Abstract
Purpose
Although many studies have linked obesity with increased risk of thyroid cancer, few have investigated the role of obesity-related lifestyle characteristics and medical conditions in the etiology of this disease. We examined the associations of self-reported physical activity and diabetes history with thyroid cancer risk in a large pooled analysis of prospective cohort studies.
Methods
Data from five prospective studies in the U.S. (n=362,342 men, 312,149 women) were coded using standardized exposure, covariate, and outcome definitions. Hazard ratios (HR) and 95% confidence intervals (CI) for thyroid cancer were estimated using age as the time metric and adjusting for sex, education, race, marital status, cigarette smoking, body mass index, alcohol intake, and cohort. Effect modification by other risk factors (e.g. age, sex, body mass index) and differences by cancer subtype (e.g. papillary, follicular) were also examined.
Results
Over follow-up (median=10.5 years), 308 men and 510 women were diagnosed with a first primary thyroid cancer. Overall, subjects reporting the greatest amount of physical activity had an increased risk of the disease (HR=1.18, 95% CI:1.00-1.39); however, this association was restricted to participants who were overweight/obese (≥25 kg/m2; HR=1.34, 95% CI:1.09-1.64) as opposed to normal-weight (<25 kg/m2; HR=0.92, 95% CI:0.69-1.22; P-interaction=0.03). We found no overall association between self-reported history of diabetes and thyroid cancer risk (HR=1.08, 95% CI:0.83-1.40).
Conclusion
Neither physical inactivity nor diabetes history was associated with increased risk of thyroid cancer. While it may have been a chance finding, the possible increased risk associated with greater physical activity warrants further investigation.
Keywords: physical activity, energy expenditure, type 2 diabetes, insulin resistance, thyroid neoplasms, prospective study
Thyroid cancer incidence has been increasing in the U.S. since the early 1980s, most dramatically in the past decade [1]. These trends may be partially attributable to medical surveillance and more widespread use of sensitive diagnostic tools as well as changes in exposure to certain environmental factors. Obesity, which has become increasingly prevalent in the U.S. during the same time period [2], could be one of few modifiable risk factors for thyroid cancer considering that case-control and prospective studies have generally found positive associations between body mass index (BMI) and risk of this malignancy [3-11], albeit with some inconsistencies (i.e. null results in men and/or women) [9-14]. However, few studies have investigated the associations of obesity-related behaviors and medical conditions with thyroid cancer risk.
In the present study, we combined data from five prospective U.S. studies to examine the associations for self-reported physical activity level and history of diabetes with thyroid cancer risk in men and women. We previously reported a positive association between BMI and thyroid cancer risk among both men and women in this pooled study [5].
METHODS
Study population
Study participants were enrolled in one of five U.S.-based prospective cohorts from the National Cancer Institute: NIH-AARP Diet and Health Study (NIH-AARP), Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO), Breast Cancer Detection and Demonstration Project (BCDDP), Agricultural Health Study (AHS), and U.S. Radiologic Technologists Study (USRT). The institutional review boards from the National Cancer Institute and all participating institutions approved the use of these data. Details of this pooled study, including methods for data standardization, outcome ascertainment, and statistical analysis were published previously [5].
For this analysis, we redefined the USRT and BCDDP study baselines as the dates responding to the second questionnaire, when participants were first asked to provide information on medical history and physical activity level. We also restricted the PLCO cohort to the intervention arm of the trial, as information on physical activity was not collected from control arm participants. In total, there were 362,342 men and 312,149 women who responded to a baseline questionnaire, accrued follow-up time, did not have a history of cancer other than non-melanoma skin cancer, were not missing information on the date of diagnosis of incident cancer, physical activity level, or history of diabetes, and did not have missing or extreme (<15 or >50 kg/m2) data on BMI. Details of the study population are shown in Table 1.
Table 1.
General characteristics of the cohorts
| Cohort | No. Participants | No. Cases | Study period | Age at baseline (mean [range]) | Male (%) | BMI ≥30 (%) | History of diabetes (%) |
|---|---|---|---|---|---|---|---|
| NIH-AARP Diet and Health Study (NIH-AARP) | 493,628 | 576 | 1995-2006 | 62.0 (50-72) | 61 | 22 | 9 |
| US Radiologic Technologists Study (USRT) | 58,325 | 121 | 1994-2006 | 47.5 (31-94) | 22 | 16 | 3 |
| Prostate, Lung, Colorectal, and Ovarian Screening Study (PLCO) | 48,497 | 51 | 1993-2009 | 62.5 (52-75) | 52 | 22 | 7 |
| Agricultural Health Study (AHS) | 44,629 | 48 | 1993-2005 | 47.3 (14-92) | 51 | 21 | 3 |
| Breast Cancer Detection Demonstration Project (BCDDP) | 29,412 | 22 | 1987-1997 | 61.0 (40-90) | 0 | 13 | 5 |
| Total | 674,491 | 818 | 1987-2009 | 59.8 (14-94) | 54 | 21 | 8 |
Exposure assessment and data standardization
Each study utilized self-administered questionnaires, which elicited information on general demographics, certain health behaviors and lifestyle characteristics, including physical activity level, and personal medical history, including medical history of diabetes. The level of detail elicited for most of these exposures was similar for each cohort. Only the AHS, BCDDP, and USRT cohorts had information on age or date of diagnosis of diabetes. Height and weight were self-reported at each cohort baseline. The level of detail on light, moderate, or vigorous activities, including whether the questionnaire specified or differentiated between leisure-time or occupational activity, and whether the directions inquired about current physical activity level or the average level over the previous 12 months, differed by cohort (Table 2). Where possible, weighted summary variables for total physical activity were calculated in cohorts that assessed various recreational, household, or occupational activities separately (USRT and BCDDP). In three of the cohorts (NIH-AARP, PLCO, and AHS), physical activity was defined as the average time spent engaging in vigorous or strenuous leisure-time or occupational activity; these cohorts did not inquire about low-intensity or moderate activity. We assigned study subjects to one of three categories of physical activity, either “low,” “medium,” or “high,” based on cohort-specific tertiles.
Table 2.
Cohort-specific definitions for physical activity and derived tertiles of physical activity level
| Cohort | Cohort-specific definition | Cohort-specific tertiles |
|---|---|---|
|
| ||
| NIH-AARP | Over the previous 12 months, the number of periods of at least 20 minutes participating in vigorous activities at home or at work that cause sweating or an increase in breathing or heart rate | Low: <1 time per week |
| Medium: 1-2 times per week | ||
| High: ≥3 times per week | ||
|
| ||
| USRT | Metabolic equivalent task (MET) units of 3, 4, and 7 were multiplied by the number of hours spent each week walking at home or at work, walking or hiking for exercise, and exercising strenuously (eg, aerobics, jogging, swimming), respectively, over the previous 12 months. | Low: <18 MET-hours/week (men), <20 MET-hours/week (women) |
| Medium: 18-49 MET-hours/week (men), 20-55 MET-hours/week (women) | ||
| High: ≥50 MET-hours/week (men), ≥56 MET-hours/week (women) | ||
|
| ||
| PLCO | The number of hours spent doing vigorous activities (eg, swimming, brisk walking) | Low: <3 hours per week |
| Medium: 3-4 hours per week | ||
| High: ≥5 hours per week | ||
|
| ||
| AHS | The number of hours per week spent doing strenuous exercise that causes rapid heart rate during leisure time, averaged over summer and winter | Low: ≤0.5 hours per week |
| Medium: 0.51-2.75 hours per week | ||
| High: >2.75 hours per week | ||
|
| ||
| BCDDP | MET units of 1, 2, 4, and 6 were multiplied by the number of hours of sleep and light, moderate, and vigorous activity, respectively, based on a comprehensive list of various household, occupational, and recreational activities during the week and weekends over the previous 12 months. A weighted daily average MET value was created by weighting self-reported weekday and weekend activities. | Low: <52.3 MET-hours/day |
| Medium: 52.3-60.86 MET-hours/day | ||
| High: >60.86 MET-hours/day | ||
Outcome assessment
Participants were followed from the date of questionnaire completion to the date of any cancer diagnosis other than non-melanoma skin cancer, death, or last date of follow-up, whichever came first. Thyroid cancer cases were defined as participants who were diagnosed with a malignant first primary thyroid neoplasm during follow-up. Cancer information was obtained through different sources: self-report (USRT, PLCO, BCDDP), cancer registry linkage (NIH-AARP, USRT, AHS, BCDDP), death certificates (USRT, PLCO, BCDDP), and/or the National Death Index (NIH-AARP, USRT, BCDDP). Any self-reported thyroid cancers that were de-confirmed during medical record or pathology review (USRT, PLCO, BCDDP) were excluded from our case definition. Using information from medical and pathology records and cancer registry linkage, we further classified cases by histology according to the International Classification of Diseases for Oncology morphology codes: papillary (8050, 8052, 8130, 8260, 8340-8344, 8450, and 8452) and follicular (8290, 8330, 8331, 8332, 8335) [15].
Statistical analysis
We used Cox proportional hazards models to calculate study-specific hazard ratios (HR) and 95% confidence intervals (CI) for thyroid cancer using attained age as the underlying time metric as an adjustment for age. Minimally-adjusted models were adjusted for sex, and multivariable-adjusted models were adjusted for sex, education (high school or less, post-high school or college, post-college, missing), race (white, black, American Indian/Alaskan Native, Asian/Pacific Islander, other, missing), marital status (married/living together, divorced/separated, widowed, single/never married, missing), BMI (15.0-18.5, 18.5-24.9, 25.0-29.9, 30.0-50.0 kg/m2), cigarette smoking (never, former, current, missing), and usual alcohol intake during the previous 12 months (none, <1 drink/week, 1-6 drinks/week, ≥7 drinks/week, missing). Heterogeneity in the HRs between studies was assessed using the I2 index [16, 17].
Data from all five cohorts were subsequently combined into one aggregate dataset to estimate the pooled HR for thyroid cancer, overall, by histological type, and by age at diagnosis. These models were additionally adjusted for cohort. We evaluated effect modification by other thyroid cancer risk factors, including baseline age, sex, education, BMI, smoking status, and alcohol intake, by comparing models with cross-product terms to models without cross-product terms using the likelihood ratio test. Differences by thyroid cancer histology (e.g., papillary and follicular) and age at diagnosis were evaluated using the Mantel-Haenszel test for heterogeneity. We also excluded the first two years of follow-up to evaluate possible bias due to changes in physical activity level among participants with undiagnosed disease at baseline.
All analyses were conducted using Stata software (version 9.2, College Station, TX). All statistical tests were two-sided and were considered statistically significant if P<0.05.
RESULTS
In total, 308 men and 510 women were diagnosed with a first primary thyroid cancer over a median follow-up of 10.5 years. Of the 285 cases in men and 462 cases in women with complete histology, papillary thyroid cancer accounted for 200 (70%) of the cases in men and 374 (81%) of the cases in women, while follicular thyroid cancer accounted for 68 (24%) and 63 (14%) of the cases in men and women, respectively.
In the four largest cohorts (NIH-AARP, USRT, PLCO, and AHS), HRs comparing the highest to lowest cohort-specific tertile of physical activity were above unity in both the minimally- and multivariable-adjusted models (Table 3). For multivariable-adjusted models, the test for heterogeneity between studies was borderline-significant (I2=53%, P-heterogeneity=0.07). We found no clear pattern between the level of detail on physical activity collected at baseline (Table 2) and the cohort-specific results (Table 3). There was less heterogeneity after excluding BCDDP, the cohort with the smallest number of cases (multivariable-adjusted HR=1.21, 95% CI: 1.02-1.43; I2=30%, P-heterogeneity=0.23). After combining all five cohorts, the overall minimally-adjusted HR for “high” versus “low” physical activity was 1.12 (95% CI: 0.96-1.32, P-trend=0.16). The magnitude of this association was slightly stronger in the multivariable-adjusted model (HR=1.18, 95% CI: 1.00-1.39, P-trend=0.06). The influence of finer adjustment for BMI as well as smoking intensity (i.e., number of cigarettes smoked per day) and smoking duration (i.e., number of years smoked) on these results was negligible (data not shown). Exclusion of the first two years of follow-up also had little influence on the results (data not shown).
Table 3.
HRs and 95% CIs for self-reported physical activity level and thyroid cancer risk by cohort
| Physical activity level (cohort-specific tertiles) | ||||
|---|---|---|---|---|
| Low | Medium | High | P-trend* | |
|
| ||||
| NIH-AARP | ||||
| Cases | 181 | 126 | 269 | |
| HR (95% CI)a | 1.00 (Reference) | 1.04 (0.83-1.31) | 1.06 (0.87-1.28) | 0.58 |
| HR (95% CI)b | 1.00 (Reference) | 1.07 (0.85-1.34) | 1.11 (0.91-1.35) | 0.30 |
| USRT | ||||
| Cases | 36 | 39 | 46 | |
| HR (95% CI)a | 1.00 (Reference) | 1.18 (0.75-1.86) | 1.35 (0.87-2.09) | 0.17 |
| HR (95% CI)b | 1.00 (Reference) | 1.21 (0.77-1.91) | 1.41 (0.91-2.19) | 0.13 |
| PLCO | ||||
| Cases | 16 | 19 | 16 | |
| HR (95% CI)a | 1.00 (Reference) | 1.09 (0.56-2.11) | 1.32 (0.66-2.64) | 0.44 |
| HR (95% CI)b | 1.00 (Reference) | 1.15 (0.59-2.26) | 1.47 (0.72-2.99) | 0.30 |
| AHS | ||||
| Cases | 13 | 14 | 21 | |
| HR (95% CI)a | 1.00 (Reference) | 1.33 (0.62-2.83) | 2.15 (1.08-4.30) | 0.03 |
| HR (95% CI)b | 1.00 (Reference) | 1.35 (0.63-2.90) | 2.19 (1.09-4.40) | 0.03 |
| BCDDP | ||||
| Cases | 9 | 10 | 3 | |
| HR (95% CI)a | 1.00 (Reference) | 1.07 (0.43-2.62) | 0.32 (0.09-1.20) | 0.11 |
| HR (95% CI)b | 1.00 (Reference) | 1.04 (0.42-2.56) | 0.33 (0.09-1.23) | 0.12 |
| Overall | ||||
| Total Cases | 255 | 208 | 355 | |
| HR (95% CI)c | 1.00 (Reference) | 1.08 (0.90-1.30) | 1.12 (0.96-1.32) | 0.16 |
| HR (95% CI)d | 1.00 (Reference) | 1.11 (0.92-1.33) | 1.18 (1.00-1.39) | 0.06 |
| Test for between-study heterogeneity: I2= 53%, P=0.07 | ||||
Calculated by modeling the categorical variables as continuous in the multivariable models
Adjusted for sex
Adjusted for sex, education, race, marital status, cigarette smoking, body mass index, and alcohol intake
Adjusted for sex and cohort
Adjusted for sex, education, race, marital status, cigarette smoking, body mass index, alcohol intake, and cohort
We found some differences in the multivariable-adjusted results within strata of certain thyroid cancer risk factors, as well as by histology (Figure 1). For instance, the association for “high” versus “low” physical activity was significantly stronger among participants who were overweight (BMI ≥25 kg/m2, HR=1.34, 95% CI: 1.09-1.64) compared to those who were normal-weight (BMI <25 kg/m2, HR=0.92, 95% CI: 0.69-1.22; P-interaction=0.03). The association was non-significantly stronger in men (HR=1.40, 95% CI: 1.06-1.86) compared to women (HR=1.07, 95% CI: 0.87-1.32; P-interaction=0.21), in subjects who had a post-high school education (HR=1.29, 95% CI: 1.29, 95% CI: 1.05-1.58) compared to those who did not (HR=0.93, 95% CI: 0.67-1.29; P-interaction=0.10), and current smokers (HR=1.89, 95% CI: 1.11-3.22) compared to former (HR=1.19) or never smokers (HR=1.07; P-interaction=0.12). The association was also non-significantly stronger for follicular (HR=1.55, 95% CI: 1.03-2.35) compared to papillary (HR=1.18, 95% CI: 0.97-1.44; P-interaction=0.24) thyroid cancer. We also observed significant differences according to age at diagnosis (P-interaction=0.03), whereby the association was strongest for thyroid cancers diagnosed before age 50 (80 cases, HR=2.58, 95% CI: 1.41-4.74, P-trend=0.002) compared to thyroid cancers diagnosed at ages 50 to 59 (127 cases, HR=1.09, 95% CI: 0.72-1.66, P-trend=0.68) or at ages 60 or older (611 cases, HR=1.11, 95% CI: 0.92-1.34, P-trend=0.28).
Figure 1.
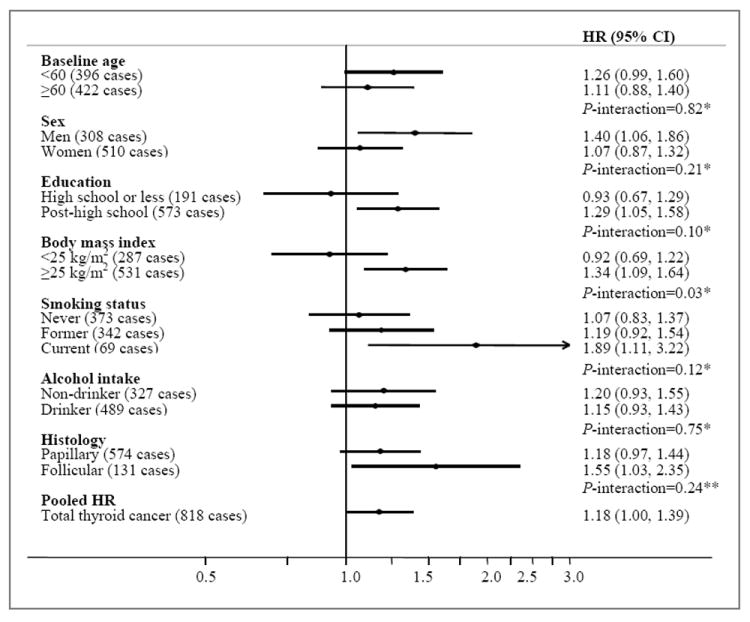
Multivariable-adjusted HRs (adjusted for sex, education, race, marital status, cigarette smoking, body mass index, alcohol intake, and cohort) and 95% CIs for self-reported physical activity level (“high” versus “low”) and thyroid cancer risk, stratified by select risk factors: aggregate analysis of the five cohorts. *Test for interaction calculated using the likelihood ratio test comparing a model with a cross-product term to a model without. **Test for interaction calculated using the Mantel-Haenszel test for heterogeneity.
Overall, we found a non-significant positive association between history of diabetes and thyroid cancer risk in minimally-adjusted models (HR=1.22, 95% CI: 0.95-1.58) (Table 4). After additional covariate adjustment, this association became attenuated (HR=1.08, 95% CI: 0.83-1.40), and no statistically-significant results were observed within any of the five cohorts. There was no evidence of significant heterogeneity between cohorts (I2=11%, P-heterogeneity=0.33). There were also no clear or suggestive differences according to baseline age, sex, education, BMI, smoking status, or alcohol intake, by age at diagnosis, or by thyroid cancer histology. The influence of finer adjustment for BMI as well as smoking intensity (i.e., number of cigarettes smoked per day) and smoking duration (i.e., number of years smoked) on these results was negligible (data not shown). Exclusion of the first two years of follow-up also had little influence on the results (data not shown).
Table 4.
HRs and 95% CIs for self-reported medical history of diabetes and thyroid cancer risk by cohort
| Medical history of diabetes | ||
|---|---|---|
| Never diagnosed | Ever diagnosed | |
|
| ||
| NIH-AARP | ||
| Cases | 516 | 60 |
| HR (95% CI)a | 1.00 (Reference) | 1.36 (1.04-1.77) |
| HR (95% CI)b | 1.00 (Reference) | 1.19 (0.90-1.57) |
| USRT | ||
| Cases | 117 | 4 |
| HR (95% CI)a | 1.00 (Reference) | 1.37 (0.51-3.72) |
| HR (95% CI)b | 1.00 (Reference) | 1.16 (0.42-3.20) |
| PLCO | ||
| Cases | 50 | 1 |
| HR (95% CI)a | 1.00 (Reference) | 0.32 (0.04-2.29) |
| HR (95% CI)b | 1.00 (Reference) | 0.26 (0.04-1.94) |
| AHS | ||
| Cases | 48 | 0 |
| HR (95% CI)a | 1.00 (Reference) | Not estimatable |
| HR (95% CI)b | 1.00 (Reference) | Not estimatable |
| BCDDP | ||
| Cases | 22 | 0 |
| HR (95% CI)a | 1.00 (Reference) | Not estimatable |
| HR (95% CI)b | 1.00 (Reference) | Not estimatable |
| Overall | ||
| Total Cases | 753 | 65 |
| HR (95% CI)c | 1.00 (Reference) | 1.22 (0.95-1.58) |
| HR (95% CI)d | 1.00 (Reference) | 1.08 (0.83-1.40) |
| Test for between-study heterogeneity: I2=11%, P=0.33 | ||
Adjusted for sex
Adjusted for sex, education, race, marital status, cigarette smoking, body mass index, and alcohol intake
Adjusted for sex and cohort
Adjusted for sex, education, race, marital status, cigarette smoking, body mass index, alcohol intake, and cohort
DISCUSSION
Many [3-11], though not all [12-14], case-control and prospective studies have shown positive associations between BMI and thyroid cancer risk. Few studies have examined the associations between other modifiable lifestyle exposures and medical conditions associated with BMI and the risk of this disease. In this pooled analysis of five large prospective studies, we found an overall positive association with greater physical activity, though the results were heterogeneous across cohorts, and no evidence of an association with history of diabetes.
We had hypothesized that greater physical activity would be associated with reduced risk of thyroid cancer, while a previous diagnosis of diabetes would be associated with increased risk. Physical activity improves insulin sensitivity and subsequent risk of diabetes through several different, independent mechanisms, including through the enhancement of glucose uptake in skeletal muscle and reduction in the amount of body fat mass [18]. Insulin resistance, a common metabolic consequence of both obesity and physical inactivity, is characterized by elevated levels of both insulin and glucose due to a reduction in tissue responsiveness to the physiologic effects of insulin [19]. Insulin could conceivably promote thyroid cancer growth directly through enhanced cancer cell proliferation or reduced apoptosis or indirectly by stimulating the production of other hormones, including insulin-like growth factor-1, estrogen, or thyroid stimulating hormone [19, 20]. This hypothesis is supported by previous evidence from a large prospective study in Austria showing that higher fasting glucose levels were associated with increased risk of thyroid cancer independent of BMI [21].
That our results failed to support this hypothesis is consistent with the limited number of epidemiologic studies of thyroid cancer that have utilized self-reported information on physical activity and diabetes. Previous, more detailed analyses conducted in the NIH-AARP and USRT cohorts, based on smaller sample sizes, showed no clear association between physical activity and thyroid cancer risk [22, 23]. One U.S. case-control study found that regular versus no recreational physical activity two years before diagnosis was associated with a significant decreased risk of papillary thyroid cancer, but no clear associations with number of hours of exercise were observed [24]. No association was previously observed between history of diabetes and thyroid cancer risk in the USRT cohort [23], while a significant positive association was previously shown in women but not men in the NIH-AARP cohort [25].
While there were several strengths of this pooled analysis, including the large sample size, ability to compare the results across five different types of cohorts, and the availability of data on several potential confounding factors, there were some important limitations. In particular, the pooled estimate for the association between physical activity and thyroid cancer risk should be interpreted cautiously considering the differences in the definition and range of physical activity levels across the five cohorts and the between-cohort heterogeneity that was observed. This heterogeneity could be explained, in part, by the degree of measurement error in self-reported physical activity for each of the cohorts [26]. For instance, there may have been greater potential for measurement error for physical activity level in cohorts for which information on light, moderate, and vigorous activity was combined into an overall activity score, or which combined leisure-time and occupational activity, as opposed to those inquiring about only one type of activity. Interestingly, the strongest positive association was observed in the AHS cohort, which may be considered to have the most narrow physical activity assessment, focusing only on vigorous physical activity in leisure-time. Other sources of heterogeneity may include differences in exposures specific to, or the type and amount of physical activity conducted by, participants belonging to a particular type of cohort (i.e. occupational [AHS, USRT] or screening [PLCO, BCDDP]) or differences in demographics or other characteristics of the study populations, particularly those that were found to modify the associations between physical activity and thyroid cancer risk in the aggregate dataset (e.g. BMI, smoking, education, age at diagnosis). The stronger positive associations observed among participants with greater education, those who were overweight/obese or current smokers at baseline, or who were diagnosed at younger ages may be attributable to greater residual confounding by these factors (i.e. if they imperfectly measured) or by other, related factors that were either not measured or were not considered for inclusion in the analysis. For instance, the positive association for physical activity, particularly among more highly-educated participants in this study, may be attributable to greater likelihood of detection of thyroid cancer in physically active versus inactive adults. However, we lacked information on frequency of thyroid check-ups, other socioeconomic indicators besides education, and stage at tumor diagnosis, all of which could have been used to evaluate the possible influence of a detection bias. Misclassification of diabetes history (i.e. due to undiagnosed disease) may explain the lack of association with thyroid cancer risk in this study. In addition, self-reported history of diabetes may not have been an adequate surrogate for insulin resistance and hyperinsulinemia, conditions that may long precede the onset of type 2 diabetes and may be more germane to the development of thyroid cancer [27]. We were also unable to investigate the association between diabetes and thyroid cancer risk by age at diabetes diagnosis, which may act as a proxy for exposure to circulating insulin or glucose, due to the small number of exposed cases identified in cohorts that collected these data (AHS, BCDDP, and USRT). The reliance on self-reported diabetes may explain the difference in results between this pooled analysis and the strong positive dose-response relationship between fasting glucose and thyroid cancer risk observed in a prospective study in Austria [21]. Additional studies with more objective measures of both physical activity and diabetes and with more comprehensive data on potential confounding factors, including screening practices, are needed before a potentially causal relationship with thyroid cancer can be ruled out.
In addition, it is not clear whether the results from this study are applicable to thyroid cancers diagnosed in young-to-middle adulthood, which may differ etiologically, or which may have been diagnosed at earlier stages on average, compared to cancers diagnosed later in life. For instance, because the majority of participants enrolled in the cohorts during middle-to-older adulthood, the median age of thyroid cancer diagnosis in this population was 65 in women and 68 in men, in contrast to median ages at diagnosis of 47 in women and 53 in men in the general U.S. population [28]. While we found that the positive association for physical activity was strongest for thyroid cancers diagnosed before age 50, the number of cases belonging to this age group was small. Studies with larger numbers of thyroid cancers diagnosed at earlier ages will be needed to more precisely evaluate effect modification by age at diagnosis.
In summary, despite generally consistent evidence of a positive association between BMI and thyroid cancer risk in this and previous studies, we found that neither physical inactivity nor a history of diabetes increased the risk of this disease. In contrast, we observed an increased risk of thyroid cancer for subjects reporting greater physical activity. Considering some of the limitations of this analysis, particularly the reliance on self-reported physical activity level and medical history, studies with more accurate exposure information on physical activity, energy expenditure, and obesity-related medical conditions, including hyperinsulinemia and diabetes, are still needed and may help to address a potential role of insulin resistance in thyroid cancer etiology.
Figure 2.
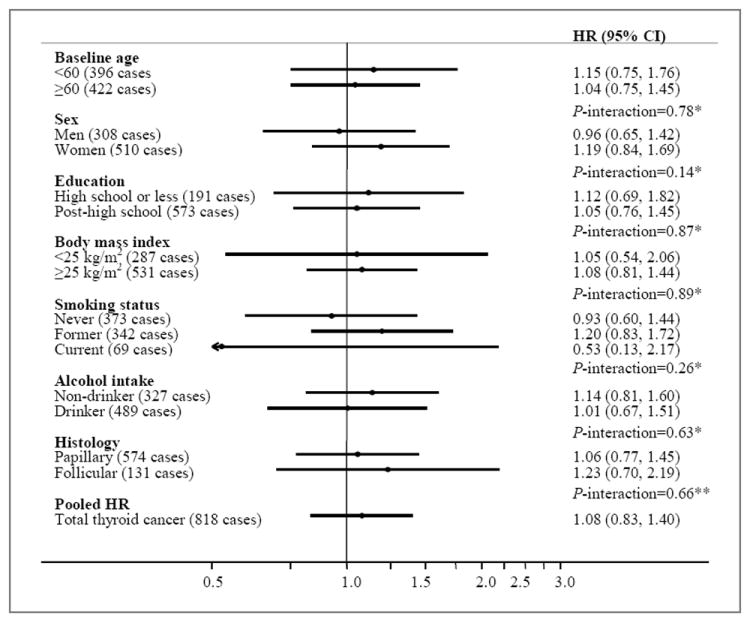
Multivariable-adjusted HRs (adjusted for sex, education, race, marital status, cigarette smoking, body mass index, alcohol intake, and cohort) and 95% CIs for self-reported medical history of diabetes (ever versus never) and thyroid cancer risk, stratified by select risk factors: aggregate analysis of the five cohorts. *Test for interaction calculated using the likelihood ratio test comparing a model with a cross-product term to a model without. **Test for interaction calculated using the Mantel-Haenszel test for heterogeneity.
Acknowledgments
Specific to NIH-AARP: Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University. Cancer incidence data from California were collected by the California Department of Health Services, Cancer Surveillance Section. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, State of Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (FCDC) under contract with the Florida Department of Health (FDOH). The views expressed herein are solely those of the authors and do not necessarily reflect those of the FCDC or FDOH. Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Medical Center in New Orleans. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey State Department of Health and Senior Services. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services. We are indebted to the participants in the NIH-AARP Diet and Health Study for their outstanding cooperation. We also thank Sigurd Hermansen and Kerry Grace Morrissey from Westat for study outcomes ascertainment and management and Leslie Carroll at Information Management Services for data support and analysis. In memory of Dr. Arthur Schatzkin, visionary investigator who founded the NIH-AARP Diet and Health Study.
This work was supported in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
Financial support: This work was supported in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health
Footnotes
Conflict of interest: none
References
- 1.Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomarkers Prev. 2009;18:784–91. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki T, Matsuo K, Hasegawa Y, Hiraki A, Kawase T, Tanaka H, et al. Anthropometric factors at age 20 years and risk of thyroid cancer. Cancer Causes Control. 2008;19:1233–42. doi: 10.1007/s10552-008-9194-x. [DOI] [PubMed] [Google Scholar]
- 4.Brindel P, Doyon F, Rachédi F, Boissin JL, Sebbag J, Shan L, et al. Anthropometric factors in differentiated thyroid cancer in French Polynesia: a case-control study. Cancer Causes Control. 2009;20:581–90. doi: 10.1007/s10552-008-9266-y. [DOI] [PubMed] [Google Scholar]
- 5.Kitahara CM, Platz EA, Beane Freeman LE, Hsing AW, Linet MS, Park Y, et al. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev. 2011;20:464–72. doi: 10.1158/1055-9965.EPI-10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tulinius H, Sigfusson N, Sigvaldason H, Bjarnadottir K, Tryggvadottir L. Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev. 1997;6:863–73. [PubMed] [Google Scholar]
- 7.Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23:4742–54. doi: 10.1200/JCO.2005.11.726. [DOI] [PubMed] [Google Scholar]
- 8.Clavel-Chapelon F, Guillas G, Tondeur L, Kernaleguen C, Boutron-Ruault MC. Risk of differentiated thyroid cancer in relation to adult weight, height and body shape over life: the French E3N cohort. Int J Cancer. 2010;126:2984–90. doi: 10.1002/ijc.25066. [DOI] [PubMed] [Google Scholar]
- 9.Dal Maso L, La Vecchia C, Francheschi S, Preston-Martin S, Ron E, Levi F, et al. A pooled analysis of thyroid cancer studies. V. Anthropometric factors. Cancer Causes Control. 2000;11:137–44. doi: 10.1023/a:1008938520101. [DOI] [PubMed] [Google Scholar]
- 10.Guignard R, Truong T, Rougier Y, Baron-Dubourdieu D, Guénel P. Alcohol drinking, tobacco smoking, and anthropometric characteristics as risk factors for thyroid cancer: a countrywide case-control study in New Caledonia. Am J Epidemiol. 2007;166:1140–9. doi: 10.1093/aje/kwm204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engeland A, Tretli S, Akslen LA, Bjørge T. Body size and thyroid cancer in two million Norwegian men and women. Br J Cancer. 2006;95:366–370. doi: 10.1038/sj.bjc.6603249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iribarren C, Haselkorn T, Tekawa IS, Friedman GD. Cohort study of thyroid cancer in a San Francisco Bay area population. Int J Cancer. 2001;93:745–50. doi: 10.1002/ijc.1377. [DOI] [PubMed] [Google Scholar]
- 13.Rapp K, Schroeder J, Klenk J, Stoehr S, Ulmer H, Concin H, et al. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005;93:1062–7. doi: 10.1038/sj.bjc.6602819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006;17:901–9.2. doi: 10.1007/s10552-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 15.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International Classification of Diseases for Oncology (ICD-O) 3. Geneva: WHO; 2000. [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 18.Balkau B, Mhamda L, Oppert JM, Nolan J, Golay A, Porcellati F, et al. Physical activity and insulin sensitivity: the RISC study. Diabetes. 2008;57:2613–8. doi: 10.2337/db07-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hursting SD, Lashinger LM, Wheatley KW, Rogers CJ, Colbert LH, Nunez NP, et al. Reducing the weight of cancer: mechanistic targets for breaking the obesity-carcinogenesis link. Best Pract Res Clin Endocrinol Metab. 2008;22:659–69. doi: 10.1016/j.beem.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Hard GC. Recent developments in the investigation of thyroid regulation and thyroid carcinogenesis. Environ Health Perspect. 1998;106:427–36. doi: 10.1289/ehp.106-1533202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rapp K, Schroeder J, Klenk J, Ulmer H, Concin H, Diem G, et al. Fasting blood glucose and cancer risk in a cohort of more than 140,000 adults in Austria. Diabetologia. 2006;49:945–52. doi: 10.1007/s00125-006-0207-6. [DOI] [PubMed] [Google Scholar]
- 22.Leitzmann MF, Brenner A, Moore SC, Koebnick C, Park Y, Hollenbeck A, et al. Prospective study of body mass index, physical activity, and thyroid cancer. Int J Cancer. 2010;126:2947–56. doi: 10.1002/ijc.24913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meinhold CL, Ron E, Schonfeld SJ, Alexander BH, Freedman DM, Linet MS, et al. Nonradiation risk factors for thyroid cancer in the US Radiologic Technologists Study. Am J Epidemiol. 2010;171:242–52. doi: 10.1093/aje/kwp354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossing MA, Remler R, Voigt LF, Wicklund KG, Daling JR. Recreational physical activity and risk of papillary thyroid cancer (United States) Cancer Causes Control. 2001;12:881–5. doi: 10.1023/a:1013757030600. [DOI] [PubMed] [Google Scholar]
- 25.Aschebrook-Kilfoy B, Sabra MM, Brenner A, Moore SC, Ron E, Schatzkin A, et al. Diabetes and thyroid cancer risk in the National Institutes of Health-AARP Diet and Health Study. Thyroid. 2011;21:957–63. doi: 10.1089/thy.2010.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrari P, Friedenreich C, Matthews CE. The role of measurement error in estimating levels of physical activity. Am J Epidemiol. 2007;166:832–40. doi: 10.1093/aje/kwm148. [DOI] [PubMed] [Google Scholar]
- 27.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–85. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surveillance Epidemiology and End Results (SEER) Bethesda, MD: National Cancer Institute, National Institutes of Health; 2009. [December 29, 2011]. SEER stat fact sheet—cancer of the thyroid. ( http://seer.cancer.gov/csr/1975_2006/results_single/sect_01_table.11_2pgs.pdf) [Google Scholar]


