Abstract
Obesity, high-fat diets, and subsequent type 2 diabetes (T2DM) are associated with cognitive impairment. Moreover, T2DM increases the risk of Alzheimer’s disease (AD) and leads to abnormal elevation of brain beta-amyloid levels, one of the hallmarks of AD. The psychoactive alkaloid caffeine has been shown to have therapeutic potential in AD but the central impact of caffeine has not been well-studied in the context of a high-fat diet. Here we investigated the impact of caffeine administration on metabolism and cognitive performance, both in control rats and in rats placed on a high-fat diet. The effects of caffeine were significant: caffeine both (i) prevented the weight-gain associated with the high-fat diet and (ii) prevented cognitive impairment. Caffeine did not alter hippocampal metabolism or insulin signaling, likely because the high-fat-fed animals did not develop full-blown diabetes; however, caffeine did prevent or reverse a decrease in hippocampal brain-derived neurotrophic factor (BDNF) seen in high-fat-fed animals. These data confirm that caffeine may serve as a neuroprotective agent against cognitive impairment caused by obesity and/or a high-fat diet. Increased hippocampal BDNF following caffeine administration could explain, at least in part, the effects of caffeine on cognition and metabolism.
1. INTRODUCTION
Human obesity continues to increase [1], associated with consumption of high-fat diets; both obesity and high fat consumption are linked to cognitive impairment [2-12] and causal factors for the current type 2 diabetes mellitus (T2DM) pandemic [13]. T2DM is a metabolic disorder characterized by hyperglycemia, hyperinsulinemia and subsequent insulin resistance [14] as well as by cognitive impairment and, specifically, hippocampal dysfunction [12, 15-22] so that high dietary fat has multiple associations with cognitive impairment. Caffeine, the most popular psychoactive drug in the US [23] with 80% of the American population consuming this stimulant [24], has recently received attention as a potential therapeutic agent to prevent and/or ameliorate T2DM [25-29], including a recent spatial memory study using very high levels of caffeine given to aged, mutant mice [30]. However, studies of the effect of caffeine on brain insulin signalling have not been consistent and have often been performed in vitro [23, 25, 31-35]. Of note, caffeine has also been shown to offer protection against neurodegenerative conditions such as Alzheimer’s disease (AD), for which T2DM is a major risk factor [7, 31, 36-43], as well as e.g. Parkinson’s disease, by mechanisms that include stimulation of insulin signalling [32, 44, 45]; however, the impact of caffeine in ameliorating the impact of a high-fat diet per se has been less studied. Impairment of central insulin signalling is a likely cause of cognitive impairment associated with obesity, a high-fat diet, and/or T2DM [22, 46] and we recently showed such signalling to be a critical, mandatory component of hippocampal memory processes [47].
Here, we investigated in vivo the cognitive and brain-metabolic effects of caffeine administration both alone and in the context of a potentially diabetogenic high-fat diet, with hippocampal microdialysis both at baseline and during cognitive (hippocampally-dependent, spatial working memory) testing. Unlike our previous work and that of others [47-49], in this study the high-fat diet did not induce a hyperglycemic, diabetic state, although plasma insulin levels were elevated. Likely as a result of this, no effect of caffeine treatment on hippocampal glucose metabolism or insulin signalling was seen, despite prevention of both weight gain and cognitive impairment associated with the high-fat diet by caffeine, and reversal of the elevation in plasma insulin. Interestingly, however, we identified a possible novel effector mechanism for caffeine, as hippocampal BDNF (which has previously been linked to enhanced mnemonic processing [50-52]) was increased by caffeine treatment.
2. METHODS
2.1 Animals
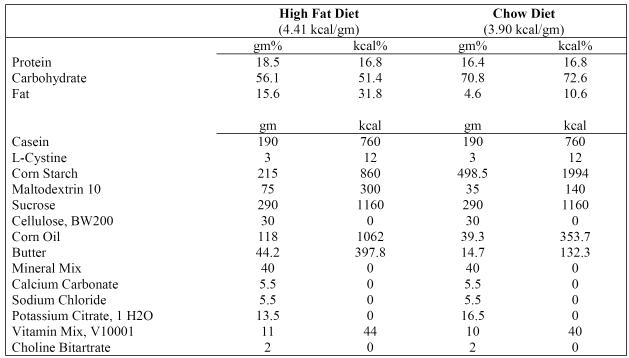
32 male Sprague–Dawley rats (Charles River, Wilmington MA) were pair housed with food and water ad lib, on a 12-hr light–dark schedule (lights on at 07:00 hr). All procedures were approved by the University at Albany Animal Care and Use Committee (IACUC). Rats entered the facility at 4 weeks and at 5 weeks were pseudorandomly assigned to one of four groups: high fat diet )control, high fat diet with caffeine, regular chow diet with caffeine, or regular chow diet control, n=8 each. The high-fat diet was research Diets D12266B, as used previously [22, 49]. All animals received either caffeine (20 mg/kg) or saline (volume-matched), i.p., once weekly. Each animal was handled every day for a minimum of 5 min to prevent handling or treatment stress.
2.2 Surgery
At 17 weeks of age, standard sterile stereotaxic procedures [22, 53-55] under isoflurane anesthesia were used to implant a microdialysis guide cannula (outer diameter 0.8 mm; BASi Microdialysis) aimed at the left dorsal lateral hippocampus. The nose bar was set at 4.6 mm above the interaural line and coordinates were +5.6 mm posterior from bregma, +4.6 mm lateral from the midline, and -3.0 mm ventral from the dura mater. Rats were allowed to recover for 1 week prior to testing, and handled extensively each day.
2.3 Microdialysis
Methods as published previously [22, 53-55]. The probe membrane projected 4mm beyond the guide cannula and thus sampled across several regions of the hippocampal formation. Probe insertion was timed to give optimum measurement conditions and to avoid glial scarring at the probe site. Each animal was used only once. Rats were allowed to move freely throughout, minimizing any effect of restraint stress. The microdialysis probes were perfused with an artificial extracellular fluid (aECF; 132 mM NaCl, 4.3 mM KCl, 0.9 mM MgCl2, 0.7 mM CaCl2, 10 mM Na2HPO4, 620 nM NaH2PO4, 1.25 mM D-glucose, pH 7.4 [54]) at a flow rate of 1.5 μL/min. To avoid either supply or drainage of glucose from ECF, the microdialysis perfusate contained 1.25 mM glucose, the basal level in the hippocampal ECF [54, 56]. Samples were collected every 20 min after equilibration and frozen immediately for later analysis (using a CMA600, CMA/Microdialysis). Concentration in the samples was corrected for in vivo probe recovery using the slope of a hippocampal ECF zero-net-flux plot under the same experimental conditions.
2.4 Spontaneous alternation testing
Also as previously published [47, 53, 57]. Rats are placed into a novel control chamber of clear Plexiglas for baseline measurements, with baseline for ECF glucose, lactate and pyruvate determined for each rat by averaging the values in the three 20 min samples immediately before testing and defined as 100%. After the baseline period, rats were placed into the center of a four-arm maze, made of black Plexiglas, and allowed to explore freely for 20 min, then placed back in the control box. Samples were collected continuously before, during, and after the test period. When allowed to explore freely, rats spontaneously alternate between maze arms, using spatial working memory to retain knowledge of arms previously visited. This spontaneous alternation has been extensively used as a working memory task in our laboratory and others [57-67]. The measure of memory used was percentage 4 out of 5 alternation: an alternation is counted when the rat visits all four arms within a span of five arm choices and is converted to a percentage by dividing the number of alternations by the total possible number of alternations: chance performance level is 44%. The maze task was given in the same room to ensure identical cue availabilities across each group, and testing was conducted during the mid light-phase.
2.5 Histology
After testing, rats were immediately euthanized. Trunk blood was collected for later analysis. Brains were extracted and immediately frozen at −80°C; hippocampi were extracted and weighed, then homogenised and separated for analysis of total and plasma membrane proteins as published [68].
2.6 Western blotting
Equal amounts (20μg) of each sample were separated into sample buffer with 95% laemmli sample buffer (BIO-RAD) and 5% 2-beta mercaptoethanol (Sigma). The samples were loaded into 10% mini-protean TGX gels (BIO-RAD) at 240V for 45 min. Wet transfer of proteins from gel to PVDF membranes was run at 350 mA (constant) for 1 hour. The membrane was washed in TBS with 0.1% Tween-20 (TBST) and then blocked for 1 h at room temperature in 5% nonfat dry milk in TBST. Primary antibodies were diluted in TBST (GluT4 [Millipore] 1:1000, GluT3 [abcam] 1:3000, pAkt [cell signaling] 1:5000, and Akt [cell signaling] 1:5000) and left overnight in the membranes. After wash, membranes were incubated in biotinylated secondary antibodies [Thermo] diluted 1:20,000 in TBST on shaker for 1 hour at room temp. After wash membranes were incubated in HRP streptavidin [Pierce] at a final concentration of 1:10,000 in TBST with 1% milk blocking buffer on shaker for 1 hour at room temperature. After final washes, membranes were mixed in a chemiluminescent substrate of super signal west pico stable peroxide solution and luminal enhancer solution in a 1:1 ratio and signals were detected on film using high sensitivity chemiluminescence. All gels were transferred simultaneously, immunoblotted in the same solutions, and exposed to film in parallel. Exposures in the linear range of the film were analyzed by densitometry. Films were imaged by transillumination on a Chemi-Doc XRS scanner (BIO-RAD) driven by QuantityOne-4.6.1 software. Images were acquired at 16 bit pixel depth, and linear gamma was maintained throughout. Quantification used ImageQuant TLv2005 and local background was subtracted for each band.
2.7 Enzyme linked immunosorbent assay
For hippocampal BDNF quantification, equal amounts (120μg) of each hippocampal sample were mixed 1:2 with diluent and run in duplicate to measure BDNF. Samples and standards were loaded in ChemiKine BDNF strips (Millipore), the plate was sealed and incubated at 4°C overnight. Diluted biotinylated mouse anti-BDNF monoclonal antibody was added and incubated at room temperature for 3 hours, followed by, diluted HRP-streptavidin for 1 hour and warm TMB/E substrate for 15 min, washing thoroughly between each. Stop solution was added and the plate was read immediately at 450nm. Samples were processed without acid pretreatment, for measurement of mature BDNF.
For blood insulin measurement, 10uL of blood serum was loaded in duplicate onto strips coated with mouse monoclonal anti-rat insulin (Millipore), mixed with 80uL of detection antibody and 10uL of assay buffer, and incubated at room temperature for 2 hours. Diluted HRP-streptavidin was added for 1 hour, then warm TMB/E substrate for 15 min, washing before each, before stopping the reaction and reading the plates.
2.8 Statistical Analysis
All tests were conducted in either SPSSv18 or GraphPad Prism5 using one-way analysis of variance (ANOVA) with individual cohort differences determined by Bonferroni multiple comparison post hoc. Ns for behavioral measures were 6-7. Data from a single animal with a misplaced cannula were not included in the ECF glucose dataset.
3. RESULTS
3.1 Caffeine prevented weight gain associated with a high fat diet
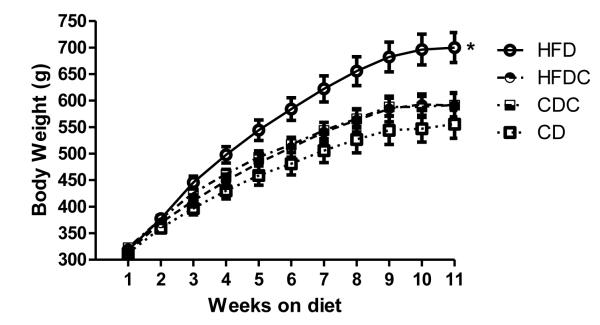
As expected, animals fed a high-fat diet gained significantly more weight than their chow-fed counterparts (Figure 2). However, this difference in weight gain was entirely prevented by caffeine administration: animals receiving both the high-fat diet and caffeine treatment did not differ in weight from chow-fed controls. Caffeine treatment did not significantly alter weight gain in animals fed a regular chow diet.
Figure 2.
Average group weights. Animals underwent surgery immediately following weighing in the 10th week, and were tested after the week 11 weighing. Error bars = SEM. HFD animals gained significantly more weight, so that they were significantly heavier after 11 weeks on the diet [t(14) = 3.72, p<.05 vs chow-fed animals] whereas caffeine prevented this weight gain [t(14) = 2.92, p <.05 for comparison of high-fat and high-fat-caffeine groups]. HFD = high fat diet, HFDC = high fat diet plus caffeine, CD = control diet and CDC = control diet plus caffeine.
3.2 Caffeine prevented spatial memory impairment associated with the high fat diet
Consistent with previous findings [47], high-fat-fed animals had impaired spatial working memory compared to their chow-fed counterparts (49.0 +/− 4.7% vs. 66.6 +/− 1.6%, t(12)=3.51, p< .05). Caffeine administration did not affect performance in chow-fed animals. However, spatial memory in animals on the high-fat diet who also received caffeine was enhanced compared to that in animals receiving the high-fat diet alone [70.8 +/− 3.6% vs. 49.0 +/− 4.7%, t(11) = 3.56, p <.05], with caffeine fully preventing the diet-induced impairment and restoring spatial memory to the same level seen in the chow-fed control animals (Figure 3A). Neither diet nor caffeine treatment affected motor activity or motivation to perform the task, assessed by total number of arms entered during the 20 min task period (Figure 3B).
Figure 3.
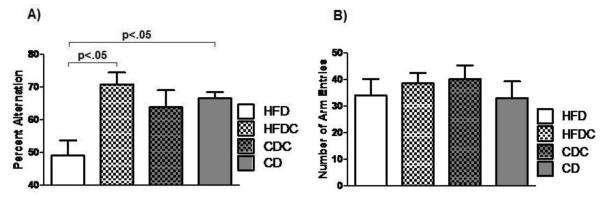
(A) Percent alternation performance. Error bars = SEM. (B) Mean arm entries during maze performance. Error bars = SEM. HFD animals had significantly worse alternation performance than either control animals or animals receiving both a high-fat diet and caffeine treatment, but there were no differences in number of arms entered.
3.3 Caffeine did not affect hippocampal insulin signalling proteins
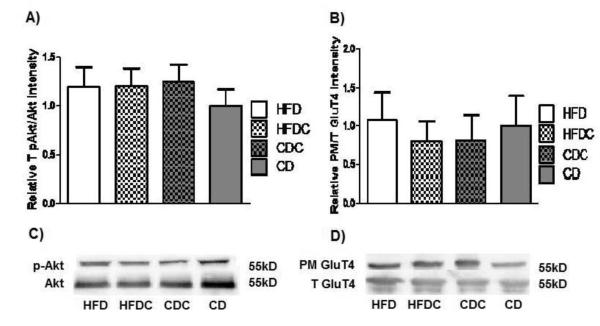
We had hypothesised that caffeine might prevent an impairment in hippocampal insulin signalling in high-fat-fed animals, given that impaired insulin signalling was seen in a similarly-treated group in our previous work [47]. However, consistent with the failure in this study to induce diabetes or alter plasma glucose, the high-fat fed group showed no decrease in hippocampal insulin signalling: neither diet nor caffeine treatment affected hippocampal Akt phosphorylation (Figure 4A-B) nor hippocampal GluT4 translocation (Figure 4C-D). There was (as expected) also no effect on the constitutive glucose transporter GluT3 (data not shown). The fact that the high-fat-fed animals were cognitively impaired but showed no decrease in Akt phosphorylation or GluT4 translocation supports the suggestion [2, 6, 11, 12, 69] that obesity-linked cognitive impairment may occur even before impairment to hippocampal insulin signalling.
Figure 4.
A. Mean density ratio of hippocampal pAkt (Ser473) to total Akt. Ratios were normalised to beta-actin. B. Mean density ratio of plasma membrane GluT4 to total GluT4. (C & D) Representative blots for Akt and GluT4, respectively.
3.4 Plasma insulin, but not plasma glucose nor hippocampal glucose, was elevated by the high-fat diet; this increase was prevented by caffeine treatment
Consistent with our hypothesis that caffeine might attenuate the impact of a high-fat diet, high-fat-fed animals had significantly elevated plasma insulin (2.94 +/− 0.50 ng/ml, compared to 1.20 +/− 0.17 in the control-fed animals, t(14)=3.29, p<.05), and this elevation was prevented by caffeine administration (high-fat-caffeine animals had plasma insulin of 1.46 +/− 0.29 ng/ml: comparison to high-fat animals t(14)=2.54, p<.05). Plasma insulin in high-fat-caffeine animals was not different from that of control animals (t(14)=0.77, p = n.s.), and caffeine treatment did not affect plasma insulin in animals on control chow (t(14)=0.07, p = n.s.). However, in contrast to our previous work [47], the high-fat diet did not lead to hyperglycemia, with no group differing from control animals in plasma glucose (all p = n.s., data not shown), which we interpret as a failure to induce diabetes; the hyperinsulinemia observed in this group suggests a pre-diabetic state. Unexpectedly, but consistent with the lack of effect of treatment on insulin signalling (including GluT4 translocation) or plasma glucose, neither caffeine treatment nor the high-fat diet had any effect on hippocampal glucose, lactate, or pyruvate levels either before, during, or following testing, nor on plasma glucose (data not shown).
3.5 Caffeine treatment prevents or reverses the reduction in hippocampal BDNF seen in high-fat-fed animals
A lead candidate mechanism by which caffeine has been suggested to modulate hippocampal processing, including long-term potentiation and memory performance, is via elevation in local brain-derived neurotrophic factor (BDNF) [70-75]. The high-fat diet reduced hippocampal BDNF compared to that of chow-fed animals (t(13) = 2.4, p< .05, Figure 5); high-fat-caffeine treated animals had hippocampal BDNF levels not different from those of chow-fed controls. Caffeine treatment did not significantly alter hippocampal BNDF in chow-fed animals.
Figure 5.
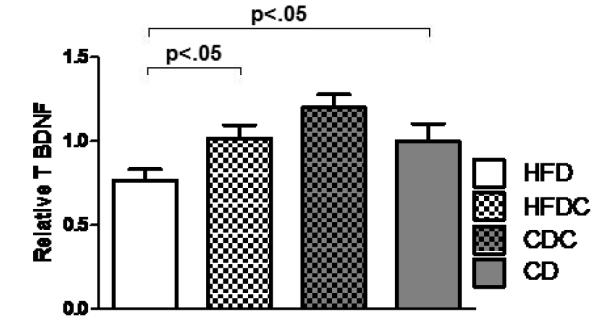
Mean hippocampal BDNF protein. Error bars = SEM
4. DISCUSSION
Here, we show that not only did caffeine administration prevent hippocampally-mediated cognitive impairment associated with a high-fat diet, but the caffeine treatment also prevented weight gain. No effect on either weight or memory was seen with caffeine treatment in the chow-fed control animals, suggesting a specific interaction with the effects of the high-fat diet; similarly, a significant effect of caffeine on hippocampal BDNF was seen only in the context of a high-fat diet. The fact that neither diet nor caffeine treatment affected motor activity, as measured by number of arms entered during the alternation testing, suggests that effects of diet and caffeine on weight were likely due to metabolic alterations. Because detailed food intake and home-cage activity measurements were not taken, however, the mechanism by which caffeine acts to prevent weight gain associated with the high-fat diet will require further study: we cannot exclude potential effects on either caloric consumption or expenditure (or both). Caffeine can affect neural activity via several routes: in addition to effects on BDNF shown here and effects on e.g. insulin signalling, caffeine can for instance increase neural excitability via antagonism of adenosine and regulate blood supply; much additional work will be required to fully characterise the central effects of caffeine.
Unlike our previous work [76], here the high-fat diet did not induce diabetes nor impair hippocampal insulin signalling or metabolism, suggesting that at least some of the cognitive impairments associated with a high- and/or saturated-fat diet are likely to occur prior to any metabolic impairment, as others have also suggested [11, 69, 77, 78]. Our data are consistent with the possibility that obesity per se may be linked to cognitive impairment, and that reduction in body mass (whether by caffeine consumption or other intervention) may attenuate that impairment. We identified a potential effector for the impairment associated with high fat consumption, which caused a decrease in hippocampal BDNF (a neurotrophin well-established to be important for memory processing), that was prevented by caffeine treatment, a novel result. One speculative possibility for BDNF’s mechanism of action might center on inflammation: one of insulin’s less-studied roles is as an anti-inflammatory agent [79], and both T2DM and AD are characterised in their early stages by increased central inflammation; caffeine may inhibit proinflammatory cytokines [80] concomitant with increases in BDNF [81]. The choice of BDNF as a candidate effector molecule to measure was driven by literature suggestions of a link between caffeine and BDNF, but there is again scope for further study of additional neurotrophins and additional potential effector pathways. Recent data [82] suggest that chronic caffeine consumption may prevent age-related cognitive decline while increasing hippocampal CA1 dendritic connections; conversely, studies of caffeine as a synaptic potentiator have identified specific effects in the relatively less-studied CA2 region of the hippocampus [83], so that caffeine may affect multiple hippocampal subfields in modulating cognitive performance. Our data suggest that further study of caffeine’s effects on the hippocampus is merited, especially in the context of obesity and/or a high-fat diet. Here, we extend the literature on caffeine as a cognitive enhancer, and support the possibility of caffeine use as a therapeutic intervention not only for T2DM and AD but also in patients with poor diet and/or obesity prior to development of either of those frank disease states. Additionally, given the widespread human consumption of caffeine, it is interesting to speculate whether current obesity rates might be even higher in the absence of such caffeine intake.
Highlights.
Caffeine prevented both weight-gain and cognitive impairment associated with a high-fat diet
Unexpectedly, these effects were not associated with any change in brain insulin signalling
Caffeine prevented or reversed a decrease in hippocampal BDNF seen in high-fat-fed animals
Figure 1.
Compositions of high-fat diet (D12266B) and chow diet (D12489B).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and Trends in Obesity Among US Adults, 1999-2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Banas SM, et al. A dietary fat excess alters metabolic and neuroendocrine responses before the onset of metabolic diseases. Cell Mol Neurobiol. 2009;29(2):157–68. doi: 10.1007/s10571-008-9307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis JF, et al. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci. 2008;122(6):1257–63. doi: 10.1037/a0013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farr SA, et al. Obesity and Hypertriglyceridemia Produce Cognitive Impairment. Endocrinology. 2008;149(5):2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerges NZ, Aleisa AM, Alkadhi KA. Impaired long-term potentiation in obese zucker rats: possible involvement of presynaptic mechanism. Neuroscience. 2003;120(2):535–9. doi: 10.1016/s0306-4522(03)00297-5. [DOI] [PubMed] [Google Scholar]
- 6.Lindqvist A, et al. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol. 2006;13(12):1385–8. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- 7.Moroz N, et al. Limited Alzheimer-type neurodegeneration in experimental obesity and type 2 diabetes mellitus. J Alzheimers Dis. 2008;15(1):29–44. doi: 10.3233/jad-2008-15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souza CG, et al. Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety-like behavior. Life Sciences. 2007;81(3):198–203. doi: 10.1016/j.lfs.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Stranahan AM, Mattson MP. Impact of energy intake and expenditure on neuronal plasticity. Neuromolecular Med. 2008;10(4):209–18. doi: 10.1007/s12017-008-8043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Berg E, et al. Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: A systematic comparison of their impact on cognition. Biochim Biophys Acta. 2009;1792(5):470–81. doi: 10.1016/j.bbadis.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol Aging. 2005;26(Suppl 1):46–9. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Winocur G, et al. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behavioral Neuroscience. 2005;119(5):1389–95. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- 13.Harris MI, Flegal K.M, Cowie, C.C., Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of Diabetes, Impaired Fasting Glucose, and Impaired Glucose Tolerance in U.S. Adults: The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21(4):518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RAF, E. Insulin Resistance: A Multifaceted Syndrome Responsible for NIDDM, Obesity, Hypertension, Dyslipidemia, and Atherosclerotic Cardiovascular Disease. Diabetes Care. 1991;14(3):173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 15.Akisaki T, et al. Cognitive dysfunction associates with white matter hyperintensities and subcortical atrophy on magnetic resonance imaging of the elderly diabetes mellitus Japanese elderly diabetes intervention trial (J-EDIT) Diabetes Metab Res Rev. 2006;22(5):376–84. doi: 10.1002/dmrr.632. [DOI] [PubMed] [Google Scholar]
- 16.Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol. 2004;26(8):1044–80. doi: 10.1080/13803390490514875. [DOI] [PubMed] [Google Scholar]
- 17.Brands AM, et al. Cognitive functioning and brain MRI in patients with type 1 and type 2 diabetes mellitus: a comparative study. Dement Geriatr Cogn Disord. 2007;23(5):343–50. doi: 10.1159/000100980. [DOI] [PubMed] [Google Scholar]
- 18.Cosway R, et al. Cognitive function and information processing in type 2 diabetes. Diabetic Medicine. 2001;18(10):803–10. doi: 10.1046/j.1464-5491.2001.00577.x. [DOI] [PubMed] [Google Scholar]
- 19.den Heijer T, et al. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46(12):1604–10. doi: 10.1007/s00125-003-1235-0. [DOI] [PubMed] [Google Scholar]
- 20.Gold SM, et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50(4):711–9. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- 21.Hassing L, et al. Type 2 diabetes mellitus contributes to cognitive decline in old age: a longitudinal population-based study. Journal of the International Neuropsychological Society. 2004;10(4):599–607. doi: 10.1017/S1355617704104165. [DOI] [PubMed] [Google Scholar]
- 22.McNay EC, et al. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem. 2010;93(4):546–53. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Review. 1999;51(1):83–153. [PubMed] [Google Scholar]
- 24.Julien RM. A Primer of Drug Action: A comprehensive guide to the actions, uses, and side effects of psychoactive drugs. eleventh edition Worth Publishers; New York, NY: 2007. [Google Scholar]
- 25.Akiba T, Yaguchi K, Tsutsumi K, Nishioka T, Koyama I, Nomura M, Yokogawa K, Moritani S, Miyamoto KI. Inhibitory mechanism of caffeine on insulin-stimulated glucose uptake in adipose cells. Biochemical pharmacology. 2004;68(10):1929–1937. doi: 10.1016/j.bcp.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 26.Duarte JMN, Carvalho RA, Cunhn RA, Gruetter R. Caffeine consumption attenuates neurochemical modifications in the hippocampus of streptozotocin-induced diabetic rats. J Neurochemistry. 2009;111(2):368–379. doi: 10.1111/j.1471-4159.2009.06349.x. [DOI] [PubMed] [Google Scholar]
- 27.Kerr D, Sherwin RS, Pavalkis F, Fayad PB, Sikorski L, Rife F, Tamborlane WV, During MJ. Effect of Caffeine on the Recognition of and Responses to Hypoglycemia in Humans. Annals of Internal Med. 1993;119(8):799–804. doi: 10.7326/0003-4819-119-8-199310150-00005. [DOI] [PubMed] [Google Scholar]
- 28.Van Dam RM, Willett WC, Manson JE, Hu FB. Coffee, Caffeine, and Risk of Type 2 Diabetes. Diabetes Care. 2006;19(2):398–403. doi: 10.2337/diacare.29.02.06.dc05-1512. [DOI] [PubMed] [Google Scholar]
- 29.Pimentel GD, Zemdegs JCS, Theodoro JA, Mota JA. Does long-term coffee intake reduce type 2 diabetes mellitus risk? Diabetology and metabolic syndrome. 2009;1(6):1–8. doi: 10.1186/1758-5996-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duarte JM, et al. Caffeine Consumption Prevents Diabetes-Induced Memory Impairment and Synaptotoxicity in the Hippocampus of NONcZNO10/LTJ Mice. PLoS One. 2012;7(4):e21899. doi: 10.1371/journal.pone.0021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Gawryluk JW, Wagener JF, Ghribi O, Geiger JD. Caffeine blocks disruption of blood brain barrier in a rabbit model of Alzheimer’s disease. J Neuroinflammation. 2008;5(12) doi: 10.1186/1742-2094-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakaso K, Ito S, Nakashima K. Caffeine activates the PI3K/Akt pathway and prevents apoptotic cell death in a Parkinson’s disease model of SH-SY5Y cells. Neuroscience Letters. 2008;43(2):146–150. doi: 10.1016/j.neulet.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto T, He Z, Ma WY, Schmid PC, Bode AM, Yang CS, Dong Z. Caffeine Inhibits Cell Proliferation by G0/G1 Phase Arrest in JB6 Cells. Cancer Res. 2004;64(9):3344–3349. doi: 10.1158/0008-5472.can-03-3453. [DOI] [PubMed] [Google Scholar]
- 34.Loke WH. Effects of caffeine on mood and memory. Physiology & Behavior. 1988;44(3):367–372. doi: 10.1016/0031-9384(88)90039-x. [DOI] [PubMed] [Google Scholar]
- 35.Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Research Reviews. 1992;17(2):139–170. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- 36.Arendash GW, Schleif W, Rezai-Zadeh K, Jackson EK, Zacharia LC, Cracchiolo JR, Shippy D, Tan J. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain β-amyloid production. Neuroscience. 2006;142(4):941–952. doi: 10.1016/j.neuroscience.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 37.Arendash GW, Mori T, Cao C, Mamcarz M, Runfeldt M, Dickson A, Rezai-Zadeh K, Tane J, Citron BA, Lin X, Echeverria V, Potter H. Caffeine Reverses Cognitive Impairment and Decreases Brain Amyloid-β Levels in Aged Alzheimer’s Disease Mice. J Alzheimer’s Disease. 2009;17(3):661–680. doi: 10.3233/JAD-2009-1087. [DOI] [PubMed] [Google Scholar]
- 38.de la Monte SM. Insulin resistance and Alzheimer’s disease. BMB Rep. 2009;42(8):475–81. doi: 10.5483/bmbrep.2009.42.8.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irie F, et al. Enhanced Risk for Alzheimer Disease in Persons With Type 2 Diabetes and APOE {varepsilon}4: The Cardiovascular Health Study Cognition Study. Arch Neurol. 2008;65(1):89–93. doi: 10.1001/archneurol.2007.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janson J, et al. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53(2):474–81. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 41.Nelson PT, et al. Human cerebral neuropathology of Type 2 diabetes mellitus. Biochim Biophys Acta. 2009;1792(5):454–69. doi: 10.1016/j.bbadis.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzanne M.d.l.M., Jack RW. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: Relevance to Alzheimer’s disease. Journal of Alzheimer’s Disease. 2005;7(1):45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- 43.Zhao WQ, Townsend M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochim Biophys Acta. 2009;1792(5):482–96. doi: 10.1016/j.bbadis.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N, Schwarzschild MA. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson’s disease. J Neuroscience. 2001;21(10):RC143. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prediger RD. Effects of caffeine in Parkinson’s disease: from neuroprotection to the management of motor and non-motor symptoms. J Alzheimer’s Disease. 2010;20(1):205–220. doi: 10.3233/JAD-2010-091459. [DOI] [PubMed] [Google Scholar]
- 46.McNay EC, Recknagel AK. Brain insulin signaling: A key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiol Learn Mem. 2011;96(3):432–42. doi: 10.1016/j.nlm.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McNay E, et al. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiology of Learning and Memory. 2010;93(4):546–53. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levin BE, et al. Selective breeding for diet-induced obesity and insulin resistance in Sprague-Dawley rats. American Journal of Physiology. 1997;273:R725–730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 49.Clegg DJ, et al. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol. 2005;288(4):R981–6. doi: 10.1152/ajpregu.00675.2004. [DOI] [PubMed] [Google Scholar]
- 50.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberge DR. The BDNF val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 51.Yamada K, Mizuno M, Nabeshima T. Role for brain-derived neurotrophic factor in learning and memory. Life Sci. 2002;70(7):735–744. doi: 10.1016/s0024-3205(01)01461-8. [DOI] [PubMed] [Google Scholar]
- 52.Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nature Neuroscience. 2000;3(6):533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- 53.McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(6):2881–5. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNay EC, Sherwin RS. From artificial cerebro-spinal fluid (aCSF) to artificial extracellular fluid (aECF): microdialysis perfusate composition effects on in vivo brain ECF glucose measurements. Journal of Neuroscience Methods. 2004;132(1):35–43. doi: 10.1016/j.jneumeth.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 55.McNay EC, et al. Cognitive and neural hippocampal effects of long-term moderate recurrent hypoglycemia. Diabetes. 2006;55(4):1088–95. doi: 10.2337/diabetes.55.04.06.db05-1314. [DOI] [PubMed] [Google Scholar]
- 56.McNay E, Gold P. Extracellular glucose concentrations in the rat hippocampus measured by zero-net-flux: effects of microdialysis flow rate, strain, and age. J Neurochem. 1999;72:785–790. doi: 10.1046/j.1471-4159.1999.720785.x. [DOI] [PubMed] [Google Scholar]
- 57.McNay EC, Gold PE. Memory modulation across neural systems: intra-amygdala glucose reverses deficits caused by intraseptal morphine on a spatial task but not on an aversive task. Journal of Neuroscience. 1998;18(10):3853–8. doi: 10.1523/JNEUROSCI.18-10-03853.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dember WN, Fowler H. Spontaneous alternation behavior. Psychological Bulletin. 1958;55(6):412–428. doi: 10.1037/h0045446. [DOI] [PubMed] [Google Scholar]
- 59.Stevens R, Cowey A. Effects of dorsal and ventral hippocampal lesions on spontaneous alternation, learned alternation and probability learning in rats. Brain Research. 1973;52:203–224. doi: 10.1016/0006-8993(73)90659-8. [DOI] [PubMed] [Google Scholar]
- 60.Richman C, Dember W, Kim P. Spontaneous alternation behavior in animals: A review. Current Psychology. 1986;5(4):358–391. [Google Scholar]
- 61.Dember WN. In: Spontaneous Alternation. CL R, editor. Springer; New York: 1989. [Google Scholar]
- 62.Stefani MR, Nicholson GM, Gold PE. ATP-sensitive potassium channel blockade enhances spontaneous alternation performance in the rat: a potential mechanism for glucose-mediated memory enhancement. Neuroscience. 1999;93(2):557–563. doi: 10.1016/s0306-4522(99)00128-1. [DOI] [PubMed] [Google Scholar]
- 63.Winocur G, Greenwood CE. The effects of high fat diets and environmental influences on cognitive performance in rats. Behav Brain Res. 1999;101(2):153–61. doi: 10.1016/s0166-4328(98)00147-8. [DOI] [PubMed] [Google Scholar]
- 64.Lalonde R. The neurobiological basis of spontaneous alternation. Neuroscience & Biobehavioral Reviews. 2002;26(1):91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- 65.Shah AA, Parent MB. Septal infusions of glucose or pyruvate with muscimol impair spontaneous alternation. Brain Research. 2004;996(2):246–250. doi: 10.1016/j.brainres.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 66.Pych J, et al. Acetylcholine release in hippocampus and striatum during testing on a rewarded spontaneous alternation task. Neurobiology of Learning & Memory. 2005;84:93–101. doi: 10.1016/j.nlm.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 67.Krebs-Kraft DL, Parent MB. Hippocampal infusions of glucose reverse memory deficits produced by co-infusions of a GABA receptor agonist. Neurobiol Learn Mem. 2008;89(2):142–52. doi: 10.1016/j.nlm.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pearson-Leary J, McNay EC. Intrahippocampal administration of amyloid-β1-42 oligomers acutely impairs spatial working memory, insulin signalling, and hippocampal metabolism. Journal of Alzheimer’s Disease. 2012;29:1–10. doi: 10.3233/JAD-2012-112192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farr SA, et al. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149(5):2628–36. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Costa MS, et al. Caffeine improves adult mice performance in the object recognition task and increases BDNF and TrkB independent on phospho-CREB immunocontent in the hippocampus. Neurochem Int. 2008;53(3-4):89–94. doi: 10.1016/j.neuint.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 71.Costa MS, et al. Caffeine prevents age-associated recognition memory decline and changes brain-derived neurotrophic factor and tirosine kinase receptor (TrkB) content in mice. Neuroscience. 2008;153(4):1071–8. doi: 10.1016/j.neuroscience.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 72.Alhaider IA, et al. Sleep deprivation prevents stimulation-induced increases of levels of P-CREB and BDNF: protection by caffeine. Mol Cell Neurosci. 2011;46(4):742–51. doi: 10.1016/j.mcn.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 73.Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22(23):10399–407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Connolly S, Kingsbury TJ. Caffeine modulates CREB-dependent gene expression in developing cortical neurons. Biochem Biophys Res Commun. 2010;397(2):152–6. doi: 10.1016/j.bbrc.2010.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santi S, et al. Hippocampal neurons recycle BDNF for activity-dependent secretion and LTP maintenance. EMBO J. 2006;25(18):4372–80. doi: 10.1038/sj.emboj.7601303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiology of learning and memory. 2010;93(4):546–553. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Julien C, et al. High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiology of Aging. 2008;31(9):1516–1531. doi: 10.1016/j.neurobiolaging.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 78.Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci. 2004;19(7):1699–707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- 79.Dandona P, et al. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab. 2001;86(7):3257–65. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 80.Merighi S, Benini A, Mirandola P, Gessi S, Varani S, Simioni C, Leung E, Maclennan S, Baraldi PG, Borea PA. Caffeine Inhibits Adenosine-Induced Accumulation of Hypoxia-Inducible Factor-1α, Vascular Endothelial Growth Factor, and Interleukin-8 Expression in Hypoxic Human Colon Cancer Cells. Molecular Pharmacology. 2007;72(2):395–406. doi: 10.1124/mol.106.032920. [DOI] [PubMed] [Google Scholar]
- 81.Tong L, Balazs R, Soiampornkul R, Thangnipon W, Cotman CW. Interleukin-1β impairs brain derived neurotrophic factor-induced signal transduction. Neurobiology of Aging. 2008;29(9):1380–1393. doi: 10.1016/j.neurobiolaging.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vila-Luna S, et al. Chronic caffeine consumption prevents cognitive decline from young to middle age in rats, and is associated with increased length, branching, and spine density of basal dendrites in CA1 hippocampal neurons. Neuroscience. 2012;202:384–95. doi: 10.1016/j.neuroscience.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 83.Simons SB, et al. Caffeine-induced synaptic potentiation in hippocampal CA2 neurons. Nat Neurosci. 2012;15(1):23–5. doi: 10.1038/nn.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]