatostatin receptor. Neuron. 2006;51:157–170. doi: 10.1016/j.neuron.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiouris JA, Brown WT. Neuropsychiatric symptoms of fragile X syndrome: pathophysiology and pharmacotherapy. CNS Drugs. 2004;18:687–703. doi: 10.2165/00023210-200418110-00001. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci. 2009;12:1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.