Abstract
In a feather, there are distinct morphologies along the proximal-distal axis. The proximal part is a cylindrical stalk (calamus), whereas the distal part has barb and barbule branches. Here we focus on what molecular signaling activity can modulate feather stem cells to generate these distinct morphologies. We demonstrate the drastic tissue remodeling during feather cycling which includes initiation, growth and resting phases. In the growth phase, epithelial components undergo progressive changes from the collar growth zone to the ramogenic zone, to maturing barb branches along the proximal- distal axis. Mesenchymal components also undergo progressive changes from the dermal papilla, to the collar mesenchyme, to the pulp along the proximal- distal axis. Over-expression of Spry4, a negative regulator of receptor tyrosine kinases, promotes barb branch formation at the expense of the epidermal collar. It even induces barb branches from the follicle sheath (equivalent to the outer root sheath in hair follicles). The results are feathers with expanded feather vane regions and small or missing proximal feather shafts (the calamus). Spry4 also expands the pulp region while reducing the size of dermal papillae, leading to a failure to regenerate. In contrast, over-expressing Fgf10 increases the size of the dermal papillae, expands collar epithelia and mesenchyme, but also prevents feather branch formation and feather keratin differentiation. These results suggest that coordinated Sprouty/FGF pathway activity at different stages is important to modulate feather epidermal stem cells to form distinct feather morphologies along the proximal-distal feather axis.
Keywords: branching, pattern formation, stem cells, skin appendages, regeneration
Introduction
The robust regenerative power and diverse feather morphologies produced over the body surface of a single bird have made the feather follicle a major model for morphogenesis. Analyses of its cellular/molecular mechanisms help us understand the language of morphogenesis during development and evolution. Feather follicles cycle continuously throughout a bird’s life. In early studies, it was reported that the dermal papilla (DP) at the follicle base is critical for feather regeneration (Lillie and Wang 1941; 1943). The feather cycle consists of initiation, growth and resting phases (Lucas and Stettenheim, 1972). Epithelial-mesenchymal interactions are essential for initiation of a new feather cycle (Yu et al., 2004; Chuong et al., 2012). The molt and regeneration processes are different from those occurring in hair follicles. For example, in hair cycling, at catagen the lower follicle is gone. The DP is shifted to a higher position at telogen. In feather cycling, the feather cycles by pulp degeneration and the expanded keratinization into the collar epithelia. The follicle becomes empty inside but the overall external structure of the follicle (follicle sheath and dermal sheath) and the position of the DP remain unchanged (Fig. 1A, B).
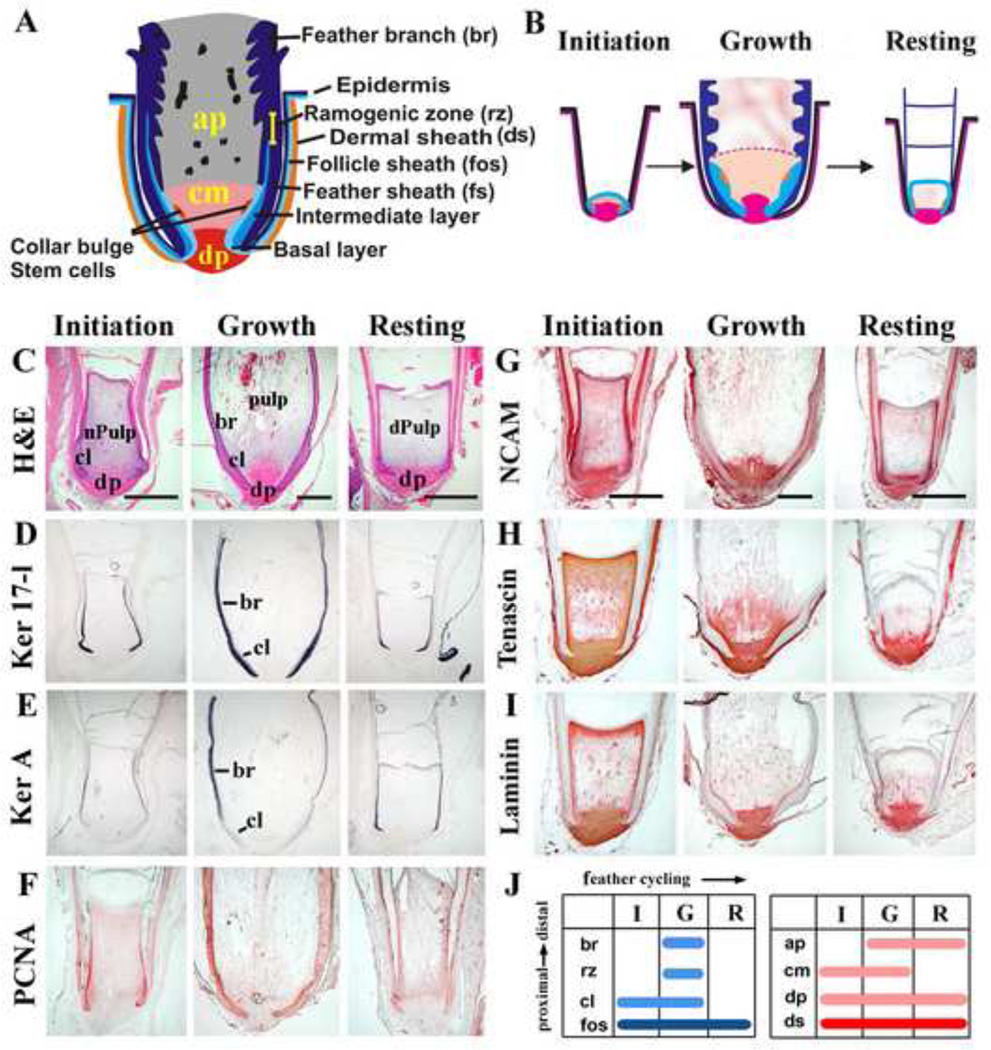
Figure 1. Components of feather follicles along the proximal-distal axis and changes during the feather cycle.
A, Schematic diagram of a feather follicle in growth phase. B, Schematic diagram of feather follicles in Initiation, Growth and Resting phases of the feather cycle. C, H&E shows drastically different follicle architectures in the initiation (left), growth (middle) and resting (right) phases. Dynamic changes are seen in both epithelia and mesenchyme. D-I, Molecular expression during feather cycles. D, Cytokeratin 17-l (in situ hybridization) is in both the epithelial collar and feather branch region. E, Keratin A (in situ hybridization) is only in the differentiated feather branch region. F, PCNA (immunostaining) is in the proliferating cells. G, NCAM stains the DP and weakly in the pulp and the epithelium. H, Tenascin C (immunostaining) is in the DP and mesenchyme facing the collar. I, Laminin (immunostaining) is in the DP, basement membrane and the blood vessels. For G-I, Please refer to panel C for annotation of components. J, diagram summary of the different components during the feather cycle. The constant portion includes the collar with variable size (epithelium), follicle sheath, dermal sheath and DP (mesenchyme). The cyclic portion includes the collar (variable in size), ramogenic zone, feather branches, feather sheath (epithelium), collar mesenchyme, and angiogenic pulp (mesenchyme). Please refer to Panel A for follicle structures.
ap, angiogenic pulp; br, feather branches; cl, collar; cm, collar mesenchyme; dp, dermal papilla; dPulp, degenerating pulp; ds, dermal sheath; fos, follicle sheath; fs, feather sheath; nPulp, new pulp; rz, ramogenic zone. Bar: 1mm.
The growing feather follicle is a cylindrical epithelial structure, surrounding a mesenchymal core. The outer layer of the epithelium is the "feather sheath" (Fig. 1). This feather sheath layer and inner basal layer later distinegrates. The follicle also has an epidermal follicle sheath (fos, Fig. 1) and dermal sheath which remain intact throughout feather cycling. Along the epithelial wall, new cells are added at the proximal end in the collar region. Branching morphogenesis occurs in the ramogenic zone and differentiation into feather filaments takes place in the distal part (see Fig. 1A, B for terminologies; Prum and Brush, 2002; Chuong et al., 2000). Thus the distal feather is formed first, and the proximal end forms later. During the feather cycle, there is a sequential expansion and regression of distinct proximal—distal epithelial and mesenchymal structures within the follicles. For epithelial components, during the growth phase, stem cells were found to be configured as a ring, sitting in the follicle base, but above the DP (Yue et al., 2005). The collar epidermis, around the stem cells and in the proximal follicle, gives rise to new cells continuously. These cells are displaced upward (distally) and undergo branching morphogenesis in the ramogenic zone to form barb ridges. The signaling micro-environment at this stage can determine the symmetry and shape of feathers. For example, a Wnt 3a gradient is shown to be important for bilaterally versus radially symmetric feathers (Yue et al. 2006). BMP activity is shown to be important for rachis formation, but suppresses barb branching (Yu et al., 2002). During resting phase, the collar epidermis shrinks, and the ramogenic zone disappears (Alibardi, 2009). For mesenchymal components, during the growth phase, the dermal papilla gives rise to pulp which expands to become the center portion of the feather cylinder. However, the molecular mechanisms that specify this proximal-distal fate in both epidermal and dermal components are largely unknown.
In vertebrates, Sprouty genes (spry) can act as both agonists and antagonists of FGF signaling (Minowada et al., 1999; Furthauer et al., 2001; Mailleux et al., 2001; Guy et al., 2003; Shim et al., 2005; Suzuki-Hirano et al., 2005), as well as other receptor tyrosine kinase pathways (Kramer et al., 1999; Rubin et al., 2003; Mason et al., 2006; Cabrita and Christofori, 2008). For example, in some contexts Sprouty inhibits signaling through receptor tyrosine kinase pathways but in other contexts, Sprouty may potentiate MAP kinase signaling by inhibiting the degradation of EGF/FGF receptors (Egan et al., 2002; Wong et al., 2002; Guy et al., 2003; Guy et al., 2009).
While the differential effects may remain obscure, Sprouty genes nonetheless are important mediators of growth factor signaling during embryogenesis. In the Drosophila trachea, FGF signaling was shown to promote epithelial branching, while sprouty inhibits this process by antagonizing FGF signaling (Sutherland et al., 1996; Hacohen et al., 1998). Similar events were also described in mammalian lung branching morphogenesis (Bellusci et al., 1997; Tefft et al., 2002; Perl et al., 2003). Furthermore, the lungs of Spry2 null mice had ectopic branches (Metzger et al., 2008) consistent with the above observations. FGF signaling was also found to be critical for chicken and mouse limb formation and help to maintain the apical ectodermal ridge (Lewandoski, 2000; Dudley et al., 2002; Sun et al., 2002). Fgf8 is present in the AER and Fgf10 is present in the underlying dermal progress zone. Fgf10 signaling is required to maintain AER function (Sekine et al., 1999). Here too Sprouty was found to modulate FGF signaling. Spry4 null mice showed polysyndactyly of the forelimbs with fusion and duplication of their digits (Taniguchi et al., 2007).
FGF signaling has been shown to be involved in feather bud initiation (Widelitz et al., 1996; Song et al., 1996; Mandler and Neubuser, 2004; Song et al., 2004; Wells et al., 2012). Here chemotaxis toward centers of FGF signaling is mediated through ERK activity (Lin et al., 2009). FGF signaling has also been implicated in the regulation of hair growth and cycling (Hebert et al., 1994; Rosenquist and Martin, 1996; Petiot et al., 2003; Kawano et al., 2005; Kimura-Ueki et al., 2012). Four Spry genes have been identified in mammals. The roles of each of these genes in hair /feather biology are largely unknown.
To explore the role of the FGF signaling pathways and their modulation by Sprouty we used RCAS virus to deliver exogenous genes into the regenerating feather follicles and investigate their roles in feather formation (Yu et al., 2002). Here we report that over-expression of Spry4 in feather follicles promotes the formation of distal follicle components, while the proximal structures such as the collar epithelium or DP are diminished. As a result, the formed feathers have extensive branches. In contrast, over-expression of Fgf10 promotes the expansion of proximal follicle structures and inhibits distal branching. These striking results suggest that Sprouty / FGF signaling is important for regulating feather branch formation along the proximal-distal axis of the feather shaft.
Results
Both epidermal and mesenchymal follicular components change dynamically during the feather cycle
Feather follicles undergo natural cycles of Resting (R), Initiation (I) and Growth (G) phases. To characterize the various components of follicles during the feather growth cycle, we examined the dynamic expression patterns of molecular markers. The epithelial components of the feather follicles were characterized by cell proliferation and differentiation markers. Chicken keratin 17-like (Krt 17-l) was used to represent type I alpha keratin. It showed ubiquitous expression throughout the feather growth cycle and thus serves as a good epithelial marker at all feather follicle cycle stages (Fig 1D). In contrast, feather Ker A, a beta keratin marker of further differentiation, is not expressed during initiation phase, becomes expressed distally during growth phase and extends proximally during resting phase (Fig 1E). The cell proliferation marker PCNA showed strong activity in the proximal follicle during initiation and growth phases but weaker expression during resting phase (Fig 1F). The mesenchymal components of the feather follicles are characterized by a set of adhesion molecules (Chuong and Edelman, 1985). NCAM is in the DP and is weakly expressed in the epithelial collar (Fig 1G). Tenascin C is in the DP, and is particularly enriched in a special region above the DP, beneath the ramogenic zone, which we termed the “collar mesenchyme”. The collar mesenchyme expanded in I / G phase, but diminished in R phase (Fig 1H). Laminin stains the DP and the basement membrane. Also the pulp is marked by speckled laminin staining (blood vessels), which occupies the mesenchyme facing barb ridges at G phase, and regresses in R phase (Fig 1I). We term this portion of the pulp “angiogenic pulp”.
Changes of molecular expression can readily be observed to accompany the reduction and expansion of epithelial / mesenchymal components. For example, the variation of DP size through the feather cycle can be easily seen in specimens that are H&E stained as well as in those stained for NCAM, where the DP is smallest in R phase and largest in G phase (Fig. 1C, G). These dynamic changes are diagrammatically summarized in Fig 1B (structural components) and in Fig 1J (molecular expression).
Expression of FGF/sprouty pathway members during feather cycling
Since FGFs were previously shown to play a significant role in feather morphogenesis (Song et al., 1996, Widelitz et al., 1996; Mandler and Neubuser et al., 2004) we decided to further explore Fgfs, their antagonist, Sprys and related factors during the feather growth cycle using in situ hybridization. Fgf10 was shown to regulate embryonic feather development (Tao et al., 2002; Mandler and Neubuser, 2004). In adult feather follicles, Fgf10 is expressed mainly in the feather epithelium; high in I / G phase and reduced in R phase. Weak expression was also seen in the pulp in I phase (Fig 2A). Fgf4 showed a similar expression pattern (data not shown). Fgf2/8 were not detected in adult feather follicles (data not shown). EGF, which was shown to be expressed in the interbud region during feather development and promotes interbud fate of embryonic skin (Atit et al., 2003), was expressed weakly in the lower collar region only in G phase (data not shown). Among the known FGF receptors, Fgfr1 was widely expressed inside the feather follicle epithelium, including the collar, ramogenic zone, feather branches, and the follicle sheath. Weak expression was also seen in the mesenchymal pulp in I phase. Fgfr1 expression was the highest in G phase, and reduced in R phase (Fig 2B). Fgfr2 expression was also cycle-dependent, present mainly in the basal layer and with greatly diminished expression in R phase (Fig 2C).
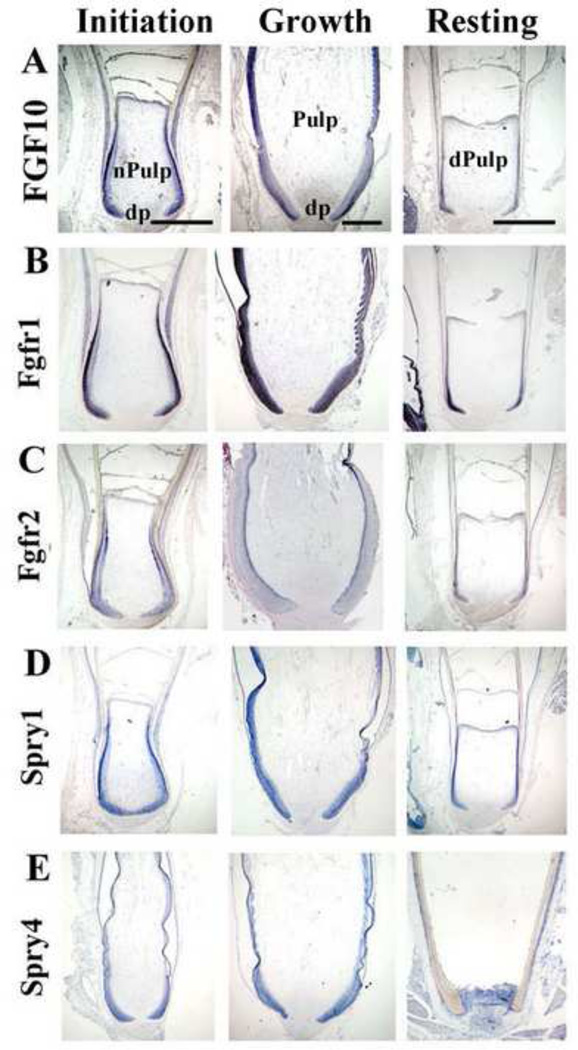
Figure 2. Expression of FGF/sprouty signaling members in feather follicles.
In situ hybridization was used to detect the mRNA expression of FGF signaling members during the feather cycle. In each panel, initiation phase (I) is to the left, growth phase (G) is in the middle, and resting phase (R) is to the right. Some follicle structures are labeled in row A. For simplicity, please refer to Fig. 1 for more annotation of components in row B–E,
A, Fgf10 is expressed strongly in the epithelium in I / G phase, and diminished in R phase. Weak expression is also seen in the mesenchymal pulp. B, Fgfr1 expression is detected in all epithelial components of the feather follicle, strongest in growth phase. Mesenchymal expression is seen in I / G phase as well. C, Fgfr2 expression starts in the ramogenic zone in I phase, becomes stronger in G phase, and disappears in R phase. D, Spry1 is expressed mainly in feather epithelium during all phases of the feather cycle. E, Spry4 is expressed in the feather epithelium, and strongly in the pulp during R phase.
dp, dermal papilla; dPulp, degenerating pulp; nPulp, new pulp. Bar: 1mm.
Spry1 / 2 / 4 genes also were expressed in the feather follicles in a cycle-dependent manner. Spry1 / 2 were mainly expressed in the epithelium (Fig 2D and data not shown). A chicken Spry4 probe was cloned by degenerative RT-PCR from known Spry4 sequences in GenBank. Spry4 expression was increased during feather growth, and strongly expressed in R phase follicles both in the epithelium and mesenchyme (Fig 2E), which is different from Spry1 / 2. These dynamic expression patterns suggest FGF / Sprouty signaling may regulate the size and shape of feather structural components during the growth cycle which may feedback to regulate the cycle itself.
Spry 4 over-expressing feather follicles show extensive disruption of proximal follicle structures and a lost ability to regenerate
Mis-expression of RCAS-Spry4 in feather follicles produced smaller, defective feathers (Fig 3A). There was diminished growth in both the barbs and rachis. The thickness of the epithelium was reduced, leading to a thin rachis. Sometimes, part of the feather vane did not form. In some specimens, the rachis disappeared and only a few feather branches formed. After a few short cycles (1~ 5 cycles of 2–3 weeks, versus cycles of ≥ 6 months in normal wing feathers; n = 20), these follicles were not able to grow back, thus their ability to regenerate was depleted.
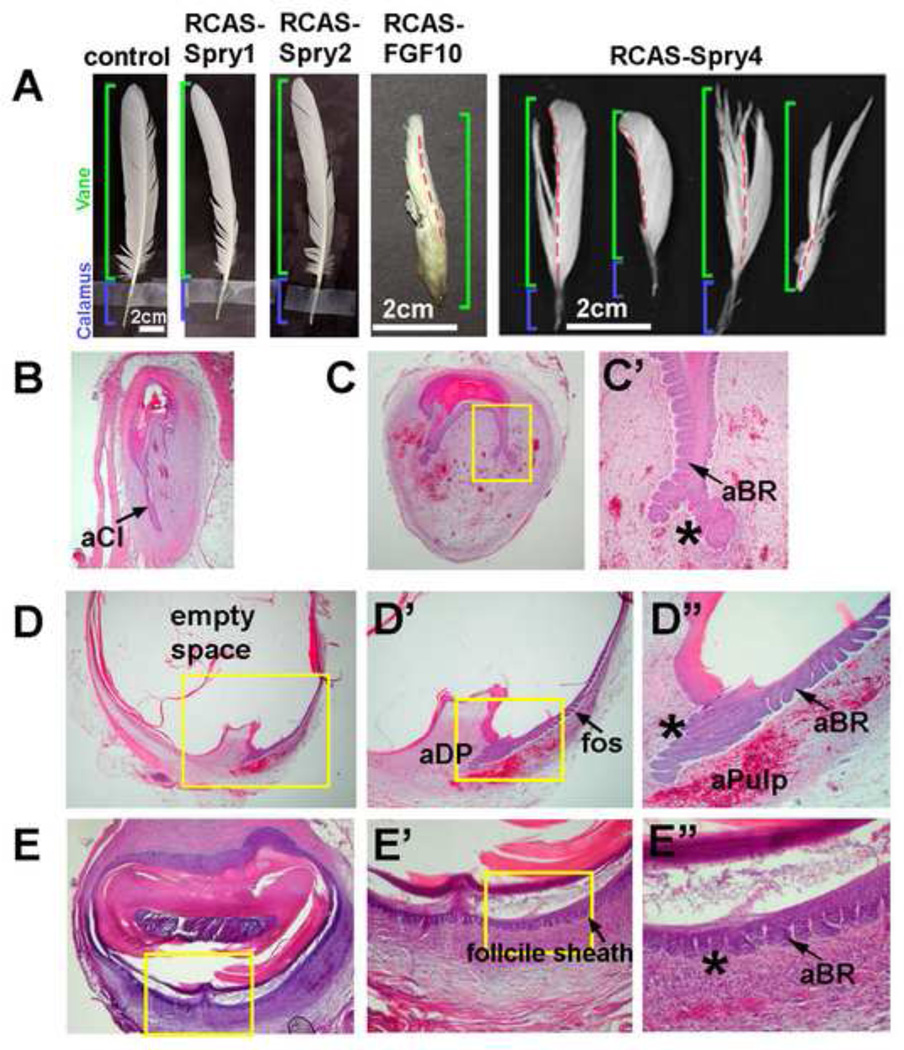
Figure 3. Over-expression of spry4 disrupts proximal follicle structure and abrogates feather regeneration ability.
A, Gross morphology of control, RCAS-Spry1, RCAS-Spry2, RCAS-Fgf10 and RCAS-Spry4 perturbed feathers. Red dotted line indicates the position of the rachis. B-E, H&E staining of RCAS-Spry4 perturbed feather follicles after 3 weeks. B, D, Longitudinal section. The collar is diminished or missing in the follicle. Ectopic epithelial branches form in the follicle sheath region. Notice the DP is missing in B and abnormal in D. Ectopic induction of angiogenic pulp is present in the lower follicle. C, E, Cross sections. Ectopic epithelial branches in the follicle sheath region. C, the follicle was not a closed circle. The rachis shrank and abnormal keratinization was observed. E, The inside of the follicle was filled with a hard keratin core. Also notice the abnormal epithelial branches in C’, D” and E” (marked by *).
aBR, abnormal barb ridge; aCl, abnormal collar; aDP, abnormal dermal papilla; aPulp; abnormal pulp.
Histological sections showed extensive barb ridge formation in the feather (Fig 3B–C'). Another obvious phenotype is that the DP is reduced in size or missing in these follicles even when every section was collected and screened for the DP (Fig 3B, D). In addition, the collar epithelia were remarkably reduced in thickness (Fig 3D). Surprisingly, abnormal epithelial branching was also observed in the follicle sheath. This occurred only in the lower, but not the upper part of the follicle sheath (Fig 3D, E), suggesting that the lower follicle sheath epithelium has higher plasticity to respond to spry4 signals. In cross sections, we observed that in many cases the follicle did not retain its normal cylinder structure. The follicle epithelium shrank, presumably due to reduced cell number and improper differentiation of the rachis (Fig 3C). The follicles were filled with a hard keratinized core (Fig 3E), suggesting abnormal epithelial differentiation / keratinization, and diminished mesenchymal components.
Virus expression was monitored by in situ hybridization to an RCAS polymerase probe. The virus mostly transduced epithelial cells, suggesting an active epithelial—mesenchymal interaction leads to the mesenchymal phenotypes (Fig 4B, E, 5B). This effect is specific for RCAS-Spry4. Over-expression of RCAS-Spry1 or RCAS-Spry2 produced normal feathers (Fig 3A). We have performed over-expression experiments with many other molecules, including RCAS-Shh, -BMP2 /4, -noggin (Yu et al., 2002), -Wnt3a (Yue et al., 2006), -beta-catenin (Widelitz et al., 2000), etc. Only RCAS-Spry4 produced these phenotypes.
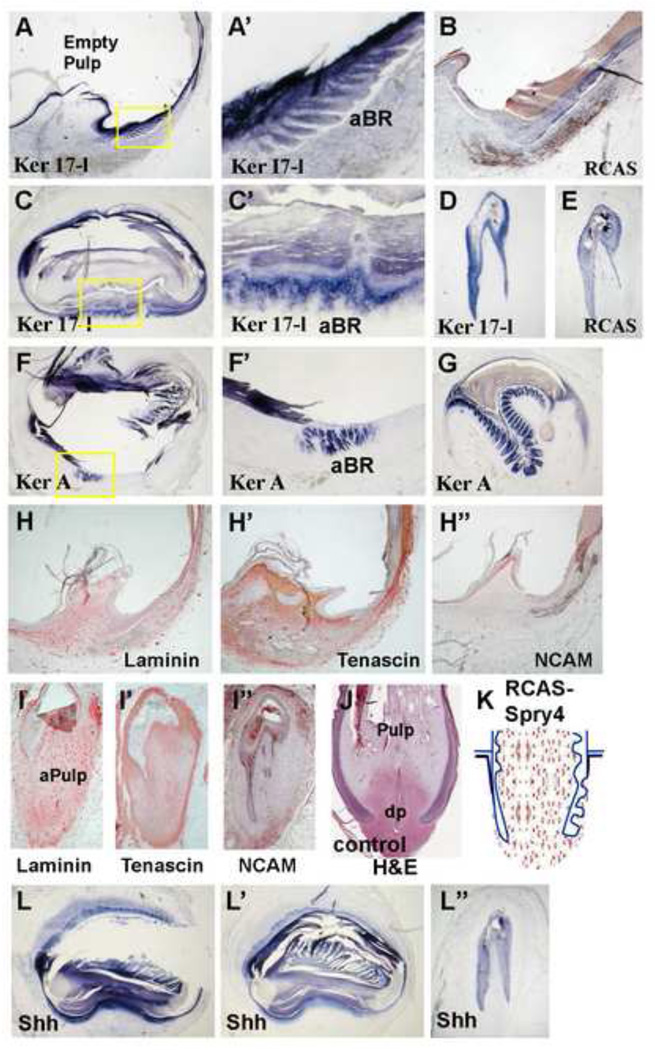
Figure 4. Molecular characterization of spry4 overexpressing feather follicles.
A–G, Epithelial marker analysis showing Ker 17-l and Ker A were expressed in the ectopically induced follicle sheath branches. RCAS polymerase in situ hybridization showing virus were mainly expressed in the follicle epithelium (B, E). H–I', Mesenchymal marker analysis. Loss of DP and expansion of angiogenic pulp were shown by laminin (H, I), Tenascin C (H', I') and NCAM (H", I") staining. J, control sample with H&E staining. The resulting follicle structure from Spry4 overexpression was schematized in K. L-L", In situ hybridization staining for Shh shows that the abnormal, ectopic barb ridges which form in the Spry4 over expressing feather follicles are capable of inducing Shh in the feather branches. aBR, abnormal barb ridge; aPulp, abnormal pulp; dp, dermal papilla.
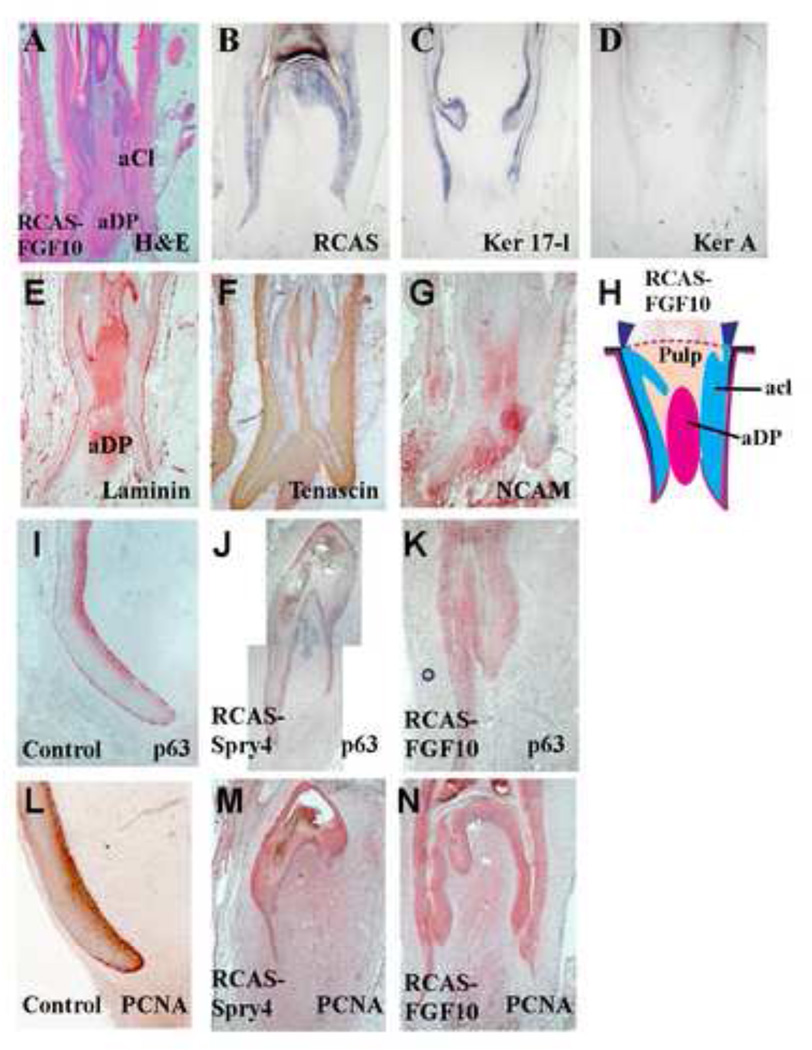
Figure 5. Over-expression of Fgf10 promotes proximal follicle structures.
A, H&E staining of perturbed feather follicles after RCAS-Fgf10 transduction for 3 weeks. The DP and collar expanded, and feather branching is not seen.
B, In situ hybridization for RCAS shows that virus expression is mainly in the epithelium (RCAS polymerase). C, There is reduced expression of Ker 17-l. D, There is a loss of the differentiation marker Ker A. E, Laminin staining of the dermal papilla is expanded. F, Tenascin C staining shows loss of the feather pulp. G, NCAM shows expansion of the DP. H. A diagram summarizing the affects of Sprouty on feather branching morphogenesis.
I, J, K. P63 staining of the normal and perturbed feathers. P63 stains strongly in the basal layer of the collar region, and was expanded in RCAS-Fgf10 samples (K), and diminished in RCAS-Spry4 samples (J). L, M, N. PCNA staining of the normal and perturbed feathers. The expression domain was expanded in RCAS-Fgf10 samples (N) and diminished in RCAS-Spry4 samples (M).
aCl, abnormal collar; aDP, abnormal dermal papilla.
Molecular Characterization of Spry4 perturbed feathers
To characterize the phenotypes produced by Spry4 over-expression, we examined the expression pattern of molecular markers in these follicles. Ker 17-l and Ker A were expressed in the abnormal epithelial branches. These molecules normally do not appear in the follicle sheath, suggesting that these abnormal epithelial structures resembled real feather branches at a molecular level (Fig. 4A, C, D, F, G). Immunohistochemistry revealed the abnormality also included mesenchymal components: a loss of the DP structure was obvious by laminin, Tenascin C, and NCAM staining (Fig. 4H–I"). The widespread laminin staining suggested that most of the mesenchyme was turned into angiogenic pulp. There is also a lack of tenascin. The DP became atrophic. A normal growth stage feather follicle is now shown in Fig. 4J for comparison. Overall, the sprouty over-expressing follicle appeared to be more distalized and was missing the proximal components (Fig 4K).
To further characterize the role of spry4 overexpression in feather follicles, we analyzed the expression of other genes known to be involved in feather morphogenesis. Shh is a molecular marker for branching epithelium, expressed in the marginal plate cells, and may be involved in regulating apoptosis of the feather epithelium (Ting-Berreth and Chuong, 1996; Yu et al., 2002; Chang et al., 2004). In spry4 transduced feather follicles, Shh was ectopically induced in the epithelial branches of the follicle sheath (Fig 4L–L"), suggesting these branches originated from a similar branching program as is responsible for the normal ramogenic zone activity. These results suggest that the ramogenic branching program can be ectopically induced by excessive spry signaling.
Fgf10 over-expressing feather follicles show extensive formation of collar epithelia and expanded dermal papilla mesenchyme
Fgf10 is endogenously expressed in feather follicles. Ectopic expression of Fgf10 was used to tilt the balance of endogenous sprouty activity. When RCAS-Fgf10 was overexrepssed in feather follicle, it generated a "fat" follicle but the feather growth stopped precociously (Fig. 3A). Fgf10 over-expression leads to expanded proximal follicle structures, including extensive formation of collar epithelia and expansion of the dermal papilla-like mesenchyme (Fig 5A). Molecular characterization showed Ker 17-l expression in the follicle epithelium, but not Ker A, suggesting the Fgf10 over expressing epithelium assumed a very proximal fate (Fig 5C–D) and failed to form differentiated barb ridges. P63 was suggested to be a molecular marker for epithelial stem / progenitor cells that is required for epithelial stratification (Mills et al., 1999; Pellegrini, et al., 2001; Senoo et al., 2007). In normal feather follicles, P63 was strongly expressed in the basal collar region of the collar epithelium (Fig 5I). In Fgf10 transduced follicle epithelium (Fig 5K), P63 remained in a similar region; whereas the region was diminished in Spry4 transduced follicles (Fig 5J). The cell proliferation marker, PCNA, showed similar staining patterns. Strong expression of PCNA was observed in the basal layer of collar epithelium in normal follicles (Fig. 5L). Similar staining was seen in Fgf10 transduced follicles (Fig 5N), but the staining is reduced in Spry4 transduced follicles (Fig 5M). Consistent with the notion that the expanded mesenchyme has a proximal fate, the expanded mesenchyme in Fgf10 transduced follicles shows positive immunostaining for laminin, tenascin and NCAM (Fig 5E–G). NCAM expression is a characteristic of the DP (Chuong and Edelman, 1985; Jiang and Chuong, 1992).
Originally, we thought that the FGF pathway would be important for the growth phase follicle. If so, tilting molecular homeostasis further toward FGF may lengthen the growth phase of feather follicles and feathers may grow longer. Our data suggest that we are only partially right. Regenerating feathers over expressing Fgf4 (not shown) or Fgf10 were short in length with a large diameter.
These results suggest that un-differentiated cell types and proximal follicle fate is enhanced in Fgf10 transduced feather follicles. On the other hand, differentiated cell types with barb ridge formation, a fate usually assumed by distal feathers, is enhanced in spry4 transduced feather follicles. Thus, tilting the balance of Sprouty / FGF activity can modulate the fate of feather follicle stem cells. These results were summarized in Fig 5H.
Discussion
In this paper we explored the expression and downstream consequences of FGF / Sprouty signaling during the initiation, growth and resting phases of the feather cycle. FGFs have been implicated in feather bud initiation (Song et al., 1996; Widelitz et al., 1996; Mandler and Neubuser, 2004; Song et al., 2004; Wells et al., 2012). We have recently shown that FGF activity is mediated by ERK activity within the mesenchyme and have devised a mathematical model based on the observed short-range activation, long-range inhibition and chemotaxis at early stages of feather bud formation (Lin et al., 2009). FGF / Sprouty signaling pathway family members showed dynamic, overlapping endogenous expression patterns throughout this morphogenetic process suggesting that sprouty modulation of FGF activity is essential for normal feather development. FGF pathway member expression patterns largely overlap with regions of proliferation. The exception to this observation was that Spry4 was uniquely expressed in the dermal papilla at resting stage. It may function at this location to modulate the affects of a different FGF family member, not studied here.
To explore the function of Sprouty in feather morphogenesis, Spry4 was constitutively expressed from a replication competent avian sarcoma viral vector. Ectopic Spry4 expression within epithelia produced smaller feathers. Portions of the feather vane from transduced feather follicles were often missing. Their capacity to regenerate was drastically reduced. The normal branching structures, the barbs and rachis, were diminished in size. Surprisingly, there was increased barb ridge formation leading to abnormal epithelial branching inappropriately in the follicle sheath. Changes in structural components were verified by molecular characterization of perturbed feather follicles. Alterations in the phenotypes were specific to Spry4 and were not observed when Spry1 or Spry2 were similarly misexpressed. The phenotype of RCAS-Spry4 is quite severe, possibly due to the broader range of its inhibitory effects.
In contrast to the effects of Sprouty misexpression, ectopic expression of Fgf10 expanded proximal feather follicle structures (collar epithelium and dermal papilla-like mesenchyme). While the detailed biochemical mechanism remains to be worked out in the feather follicles, the paired study shown here suggests that Fgf10 and Spry4 antagonize each other and this pathway is involved in the incremental specification of the P-D fates of feather keratinocytes.
Sprouty can modulate FGF signaling during development
In development, Spry has been shown to regulate morphogenesis during Xenopus gastrulation through Ca++ and PKC signaling (Nutt et al., 2001; Sivak et al., 2005; Hanafusa et al., 2009). However, the function of Spry is more often linked to FGF signaling, partly because of their close expression domain during development. For example, In feather morphogenesis, FGF activity is enriched in the feather primordia region, while EGF is in the inter-primordia region (Atit et al., 2003).
Functional testing of Sprouty has been consistent with the regulation of FGF activity. In the inner ear, signaling through Fgfr1 was shown to decrease the size of sensory auditory hair progenitor cell pools (Pirvola et al., 2002). Spry1, 2 double knockout mice had enlarged otic placodes (Mahoney Rogers et al., 2011). Fgfr3 signaling is involved in the formation of the tunnel of Corti in the inner ear and pillar cell specification (Colvin et al., 1996). More recently, conditional Spry2 null mice were found to have an extra row of hair cells in the tunnel of Corti. This phenotype was partially rescued by reducing Fgf8 expression (Shim et al., 2005). In the mouth, Fgf10 signaling retains the tooth stem cell compartment for mouse incisors (Harada et al., 2002). The epithelial expression of Spry2 and mesenchymal expression of Spry4 was shown to prevent tooth formation in the normally toothless, diastema region (Klein et al., 2006). The removal of Spry2 from the mouths of knockout mice cause cleft palate formation. Expression of Spry2 from a Spry2-BAC clone partially rescues the phenotype demonstrating a dose dependent dependence for Spry2 in palate development (Welsh et al., 2007). More recently, mice expressing functionally null Spry1, 2 and 4 showed an inverse gene dosage effect on the number of incisors formed (Charles et al., 2011). Furthermore, FGFs modulated by Sprouty activity regulates the enamel producing ameloblasts. Spry4−/−; Spry2+/− mice abnormally develop enamel on the tooth lingual surface (Klein et al., 2008).
Dynamic and coordinated changes of FGF/Sprouty and other signaling may modulate stem cells to form distinct feather morphologies along the P-D axis
In the branching morphogenesis of feathers, the multilayer epidermal cylinder, with the basal layer facing the inside mesenchyme, starts to form periodically arranged barb ridges and marginal plates in the ramogenic zone (Lucas and Stettenheim, 1972; Yu et al., 2002). A Shh-BMP2 module has been proposed to regulate feather branch and rachis formation in feather follicles (Harris et al., 2002). Over-expression of BMP 2, 4 leads to the formation of a giant rachis, while over-expression of noggin causes barb ridges to branch elaborately (Yu et al., 2002). On the other hand, localized apoptotic activity is essential to open feather follicles (Chang et al., 2004). Suppression of Shh signaling, expressed in the marginal plate epithelium, leads to remnants of an epithelia "web" between barbs (Yu et al., 2002). We showed that radial downy feathers and bilaterally symmetric flight feathers differ in that the latter exhibits a Wnt 3a gradient (high toward the rachis) while the former does not. Further, flattening such a gradient by over expression or suppression of Wnt 3a agonists or antagonists lead to the conversion of bilaterally to radially symmetric feathers (Yue et al., 2006).
While this work provides an understanding as to how the morphogenesis of feather barb branches takes place, how the fate of feather stem cells are specified to assume the branching fate is not known. Here we provide molecular evidence that feathers are not "pre-patterned" as suggested by Cohen and Espinasse (1961). Rather, morphogenesis takes place by guiding the fates of epithelial stem cells (Yue et al., 2005) according to the follicle micro-environments. As shown in Fig. 1, the proximal to distal feather follicle mesenchyme fate is determined over time to become the dermal papilla, collar mesenchyme, ramogenic mesenchyme and pulp in progressive feather cycle stages. Epihelial cell fate is also determined over time. One of the molecular signals which guide epithelial cell fate is Fgf10 (Tao et al., 2002; Mandler and Neubuser, 2004). When there is excess Fgf10, cells take the proximal collar epithelial fate and do not differentiate or form feather branches. Barb branch formation is almost completely suppressed. When Fgf10 activity is reduced physiologically toward the ramogenic zone, or suppressed experimentally by Spry4, cells switch their fate and start to form barb ridges. The incremental feather morphology formed at any particular moment is determined by the follicle micro-environment adjacent to the stem cells at that time. When Sprouty signaling is over-whelming, many epidermal cells within the cylinder, including the collar and stem cells are induced to form barb ridges. This conversion even extends beyond the follicle, to include follicle sheath cells. A similar ability of outer root sheath cells to be induced was reported in hair follicles (Horne and Jahoda, 1992). The fact that Sprouty / FGF can re-specify epidermal cell fates also supports the idea that feather epidermal stem cells in the collar bulge are indeed stem cells (Yue et al., 2005) that can be modulated to form different feather morphologies (Yue et al., 2006).
FGF / Sprouty signaling have been implicated in the branching morphogenesis of other organ systems. For instance, villous outgrowth was increased by siRNA mediated suppression of Spry2 in cultured explants of human placenta extravillous trophoblasts (Natanson-Yaron et al., 2007). In another example, ectopic Spry2 expression in the ureteric bud inhibited branching morphogenesis (Chi et al., 2004). Transgenic mice expressing Spry2 in the peripheral lung epithelium decreased branching as did mouse lungs transduced with virus expressing Spry2 (Mailleux et al., 2001) while Spry2 null mice had increased branching (Metzger et al., 2008). Similarly, transgenic mice expressing Spry4 during lung development had reduced branching (Perl et al., 2003). It may appear strange that Spry4 increases branching in feathers. This is because lung branching occurs through differential proliferation, while feather branching occurs through differential apoptosis. In both cases, Sprouty works to suppress proliferation but leads to different morphological phenotypes.
Another major effect of Spry4 is on the formation of proximal follicle morphology, i.e. the collar. Several proximal follicular components are lost: the collar epidermis is diminished, the dermal papilla is greatly reduced in size, and the follicle sheath is also turned into barb ridges. The collar mesenchyme shows characteristics of pulp (loose mesenchyme, with a lot of laminin positive blood vessels, which are devoid of tenascin-C). In contrast, over-expression of Fgf10 further promotes the expansion of proximal components in the follicle, including the collar and the DP. Distal components including the branching epithelium, the collar mesenchyme and angiogenic pulp are diminished or do not form. Tissue sections show that the follicle is full of epithelia and express characteristics of the collar and DP like the mesenchyme (Fig. 5A). The mesenchyme is positive for NCAM, as if most of the follicle mesenchyme was converted into a giant DP.
One of the novel developmental mechanisms in the evolution of feathers is to form an epidermal cylinder and to convert part of it into barb ridges (Prum, et al., 2005). In this aspect, it is interesting to look into the feathers of Mesozoic birds from the Jehol Biota, China (Zhou, 2008; Hou et al., 2003). Some of the tail feathers of Sinornithosaurus millenii have a long (up to 45 mm), narrow (1–3 mm) membranous shaft-like structure with branched tufts only at the distal end (Xu et al., 2001). One can speculate that sprouty 4 was expressed transiently when that branched region was made. By modulating the level and duration of Spry4 activities, feathers can form different degrees of branching regions along the proximal - distal axis. Thus modulations of the temporal micro-environmental changes within the follicle are "cast" as distinct morphologies along the spatial axis of feathers (with early events located at the distal end). The topological organization of feather follicles provides a linear platform for a great range of variable morphologies to develop and evolve.
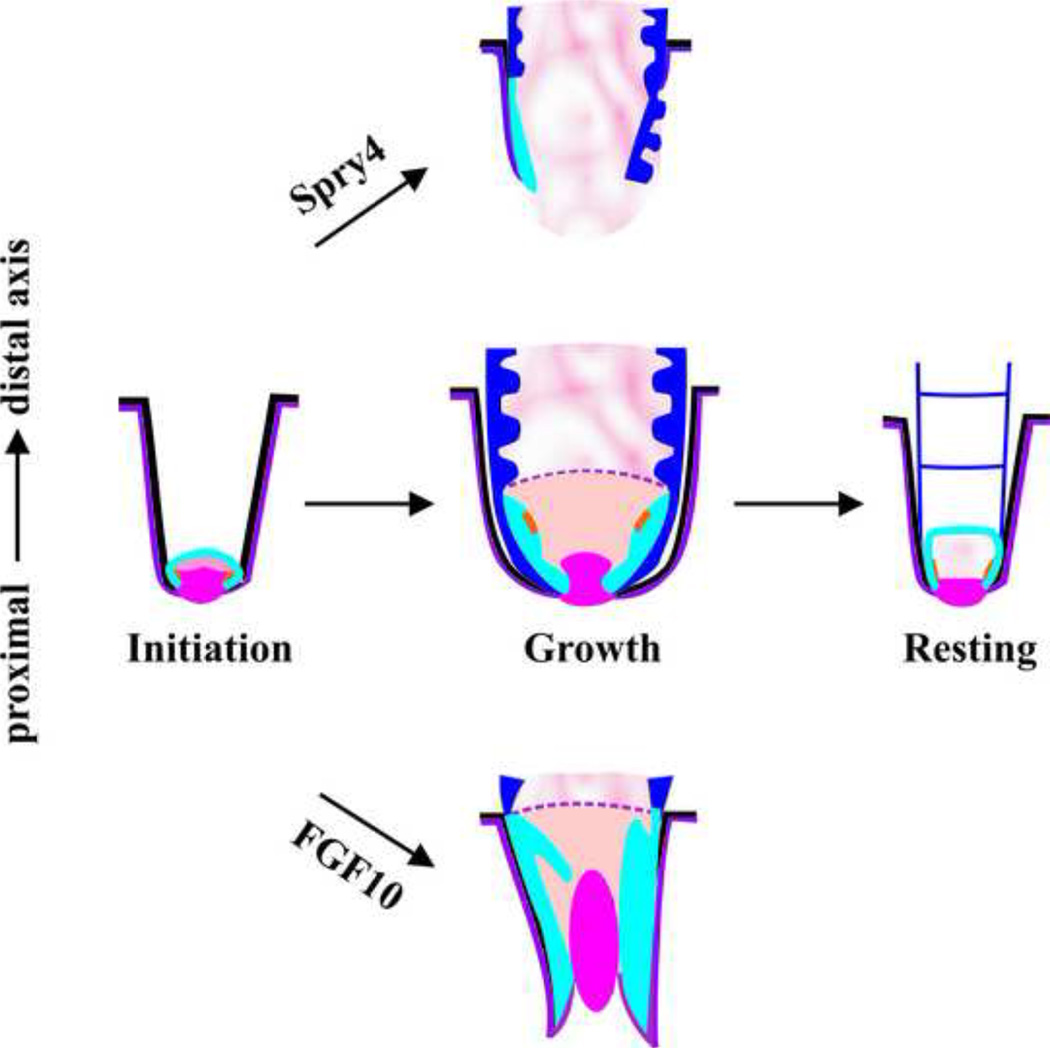
In summary from these and previous results, we propose that a combination of well-coordinated temporal molecular gradients work together to shape a feather. As the feather structure is made sequentially, tissues which are regulated by early events will become the distal end whereas tissues regulated by later events will become the proximal end. Hence, the temporal axis is projected onto a spatial axis from the distal to the proximal end. In the growth phase, high FGF may initiate TA cell formation and set up the collar region complete with a dermal papilla (Fig. 6). In contrast, the addition of Spry4 (expressed in the epithelium) and noggin (expressed in the mesenchyme, Yu et al., 2002) promotes branching and starts the formation of the feather vane (Fig. 6). BMP is shown to increase the size of the rachis (major feather shaft, Yu et al., 2002), and BMP activity may gradually increase. Eventually, the proximal follicle is dominated by an environment of high BMP and minimal FGF activity, allowing the generation of a cylindrical calamus (Fig. 6). In later stages, Spry4 is enriched in the dermal papilla. This may help to exhaust TA cells and promote entry into the resting phase (Fig. 6). Further increases in Spry4, among other factors, may make further reductions in the size of the dermal papilla, and cause feather molting to take place.
Figure 6.
A schematic summary showing the role of FGF/Sprouty signaling in feather cycling and on the specification of proximal or distal feather fates
The basic message we have learned here is that FGF receptor pathway activity plays an important role in regulating the dynamic morphology of growing feather follicles. We used Spry4 and Fgf10 to modulate this activity and obtained phenotypes of extensive branching or excess proliferation. Future work will be required to investigate further the molecular mechanisms that regulate this process.
Materials and Methods
Experimental Animal
In our colony, flight feathers molt at about 2 months of age, and the timing was verified by vascularity in the calamus region. Since remiges cycle in order from the proximal to distal, this also helps us predict the feather cycle stage that a feather will be in. Chickens were anesthetized with ketamine and xylocine (2:1, 10 mg/kg) and feather follicles were collected with small surgery on the body surface.
Immunostaining and in situ hybridization
Immunostaining and in situ hybridizations were processed as described (Jiang et al., 1999). We used antibodies for PCNA (chemicon), P63 (Santa Cruz Biotech), NCAM, laminin and tenascin C (Developmental Study Hybridoma Bank). RNA probes used in this study: Shh (Riddle et al., 1993), BMP2 (Jiang et al., 1999), feather keratin A, a kind of beta keratin (nt 571-1099, X17511; Presland et al., 1989), Fgfr1 / 2 (Patstone et al., 1993), Fgf10 (Tao et al., 2002), RCAS polymerase (Crowe et al, 1998), Spry1 / 2 (Minowada et al., 1999). Spry4 was cloned by degenerative PCR, primers: forward, 5’-CCNATHGAYCARATGAAR, reverse, 5’-YTCYTGRTTRCANACCCA) and was 99.7% identical to the chicken Spry4 (Genbank accession number: NM_001079735).
Feather keratin A respresents beta keratin (Presland et al., 1989). Alpha keratin 17-like was cloned by degenerative PCR, primers: forward, 5’-ATGCARAAYYTNAAYGAYMG, reverse, 5’-YTCYTCYTCRTGRTTYTTYTN). We had previously named this Cytokeratin I in Chodankar et al., 2003 because it was cloned using degenerate primers to mouse type I keratins. However, we sequenced this clone and a blast to the current Genbank database showed it was 99% identical to type I keratin 17-like (XM_001233971.2).
Retrovirus production and mis-expression
RCAS viruses were cultured and harvested as described. RCAS, RCAS-Fgf2 / 4 / 10 (Tao et al., 2002), RCAS-Spry1 / 2 / 4 (Minowada et al., 1999) virus transfection of regenerative feathers and sample processing were performed as described (Yu et al., 2002). Fgf 2, 4, 10 and Spry1, 2, 4 are from mouse. The presence of virus was detected by in situ hybridization using a probe to the viral polymerase gene (Crowe et al., 1998).
Highlights.
-
○
Feather follicles undergo drastic tissue remodeling during feather cycling
-
○
Molecules & cell organization differ along the proximal – distal axis of feathers
-
○
Spry-4 promotes barb branch formation and diminishes proximal follicle structures
-
○
FGF10 suppresses distal barb branching but expands the proximal feather follicle
Acknowledgement
This work is supported by grants from NIAMS AR 42177 and AR47364, and AR 60306. We are grateful to Drs. G. Martin (RCAS-spry1 / 2 / 4), P. Maher (Fgfr1 / 2 probes), S. Noji (RCAS-Fgf10, Fgf10 probe) for providing reagents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Alibardi L. Cornification of the pulp epithelium and formation of pulp cups in downfeathers and regenerating feathers. Anat Sci Int. 2009;84:269–279. doi: 10.1007/s12565-009-0033-2. [DOI] [PubMed] [Google Scholar]
- Atit R, Conlon RA, Niswander L. EGF Signaling patterns the feather array by promoting the interbud fate. Dev. Cell. 2003;4:231–240. doi: 10.1016/s1534-5807(03)00021-2. [DOI] [PubMed] [Google Scholar]
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–4878. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- Cabrita MA, Christofori G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis. 2008;11:53–62. doi: 10.1007/s10456-008-9089-1. [DOI] [PubMed] [Google Scholar]
- Chang CH, Yu M, Wu P, Jiang TX, Yu HS, Widelitz RB, Chuong CM. Sculpting skin appendages out of epidermal layers via temporally and spatially regulated apoptotic events. J. Invest. Dermatol. 2004;122:1348–1355. doi: 10.1111/j.0022-202X.2004.22611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles C, Hovorakova M, Ahn Y, Lyons DB, Marangoni P, Churava S, Biehs B, Jheon A, Lesot H, Balooch G, Krumlauf R, Viriot L, Peterkova R, Klein OD. Regulation of tooth number by fine-tuning levels of receptor-tyrosine kinase signaling. Development. 2011;138:4063–4073. doi: 10.1242/dev.069195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Zhang S, Lin Y, Prunskaite-Hyyryläinen R, Vuolteenaho R, Itäranta P, Vainio S. Sprouty proteins regulate ureteric branching by coordinating reciprocal epithelial Wnt11, mesenchymal Gdnf and stromal Fgf7 signalling during kidney development. Development. 2004;131:3345–3356. doi: 10.1242/dev.01200. [DOI] [PubMed] [Google Scholar]
- Chodankar R, Chang C-H, Yue Z, Suksaweang S, Burrus L, Chuong C-M, Widelitz RB. Shift of Localized Growth Zones Contributes to Skin Appendage Morphogenesis: Role of the Wnt/Beta-catenin Pathway. J Inv Dermatol. 2003;120:19–26. doi: 10.1046/j.1523-1747.2003.12008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofori G. Split personalities: the agonistic antagonist Sprouty. Nat. Cell Biol. 2003;5:377–379. doi: 10.1038/ncb0503-377. [DOI] [PubMed] [Google Scholar]
- Chuong CM, Chodankar R, Widelitz RB, Jiang TX. Evo-Devo of feathers and scales: Building complex epithelial appendages. Curr. Opin. Genet. Dev. 2000;10:449–456. doi: 10.1016/s0959-437x(00)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Edelman GM. Expression of cell-adhesion molecules in embryonic induction. II. Morphogenesis of adult feathers. J. Cell Biol. 1985;101:1027–1043. doi: 10.1083/jcb.101.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Randall VA, Widelitz RB, Wu P, Jiang T-X. Physiological regeneration of skin appendages and implications for regenerative medicine. Physiology. 2012 doi: 10.1152/physiol.00028.2011. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Espinasse PG. On the normal and abnormal development of the feather. J. Embryol. Exp. Morphol. 1961;9:223–251. [PubMed] [Google Scholar]
- Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–397. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- Crowe R, Niswander L. Disruption of scale development by Delta-1 misexpression. Dev. Biol. 1998;195:70–74. doi: 10.1006/dbio.1997.8844. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Ros MA, Tabin CJ. A re-examination of proximodistal patterning during vertebrate limb development. Nature. 2002;418:539–544. doi: 10.1038/nature00945. [DOI] [PubMed] [Google Scholar]
- Egan JE, Hall AB, Yatsula BA, Bar-Sagi D. The bimodal regulation of epidermal growth factor signaling by human Sprouty proteins. Proc. Natl. Acad. Sci. USA. 2002;99:6041–6046. doi: 10.1073/pnas.052090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürthauer M, Reifers F, Brand M, Thisse B, Thisse C. sprouty4 acts in vivo as a feedback-induced antagonist of FGF signaling in zebrafish. Development. 2001;128:2175–2186. doi: 10.1242/dev.128.12.2175. [DOI] [PubMed] [Google Scholar]
- Guy GR, Wong ES, Yusoff P, Chandramouli S, Lo TL, Lim J, Fong CW. Sprouty: how does the branch manager work? J. Cell Sci. 2003;116:3061–3068. doi: 10.1242/jcs.00652. [DOI] [PubMed] [Google Scholar]
- Guy G, Jackson RA, Yusoff P, Chow SY. Sprouty proteins: modified modulators, matchmakers or missing links? J Endocrinol. 2009;203:191–202. doi: 10.1677/JOE-09-0110. [DOI] [PubMed] [Google Scholar]
- Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell. 1998;92:253–263. doi: 10.1016/s0092-8674(00)80919-8. [DOI] [PubMed] [Google Scholar]
- Hanafusa H, Matsumoto K, Nishida E. Regulation of ERK activity duration by Sprouty contributes to dorsoventral patterning. Nat Cell Biol. 2009;11:106–109. doi: 10.1038/ncb1820. [DOI] [PubMed] [Google Scholar]
- Harada H, Toyono T, Toyoshima K, Yamasaki M, Itoh N, Kato S, Sekine K, Ohuchi H. FGF10 maintains stem cell compartment in developing mouse incisors. Development. 2002;129:1533–1541. doi: 10.1242/dev.129.6.1533. [DOI] [PubMed] [Google Scholar]
- Harris MP, Fallon JF, Prum RO. Shh-Bmp2 signaling module and the evolutionary origin and diöersification of feathers. J. Exp. Zool. 2002;294:160–176. doi: 10.1002/jez.10157. [DOI] [PubMed] [Google Scholar]
- Hebert JM, Rosenquist T, Gotz J, Martin GR. FGF5 as a regulator of the hair growth cycle: evidence from targeted and spontaneous mutations. Cell. 1994;78:1017–1025. doi: 10.1016/0092-8674(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Horne KA, Jahoda CA. Restoration of hair growth by surgical implantation of follicular dermal sheath. Development. 1992;116:563–571. doi: 10.1242/dev.116.3.563. [DOI] [PubMed] [Google Scholar]
- Jiang TX, Chuong CM. Mechanism of skin morphogenesisIAnalyses with antibodies to adhesion molecules tenascin, N-CAM, and integrin. Dev Biol. 1992;150:82–98. doi: 10.1016/0012-1606(92)90009-6. [DOI] [PubMed] [Google Scholar]
- Jiang T-X, Jung H-S, Widelitz RB, Chuong C-M. Self organization is the initial event in periodic feather patterning: Roles of signaling molecules and adhesion molecules. Development. 1999;126:4997–5009. doi: 10.1242/dev.126.22.4997. [DOI] [PubMed] [Google Scholar]
- Kawano M, Komi-Kuramochi A, Asada M, Suzuki M, Oki J, Jiang J, Imamura T. Comprehensive analysis of FGF and FGFR expression in skin: FGF18 is highly expressed in hair follicles and capable of inducing anagen from telogen stage hair follicles. J Invest Dermatol. 2005;124:877–885. doi: 10.1111/j.0022-202X.2005.23693.x. [DOI] [PubMed] [Google Scholar]
- Kimura-Ueki M, Oda Y, Oki J, Komi-Kuramochi A, Honda E, Asada M, Suzuki M, Imamura T. Hair Cycle Resting Phase Is Regulated by Cyclic Epithelial FGF18 Signaling. J. Invest. Dermatol. 2012 doi: 10.1038/jid.2011.490. In Press. [DOI] [PubMed] [Google Scholar]
- Klein OD, Lyons DB, Balooch G, Marshall GW, Basson MA, Peterka M, Boran T, Peterkova R, Martin GR. An FGF signaling loop sustains the generation of differentiated progeny from stem cells in mouse incisors. Development. 2008;135:377–385. doi: 10.1242/dev.015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein OD, Minowada G, Peterlova R, Kangas A, Yu BD, Lesot H, Peterka M, Jernvall J, Martin GR. Sprouty genes control diastema tooth development via bidiretional antagonism of epithelial-mesenchymal FGF signaling. Dev. Cell. 2006;11:181–190. doi: 10.1016/j.devcel.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer S, Okabe M, Hacohen N, Krasnow MA, Hiromi Y. Sprouty: a common antagonist of FGF and EGF signaling pathways in Drosophila. Development. 1999;126:2515–2525. doi: 10.1242/dev.126.11.2515. [DOI] [PubMed] [Google Scholar]
- Lewandoski M, Sun X, Martin GR. Fgf8 signalling from the AER is essential for normal limb development. Nat Genet. 2000;26:460–463. doi: 10.1038/82609. [DOI] [PubMed] [Google Scholar]
- Lillie FR, Wang H. Physiology of development of the featherVExperimental morphogenesis. Physiol. Zool. 1941;14:103–135. [Google Scholar]
- Lillie FR, Wang H. Physiology of development of the feather. VI. The production and analysis of feather-chimerae in fowl. Physiol. Zool. 1943;16:1–21. [Google Scholar]
- Lin CM, Jiang TX, Baker RE, Maini PK, Widelitz RB, Chuong CM. Spots and stripes: Pleomorphic patterning of stem cells via p-ERK-dependent cell chemotaxis shown by feather morphogenesis and mathematical simulation. Dev. Biol. 2009;334:369–382. doi: 10.1016/j.ydbio.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas AM, Stettenheim PR. Avian Anatomy: Integument. Agriculture Handbook. 1972;362 [Google Scholar]
- Mahoney Rogers AA, Zhang J, Shim K. Sprouty1 and Sprouty2 limit both the size of the otic placode and hindbrain Wnt8a by antagonizing FGF signaling. Dev. Biol. 2011;353:94–104. doi: 10.1016/j.ydbio.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux AA, Tefft D, Ndiaye D, Itoh N, Thiery JP, Warburton D, Bellusci S. Evidence that Sprouty2 functions as an inhibitor of mouse embryonic lung growth and morphogenesis. Mech. Dev. 2001;102:81–94. doi: 10.1016/s0925-4773(01)00286-6. [DOI] [PubMed] [Google Scholar]
- Mandler M, Neubuser A. FGF signaling is required for initiation of feather placode development. Development. 2004;131:3333–3343. doi: 10.1242/dev.01203. [DOI] [PubMed] [Google Scholar]
- Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453:745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, Hacohen N, Krasnow MA, Martin GR. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–4475. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- Natanson-Yaron S, Anteby EY, Greenfield C, Goldman-Wohl D, Hamani Y, Hochner-Celnikier D, Yagel S. FGF 10 and Sprouty 2 modulate trophoblast invasion and branching morphogenesis. Mol Hum Reprod. 2007;13:511–519. doi: 10.1093/molehr/gam034. [DOI] [PubMed] [Google Scholar]
- Nutt SL, Dingwell KS, Holt CE, Amaya E. Xenopus Sprouty2 inhibits FGFmediated gastrulation movements but does not affect mesoderm induction and patterning. Genes Dev. 2001;15:1152–1166. doi: 10.1101/gad.191301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patstone G, Pasquale EB, Maher PA. Different members of the fibroblast growth factor receptor family are specific to distinct cell types in the developing chicken embryo. Dev. Biol. 1993;155:107–123. doi: 10.1006/dbio.1993.1011. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. p63 identifies keratinocyte stem cells. Pro. Natl. Acad. Sci. USA. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl AK, Hokuto I, Impagnatiello MA, Christofori G, Whitsett JA. Temporal effects of Sprouty on lung morphogenesis. Dev Biol. 2003;258:154–168. doi: 10.1016/s0012-1606(03)00106-4. [DOI] [PubMed] [Google Scholar]
- Petiot A, Conti FJ, Grose R, Revest JM, Hodivala-Dilke KM, Dickson C. A crucial role for Fgfr2-IIIb signalling in epidermal development and hair follicle patterning. Development. 2003;130:5493–5501. doi: 10.1242/dev.00788. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Trokovic R, Hébert JM, McConnell SK, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671–680. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Presland RB, Gregg K, Molloy PL, Morris CP, Crocker LA, Rogers GE. Avian keratin genesIA molecular analysis of the structure and expression of a group of feather keratin genes. J. Mol. Biol. 1989;209:549–559. doi: 10.1016/0022-2836(89)90593-7. [DOI] [PubMed] [Google Scholar]
- Prum RO. Evolution of the morphological innovations of feathers. J. Exp. Zool. B Mol. Dev. Evol. 2005;304:570–579. doi: 10.1002/jez.b.21073. [DOI] [PubMed] [Google Scholar]
- Prum RO, Brush AH. The evolutionary origin and diversification of feathers. Q Rev Biol. 2002;77:261–295. doi: 10.1086/341993. [DOI] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Rosenquist TA, Martin GR. Fibroblast growth factor signalling in the hair growth cycle: expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev Dyn. 1996;205:379–386. doi: 10.1002/(SICI)1097-0177(199604)205:4<379::AID-AJA2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Rubin C, Litvak V, Medvedovsky H, Zwang Y, Lev S, Yarden Y. Sprouty fine-tunes EGF signaling through interlinked positive and negative feedback loops. Curr. Biol. 2003;13:297–307. doi: 10.1016/s0960-9822(03)00053-8. [DOI] [PubMed] [Google Scholar]
- Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21:138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, McKeon F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- Shim K, Minowada G, Coling DE, Martin GR. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev Cell. 2005;8:553–564. doi: 10.1016/j.devcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Sivak JM, Petersen LF, Amaya E. FGF signal interpretation is directed by Sprouty and Spred proteins during mesoderm formation. Dev. Cell. 2005;8:689–701. doi: 10.1016/j.devcel.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Song H, Wang Y, Goetinck PF. Fibroblast growth factor 2 can replace ectodermal signaling for feather development. Proc Natl Acad Sci U S A. 1996;93:10246–10249. doi: 10.1073/pnas.93.19.10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HK, Lee SH, Goetinck PF. FGF-2 signaling is sufficient to induce dermal condensations during feather development. Dev Dyn. 2004;231:741–749. doi: 10.1002/dvdy.20243. [DOI] [PubMed] [Google Scholar]
- Sun X, Mariani FV, Martin GR. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature. 2002;418:501–508. doi: 10.1038/nature00902. [DOI] [PubMed] [Google Scholar]
- Sutherland D, Samakovlis C, Krasnow MA. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 1996;87:1091–1101. doi: 10.1016/s0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]
- Suzuki-Hirano A, Sato T, Nakamura H. Regulation of isthmic Fgf8 signal by sprouty2. Development. 2005;132:257–265. doi: 10.1242/dev.01581. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Ayada T, Ichiyama K, Kohno R, Yonemitsu Y, Minami Y, Kikuchi A, Maehara Y, Yoshimura A. Sprouty2 and Sprouty4 are essential for embryonic morphogenesis and regulation of FGF signaling. Biochem Biophys Res Commun. 2007;352:896–902. doi: 10.1016/j.bbrc.2006.11.107. [DOI] [PubMed] [Google Scholar]
- Tao H, Yoshimoto Y, Yoshioka H, Nohno T, Noji S, Ohuchi H. FGF10 is a mesenchymally derived stimulator for epidermal development in the chick embryonic skin. Mech. Dev. 2002;116:39–49. doi: 10.1016/s0925-4773(02)00131-4. [DOI] [PubMed] [Google Scholar]
- Tefft D, Lee M, Smith S, Crowe DL, Bellusci S, Warburton D. mSprouty2 inhibits FGF10-activated MAP kinase by differentially binding to upstream target proteins. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;283:L700–706. doi: 10.1152/ajplung.00372.2001. [DOI] [PubMed] [Google Scholar]
- Ting-Berreth SA, Chuong C-M. Sonic hedgehog in feather morphogenesis: Induction of mesenchymal condensation and association with cell death. Dev. Dyn. 1996;207:157–170. doi: 10.1002/(SICI)1097-0177(199610)207:2<157::AID-AJA4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Wells KL, Hadad Y, Ben-Avraham D, Hillel J, Cahaner A, Headon DJ. Genome-wide SNP scan of pooled DNA reveals nonsense mutation in FGF20 in the scaleless line of featherless chickens. BMC Genomics. 2012;13:257. doi: 10.1186/1471-2164-13-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh IC, Hagge-Greenberg A, O'Brien TP. A dosage-dependent role for Spry2 in growth and patterning during palate development. Mech Dev. 2007;124:746–761. doi: 10.1016/j.mod.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widelitz RB, Jiang TX, Noveen A, Chen CW, Chuong CM. FGF induces new feather buds from developing avian skin. J. Invest. Dermatol. 1996;107:797–803. doi: 10.1111/1523-1747.ep12330553. [DOI] [PubMed] [Google Scholar]
- Widelitz RB, Jiang TX, Lu J, Chuong CM. beta-catenin in epithelial morphogenesis: conversion of part of avian foot scales into feather buds with a mutated beta-catenin. Dev Biol. 2000;219:98–114. doi: 10.1006/dbio.1999.9580. [DOI] [PubMed] [Google Scholar]
- Wong ES, Fong CW, Lim J, Yusoff P, Low BC, Langdon WY, Guy GR. Sprouty2 attenuates epidermal growth factor receptor ubiquitylation and endocytosis, and consequently enhances Ras/ERK signalling. EMBO J. 2002;21:4796–4808. doi: 10.1093/emboj/cdf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zhou Z, Prum RO. Branched integumental structures in Sinornithosaurus and the origin of feathers. Nature. 2001;410:200–204. doi: 10.1038/35065589. [DOI] [PubMed] [Google Scholar]
- Yu M, Wu P, Widelitz RB, Chuong CM. The morphogenesis of feathers. Nature. 2002;420:308–312. doi: 10.1038/nature01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Yue Z, Wu P, Wu D-Y, Mayer JA, Medina M, Widelitz RB, Jiang T-X, Chuong C-M. The developmental biology of feather follicles. Int. J. Dev. Biol. 2004;48:181–191. doi: 10.1387/ijdb.031776my. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jiang T-X, Widelitz RB, Chuong CM. The feather follicle stem cells. Nature. 2005;438:1026–1029. doi: 10.1038/nature04222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jiang T-X, Widelitz RB, Chuong CM. A Wnt3a gradient controles feather morphogenesis. PNAS. 2006;103:951–955. doi: 10.1073/pnas.0506894103. [DOI] [PMC free article] [PubMed] [Google Scholar]