Abstract
Background
The efficacy of combining carbohydrate quality with exercise on metabolic syndrome risk is unclear. Thus, we determined the effects of exercise training with a low or high glycemic diet on metabolic syndrome severity (Z-score).
Methods
Twenty-one adults (66.2 ± 1.1 yr; BMI = 35.3 ± 0.9 kg/m2) with metabolic syndrome were randomized to 12 weeks of exercise (60 minutes/d for 5 d/week at ~85% HRmax) and provided a low-glycemic (n=11; LoGIx) or high glycemic (n=10; HiGIx) diet. Z-scores were determined from: blood pressure, triglycerides (TG), high-density lipoproteins (HDL), fasting plasma glucose (FPG), and waist circumference (WC) before and after the intervention. Body composition, aerobic fitness, insulin resistance, and non-esterfied fatty acid (NEFA) suppression were also assessed.
Results
LoGIx and HiGIx decreased body mass and insulin resistance and increased aerobic fitness comparably (p < 0.05). LoGIx and HiGIx decreased the Z-score similarly, as each intervention decreased blood pressure, TG, FPG, and WC (p < 0.05). HiGIx tended to suppress NEFA during insulin stimulation compared to LoGIx (p = 0.06).
Conclusions
Our findings highlight that exercise with weight loss reduces metabolic syndrome severity whether individuals were randomized to a high or low glycemic index diet.
Keywords: aging, obesity, lifestyle modification, diabetes, impaired glucose tolerance
Introduction
Approximately 35 million adults in the U.S. over the age of 60 years have metabolic syndrome [1]. The components of metabolic syndrome (i.e. central obesity, hypertension, dyslipidemia and hyperglycemia) increase risk for type 2 diabetes and cardiovascular disease. Given that nearly 20% of the U.S. population is expected to be over the age of 65 years by 2030, the number of individuals with metabolic syndrome will rise. Thus, there is a need to identify optimal treatments to prevent metabolic syndrome, and reduce the associated health and economic burden.
The U.S. Diabetes Prevention Program demonstrated that lifestyle modification, consisting of increased physical activity and low-fat diet, reduced metabolic syndrome prevalence compared to metformin treatment or placebo [2]. Exercise training reduces cardiometabolic risk factors, including blood pressure, lipids and insulin resistance, in older adults with metabolic syndrome [3,4]. Despite reports that greater weight loss reverses the constellation of metabolic syndrome risk factors [5], exercise training with hypocaloric diet has not translated into greater reductions in cardiometabolic risk factors compared to exercise training alone [6,7]. This suggests that exercise may be sufficient to correct many of the underlying metabolic syndrome risk factors. However, it may be possible to enhance the effect of exercise on these risk factors by adjusting the glycemic content of the diet.
Although lifestyle interventions often recommend carbohydrate-based diets, few studies have determined the role of carbohydrate type in combination with exercise on metabolic syndrome severity. High glycemic index carbohydrates increase plasma glucose concentrations, blood lipids, ectopic lipid storage, and the demand for insulin [8], whereas low-glycemic index diets improve glycemic control and lower triglycerides, blood pressure, and hyperinsulinemia [9,10]. We have shown that a low-glycemic index diet combined with exercise training raised insulin sensitivity [11] and lowered glucose concentrations, inflammation, and systolic blood pressure to a greater extent than a high glycemic index diet in older adults [12,13]. To date, however, no study has determined the efficacy of a low glycemic index diet combined with exercise on metabolic syndrome severity (i.e. Z-score), as opposed to metabolic syndrome being present or absent. Considering metabolic syndrome in this way has clinical relevance as it allows a change in cardiometabolic health to be measured across a continuum. Therefore, the purpose of this study was to determine the effect of exercise training with a low glycemic diet, compared to a high glycemic diet, on metabolic syndrome severity and insulin resistance in older adults. We hypothesized that a low glycemic diet with exercise training would reduce metabolic syndrome severity and insulin resistance more than a high glycemic diet.
Methods
Subjects
Twenty-one older (66.2 ± 1.1 yr) obese (BMI = 35.5 ± 0.9 kg/m2) adults (see Table 2) who met the National Cholesterol Education Program Adult Treatment Panel (ATP) III criteria for metabolic syndrome were used in this analysis from our past work [13,14,15]. Subjects were non-smoking, weight stable (<2 kg in previous 6 months), sedentary (less than <30 min/d <3 d/week), and free of chronic disease (i.e. hematological, renal, hepatic, cardiovascular) or medications known to affect the primary outcomes. Participants were randomly assigned to exercise training with a low-glycemic index diet (LoGIx; n=11) or a high glycemic index diet (HiGIx; n=10). Post-menopausal women were not on hormone replacement therapy, and all subjects provided signed informed consent approved by our Institutional Review Board.
Table 2.
Effects of HiGIx and LoGIx on body composition, blood pressure, lipids, insulin resistance, and aerobic fitness.
| Metabolic Syndrome - HiGIx | Metabolic Syndrome - LoGIx | ANOVA (p-value) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Test | Group x Test | |
| n (M,F) | 10 (3F, 7M) | - | 11 (7F, 4M) | - | - | - |
| Age (years) | 65.6 ± 1.3 | - | 67.2 ± 1.6 | - | - | - |
| Weight (kg) | 107.9 ± 4.3 | 96.9 ± 3.8 | 93.6 ± 4.0 | 86.8 ± 3.5 | ≤ 0.001 | ≤ 0.09 |
| Body Mass Index (kg/m2) | 36.7 ± 0.9 | 33.1 ± 1.2 | 33.8 ± 1.3 | 31.4 ± 1.3 | ≤ 0.001 | ≤ 0.10 |
| Fat Mass (kg) | 45.1 ± 2.1 | 36.2 ± 3.2 | 41.6 ± 2.1 | 35.9 ± 2.2 | ≤ 0.001 | ≤ 0.07 |
| Fat Free Mass (kg) | 62.4 ± 4.0 | 60.7 ± 3.7 | 52.1 ± 3.3 | 50.9 ± 2.8 | ≤ 0.01 | ≤ 0.67 |
| Hemoglobin A1c (%) | 5.6 ± 0.2 | 5.4 ± 0.1 | 5.8 ± 0.2 | 5.7 ± 0.2 | ≤ 0.02 | ≤ 0.97 |
| 2-hour Glucose (mg/dl) | 137.4 ± 11.6 | 131.5 ± 16.8 | 152.5 ± 8.7 | 142.9 ± 9.8 | ≤ 0.18 | ≤ 0.39 |
| Fasting Plasma Insulin (μU/ml) | 21.9 ± 5.8 | 11.9 ± 1.4 | 31.2 ± 8.5 | 15.2 ± 2.0 | ≤ 0.01 | ≤ 0.53 |
| 2-hour Plasma Insulin (μU/ml) | 111.6 ± 27.7 | 88.1 ± 24.2 | 164.4 ± 34.8 | 94.0 ± 31.9 | ≤ 0.02 | ≤ 0.22 |
| HOMA-IR | 4.3 ± 0.6 | 2.7 ± 0.3 | 5.2 ± 0.7 | 3.2 ± 0.5 | ≤ 0.003 | ≤ 0.80 |
| TC (mg/dL) | 205.6 ± 9.5 | 183.5 ± 9.7 | 200.8 ± 12.0 | 180.6 ± 10.3 | ≤ 0.002 | ≤ 0.87 |
| LDL (mg/dL) | 136.2 ± 6.8 | 123.0 ± 7.5 | 120.3 ± 9.3 | 112.1 ± 9.9 | ≤ 0.05 | ≤ 0.63 |
| VO2max (ml/kg/min) | 22.2 ± 1.5 | 28.3 ± 2.7 | 19.6 ± 0.9 | 25.2 ± 1.9 | ≤ 0.0001 | ≤ 0.87 |
Data are mean ± standard error of mean. There were no statistical differences at baseline between groups for any outcome. HiGIx = high glycemic index; LoGIx = low-glycemic index.
Aerobic Fitness
Maximum oxygen consumption (VO2max) was determined using a continuous incremental treadmill exercise test (Jaeger Oxygcon Pro; Viasys, Yorba Linda, CA). VO2max was determined as previously described (12). Maximal heart rate (HRmax) obtained during this test was used during exercise training.
Body Composition
Height was measured without shoes using a wall-mounted stadiometer and weight was recorded on a digital scale in a hospital gown. Dual-x-ray absorptiometry (DEXA; Lunar Prodigy, Madison, WI) was used to quantify total fat mass and fat free-mass.
Inpatient Control Period
Pre and post treatment assessments of cardiometabolic risk factors and insulin resistance were conducted during a 3-day inpatient stay in the Clinical Research Unit. Subjects were provided weight-maintenance meals (resting metabolic rate x 1.2 activity factor; 55% CHO, 30% fat, 15% protein). Following the intervention, cardiometabolic risk factor measurements and insulin resistance were made approximately 16–18 hours after the last exercise bout. Subjects maintained their respective dietary intervention at the time of post-testing.
Exercise Training and Nutritional Intervention
All participants underwent a 12-week supervised exercise training program, consisting of treadmill-walking and cycle ergometer exercise at approximately 85% of HRmax. All meals and fluids were provided to the participants throughout the intervention. A registered dietitian (H.B.) created the diets to be isocaloric to the individual requirements at baseline (i.e. resting metabolic rate measurement x 1.2 activity factor). As a result, weight loss during the interventions was primarily due to exercise energy expenditure, not caloric restriction. The macronutrient composition, including fiber, was matched between groups. However, the LoGIx subjects received a diet corresponding to a GI of 40 arbitrary units (au), while the HiGIx subjects were provided an 80 au GI diet (see Table 1). Recipes were identical between diets, with only substitutions made between the types of carbohydrate. Dietary compliance was determined by daily food container weigh backs, and diet analysis was performed with the Nutritionist Pro software (Axxya systems, Stafford, TX).
Table 1.
Low and high glycemic index diets while exercise training.
| Metabolic Syndrome- HiGIx | Metabolic Syndrome - LoGIx | |
|---|---|---|
| GI (au) | 80.3 ± 0.6* | 40.3 ± 0.2 |
| GL (au) | 241.7 ± 16.9* | 105.0 ± 4.8 |
| Energy Intake (kcal/d) | 2082.0 ± 131.7 | 1827.7 ± 81.7 |
| Carbohydrate (gram/d) | 301.0 ± 20.2 | 256.7 ± 11.8 |
| Fiber (gram/d) | 30.5 ± 2.1 | 29.5 ± 1.3 |
| Fat (gram/d) | 88.6 ± 5.6 | 78.9 ± 3.5 |
| Protein (gram/d) | 66.3 ± 3.9 | 58.2 ± 2.3 |
Data are mean ± standard error of mean. GI = glycemic index. GL = glycemic load. Significant compared to LoGIx,
p < 0.05.
Cardiometabolic Risk Factors
After an overnight fast, blood pressure was measured in the seated position after 10 minutes of rest and was based on the average of 3 measurements. Mean arterial pressure (MAP) was calculated as: MAP = 2/3(DBP) + (1/3SBP). Waist circumference (WC) was measured up to 3 times using a plastic tape measure approximately 2 cm above the umbilicus. Measurements within 0.5 cm were used for analysis. It is possible that we overestimated our waist circumference results by measuring at the umbilicus compared to the NCEP boney landmark recommendation [15]. However, we believe our waist circumference data are valid since they parallel the change in visceral adiposity as measured by CT scans in our previous work [13,14,15]. Fasting plasma glucose (FPG), triglyceride (TG), and high density lipoprotein (HDL) measurements were obtained from blood sampling. Sex-specific Z-scores were calculated to indicate changes in metabolic syndrome severity before and after the intervention [16]. The equations used were: Z-scoremen = [(40-HDL)/10.3] + [(TG-150/66.5)] + [(FPG-100)/13.4] + [(WC-102)/8.5] + [(MAP-100)/10.0], and Z-scorewomen = [(50-HDL)/12.4] + [(TAG-150/66.5)] + [(FPG-100)/13.4] + [(WC-88)/11.7] + [(MAP-100)/10.03]. ATP III scores were also calculated for each subject based on the sum of risk factors meeting metabolic syndrome criteria.
Insulin Resistance
After an overnight fast, a 2-hour euglycemic-hyperinsulinemic clamp was performed as previously described [14]. In summary, a constant infusion (40 mU/m2·min−1) of insulin was administered via an indwelling catheter placed in an antecubital vein. Glucose (20%) was infused at a variable rate to maintain plasma glucose at 90 mg/dl. Arterialized plasma samples were collected from a retrograde hand vein warmed to 60°C. Blood samples were collected every 5 minutes for the analysis of glucose and every 15 minutes for analysis of insulin. Non-esterfied fatty acid (NEFA) plasma samples were collected at baseline and 120 minutes. Insulin stimulated NEFA suppression was calculated as: [1− (NEFAclamp/NEFAbase) * 100]. Respiratory gases (VO2 and VCO2) were analyzed via indirect calorimetry for 20 minutes in the fasted and insulin stimulated state (last 20 minutes of clamp) while the subject rested in the supine position. The last 10-minutes were averaged for determination of substrate oxidation [17,18]. Non-oxidative glucose disposal was calculated as: glucose infusion rate – total carbohydrate oxidation rate. Homeostatic model assessment (HOMA-IR), a surrogate for hepatic insulin resistance, was calculated as fasting glucose (mg/dl) x fasting insulin (uU/ml)/ 405.
Glucose Tolerance
After an overnight fast, blood samples were collected from an antecubital vein and a 75 gram glucose load was administered orally. Blood samples were then obtained at 120 minutes.
Biochemical Analysis
Plasma glucose was determined using a glucose oxidase assay (YSI 2300 STAT Plus, Yellow Springs, OH). Plasma insulin was measured by radioimmunoassay (Millipore, Billerica, MA). Plasma triglycerides and cholesterol were analyzed using enzymatic methods with an automated platform (Roche Modular Diagnositcs, Indianapolois, IN). Plasma NEFA was measured by enzymatic colorimetry (Wako Chemicals, Richmond, VA).
Statistical Analysis
Data are expressed as mean ± standard error of the mean (SEM). Group means were compared using the R statistical software package (Version 2.4.0, The R Foundation, Vienna, Austria, 2006). Diet and baseline variables were assessed by unpaired t-tests. Baseline variables were not different between groups. Outcomes were assessed using a two-way (group x test) repeated measures ANOVA. Unpaired t-tests were used to determine statistical differences between group mean differences (i.e. change from pre to post) when there was a significant group x test interaction. Pearson’s correlation was used to examine associations between outcomes. Significance was accepted as α ≤ 0.05.
Results
Exercise and Diet Compliance
Exercise adherence and dietary data were previously reported [13,14]. However, dietary intake data are reported here for clarity (i.e. ~55% carbohydrate, 28% fat and 16% protein; Table 1). By design, glycemic index and load were statistically different between groups (p < 0.05; Table 1).
Metabolic Syndrome Severity
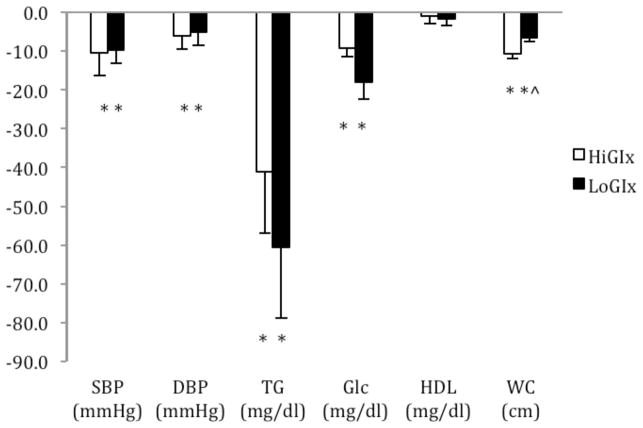
There was no statistical differences at baseline between HiGIx and LoGIx for systolic blood pressure (135.2 ± 6.0 vs. 132.9 ± 3.6 mmHg; p = 0.75), diastolic blood pressure (80.6 ± 3.4 vs. 80.5 ± 3.2 mmHg; p = 0.99), triglycerides (139.1 ± 21.1 vs. 180.9 ± 23.6 mg/dl; p = 0.20), HDL (42.4 ± 3.7 vs. 46.8 ± 3.9 mg/dl; p = 0.42) and waist circumference (121.9 ± 2.3 vs. 115.0 ± 3.9 cm; p = 0.16). HiGIx and LoGIx reduced body mass, waist circumference, and fat mass by approximately 8% each (p < 0.05; Figure 1a and 1b, Table 2). Both groups lowered the metabolic syndrome Z-score (p = 0.001; Figure 1a) and ATP III score (HiGIx = pre: 3.6 ± 0.2 vs. post: 2.4 ± 0.5; LoGIx = pre: 3.6 ± 0.2 vs. post: 2.2 ± 0.3; p = 0.001). There were no group differences between the reduction in Z-score (p ≤ 0.85), ATP III score (p ≤ 0.85) or metabolic syndrome presence (HiGIx = 5 out of 10 vs. LoGIx = 6 out of 11). Consistent with these data, systolic and diastolic blood pressure and triglycerides were lowered by HiGIx and LoGIx (Figure 1a and 1b; p < 0.05). Neither GI group had effects on HDL.
Figure 1.

Figure 1a. Effects of HiGIx and LoGIx on metabolic syndrome Z-score outcomes. Data are mean ± standard error of mean. ^LoGIx compared to HiGIx (group x test interaction; p <0.05). *Test effect (p < 0.05).
Figure 1b. Effects of HiGIx and LoGIx on metabolic syndrome criteria. Data are mean ± standard error of mean. ^LoGIx compared to HiGIx (group x test interaction; p <0.05).*Test effect (p < 0.05).
Glycemic Control
Baseline fasting glucose concentrations were not different between HiGIx and LoGIx (103.8 ± 3.5 vs. 112.3 ± 5.2 mg/dl; p = 0.19). Fasting glucose concentrations were reduced comparably between HiGIx and LoGIx (Figure 1a and 1b; p <0.05), and both groups lowered HbA1c (p < 0.05; Table 2).
Insulin Resistance and NEFA suppression
HiGIx and LoGIx reduced insulin resistance measured by clamp and HOMA-IR to a similar extent (p < 0.05; Table 2 & 3). Decreased insulin resistance was a result of both increased non-oxidative glucose disposal (p < 0.05) and carbohydrate oxidation (p < 0.03; Table 3). HiGIx showed a strong trend to increase insulin stimulated NEFA suppression, compared to LoGIx (p = 0.06) and both groups decreased lipid oxidation during insulin stimulation (p < 0.03; Table 3).
Table 3.
Effects of HiGIx and LoGIx on insulin sensitivity, carbohydrate and fat metabolism.
| Metabolic Syndrome - HiGIx | Metabolic Syndrome - LoGIx | ANOVA (p-value) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Test | Group x Test | |
| Fasted | ||||||
| NEFA (mM) | 0.56 ± 0.04 | 0.57 ± 0.03 | 0.60 ± 0.04 | 0.55 ± 0.04 | ≤ 0.19 | ≤ 0.21 |
| CHO utilization (%) | 35.7 ± 2.7 | 27.9 ± 2.9 | 26.3 ± 4.5 | 31.1 ± 4.8 | ≤ 0.68 | ≤ 0.10 |
| Lipid utilization (%) | 65.3 ± 2.8 | 72.1 ± 2.9 | 73.7 ± 4.5 | 68.9 ± 4.8 | ≤ 0.68 | ≤ 0.10 |
| Clamp | ||||||
| GDRI (mg/kg-FFM/min/μU/ml) | 0.04 ± 0.01 | 0.08 ± 0.01 | 0.04 ± 0.01 | 0.06 ± 0.01 | ≤ 0.001 | ≤ 0.95 |
| Insulin (uU/ml) | 96.6 ± 5.2 | 92.7 ± 5.2 | 101.1 ± 8.1 | 101.6 ± 7.2 | ≤ 0.66 | ≤ 0.58 |
| NOGD (mg/kg-FFM/min) | 1.4 ± 0.6 | 3.7 ± 0.7 | 1.0 ± 0.5 | 2.1 ± 0.4 | ≤ 0.003 | ≤ 0.13 |
| CHO utilization (%) | 55.8 ± 2.1 | 63.0 ± 4.0 | 52.9 ± 3.4 | 56.3 ± 2.3 | ≤ 0.03 | ≤ 0.98 |
| Lipid utilization (%) | 44.2 ± 6.8 | 36.9 ± 4.0 | 47.0 ± 3.4 | 43.7 ± 2.3 | ≤ 0.03 | ≤ 0.98 |
| NEFA (mM) | 0.15 ± 0.3 | 0.11 ± 0.3 | 0.13 ± 0.3 | 0.15 ± 0.3 | ≤ 0.73 | ≤ 0.12 |
| NEFA suppression (%) | 74.1 ± 4.8 | 80.7 ± 4.9 | 76.8 ± 6.4 | 71.9 ± 5.9 | ≤ 0.85 | ≤ 0.06 |
Data are mean ± standard error of mean. There were no statistical differences at baseline between groups for any outcome. GDRI = glucose disposal rate divided by insulin. NOGD = non-oxidative glucose disposal. CHO = carbohydrate. NEFA = non-esterfied fatty acid.
Correlations
Reductions in fasting insulin concentrations (r = −0.38; p = 0.09), total cholesterol (r = 0.49; p < 0.05), and total fat mass (r = 0.46; p < 0.04) were correlated with lower metabolic syndrome severity (i.e. Z-score). Insulin resistance calculated by HOMA-IR (r = 0.48; p < 0.05), but not the clamp (r = −0.29; p = 0.20), was correlated with lower metabolic syndrome severity. Finally, the change in NEFAsuppression correlated with the change in fat mass (r = −0.45; p < 0.05).
Discussion
The novel finding from this study is that 12-weeks of exercise can successfully reduce metabolic syndrome severity, and that a low-glycemic index diet has little added benefit on metabolic syndrome severity when compared to a high glycemic index diet matched on fiber content in older men and women. Lifestyle modification, consisting of a low-fat diet and increased physical activity, reduces metabolic syndrome prevalence by approximately 45% in glucose intolerant adults and post-menopausal women [2,6]. This is consistent with previous work from our lab demonstrating significant improvements in aerobic fitness and reductions in cardiometabolic risk factors after high intensity exercise and diet induced weight loss in older men and women with metabolic syndrome [7]. Aerobic fitness is related to reductions in many of the underlying metabolic syndrome risk factors (e.g. insulin resistance, inflammation, and blood pressure) [19,20]. In the current study, both exercise groups increased VO2max by approximately 27%, however, only reductions in body fat correlated with decreased metabolic syndrome severity. Decreased body fat was correlated with increased insulin stimulated NEFA suppression. Reduced availability of NEFA may have contributed to less inflammation and improved cardiometabolic health [21,22]. Thus, these observations suggest that increasing physical activity leads to fat loss, which decreases cardiometabolic risk in adults with metabolic syndrome.
Aerobic exercise, and to some extent resistance exercise, decreases triglyceride and increases HDL concentrations [23], reduces waist circumference [24,25], and lowers blood pressure [26]. Although exercise may not improve all ATP III criteria outcomes, the collective change in these outcomes (i.e. Z-score) has clinical utility. We found that exercise training with high or low glycemic index diet, decreased metabolic syndrome severity. Moreover, 5 out of 10 in the HiGIx group and 6 out of 10 in the LoGIx group no longer had the metabolic syndrome after the intervention. These findings are not entirely surprising given that HiGIx and LoGIx improved insulin sensitivity, aerobic fitness, and adiposity comparably [27]. Regardless, our data are consistent with previous work showing that aerobic exercise training, with or without resistance exercise, reduces metabolic syndrome severity in middle-aged men and women regardless of exercise intensity, duration, or caloric deficit [16, 28, 29]. We acknowledge the small sample size may have limited our ability to detect statistical differences between metabolic syndrome severity. Thus, future studies with larger sample sizes are warranted to examine whether exercise training without weight loss, or at lower exercise intensities, while consuming either a low or high glycemic diet yields similar results on metabolic syndrome severity [30,31].
Glucose abnormalities are found in approximately 40% of adults with metabolic syndrome [32]. Although exercise decreases/maintains glucose concentrations [7,29,33], Cheong et al. reported that a lifestyle intervention consisting of increased steps and consumption of low-glycemic foods, compared to walking only, had similar reductions in HbA1c in adults with type 2 diabetes [34]. Similarly, we show that 12 weeks of supervised exercise training, independent of strict dietary control, was effective at lowering HbA1c levels in adults with metabolic syndrome. LoGIx and HiGIx likely improved glycemic control because of reduced insulin resistance [14]. Interestingly, similar reductions in HbA1c occurred despite increased NEFA suppression in 80% of the individuals after HiGIx compared to only 20% in the LoGIx intervention. Although kinetic tracer studies would have more accurately assessed lipid flux in this study, we previously demonstrated that exercise training with approximately 8% weight loss reduced NEFA concentrations by decreasing palmitate turnover rates and increasing lipid utilization in obese older adults [35]. In the current study, individuals lost approximately 8% body weight, and we found a significant correlation between fat mass loss and NEFA suppression. Together, these observations suggest that fat mass loss, not increased fat utilization, leads to decreased NEFA mobilization [21,22]. However, we cannot rule out the possibility that exercise training while consuming a high glycemic index carbohydrate diet increased extramyocellular lipid storage [36]. Regardless of the exact mechanism, changes in NEFA concentrations after the HiGIx intervention did not translate into enhanced glycemic control. Thus, the clinical relevance of altered lipid availability after the HiGIx intervention remains unclear. Longer duration clinical trials comparing HiGIx and LoGIx are needed to confirm our observation, as differences in glycemic control may become evident after 12 months [10].
In conclusion, these findings indicate that exercise training with approximately 8% weight loss reduces metabolic syndrome severity whether individuals followed a low or high GI diet. However, similar fiber intakes between diets may reduce the “real-world” relevance of these findings because fiber intake was approximately 15 g/d more than what the typical American is currently consuming. Thus, it remains possible that a low glycemic index and high fiber diet combined with exercise may have greater efficacy in lowering metabolic severity compared to a high glycemic index and low fiber diet. More studies are needed to fully understand the utility of exercise training with different types of carbohydrates on reducing cardiovascular and type 2 diabetes risk in adults with metabolic syndrome.
Acknowledgments
S.K.M, H.B., and J.P.K developed the study hypothesis. H.B. designed the diet interventions. S.K.M was primarily responsible for data analysis and statistical integrity. S.K.M, N.F., T.P.J.S, J.M.H., K.R.K., J.F, M.R., H.B., and J.P.K assisted with data collection, analysis, editing, and study organization. S.K.M and J.P.K. wrote the manuscript. We thank the nursing staff for technical assistance and the dedicated participants for their effort. This research was supported by National Institutes of Health Grants RO1 AG-12834 (J.P.K.), and National Institutes of Health National Center for Research Resources, 1UL1RR024989, Cleveland, OH. J.M.H and K.R.K were supported by T32 HL-007887 and T32 DK007319, respectively.
Footnotes
The authors report no conflict of interest.
References
- 1.Ford E, Giles W, Dietz W. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 2.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler S Diabetes Prevention Program Research Group. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142(8):611–619. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katzmarzyk P, Leon A, Wilmore J, Skinner J, Rao DC, Rankinen T, Bouchard C. Targeting the metabolic syndrome with exercise: evidence from the HERITAGE Family Study. Med Sci Sports Exerc. 2003;35(10):1703–1709. doi: 10.1249/01.MSS.0000089337.73244.9B. [DOI] [PubMed] [Google Scholar]
- 4.Villareal D, Miller B, Banks M, Fontana L, Sinacore D, Klein S. Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J Clin Nutr. 2006;84(6):1317–1323. doi: 10.1093/ajcn/84.6.1317. [DOI] [PubMed] [Google Scholar]
- 5.Stewart K, Bacher A, Turner K, Lim J, Hees P, Shapiro E, Tayback M, Ouyang P. Exercise and risk factors associated with metabolic syndrome in older adults. Am J Prev Med. 2005;28(1):9–18. doi: 10.1016/j.amepre.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Joseph L, Prigeon R, Blumenthal J, Ryan A, Goldberg A. Weight loss and low-intensity exercise for the treatment of metabolic syndrome in obese postmenopausal women. J Gerontol Biol Sci Med Sci. 2011;66(9):1022–1029. doi: 10.1093/gerona/glr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yassine HN, Marchetti CM, Krishnan R, Vrobel T, Gonzalez F, Kirwan JP. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults--a randomized clinical trial. J Gerontol Biol Sci Med Sci. 2009;64(1):90–95. doi: 10.1093/gerona/gln032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ludwig D. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 9.Rizkalla S, Taghrid L, Laromiguiere M, Huet D, Boillot J, Rigoir A, Elgrably F, Slama G. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycemic index diet in type 2 diabetic men: a randomized controlled trial. Diabetes Care. 2004;27(8):1866–1872. doi: 10.2337/diacare.27.8.1866. [DOI] [PubMed] [Google Scholar]
- 10.Wolever TMS, Mehling C, Chiasson J, Josse RG, Leiter LA, Maheux P, Rabasa Lhoret R, Rodger NW, Ryan EA. Low glycaemic index diet and disposition index in type 2 diabetes (the Canadian trial of carbohydrates in diabetes): a randomised controlled trial. Diabetologia. 2008;51(9):1607–1615. doi: 10.1007/s00125-008-1093-x. [DOI] [PubMed] [Google Scholar]
- 11.Kirwan JP, Barkoukis H, Brooks L, Marchetti CM, Stetzer B, Gonzalez F. Exercise training and dietary glycemic load may have synergistic effects on insulin resistance in older obese adults. Ann Nutr Metab. 2009;55(4):326–333. doi: 10.1159/000248991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solomon TPJ, Haus JM, Kelly KR, Cook M, Riccardi M, Rocco M, Kashyap SR, Barkoukis H, Kirwan JP. Randomized trial on the effects of a 7-d low-glycemic diet and exercise intervention on insulin resistance in older obese humans. Am J Clin Nutr. 2009;90(5):1222–1229. doi: 10.3945/ajcn.2009.28293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly KR, Haus JM, Solomon TPJ, Patrick Melin A, Cook M, Rocco M, Barkoukis H, Kirwan JP. A low-glycemic index diet and exercise intervention reduces TNF(alpha) in isolated mononuclear cells of older, obese adults. J Nutr. 2011;141(6):1089–1094. doi: 10.3945/jn.111.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon TPJ, Haus JM, Kelly KR, Cook M, Filion J, Rocco M, Kashyap SR, Watanabe R, Barkoukis H, Kirwan JP. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr. 2010;92(6):1359–1368. doi: 10.3945/ajcn.2010.29771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundy S, Cleeman J, Daniels S, Donato K, Eckel R, Franklin B, Gordon D, Krauss R, Savage P, Smith S, Spertus J, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit Pathw Cardiol. 2005;4(4):198–203. doi: 10.1097/00132577-200512000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Johnson J, Slentz C, Houmard J, Samsa G, Duscha B, Aiken L, McCartney J, Tanner C, Kraus W. Exercise training amount and intensity effects on metabolic syndrome (from Studies of a Targeted Risk Reduction Intervention through Defined Exercise) Am J Cardiol. 2007;100(12):1759. doi: 10.1016/j.amjcard.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55(2):628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 18.Kuo CC, Fattor JA, Henderson GC, Brooks GA. Lipid oxidation in fit young adults during postexercise recovery. J Appl Physiol. 2005;99(1):349–356. doi: 10.1152/japplphysiol.00997.2004. [DOI] [PubMed] [Google Scholar]
- 19.Lakka T, Laaksonen D. Physical activity in prevention and treatment of the metabolic syndrome. Applied Physiology, Nutrition, and Metabolism. 2007;32(1):76–88. doi: 10.1139/h06-113. [DOI] [PubMed] [Google Scholar]
- 20.Hassinen M, Lakka T, Savonen K, Litmanen H, Kiviaho L, Laaksonen D, Komulainen P, Rauramaa R. Cardiorespiratory fitness as a feature of metabolic syndrome in older men and women: the Dose-Responses to Exercise Training study (DR’s EXTRA) Diabetes Care. 2008;31(6):1242–1247. doi: 10.2337/dc07-2298. [DOI] [PubMed] [Google Scholar]
- 21.Schenk S, Harber M, Shrivastava C, Burant C, Horowitz J. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. J Physiol. 2009;587(20):4949–4961. doi: 10.1113/jphysiol.2009.175489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittendorfer B, Magkos F, Fabbrini E, Mohammed BS, Klein S. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity. 2009;17(10):1872–1877. doi: 10.1038/oby.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraus W, Houmard J, Duscha B, Knetzger K, Wharton M, McCartney J, Bales C, Henes S, Samsa G, Otvos J, Kulkarni K, Slentz C. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347(19):1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 24.O’Leary VB, Marchetti CM, Krishnan R, Stetzer B, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol. 2006;100(5):1584–1589. doi: 10.1152/japplphysiol.01336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross R, Janssen I, Dawson J, Kungl A, Kuk JL, Wong SL, Nguyen-Duy T, Lee S, Kilpatrick K, Hudson R. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12(5):789–798. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 26.Fagard RH. Exercise is good for your blood pressure: effects of endurance training and resistance training. Clinical and Experimental Pharmacology Physiology. 2006;33(9):853–856. doi: 10.1111/j.1440-1681.2006.04453.x. [DOI] [PubMed] [Google Scholar]
- 27.Kahn SE, Prigeon RL, Schwartz RS, Fujimoto WY, Knopp RH, Brunzell JD, Porte D. Obesity, body fat distribution, insulin sensitivity and Islet beta-cell function as explanations for metabolic diversity. J Nutr. 2001;131(2):354S–360S. doi: 10.1093/jn/131.2.354S. [DOI] [PubMed] [Google Scholar]
- 28.Bateman L, Slentz C, Willis L, Shields AT, Piner L, Bales C, Houmard J, Kraus W. Comparison of Aerobic Versus Resistance Exercise Training Effects on Metabolic Syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise - STRRIDE-AT/RT) Am J Cardiol. 2011;108(6):838–844. doi: 10.1016/j.amjcard.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potteiger J, Claytor R, Hulver M, Hughes M, Carper M, Richmond S, Thyfault J. Resistance exercise and aerobic exercise when paired with dietary energy restriction both reduce the clinical components of metabolic syndrome in previously physically inactive males. Eur J Appl Physiol. 2011 doi: 10.1007/s00421-011-2174-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Segal KR, Edano A, Abalos A, Albu J, Blando L, Tomas MB, Pi-Sunyer FX. Effect of exercise training on insulin sensitivity and glucose metabolism in lean, obese, and diabetic men. J Appl Physiol. 1991;71(6):2402–2411. doi: 10.1152/jappl.1991.71.6.2402. [DOI] [PubMed] [Google Scholar]
- 31.Kemmler W, Von Stengel S, Engelke K, Kalender W. Exercise decreases the risk of metabolic syndrome in elderly females. Med Sci Sports Exerc. 2009;41(2):297–305. doi: 10.1249/MSS.0b013e31818844b7. [DOI] [PubMed] [Google Scholar]
- 32.Ong K, Tso AWK, Lam KSL, Cherny S, Sham P, Cheung BMY. Using glycosylated hemoglobin to define the metabolic syndrome in United States adults. Diabetes Care. 2010;33(8):1856–1858. doi: 10.2337/dc10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol. 2004;96(1):101. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- 34.Cheong S, McCargar L, Paty B, Tudor Locke C, Bell R. The First Step First Bite Program: guidance to increase physical activity and daily intake of low-glycemic index foods. J Am Diet Assoc. 2009;109(8):1411–1416. doi: 10.1016/j.jada.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Solomon TPJ, Haus JM, Marchetti CM, Stanley W, Kirwan JP. Effects of exercise training and diet on lipid kinetics during free fatty acid-induced insulin resistance in older obese humans with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2009;297(2):E552–E559. doi: 10.1152/ajpendo.00220.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haus JM, Solomon TPJ, Lu L, Jesberger J, Barkoukis H, Flask C, Kirwan JP. Intramyocellular lipid content and insulin sensitivity are increased following a short-term low-glycemic index diet and exercise intervention. Am J Physiol Endocrinol Metab. 2011;301(3):E511–E516. doi: 10.1152/ajpendo.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]