SUMMARY
Human mitochondrial transcription factor A (TFAM) is a high-mobility group (HMG) protein at the nexus of mitochondrial DNA (mtDNA) replication, transcription and inheritance. Little is known about the mechanisms underlying its post-translational regulation. Here, we demonstrate that TFAM is phosphorylated within its HMG box 1 (HMG1) by cAMP-dependent protein kinase in mitochondria. HMG1 phosphorylation impairs the ability of TFAM to bind DNA and to activate transcription. We show that only DNA-free TFAM is degraded by the Lon protease, which is inhibited by the anti-cancer drug bortezomib. In cells with normal mtDNA levels, HMG1-phosphorylated TFAM is degraded by Lon. However in cells with severe mtDNA deficits, non-phosphorylated TFAM is also degraded as it is DNA-free. Depleting Lon in these cells increases TFAM, and upregulates mtDNA content, albeit transiently. Phosphorylation and proteolysis thus provide mechanisms for rapidly fine-tuning TFAM function and abundance in mitochondria, which are crucial for maintaining and expressing mtDNA.
INTRODUCTION
TFAM is essential for mtDNA synthesis and expression as well as mtDNA packaging (Asin-Cayuela and Gustafsson, 2007; Ekstrand et al., 2004; Kaufman et al., 2007; Kukat et al., 2011; Larsson et al., 1998). In animal models, a TFAM knockout in mice severely depletes mtDNA, abolishes oxidative phosphorylation and leads to embryonic lethality (Larsson et al., 1998). A heart-specific knockout results in cardiomyopathy during embryogenesis and neonatal death (Li et al., 2000). By contrast, TFAM overproduction in transgenic mice increases mtDNA content (Ekstrand et al., 2004; Larsson et al., 1998), and also ameliorates cardiac failure (Ikeuchi et al., 2005), neurodegeneration and age-dependent deficits in brain function (Hokari et al., 2010). TFAM is the most abundant component of mitochondrial nucleoids, which are protein complexes associated with mtDNA that orchestrate genome replication, expression and inheritance (Bogenhagen, 2011; Bogenhagen et al., 2008; Kukat et al., 2011). The in vivo packaging of mtDNA by TFAM has been estimated from ~35-50 molecules (Cotney et al., 2007; Maniura-Weber et al., 2004), to ~1,000-1,700 molecules per genome (Ekstrand et al., 2004; Kanki et al., 2004; Kaufman et al., 2007; Kukat et al., 2011; Pellegrini and Scorrano, 2007). Higher TFAM:mtDNA ratios are interpreted to result in tighter compaction of mtDNA and reduced accessibility to transcription, replication or repair factors, whereas lower ratios are predicted to permit increased accessibility. Recently, a debate has emerged as to whether TFAM is required for basal transcription, and whether it functions as both an activator and a repressor of transcription (Asin-Cayuela and Gustafsson, 2007; Falkenberg et al., 2002; Litonin et al., 2010; Lodeiro et al., 2012; Shi et al., 2012; Shutt et al., 2010; Sologub et al., 2009; Zollo et al., 2012). Future experiments are required to resolve this debate. Another fundamental question that has yet to be addressed, pertains to the regulatory processes controlling the binding and release cycle of TFAM at the mitochondrial genome.
Mitochondrial Lon belongs to the AAA+ family of proteins (ATPases associated with various cellular activities) and requires ATP-hydrolysis to degrade proteins (Venkatesh et al., 2012). As a quality control protease, human Lon selectively eliminates certain abnormal proteins (Bota and Davies, 2002). However, Lon also degrades some folded (Ondrovicova et al., 2005) and regulatory proteins (Granot et al., 2007; Tian et al., 2011). Although the majority of Lon is soluble within the matrix, it is also present in mitochondrial nucleoids (Bogenhagen et al., 2008). Lon binds mtDNA in a sequence-specific and strand-specific manner, showing low affinity binding to sequences on the heavy-strand that form parallel G-quartets (Chen et al., 2008; Liu et al., 2004). In cultured mammalian cells, Lon preferentially binds to the control region of mtDNA (Lu et al., 2007), which contains origins of replication and the heavy-strand promoter (HSP) and light-strand promoter (LSP) for transcription initiation (Bonawitz et al., 2006; Falkenberg et al., 2007). Lon is thus uniquely poised at the mitochondrial genome to regulate mtDNA metabolism or to remodel nucleoid composition. In Drosophila cells with normal mtDNA content, the knockdown of Lon increases the levels of TFAM protein as well as mtDNA, whereas the overexpression of Lon decreases these levels (Matsushima et al., 2010). By contrast, in human cells with normal mtDNA content, changes in Lon expression do not alter TFAM or mtDNA levels (Lu et al., 2007) (Fig. S1A-C). Such differences between flies and humans may be linked to phylogenetic diversity in the structure and metabolism of mtDNA. Interestingly, in human tissue or cells that are depleted of mtDNA, the protein levels of TFAM are dramatically reduced even though transcript levels are the same as in control cells with mtDNA (Larsson et al., 1994; Seidel-Rogol and Shadel, 2002). These findings implicate Lon in the proteolytic turnover of TFAM in humans as well as in flies.
Here, we demonstrate that PKA-mediated phosphorylation of TFAM within HMG1 occurs inside the mitochondrion, resulting in rapid and selective degradation by the Lon protease. HMG1 phosphorylation of TFAM leads to DNA-dissociation and reduced transcriptional activation. We propose that phosphorylation of TFAM within HMG1 causes electrostatic repulsion of the DNA phosphate backbone, thereby providing a mechanism for regulating mtDNA binding and release, which are essential for the maintenance and expression of the mitochondrial genome.
RESULTS
DNA-bound TFAM is resistant to Lon-mediated proteolysis
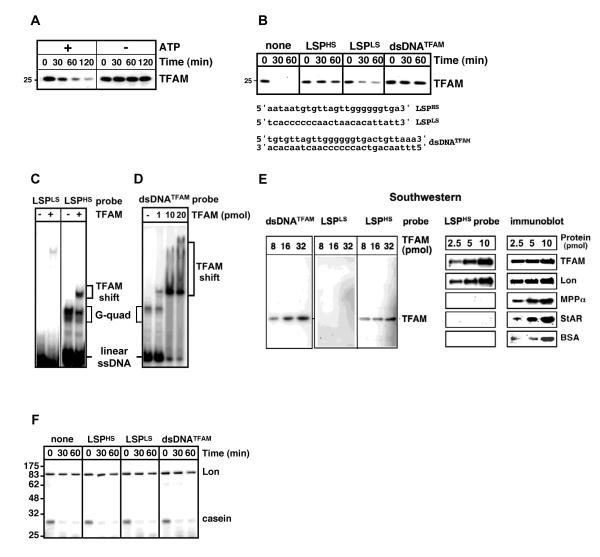
We set out to test the hypothesis that Lon selectively degrades TFAM that is not bound to mtDNA. Purified TFAM and Lon (Fig. S1D) were incubated with or without ATP/Mg2+. TFAM was rapidly degraded by Lon only when ATP was present (Fig. 1A). TFAM was not degraded by ClpXP, which is another AAA+ protease in the mitochondrial matrix (Fig. S1D-F). To determine if DNA-binding by TFAM affected its sensitivity to degradation, single-stranded or double-stranded DNA (ssDNA or dsDNA) oligonucleotides were preincubated with TFAM prior to adding Lon (Fig. 1B); a higher concentration of Lon was added to accelerate proteolysis and to more clearly observe the effect of DNA. We employed ssDNAs corresponding to heavy- and light- strand sequences upstream of LSP (LSPHS and LSPLS), as well as a dsDNA corresponding to the TFAM binding site (dsDNATFAM) (Fig. 1B) (Dairaghi et al., 1995). DNAs with greater relative binding to TFAM conferred greater resistance to Lon (Figs. 1B-E). TFAM was stabilized by LSPHS and dsDNATFAM, but only marginally by LSPLS (Fig. 1B). Correspondingly, LSPHS and dsDNATFAM showed greater relative binding to TFAM as compared to LSPLS in gel shift and Southwestern assays (Figs. 1C-E). LSPHS migrates as a fast mobility linear ssDNA and as a slow mobility G-quartet species on native gels (Fig. 1C) (Chen et al., 2008; Liu et al., 2004).
Figure 1. TFAM bound to DNA is resistant to Lon.
(A) and (B) TFAM (80 nM) was incubated with Lon (50 nM) with or without ATP (2 mM) (A), or first preincubated with DNA oligonucleotides (4 μM) for 10 min on ice, prior to adding Lon (80 nM) and ATP (2 mM) (B); TFAM was detected by immunoblotting. (C-E) TFAM (1 pmol or as indicated) incubated with radiolabeled DNA (4 pmol) was analyzed by gel shift (C and D) or Southwestern (E) assays. Southwestern membranes were probed with radiolabeled DNA or immunoblotted for TFAM, Lon, the mitochondrial processing peptidase α subunit (MPPα), steroidogenic acute regulatory protein (StAR), or bovine serum albumin (BSA). (F) Lon (66 nM) was preincubated with or without DNA (4 μM) for 10 min before adding casein (3 μM) and ATP, and analyzed by SDS-PAGE and Coomassie Blue staining.
As Lon is a ssDNA-binding protein (Chen et al., 2008; Liu et al., 2004), its binding to LSPHS or dsDNATFAM may directly block its protease activity resulting in TFAM stabilization. However, LSPHS nor dsDNATFAM failed to inhibit Lon-mediated degradation of casein (Fig. 1F). Alhough Lon shows the greatest relative affinity for LSPHS as compared to other mtDNA sequences (Chen et al., 2008; Liu et al., 2004), its binding to LSPHS is substantially weaker than that of TFAM (unpublished results). Thus, it is not possible to test whether DNA-bound Lon degrades DNA-free TFAM.
Downregulation of Lon in cells with severe mtDNA deficits blocks TFAM degradation and increases mtDNA content
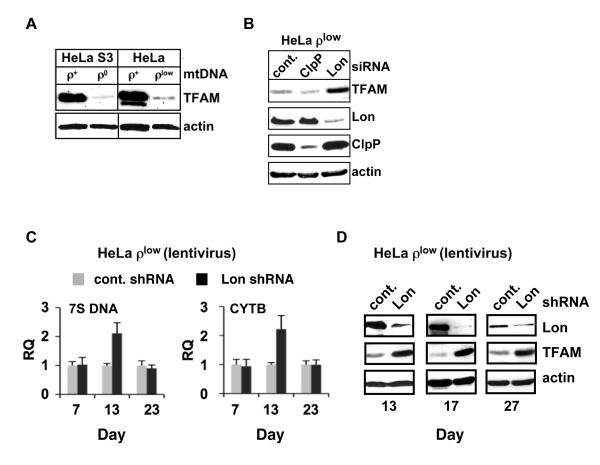
The link between TFAM levels, mtDNA copy number and Lon-mediated proteolysis was investigated in HeLa cells with either normal mtDNA content or severe mtDNA deficits. TFAM was detected by immunoblotting in cells with normal mtDNA levels (ρ+ cells) (Fig. 2A). By contrast, TFAM was barely detected in cells that were irreversibly depleted of mtDNA (ρ0 cells) (Fig. 2A). ρ0 cells were generated by culturing ρ+ cells with ethidium bromide for an extended period to deplete mtDNA, and then selecting the cells that were auxotrophic for pyruvate and uridine (King and Attardi, 1996). TFAM levels were also strikingly reduced in cells with low mtDNA content (ρlow cells) (Fig. 2A), which were generated by ethidium bromide incubation for a shorter time, resulting in cells that did not exhibit pyruvate and uridine auxotrophy. Although ρlow cells had severely reduced mtDNA levels similar to ρ0 cells (Fig. S2), they still retained residual copies of the genome (see Fig. 2C). To determine if the reduced TFAM levels in ρlow cells resulted from Lon-mediated proteolysis, we genetically knocked down Lon. A substantial increase in TFAM was observed upon transient siRNA knockdown of Lon, whereas TFAM levels remained unchanged in cells transfected with control siRNA (Fig. 2B). By contrast, no upregulation of TFAM was observed when ClpP was knocked down (Fig. 2B).
Figure 2. Lon knockdown in mtDNA-depleted cells increases TFAM and mtDNA.
(A) Extracts from HeLa ρ+, ρ0 and ρlow cells were blotted for TFAM. Overexposure permits detection of TFAM in ρ0 and ρlow cells. A lower molecular weight TFAM band in ρ+ cells is likely a processed form or breakdown product. (B) Extracts from ρlow cells transfected with control, Lon or ClpP siRNAs were blotted for TFAM, Lon, ClpP and actin. (C) Total DNA was isolated from ρlow cells transduced with control or Lon shRNA lentivirus (5 MOI) and relative quantitation (RQ) of mtDNA was determined by qPCR of 7S DNA and CYTB gene using the nuclear APP gene as an endogenous control. Data represent at least three independent experiments. Standard error of the mean is shown. (D) Extracts from ρlow cells transduced as in (C) were immunoblotted for Lon, TFAM and actin.
One potential consequence of upregulating TFAM by knocking down Lon in ρlow cells is a coordinated increase in mtDNA. Lentiviral delivery of shRNAs targeting Lon was employed as this approach showed higher efficiency, less toxicity and lower background as compared to transfections with siRNA or shRNA plasmids (data not shown). At 1 week, mtDNA copy number was essentially the same in control and Lon knockdown ρlow cells. However, by ~2 weeks, the Lon depletion led to increased mtDNA copy number, which was reproducibly observed as compared to the control (Fig. 2C, 13 days). During this time period, Lon knockdown cells showed reduced levels of Lon and upregulated levels of TFAM (Fig. 2D, 13 and 17 days). By ~3 weeks, mtDNA copy number had declined in Lon knockdown cells (Fig. 2C, 23 days), even though Lon was still depleted and TFAM remained elevated during this period (Fig. 2D, 27 days). We speculate that the lentiviral-mediated knockdown of Lon in ρlow cells only transiently increases mtDNA content even though TFAM remains elevated, because Lon is needed for mitochondrial homeostasis and genome maintenance in HeLa ρlow cells. Further experiments are required to address this issue.
Bortezomib inhibits Lon and blocks TFAM degradation
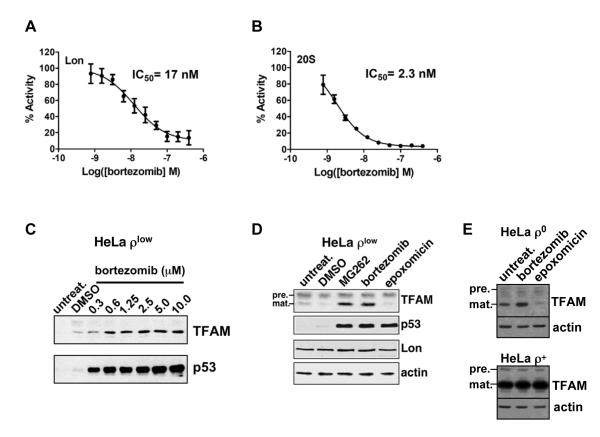
TFAM degradation was also explored by pharmacologically inactivating Lon. Previous work shows that Lon is selectively inhibited by MG132 and MG262 but not by epoxomicin, although all three are inhibitors of the 20S proteasome (Frase and Lee, 2007; Granot et al., 2007). We demonstrated that Lon is also inhibited by the MG262-related compound bortezomib (Velcade, PS-341) (Fig. 3A), which is used to treat multiple myeloma and mantle cell lymphoma (Adams, 2004). Bortezomib inhibited Lon-mediated cleavage of a dipeptide substrate with an IC50 value of 17 nM, which was comparable to its inhibition of the 20S proteasome with an IC50 value of 2.3 nM (Fig. 3A and B). Bortezomib (0.6-10 μM) also blocked the turnover of TFAM in ρlow cells at concentrations that inhibited the proteasome-mediated degradation of p53, which is constitutively unstable in HeLa cells (Fig. 3C) (Scheffner et al., 1990). Bortezomib, like MG262, selectively stabilized TFAM in ρlow cells, whereas epoxomicin did not (Fig. 3D). By contrast, all three inhibitors stabilized p53 (Fig. 3D). Although bortezomib stabilized TFAM in both ρlow and ρ0 cells, no change was observed in ρ+ cells (Fig. 3E), most likely because the vast majority of TFAM was bound to mtDNA and Lon-resistant.
Figure 3. Lon-dependent proteolysis of TFAM is blocked by bortezomib and MG262 but not epoxomicin.
(A) and (B) Lon (200 nM monomer) or 20S (3 nM complex) peptidase activities were measured using the fluorescent dipeptide substrate AA2-Rh110 (6 μM) incubated in the presence or absence of bortezomib at 37°C for 3 hr. Fluorescence was normalized to percent activity of no drug control. Results represent at least 3 independent experiments. (C) ρlow cells were incubated with or without bortezomib for 18 hr and extracts were blotted for TFAM or p53. (D) and (E) ρlow, ρ0 or ρ+ cells were treated with DMSO, bortezomib (5 μM), MG262 (1.25 μM) or epoxomicin (1 μM) for 18 hr; extracts were blotted for TFAM, p53, Lon or actin. TFAM precursor (pre.) and mature (mat.) proteins are indicated.
TFAM carrying inactivating HMG box mutations is degraded by Lon
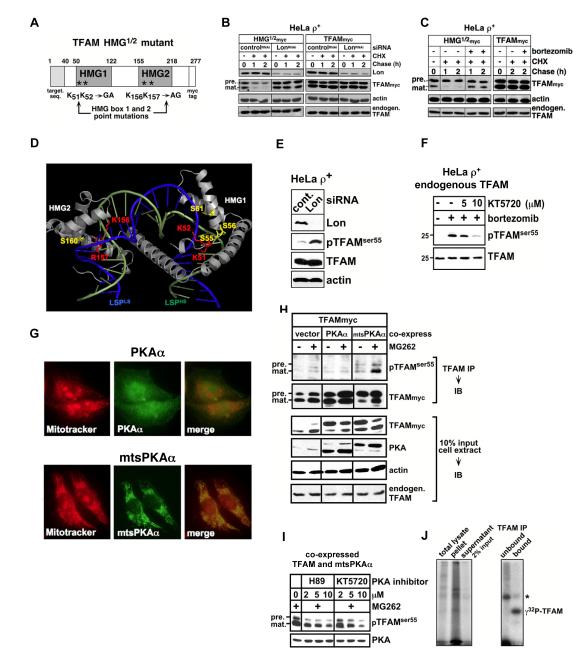
Using HeLa ρ+ cells with normal mtDNA levels, we tested whether the failure of TFAM to bind DNA led to degradation by Lon. A TFAM mutant was engineered with inactivating mutations in HMG1 and 2, in which lysines 51, 52 and 156 as well as arginine 157 were replaced by alanines or glycines (TFAMHMG1/2, Fig. 4A). The crystal structure of TFAM bound to LSP dsDNA (Ngo et al., 2011; Rubio-Cosials et al., 2011) predicts that these HMG box substitutions will reduce DNA-binding affinity by disrupting hydrogen bond formation with DNA (see Fig. 4D). When corresponding mutations are introduced in the S. cerevisiae Abf2p, which shows sequence homology to TFAM, impaired DNA-binding is observed (Zelenaya-Troitskaya et al., 1998).
Figure 4. HMG box mutation or PKA-dependent phosphorylation of TFAM leads to degradation by Lon.
(A) Diagram of HMG box mutants of TFAM. (B) HeLa ρ+ cells were transfected twice with siRNAs on Day (D) = 0 and 2; on D=3, the cells were transfected with plasmids for expressing HMG1/2myc or TFAMmyc; and on D=4, cells were chased with cycloheximide (CHX, 100 μg/ml). Extracts were blotted for TFAM, Lon or actin. (C) ρ+ cells were transfected as in (B) and chased with CHX and bortezomib (5 μM) and blotted as in (B). (D) TFAM-DNA complex (PDB ID 3TMM) showing HMG box lysines 51, 52, 156 and arginine 157 (red); HMG box serines 55, 56, 61 and 160 (yellow); LSPLS (green); and LSPHS (blue). (E, F) Endogenous pTFAMser55 in ρ+ cells knocked down for Lon for 48 hr (E), or treated with or without the PKA inhibitor KT5720 or bortezomib (5μM) for 16 hr (F). (G) Fluorescent double labeling of Mitotracker Orange and overexpressed PKAα or mtsPKAα in ρ+ cells. (H) TFAMmyc coexpressed with mtsPKAα or PKAα in ρ+ cells treated with or without MG262 (1.25 μM) for 16 hr. Anti-TFAM immunoprecipitates were blotted for pTFAMser55, TFAM or PKA. (I) TFAMmyc and mtsPKAα were coexpressed in ρ+ cells and treated with MG262 (1.25 μM) in the presence or absence of H89 or KT5720 for 16 hr. (J) Trypsin-treated mitochondria coexpressing TFAM and mtsPKAα were incubated with [32P]-ATP, then lysed (total lysate) and centrifuged (resulting in supernatant and pellet). The supernatant was immunoprecipitated for TFAM (bound and unbound). Samples were analyzed by SDS-PAGE and autoradiography. (*) Autophosphorylated PKAα coimmunoprecipitated with TFAM (Fig. S4A and H).
We examined the stability of TFAMHMG1/2 and wild type TFAM transiently expressed in ρ+ cells that were genetically or pharmacologically downregulated for Lon using RNAi or bortezomib, respectively (Figs. 4B and C). TFAM degradation was examined using a cycloheximide (CHX) chase to block cytosolic protein synthesis over a time course. Cell extracts from each time point were immunoblotted with antibodies recognizing the myc-tag fused to mutant or wild type TFAM, distinguishing overexpressed from the endogenous TFAM. In the absence of CHX (T= 0), TFAMHMG1/2 and wild type TFAM were detected as two molecular weight forms (Figs. 4B and C): the cytosolic full-length precursor protein carrying a mitochondrial targeting sequence, and the mature protein lacking its targeting sequence that is cleaved off in the matrix. When normal levels of Lon were present (controlRNAi), both the precursor and mature forms of TFAMHMG1/2 rapidly declined during the chase with half-lives <1 hr (Fig. 4B). However, when Lon was knocked down (LonRNAi), both the precursor and mature forms of TFAMHMG1/2 were stabilized, suggesting that Lon degraded both the partially translocated precursor and the mature form (Fig. 4B). By contrast, the knockdown of Lon did not alter the half-lives of either the wild type precursor or mature protein (Fig. 4B). Little conversion of the wild type precursor to the mature form was observed, most likely because the precursor was expressed at high levels and not degraded, thus saturating the import channel. Bortezomib addition during the chase stabilized mature TFAMHMG1/2 with little effect on the precursor protein (Fig. 4C). Like the Lon knockdown, bortezomib treatment had virtually no effect on the levels of the mature or processed wild type TFAM in ρ+ cells (Figs. 4B and C).
In vitro phosphorylation of TFAM by PKA
We surmised that TFAM binding to mtDNA might be antagonized by HMG phosphorylation leading to Lon-mediated degradation. As PKA activity has been shown in the mitochondrial matrix (Acin-Perez et al., 2011; Agnes et al., 2010; Robin et al., 2003; Schmidt et al., 2011), we tested whether TFAM was in vitro phosphorylated by the catalytic subunit of PKA. In the presence of γ32-ATP, TFAM was radiolabeled (Fig. S3A). LC/MS/MS identified four in vitro phosphorylated sites within TFAM- serines 55, 56 and 61 in HMG1, and serine 160 in HMG2 (Fig. S3B). HMG1 serines 55, 56 and 61 are noteworthy as these residues interact with DNA in the X-ray structures of TFAM bound to LSP DNA (Fig. 4D) (Ngo et al., 2011; Rubio-Cosials et al., 2011). The yeast homolog of TFAM- Abf2p, is also in vitro phosphorylated by PKA within HMG1 and exhibits impaired DNA-binding (Cho et al., 2001). However, Abf2p phosphorylation does not lead to proteolysis and its mechanistic significance is unclear. In vitro studies show that Abf2p weakly stimulates transcription 1.5 - 2-fold (Parisi et al., 1993), by contrast to TFAM, which stimulates transcription >1,000-fold (Litonin et al., 2010). However, Abf2p like TFAM, has a common role in mtDNA packaging and inheritance (Zelenaya-Troitskaya et al., 1998).
Phosphorylation of TFAM by PKA within mitochondria
To investigate whether TFAM phosphorylation occurs in cells and in mitochondria, we purified the overexpressed protein from 293T cells treated with or without MG262 to inhibit Lon. Phosphopeptide enrichment and LC/MS/MS identified phosphorylated sites within TFAM (Fig. S3C). Peptides phosphorylated at serines 55 and/or 56 were detected only when TFAM was isolated from MG262-treated cells (Fig. S3C). Based on this finding, we produced anti-phosphopeptide antibodies to these sites. An anti-phosphoserine 55 (pTFAMser55) antibody was comprehensively validated, which specifically recognized both the phosphopeptide and in vitro phosphorylated TFAM, as well as wild type phosphoTFAM expressed in cells, but not TFAMSSAA in which serine 55/56 were replaced by alanines (Fig. S3D). Using this antibody we showed that endogenous pTFAMser55 was present in cell and mitochondrial extracts only when Lon was genetically or pharmacologically downregulated (Fig. 4 E, F, I and Fig. S3E). The PKA inhibitors H89 or KT5720 blocked the appearance of pTFAMser55 in Lon-inhibited cells (Fig. 4F and I), implicating PKA as the kinase mediating this phosphorylation.
PKA-catalyzed phosphorylation occurs both inside and outside mitochondria (Acin-Perez et al., 2011; Agnes et al., 2010; Robin et al., 2003; Schmidt et al., 2011). A complete PKA system is present within the mitochondrial matrix, which is activated by cAMP generated by a soluble adenylyl cyclase (Acin-Perez et al., 2009). However, the signals targeting PKA to the matrix and the specific catalytic and regulatory isoforms functioning in the matrix are unknown. To discriminate whether TFAMser55 was phosphorylated in the cytosol or in the mitochondrial matrix, TFAM was coexpressed with either the PKA catalytic α subunit (PKAα), or a a mitochondrial targeted PKAα mutant (mtsPKAα). PKAα was diffusely distributed in the cytosol, whereas mtsPKAα colocalized with mitochondria (Fig. 4G). When cytosolic PKAα and TFAM were coexpressed, no increase in pTFAMser55 was observed either in the presence or absence of MG262 (Fig. 4H). The activity of PKAα was confirmed by its phosphorylation of histone deacetylase 8 (HDAC8) at serine 39 (Lee et al., 2004) (Fig. S3F). By contrast, mtsPKAα coexpression led to the appearance of pTFAMser55, which accumulated upon Lon inhibition by MG262 (Fig. 4H). The PKA inhibitors H89 or KT5720 blocked phosphorylation of overexpressed TFAM at serine 55 (Fig. 4I). We showed that pTFAMser55, mtsPKAα and Lon were present in isolated mitochondria that had been trypsin-digested to remove copurifying non-mitochondrial proteins as well as the cytosolic domains of outer membrane proteins (Fig. S3G). Thus, pTFAMser55 and mtsPKAα were protected within mitochondria. To determine if TFAM was phosphorylated within mitochondria, in organello reactions were performed by incubating trypsin-treated mitochondria expressing mtsPKAα with γ32P-ATP (Fig. 4J and Fig. S3G). We showed that phosphorylated radiolabeled TFAM was immunoprecipitated from mitochondria (Fig. 4J). Collectively, these results demonstrated that phosphorylation of TFAMser55 occurs in mitochondria and not in the cytosol.
TFAM phosphomimics within HMG1, but not HMG2, are degraded by Lon
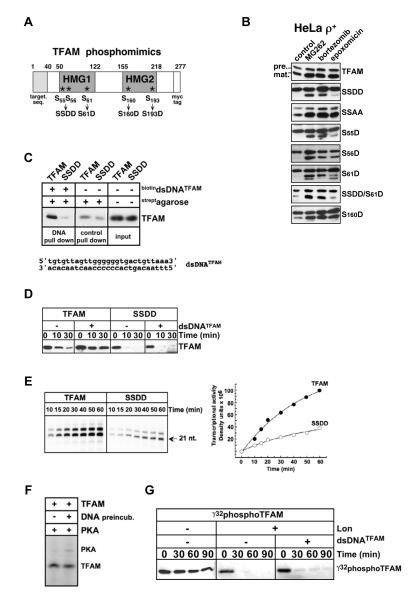
We predicted that TFAM phosphomimics unable to bind mtDNA would be degraded by Lon, and selectively stabilized by bortezomib or MG262, but only nominally by epoxomicin (see Fig. 3D). TFAM phosphomimics carrying serine to aspartate substitutions within HMG1 or 2 were expressed in ρ+ cells (Fig. 5A and B). In untreated HeLa ρ+ cells, wild type TFAM was detected as both precursor and mature proteins (Fig. 5B). MG262, bortezomib as well as epoxomicin, increased both precursor and mature forms of TFAM, suggesting that proteasome inhibition stabilized the precursor, leading to increased import and increased levels of mature protein. A markedly different inhibitor response profile was observed for the phosphomimic TFAMSSDD in which serines 55 and 56 in HMG1 were replaced by aspartates. In untreated cells, the precursor of TFAMSSDD was strongly detected, however the mature protein was virtually absent (Fig. 5B). The Lon-inhibiting compounds MG262 and bortezomib substantially increased mature TFAMSSDD, whereas only a marginal increase was observed with epoxomicin. All three inhibitors slightly increased precursor levels of TFAMSSDD. The instability of TFAMSSDD was caused by aspartate substitutions in HMG1, as the alanine-substituted TFAMSSAA was stable and resembled wild type TFAM (Fig. 5B). The single, double or triple serine to aspartate phosphomimics at serines 55, 56, and 61 were also barely detected in untreated controls and were selectively stabilized by the inhibitors to varying degrees (Figs. 5B and S4A). TFAMS55D and TFAMSSDD showed the most clear and reproducible upregulation by MG262 or bortezomib, with little effect of epoxomicin (Fig. 5B). By contrast, the HMG2 phosphomimics TFAMS160D (Fig. 5B) and TFAMS193D (Fig. S4A) were not Lon-sensitive as their inhibitor response patterns were similar to wild type TFAM.
Figure 5. TFAM phosphorylation modulates DNA-binding, transcriptional activation and Lon-sensitivity.
(A) Diagram of TFAM phosphomimic mutants at serines phosphorylated by PKA in vitro. (B) ρ+ cells transiently expressing wild type TFAM or phosphomimics were treated with DMSO, MG262 (1.25 μM), bortezomib (5 μM) or epoxomicin (1 μM); extracts were blotted with anti-myc antibodies. Actin controls are shown in Fig. S4A. (C) TFAM or TFAMSSDD were preincubated with or without biotinylated dsDNATFAM prior to incubation with streptavidin agarose. Pull-down and protein input were blotted for TFAM. (D) TFAM or TFAMSSDD (160 nM) were preincubated with or without dsDNATFAM (8 μM), before adding Lon (70 nM) and ATP (2 mM); reactions were blotted for TFAM. (E) Transcription reactions with TFAM or TFAMSSDD using the LSP promoter template with α32P-ATP. (F) TFAM (160 nM) was preincubated with a 300-fold molar excess of DNA prior to adding PKA (2500 U) and [32P]-ATP (8 μCi) at 30°C for 2 hr. (G) [32P]-labeled PKA phosphorylated TFAM was preincubated with or without dsDNATFAM before adding Lon as in (D), and visualized by autoradiography.
TFAM phosphorylation at HMG1 impairs DNA-binding and transcription, and promotes Lon-mediated degradation
We directly tested the ability of TFAMSSDD to bind DNA, which is required for transcriptional activation and resistance to Lon. Purified TFAMSSDD and wild type TFAM were incubated with biotinylated dsDNATFAM carrying the TFAM binding site. TFAMSSDD was not significantly pulled-down by contrast to wild type TFAM (Fig. 5C). Because of this DNA-binding defect, preincubating TFAMSSDD with dsDNATFAM (without biotin) failed to confer Lon-resistance, whereas wild type TFAM was fully stabilized (Fig. 5D). To analyze the effect of HMG1 phosphorylation at serines 55 and 56, we compared the transcriptional activation of TFAMSSDD with that of wild type TFAM. MtDNA transcription is catalyzed by mitochondrial RNA polymerase (mtRNAP or POLRMT), which requires TFAM as well as another initiation factor TFB2M for promoter binding and melting. Together TFAM and TFB2M operate synergistically to boost the efficiency of mtRNAP (Litonin et al., 2010). We employed a reconstituted transcription system consisting of mtRNAP, TFB2M and either TFAMSSDD or wild type TFAM, which were incubated with a DNA template carrying the LSP promoter driving the synthesis of a 21-22 nucleotide transcript (Falkenberg et al., 2002; Sologub et al., 2009). In transcription run-off experiments, TFAMSSDD showed a 3-4-fold reduction in activation from LSP as compared to wild type TFAM (Fig. 5E). Similar results were observed using a DNA template carrying HSP1 (Fig. S4B). These results suggested that phosphoserines 55 and 56 within the HMG1 prevent DNA binding, attenuate transcriptional activation and promote Lon-mediated degradation.
Biochemical experiments were carried out to test directly if TFAM already bound to DNA can be phosphorylated by PKA, and if PKA phosphorylated TFAM is degraded by Lon. TFAM was preincubated with and without a ~300-fold molar excess of dsDNATFAM before adding PKA and γ32-ATP. DNA-bound TFAM was phosphorylated and radiolabeled by PKA to nearly the same extent as DNA-free TFAM (γ32phosphoTFAM, Fig. 5F). We further showed that γ32phosphoTFAM was degraded by Lon, and was not stabilized by adding DNA (Fig. 5G). Taken together, these results suggest that HMG1 phosphorylation of TFAM affects DNA binding and release, thereby modulating transcriptional activation and sensitivity to Lon-mediated proteolysis.
DISCUSSION
This study reveals mechanisms for rapidly regulating TFAM binding to mtDNA by phosphorylation and proteolysis. We propose that PKA-mediated phosphorylation of TFAM within HMG1 causes electrostatic repulsion of the DNA phosphate backbone, thus providing a mechanism for regulating mtDNA binding and release. Results of TFAM-DNA binding experiments along with the crystal structure of the TFAM-LSP complex have proposed a mechanism by which TFAM binds to and bends DNA (Gangelhoff et al., 2009; Ngo et al., 2011; Wong et al., 2009). According to this mechanism, the high-affinity HMG1 domain of TFAM binds LSP first, and subsequent conformational changes in TFAM and mtDNA allow for the binding by HMG2, which exhibits lower affinity and less specificity for LSP. TFAM binding to non-promoter regions of mtDNA likely depends primarily on HMG1. Thus for promoter and non-promoter binding, phosphorylation of serine residues within HMG1 will play a major role in affecting the DNA-binding status of TFAM and degradation by Lon.
Mechanisms for dissociating TFAM from mtDNA are critically important as it may extensively coat the genome, potentially binding every ~20 base pairs (Kukat et al., 2011; Takamatsu et al., 2002). The coating of mtDNA by TFAM mediates the tight compaction of the genome, which affects its replication, transcription and maintenance. The process of uncoating mtDNA has not been elucidated, but likely involves the selective and processive dissociation of TFAM. One can envisage that phosphorylation-stimulated release of TFAM from mtDNA decompacts the genome, and that its degradation by Lon permits sustained decompaction. However, HMG1 phosphorylated TFAM may rebind to mtDNA if it is dephosphorylated by phosphatases prior to degradation. Alternatively, unphosphorylated and DNA-free TFAM may be sequestered within nucleoids and protected from Lon proteolysis until it is utilized for genome coating. In addition, newly imported TFAM may bind mtDNA during or soon after translocation and folding of the HMG1 domain, which is ~10 amino acids away from the mature amino terminus. The coordination of phosphorylation/dephosphorylation with proteolysis of TFAM can thus fine-tune its functions in mitochondrial maintenance, expression and inheritance.
The pathways activating PKA-mediated phosphorylation of TFAM are unknown. Previous work suggests that mitochondrial protein phosphorylation by PKA may be activated by changes in CO2 and O2 tension (e.g. ischemia, hypoxia) (Acin-Perez et al., 2011; Acin-Perez et al., 2009; Agnes et al., 2010; Papa et al., 2008; Prabu et al., 2006; Sardanelli et al., 2006). PKA within mitochondria is activated by cAMP, which is generated by a matrix-localized soluble adenylyl cyclase that responds to metabolically generated CO2/HCO3− (Acin-Perez et al., 2009; Agnes et al., 2010; Sardanelli et al., 2006). Studies also show that hypoxia and/or ischemia lead to PKA-mediated phosphorylation and functional changes in Complexes I and IV (Acin-Perez et al., 2011; Papa et al., 2008; Prabu et al., 2006). Interestingly, hypoxia and ischemia upregulate Lon expression, which assists in adapting cells to these stresses (Bota and Davies, 2001; Fukuda et al., 2007; Hori et al., 2002). Further studies are required to determine whether phosphorylation of TFAM is coordinated with that of Complex I or IV subunits.
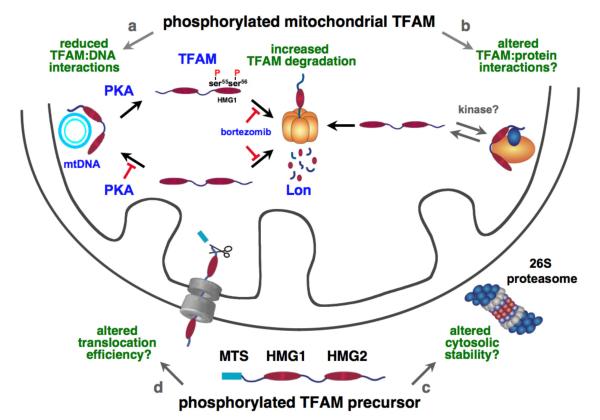
We speculate that TFAM may be phosphorylated by PKA and possibly other kinases not only in the mitochondrion but also in the cytosol (Fig. 6). In this study we show that TFAM is phosphorylated at HMG1 serines 55 and 56 by PKA inside mitochondria, which blocks DNA binding and leads to rapid degradation by Lon (Fig. 6a, 4E-J, Fig. S3). Both the precursor and mature forms of TFAM are phosphorylated within HMG1 (Fig 4H and I). In addition, our proteomics data show that TFAM isolated from HEK293 cells is also phosphorylated at sites outside HMG1, which are not linked to Lon-mediated proteolysis (unpublished data). We envisage that TFAM phosphorylation alters its activity in diverse ways. Mitochondrial phosphorylation of TFAM at sites outside HMG1 may influence interactions with proteins involved in transcription or nucleoid dynamics (Fig. 6b). Whereas, in the cytosol, phosphorylation of TFAM may alter its degradation by the proteasome (Fig. 6c), or its association with the mitochondrial protein translocation machinery (Fig. 6d). The phosphorylation of TFAM at single or multiple concurrent sites may provide temporal and spatial cues that coordinate its function and abundance within mitochondria and at mtDNA.
Figure 6. Phosphorylation potentially regulates multiple aspects of TFAM biogenesis and activity.
Mitochondrial phosphorylation of TFAM- a, Phosphorylation of TFAM at HMG1 serines 55 and 56 by PKA regulates mtDNA binding and release. b, Phosphorylation of TFAM by PKA or other protein kinases may alter its interactions with other proteins. Cytosolic phosphorylation of TFAM precursor- c, Precursor phosphorylation may alter its degradation by the proteasome; or d, may alter its binding to the protein translocation machinery.
Our finding that the anti-cancer agent bortezomib inhibits Lon as well as the 20S proteasome raises the question as to whether Lon inhibition is therapeutically beneficial in treating hematological malignancies. Recently, we showed that knocking down Lon in a mantle cell lymphoma cell line leads to cell death (Bernstein et al., 2012). Cancer cells, unlike normal cells, survive a myriad of chronic stresses linked to oncogenesis, such as mitotic, metabolic, oxidative and proteotoxic stress (Solimini et al., 2007). Previous work shows that Lon supports cell viability during hypoxia, proteotoxicity and endoplasmic reticulum (ER) stress (Bota and Davies, 2001; Fukuda et al., 2007; Hori et al., 2002). Further experiments are required to address whether inhibiting Lon is toxic for certain cancers. In addition, Lon inhibition may have unique therapeutic effects in cancers with mtDNA mutations or reduced mtDNA copy number, which have been linked to increased aggressiveness or resistance to chemotherapy (Chandra and Singh, 2011; Yu, 2011). Collectively, the results and perspectives presented here provide a gateway to exploring new pathways in mitochondrial metabolism that depend on TFAM and Lon.
EXPERIMENTAL PROCEDURES
Materials, antibodies, cell culture experiments, protein purification, biochemical assays, molecular biology, mitochondrial isolation and standard methods are detailed in Supplemental Information.
Cells and cell culture
HeLa ρ+, ρ0 cells and ρlow cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM) containing 10% fetal bovine serum and 100 units/ml of penicillin streptomycin. ρ0 and ρlow cells were supplemented with sodium pyruvate (110 μg/ml) and uridine (50 μg/ml).
Plasmid or siRNA transfection and lentivirus transduction
Wild type and mutant TFAM as well as PKAα with or without the mitochondrial targeting sequence of cytochrome c oxidase subunit 8A, were carboxyl-terminal myc-tagged. Transfections were carried out using Lipofectamine2000 (Invitrogen) following the manufacturer’s procedures. Transductions were carried out at a multiplicity of infection (MOI) of 5 following the manufacturer’s procedures.
Protein purification
Recombinant human TFAM, Lon, ClpX and ClpP (Fig. S1D) lacked their respective predicted mitochondrial targeting sequences. Lon and TFAM carried an amino-terminal his-tag, ClpX and ClpP carried a carboxyl-terminal his-tag. Recombinant proteins were purified as described (Bernstein et al., 2012; Litonin et al., 2010; Liu et al., 2004).
Peptidase and protease assays
Peptidase activities of recombinant human Lon or the 20S proteasome (Boston Biochem) were measured using rhodamine 110, bis-(CBZ-L-alanyl-L-alanine amide, Anaspec) as described (Bernstein et al., 2012). For protease assays, Lon or ClpXP were incubated with TFAM or casein with or without 2 mM ATP. Reactions were analyzed by immunoblotting or Coomassie Blue staining.
Gel-shift, Southwestern and DNA pull-down assays
Detailed procedures are described in Supplemental Methods. Gel shift reactions with TFAM and [γ-32P] labeled DNA probes were performed as described (Liu et al., 2004). For Southwestern assays, proteins were separated by SDS-PAGE and transferred to nitrocellulose, which was either immunoblotted or probed with [γ-32P] labeled DNA. For pull-down experiments, TFAM or TFAMSSDD were incubated with biotinylated dsDNATFAM and then pulled-down with streptavidin agarose, and analyzed by immunoblotting.
Quantitative PCR (qPCR)
qPCR was performed with Taqman primers and probes using total DNA isolated from cells (Lu et al., 2007) (see Supplemental Methods). Statistical significance was evaluated with Student’s unpaired t-test.
Reconstituted mitochondrial transcription assay
Transcription reactions were carried out as described (Litonin et al., 2010) (Supplemental Methods), using mutant or wild type TFAM (100 nM), TFB2M (150 nM) and mtRNAP (150 nM).
Phosphorylation site identification within recombinant PKA-phosphorylated TFAM and purified cellular TFAM
Phosphopeptide enrichment was performed using recombinant TFAM that was PKA-phosphorylated, or cellular TFAM purified from HEK293 cells (Novoprotein, NJ). Phosphorylation site identification was performed using LC/MS/MS (Center for Advanced Proteomics, New Jersey Medical School, NJ). Detailed procedures are described in Supplemental Methods and Figures S3A-C.
Immunofluorescence
Double labeling of PKAα or mtsPKAα using anti-myc antibodies and Mitotracker Orange was performed as described (Lu et al., 2003).
TFAM phosphorylation in trypsin-treated mitochondria
Mitochondria were incubated in buffer with trypsin for 10 min on ice, followed by trypsin inhibitor for 5 min on ice. Trypsin-treated mitochondria were washed and incubated in buffer with [γ-32P] ATP. See Supplemental Methods for detailed protocol.
TFAM immunoprecipitation
HeLa ρ+ cells or mitochondria were lysed in buffer with protease and phosphatase inhibitors. Cell extracts were immunoprecipitated as detailed in Supplemental Methods.
Supplementary Material
HIGHLIGHTS.
cAMP-dependent protein kinase (PKA) serine phosphorylates TFAM within HMG1.
HMG1 phosphorylation of TFAM impairs DNA binding and transcription activation.
Lon protease selectively degrades DNA-free TFAM and is inhibited by bortezomib.
Lon knockdown stabilizes TFAM in mtDNA-deficient cells and upregulates mtDNA.
ACKNOWLEDGEMENTS
We thank Drs. V. Bellofatto and M.Z. Humayun for critical review of the manuscript, and Dr. J. Sadoshima for helpful discussions. This study was supported by the National Institutes of Health R01GM084039 and 1R21NS067668 (C.K.S), and the Foundation of UMDNJ (C.K.S.). We gratefully acknowledge Drs. H. Li and T. Liu at the UMDNJ- Neuroproteomics Core Facility; the Orbitrap instrument was funded in part by NIH grant NS046593.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acin-Perez R, Gatti DL, Bai Y, Manfredi G. Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Cell Metab. 2011;13:712–719. doi: 10.1016/j.cmet.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9:265–276. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- Agnes RS, Jernigan F, Shell JR, Sharma V, Lawrence DS. Suborganelle sensing of mitochondrial cAMP-dependent protein kinase activity. J Am Chem Soc. 2010;132:6075–6080. doi: 10.1021/ja909652q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asin-Cayuela J, Gustafsson CM. Mitochondrial transcription and its regulation in mammalian cells. Trends Biochem Sci. 2007;32:111–117. doi: 10.1016/j.tibs.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Bernstein SH, Venkatesh S, Li M, Lee J, Lu B, Hilchey SP, Morse KM, Metcalfe HM, Skalska J, Andreeff M, et al. The mitochondrial ATP-dependent Lon protease: a novel target in lymphoma death mediated by the synthetic triterpenoid CDDO and its derivatives. Blood. 2012;119(14):3321–3329. doi: 10.1182/blood-2011-02-340075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenhagen DF. Mitochondrial DNA nucleoid structure. Biochim Biophys Acta. 2011;1819(9-10):914–920. doi: 10.1016/j.bbagrm.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Bogenhagen DF, Rousseau D, Burke S. The layered structure of human mitochondrial DNA nucleoids. J. Biol. Chem. 2008;283:3665–3675. doi: 10.1074/jbc.M708444200. [DOI] [PubMed] [Google Scholar]
- Bonawitz ND, Clayton DA, Shadel GS. Initiation and beyond: multiple functions of the human mitochondrial transcription machinery. Mol Cell. 2006;24:813–825. doi: 10.1016/j.molcel.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Bota DA, Davies KJ. Protein degradation in mitochondria: implications for oxidative stress, aging and disease: a novel etiological classification of mitochondrial proteolytic disorders. Mitochondrion. 2001;1:33–49. doi: 10.1016/s1567-7249(01)00005-8. [DOI] [PubMed] [Google Scholar]
- Bota DA, Davies KJA. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat. Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- Chandra D, Singh KK. Genetic insights into OXPHOS defect and its role in cancer. Biochim. Biophys. Acta. 2011;1807:620–625. doi: 10.1016/j.bbabio.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Suzuki CK, Wu SH. Thermodynamic characterization of specific interactions between the human Lon protease and G-quartet DNA. Nucleic Acids Res. 2008;36:1273–1287. doi: 10.1093/nar/gkm1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Lee YK, Chae CB. The modulation of the biological activities of mitochondrial histone Abf2p by yeast PKA and its possible role in the regulation of mitochondrial DNA content during glucose repression. Biochim Biophys Acta. 2001;1522:175–186. doi: 10.1016/s0167-4781(01)00333-5. [DOI] [PubMed] [Google Scholar]
- Cotney J, Wang Z, Shadel GS. Relative abundance of the human mitochondrial transcription system and distinct roles for h-mtTFB1 and h-mtTFB2 in mitochondrial biogenesis and gene expression. Nucleic Acids Res. 2007;35:4042–4054. doi: 10.1093/nar/gkm424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dairaghi DJ, Shadel GS, Clayton DA. Addition of a 29 residue carboxyl-terminal tail converts a simple HMG box-containing protein into a transcriptional activator. J. Mol. Biol. 1995;249:11–28. doi: 10.1006/jmbi.1995.9889. [DOI] [PubMed] [Google Scholar]
- Ekstrand MI, Falkenberg M, Rantanen A, Park CB, Gaspari M, Hultenby K, Rustin P, Gustafsson CM, Larsson NG. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum Mol Genet. 2004;13:935–944. doi: 10.1093/hmg/ddh109. [DOI] [PubMed] [Google Scholar]
- Falkenberg M, Gaspari M, Rantanen A, Trifunovic A, Larsson NG, Gustafsson CM. Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nature Genetics. 2002;31:289–294. doi: 10.1038/ng909. [DOI] [PubMed] [Google Scholar]
- Falkenberg M, Larsson NG, Gustafsson CM. DNA replication and transcription in mammalian mitochondria. Annu Rev Biochem. 2007;76:679–699. doi: 10.1146/annurev.biochem.76.060305.152028. [DOI] [PubMed] [Google Scholar]
- Frase H, Lee I. Peptidyl Boronates Inhibit Salmonella enterica serovar Typhimurium Lon Protease by a Competitive ATP-Dependent Mechanism. Biochemistry. 2007;46:6647–6657. doi: 10.1021/bi7002789. [DOI] [PubMed] [Google Scholar]
- Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- Gangelhoff TA, Mungalachetty PS, Nix JC, Churchill ME. Structural analysis and DNA binding of the HMG domains of the human mitochondrial transcription factor A. Nucleic Acids Res. 2009;37:3153–3164. doi: 10.1093/nar/gkp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot Z, Kobiler O, Melamed-Book N, Eimerl S, Bahat A, Lu B, Braun S, Maurizi MR, Suzuki CK, Oppenheim AB, et al. Turnover of mitochondrial steroidogenic acute regulatory (StAR) protein by Lon protease: the unexpected effect of proteasome inhibitors. Mol Endocrinol. 2007;21:2164–2177. doi: 10.1210/me.2005-0458. [DOI] [PubMed] [Google Scholar]
- Hokari M, Kuroda S, Kinugawa S, Ide T, Tsutsui H, Iwasaki Y. Overexpression of mitochondrial transcription factor A (TFAM) ameliorates delayed neuronal death due to transient forebrain ischemia in mice. Neuropathology. 2010;30:401–407. doi: 10.1111/j.1440-1789.2009.01086.x. [DOI] [PubMed] [Google Scholar]
- Hori O, Icinoda F, Tamatani T, Yamaguchi A, Sato N, Ozawa K, Kitao Y, Miyazaki M, Harding HP, ROn D, et al. Transmission of cell stress from endoplasmic reticulum to mitochondria: enhanced expression of Lon protease. J. Cell Biol. 2002;157:1151–1160. doi: 10.1083/jcb.200108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M, Matsusaka H, Kang D, Matsushima S, Ide T, Kubota T, Fujiwara T, Hamasaki N, Takeshita A, Sunagawa K, et al. Overexpression of mitochondrial transcription factor a ameliorates mitochondrial deficiencies and cardiac failure after myocardial infarction. Circulation. 2005;112:683–690. doi: 10.1161/CIRCULATIONAHA.104.524835. [DOI] [PubMed] [Google Scholar]
- Kanki T, Nakayama H, Sasaki N, Takio K, Alam TI, Hamasaki N, Kang D. Mitochondrial nucleoid and transcription factor A. Ann N Y Acad Sci. 2004;1011:61–68. doi: 10.1007/978-3-662-41088-2_7. [DOI] [PubMed] [Google Scholar]
- Kaufman BA, Durisic N, Mativetsky JM, Costantino S, Hancock MA, Grutter P, Shoubridge EA. The mitochondrial transcription factor TFAM coordinates the assembly of multiple DNA molecules into nucleoid-like structures. Mol Biol Cell. 2007;18:3225–3236. doi: 10.1091/mbc.E07-05-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MP, Attardi G. Isolation of human cell lines lacking mitochondrial DNA. Methods Enzymol. 1996;264:304–313. doi: 10.1016/s0076-6879(96)64029-4. [DOI] [PubMed] [Google Scholar]
- Kukat C, Wurm CA, Spåhr H, Falkenberg M, Larsson NG, Jakobs S. Super-resolution microscopy reveals that mammalian mitochondrial nucleoids have a uniform size and frequently contain a single copy of mtDNA. Proc. Natl. Acad. Sci. USA. 2011;108:13534–13539. doi: 10.1073/pnas.1109263108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson NG, Oldfors A, Holme E, Clayton DA. Low levels of mitochondrial transcription factor A in mitochondrial DNA depletion. Biochem. Biophys. Res. Commun. 1994;200:1374–1381. doi: 10.1006/bbrc.1994.1603. [DOI] [PubMed] [Google Scholar]
- Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18:231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- Lee H, Rezai-Zadeh N, Seto E. Negative regulation of histone deacetylase 8 activity by cyclic AMP-dependent protein kinase A. Mol Cell Biol. 2004;24:765–773. doi: 10.1128/MCB.24.2.765-773.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wang J, Wilhelmsson H, Hansson A, Thoren P, Duffy J, Rustin P, Larsson NG. Genetic modification of survival in tissue-specific knockout mice with mitochondrial cardiomyopathy. Proc. Natl. Acad. Sci. USA. 2000;97:3467–3472. doi: 10.1073/pnas.97.7.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litonin D, Sologub M, Shi Y, Savkina M, Anikin M, Falkenberg M, Gustafsson CM, Temiakov D. Human mitochondrial transcription revisited: only TFAM and TFB2M are required for transcription of the mitochondrial genes in vitro. J. Biol. Chem. 2010;285:18129–18133. doi: 10.1074/jbc.C110.128918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Lu B, Lee I, Ondrovicova G, Kutejova E, Suzuki CK. DNA and RNA binding by the mitochondrial Lon protease is regulated by nucleotide and protein substrate. J. Biol. Chem. 2004;279:13902–13910. doi: 10.1074/jbc.M309642200. [DOI] [PubMed] [Google Scholar]
- Lodeiro MF, Uchida A, Bestwick M, Moustafa IM, Arnold JJ, Shadel GS, Cameron CE. Transcription from the second heavy-strand promoter of human mtDNA is repressed by transcription factor A in vitro. Proc. Natl. Acad. Sci. USA. 2012;109:6513–6518. doi: 10.1073/pnas.1118710109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Liu T, Crosby JA, Thomas-Wohlever J, Lee I, Suzuki CK. The ATP-dependent Lon protease of Mus musculus is a DNA-binding protein that is functionally conserved between yeast and mammals. Gene. 2003;306:45–55. doi: 10.1016/s0378-1119(03)00403-7. [DOI] [PubMed] [Google Scholar]
- Lu B, Yadav S, Shah PG, Liu T, Tian B, Pukszta S, Villaluna N, Kutejová E, Newlon CS, Santos JH, et al. Roles for the human ATP-dependent Lon protease in mitochondrial DNA maintenance. J. Biol. Chem. 2007;282:17363–17374. doi: 10.1074/jbc.M611540200. [DOI] [PubMed] [Google Scholar]
- Maniura-Weber K, Goffart S, Garstka HL, Montoya J, Wiesner RJ. Transient overexpression of mitochondrial transcription factor A (TFAM) is sufficient to stimulate mitochondrial DNA transcription, but not sufficient to increase mtDNA copy number in cultured cells. Nucleic Acids Res. 2004;32:6015–6027. doi: 10.1093/nar/gkh921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima Y, Goto Y, Kaguni LS. Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM) Proc. Natl. Acad. Sci. USA. 2010;107:18410–18415. doi: 10.1073/pnas.1008924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo HB, Kaiser JT, Chan DC. The mitochondrial transcription and packaging factor Tfam imposes a U-turn on mitochondrial DNA. Nat Struct Mol Biol. 2011;18:1290–1296. doi: 10.1038/nsmb.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondrovicova G, Liu T, Singh K, Tian B, Li H, Gakh O, Perecko D, Janata J, Granot Z, Orly J, et al. Cleavage Site Selection within a Folded Substrate by the ATP-dependent Lon Protease. J. Biol. Chem. 2005;280:25103–25110. doi: 10.1074/jbc.M502796200. [DOI] [PubMed] [Google Scholar]
- Papa S, De Rasmo D, Scacco S, Signorile A, Technikova-Dobrova Z, Palmisano G, Sardanelli AM, Papa F, Panelli D, Scaringi R, et al. Mammalian complex I: a regulable and vulnerable pacemaker in mitochondrial respiratory function. Biochim Biophys Acta. 2008;1777:719–728. doi: 10.1016/j.bbabio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Parisi MA, Xu B, Clayton DA. A human mitochondrial transcriptional activator can functionally replace a yeast mitochondrial HMG-box protein both in vivo and in vitro. Mol Cell Biol. 1993;13:1951–1961. doi: 10.1128/mcb.13.3.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini L, Scorrano L. A cut short to death: Parl and Opa1 in the regulation of mitochondrial morphology and apoptosis. Cell Death Differ. 2007;14:1275–1284. doi: 10.1038/sj.cdd.4402145. [DOI] [PubMed] [Google Scholar]
- Prabu SK, Anandatheerthavarada HK, Raza H, Srinivasan S, Spear JF, Avadhani NG. Protein kinase A-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury. J. Biol. Chem. 2006;281:2061–2070. doi: 10.1074/jbc.M507741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin MA, Prabu SK, Raza H, Anandatheerthavarada HK, Avadhani NG. Phosphorylation enhances mitochondrial targeting of GSTA4-4 through increased affinity for binding to cytoplasmic Hsp70. J. Biol. Chem. 2003;278:18960–18970. doi: 10.1074/jbc.M301807200. [DOI] [PubMed] [Google Scholar]
- Rubio-Cosials A, Sidow JF, Jimenez-Menendez N, Fernandez-Millan P, Montoya J, Jacobs HT, Coll M, Bernado P, Sola M. Human mitochondrial transcription factor A induces a U-turn structure in the light strand promoter. Nat. Struct. Mol. Biol. 2011;18:1281–1289. doi: 10.1038/nsmb.2160. [DOI] [PubMed] [Google Scholar]
- Sardanelli AM, Signorile A, Nuzzi R, Rasmo DD, Technikova-Dobrova Z, Drahota Z, Occhiello A, Pica A, Papa S. Occurrence of A-kinase anchor protein and associated cAMP-dependent protein kinase in the inner compartment of mammalian mitochondria. FEBS Lett. 2006;580:5690–5696. doi: 10.1016/j.febslet.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Schmidt O, Harbauer AB, Rao S, Eyrich B, Zahedi RP, Stojanovski D, Schonfisch B, Guiard B, Sickmann A, Pfanner N, et al. Regulation of mitochondrial protein import by cytosolic kinases. Cell. 2011;144:227–239. doi: 10.1016/j.cell.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Seidel-Rogol BL, Shadel GS. Modulation of mitochondrial transcription in response to mtDNA depletion and repletion in HeLa cells. Nucleic Acids Res. 2002;30:1929–1934. doi: 10.1093/nar/30.9.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Dierckx A, Wanrooij PH, Wanrooij S, Larsson NG, Wilhelmsson LM, Falkenberg M, Gustafsson CM. Mammalian transcription factor A is a core component of the mitochondrial transcription machinery. Proc. Natl. Acad. Sci. USA. 2012 doi: 10.1073/pnas.1119738109. 2012 Sep 24., [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutt TE, Lodeiro MF, Cotney J, Cameron CE, Shadel GS. Core human mitochondrial transcription apparatus is a regulated two-component system in vitro. Proc. Natl. Acad. Sci. U S A. 2010;107:12133–12138. doi: 10.1073/pnas.0910581107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solimini NL, Luo J, Elledge SJ. Non-oncogene addiction and the stress phenotype of cancer cells. Cell. 2007;130:986–988. doi: 10.1016/j.cell.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Sologub M, Litonin D, Anikin M, Mustaev A, Temiakov D. TFB2 is a transient component of the catalytic site of the human mitochondrial RNA polymerase. Cell. 2009;139:934–944. doi: 10.1016/j.cell.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu C, Umeda S, Ohsato T, Ohno T, Abe Y, Fukuoh A, Shinagawa H, Hamasaki N, Kang D. Regulation of mitochondrial D-loops by transcription factor A and single-stranded DNA-binding protein. EMBO Rep. 2002;3:451–456. doi: 10.1093/embo-reports/kvf099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Li T, Hou W, Zheng J, Schrum LW, Bonkovsky HL. Lon peptidase 1 (LONP1)-dependent breakdown of mitochondrial 5-aminolevulinic acid synthase protein by heme in human liver cells. J. Biol. Chem. 2011;286:26424–26430. doi: 10.1074/jbc.M110.215772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh S, Lee J, Singh K, Lee I, Suzuki CK. Multitasking in the mitochondrion by the ATP-dependent Lon protease. Biochim Biophys Acta. 2012;1823:56–66. doi: 10.1016/j.bbamcr.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TS, Rajagopalan S, Freund SM, Rutherford TJ, Andreeva A, Townsley FM, Petrovich M, Fersht AR. Biophysical characterizations of human mitochondrial transcription factor A and its binding to tumor suppressor p53. Nucleic Acids Res. 2009;37:6765–6783. doi: 10.1093/nar/gkp750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M. Generation, function and diagnostic value of mitochondrial DNA copy number alterations in human cancers. Life Sci. 2011;89:65–71. doi: 10.1016/j.lfs.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Zelenaya-Troitskaya O, Newman SM, Okamoto K, Perlman PS, Butow RA. Functions of the high mobility group protein, Abf2p, in mitochondrial DNA segregation, recombination and copy number in Saccharomyces cerevisiae. Genetics. 1998;148:1763–1776. doi: 10.1093/genetics/148.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollo O, Tiranti V, Sondheimer N. Transcriptional requirements of the distal heavy-strand promoter of mtDNA. Proc. Natl. Acad. Sci. U S A. 2012;109:6508–6512. doi: 10.1073/pnas.1118594109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.