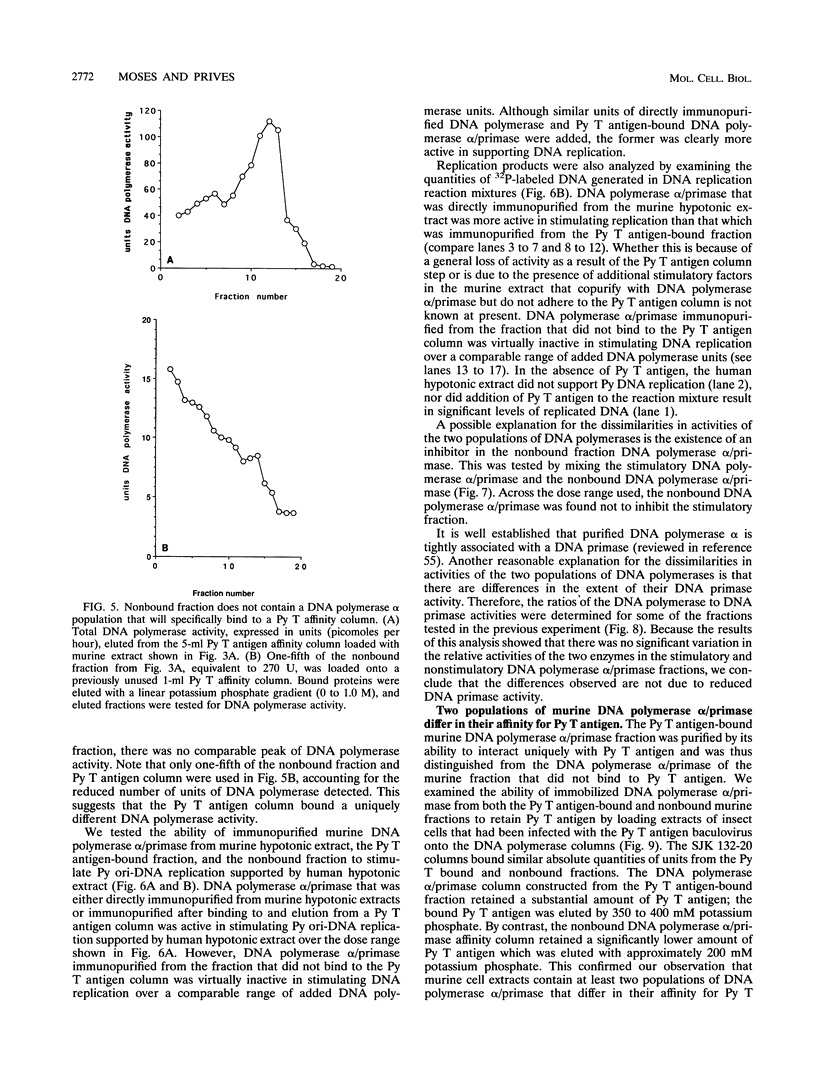
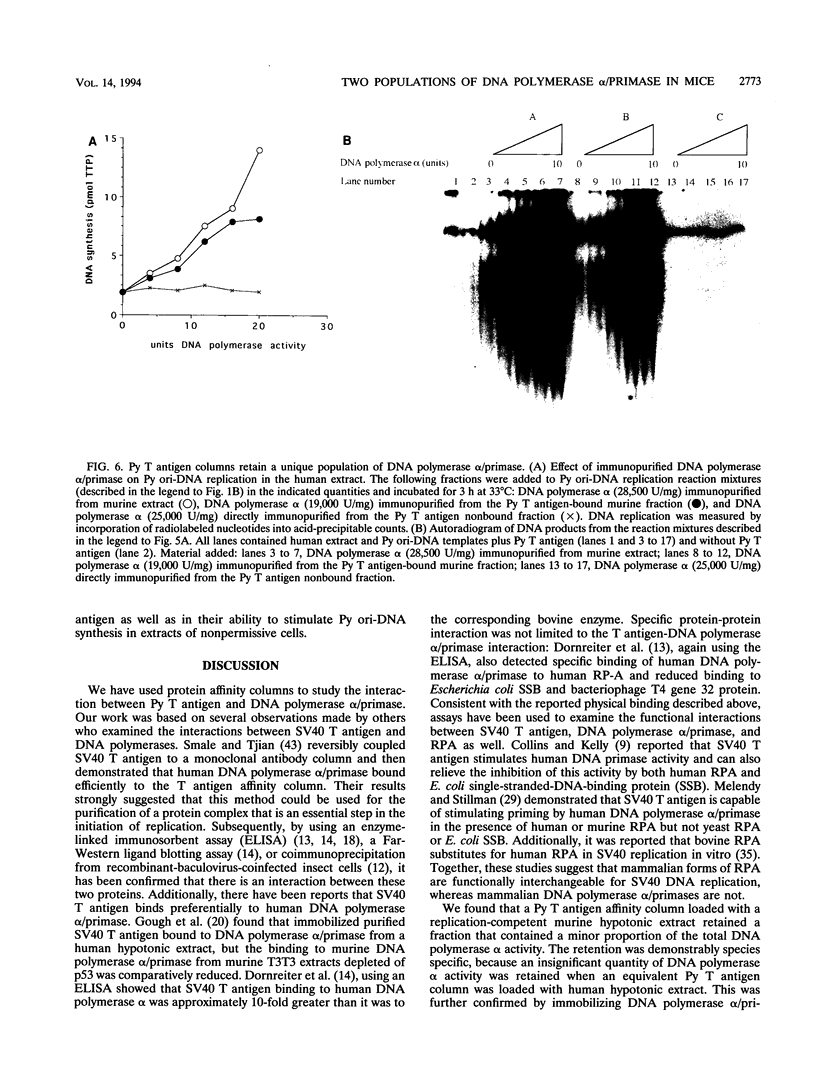
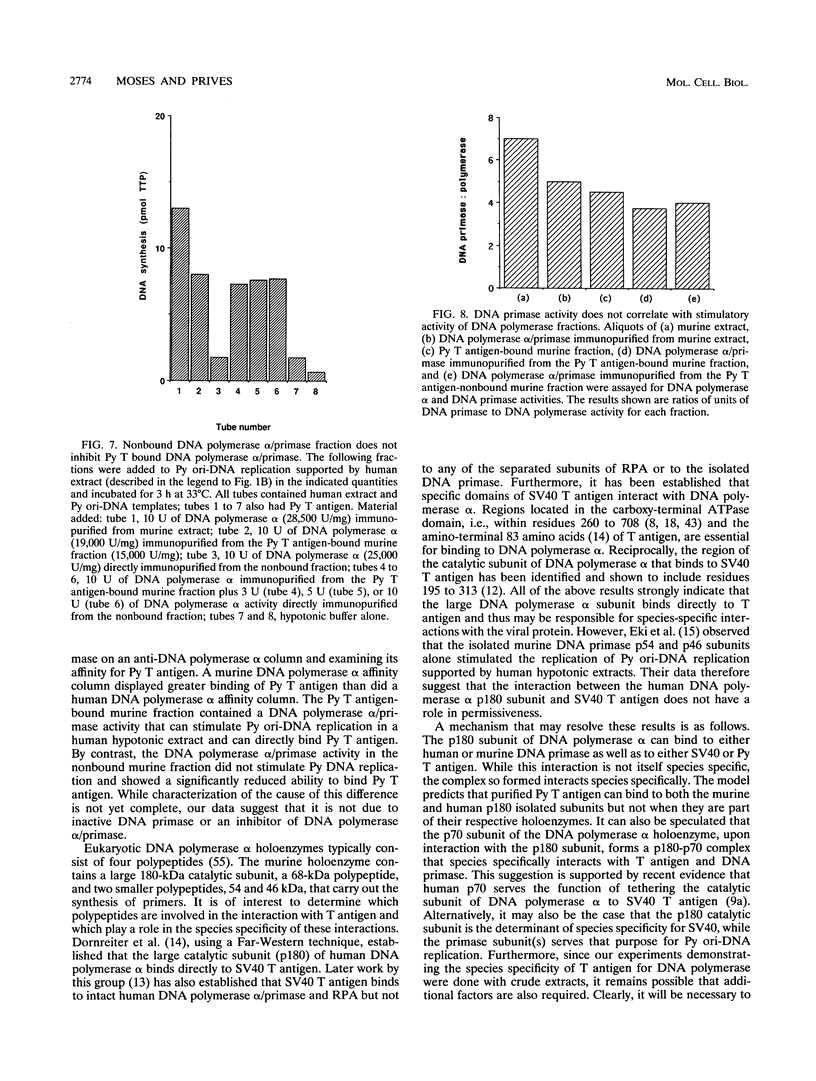
Abstract
Murine cells or cell extracts support the replication of plasmids containing the replication origin (ori-DNA) of polyomavirus (Py) but not that of simian virus 40 (SV40), whereas human cells or cell extracts support the replication of SV40 ori-DNA but not that of Py ori-DNA. It was shown previously that fractions containing DNA polymerase alpha/primase from permissive cells allow viral ori-DNA replication to proceed in extracts of nonpermissive cells. To extend these observations, the binding of Py T antigen to both the permissive and nonpermissive DNA polymerase alpha/primase was examined. Py T antigen was retained by a murine DNA polymerase alpha/primase but not by a human DNA polymerase alpha/primase affinity column. Likewise, a Py T antigen affinity column retained DNA polymerase alpha/primase activity from murine cells but not from human cells. The murine fraction which bound to the Py T antigen column was able to stimulate Py ori-DNA replication in the nonpermissive extract. However, the DNA polymerase alpha/primase activity in this murine fraction constituted only a relatively small proportion (approximately 20 to 40%) of the total murine DNA polymerase alpha/primase that had been applied to the column. The DNA polymerase alpha/primase purified from the nonbound murine fraction, although far more replete in this activity, was incapable of supporting Py DNA replication. The two forms of murine DNA polymerase alpha/primase also differed in their interactions with Py T antigen. Our data thus demonstrate that there are two distinct populations of DNA polymerase alpha/primase in murine cells and that species-specific interactions between T antigen and DNA polymerases can be identified. They may also provide the basis for initiating a novel means of characterizing unique subpopulations of DNA polymerase alpha/primase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bambara R. A., Jessee C. B. Properties of DNA polymerases delta and epsilon, and their roles in eukaryotic DNA replication. Biochim Biophys Acta. 1991 Jan 17;1088(1):11–24. doi: 10.1016/0167-4781(91)90147-e. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Israel M. A. Middle tumor antigen of polyomavirus transformation-defective mutant NG59 is associated with pp60c-src. J Virol. 1985 Jan;53(1):114–119. doi: 10.1128/jvi.53.1.114-119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec J. A., Dean F. B., Bullock P. A., Hurwitz J. Binding and unwinding--how T antigen engages the SV40 origin of DNA replication. Cell. 1990 Jan 26;60(2):181–184. doi: 10.1016/0092-8674(90)90730-3. [DOI] [PubMed] [Google Scholar]
- Bullock P. A., Seo Y. S., Hurwitz J. Initiation of simian virus 40 DNA replication in vitro: pulse-chase experiments identify the first labeled species as topologically unwound. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3944–3948. doi: 10.1073/pnas.86.11.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes J. J. Differential inhibitors of DNA polymerases alpha and delta. Biochem Biophys Res Commun. 1985 Oct 30;132(2):628–634. doi: 10.1016/0006-291x(85)91179-9. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- Clark R., Lane D. P., Tjian R. Use of monoclonal antibodies as probes of simian virus 40 T antigen ATPase activity. J Biol Chem. 1981 Nov 25;256(22):11854–11858. [PubMed] [Google Scholar]
- Collins K. L., Kelly T. J. Effects of T antigen and replication protein A on the initiation of DNA synthesis by DNA polymerase alpha-primase. Mol Cell Biol. 1991 Apr;11(4):2108–2115. doi: 10.1128/mcb.11.4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins K. L., Russo A. A., Tseng B. Y., Kelly T. J. The role of the 70 kDa subunit of human DNA polymerase alpha in DNA replication. EMBO J. 1993 Dec;12(12):4555–4566. doi: 10.1002/j.1460-2075.1993.tb06144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie A., Kamen R. Guanine nucleotide contacts within viral DNA sequences bound by polyomavirus large T antigen. J Virol. 1986 Feb;57(2):505–514. doi: 10.1128/jvi.57.2.505-514.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean F. B., Bullock P., Murakami Y., Wobbe C. R., Weissbach L., Hurwitz J. Simian virus 40 (SV40) DNA replication: SV40 large T antigen unwinds DNA containing the SV40 origin of replication. Proc Natl Acad Sci U S A. 1987 Jan;84(1):16–20. doi: 10.1073/pnas.84.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornreiter I., Copeland W. C., Wang T. S. Initiation of simian virus 40 DNA replication requires the interaction of a specific domain of human DNA polymerase alpha with large T antigen. Mol Cell Biol. 1993 Feb;13(2):809–820. doi: 10.1128/mcb.13.2.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornreiter I., Erdile L. F., Gilbert I. U., von Winkler D., Kelly T. J., Fanning E. Interaction of DNA polymerase alpha-primase with cellular replication protein A and SV40 T antigen. EMBO J. 1992 Feb;11(2):769–776. doi: 10.1002/j.1460-2075.1992.tb05110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornreiter I., Höss A., Arthur A. K., Fanning E. SV40 T antigen binds directly to the large subunit of purified DNA polymerase alpha. EMBO J. 1990 Oct;9(10):3329–3336. doi: 10.1002/j.1460-2075.1990.tb07533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eki T., Enomoto T., Masutani C., Miyajima A., Takada R., Murakami Y., Ohno T., Hanaoka F., Ui M. Mouse DNA primase plays the principal role in determination of permissiveness for polyomavirus DNA replication. J Virol. 1991 Sep;65(9):4874–4881. doi: 10.1128/jvi.65.9.4874-4881.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdile L. F., Heyer W. D., Kolodner R., Kelly T. J. Characterization of a cDNA encoding the 70-kDa single-stranded DNA-binding subunit of human replication protein A and the role of the protein in DNA replication. J Biol Chem. 1991 Jun 25;266(18):12090–12098. [PubMed] [Google Scholar]
- Fanning E., Knippers R. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- Gannon J. V., Lane D. P. p53 and DNA polymerase alpha compete for binding to SV40 T antigen. Nature. 1987 Oct 1;329(6138):456–458. doi: 10.1038/329456a0. [DOI] [PubMed] [Google Scholar]
- Gaudray P., Clertant P., Cuzin F. ATP phosphohydrolase (ATPase) activity of a polyoma virus T antigen. Eur J Biochem. 1980 Aug;109(2):553–560. doi: 10.1111/j.1432-1033.1980.tb04827.x. [DOI] [PubMed] [Google Scholar]
- Hay R. T., Russell W. C. Recognition mechanisms in the synthesis of animal virus DNA. Biochem J. 1989 Feb 15;258(1):3–16. doi: 10.1042/bj2580003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny M. K., Schlegel U., Furneaux H., Hurwitz J. The role of human single-stranded DNA binding protein and its individual subunits in simian virus 40 DNA replication. J Biol Chem. 1990 May 5;265(13):7693–7700. [PubMed] [Google Scholar]
- Kern F. G., Dailey L., Basilico C. Common regulatory elements control gene expression from polyoma early and late promoters in cells transformed by chimeric plasmids. Mol Cell Biol. 1985 Aug;5(8):2070–2079. doi: 10.1128/mcb.5.8.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro: specificity of initiation and evidence for bidirectional replication. Mol Cell Biol. 1985 Jun;5(6):1238–1246. doi: 10.1128/mcb.5.6.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer H. E., Wang E. H., Prives C. The DNA-binding properties of polyomavirus large T antigen are altered by ATP and other nucleotides. J Virol. 1991 Feb;65(2):687–699. doi: 10.1128/jvi.65.2.687-699.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo I. A., Hough P. V., Wall J. S., Dodson M., Dean F. B., Hurwitz J. ATP-dependent assembly of double hexamers of SV40 T antigen at the viral origin of DNA replication. Nature. 1989 Apr 20;338(6217):658–662. doi: 10.1038/338658a0. [DOI] [PubMed] [Google Scholar]
- Melendy T., Stillman B. An interaction between replication protein A and SV40 T antigen appears essential for primosome assembly during SV40 DNA replication. J Biol Chem. 1993 Feb 15;268(5):3389–3395. [PubMed] [Google Scholar]
- Morrison A., Araki H., Clark A. B., Hamatake R. K., Sugino A. A third essential DNA polymerase in S. cerevisiae. Cell. 1990 Sep 21;62(6):1143–1151. doi: 10.1016/0092-8674(90)90391-q. [DOI] [PubMed] [Google Scholar]
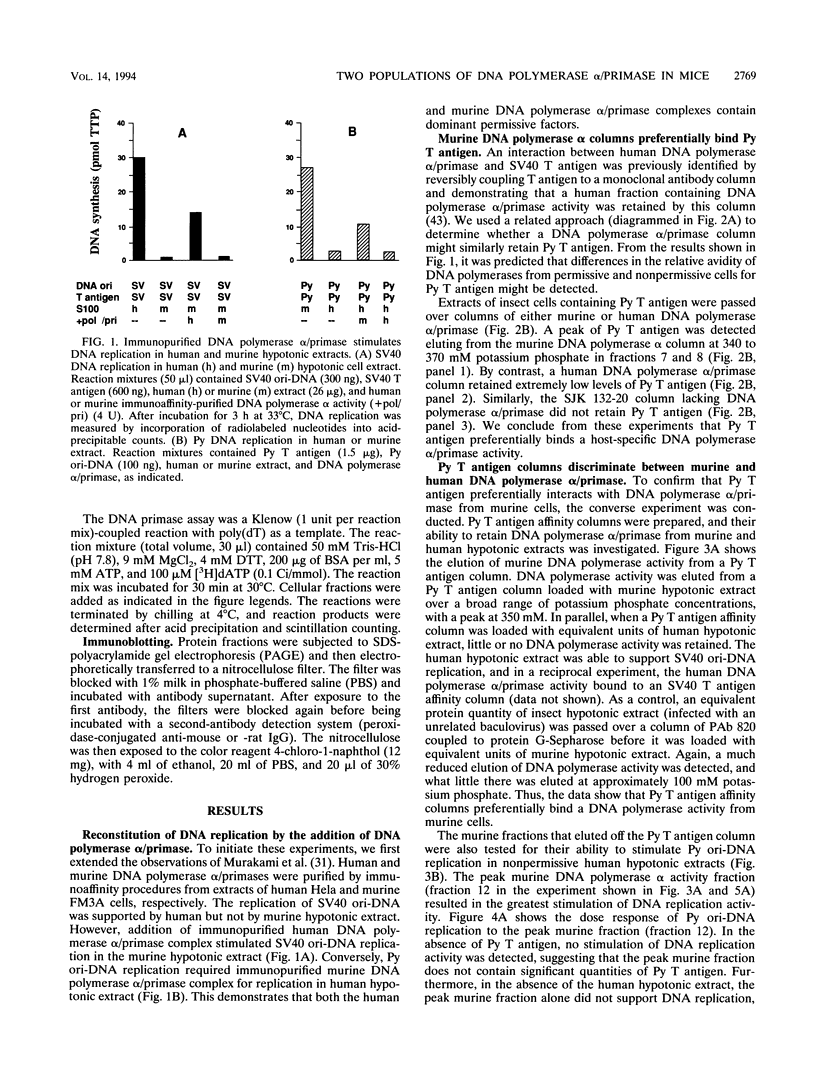
- Murakami Y., Eki T., Yamada M., Prives C., Hurwitz J. Species-specific in vitro synthesis of DNA containing the polyoma virus origin of replication. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6347–6351. doi: 10.1073/pnas.83.17.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Wobbe C. R., Weissbach L., Dean F. B., Hurwitz J. Role of DNA polymerase alpha and DNA primase in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1986 May;83(9):2869–2873. doi: 10.1073/pnas.83.9.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasheuer H. P., Grosse F. Immunoaffinity-purified DNA polymerase alpha displays novel properties. Biochemistry. 1987 Dec 15;26(25):8458–8466. doi: 10.1021/bi00399a064. [DOI] [PubMed] [Google Scholar]
- Nasheuer H. P., Moore A., Wahl A. F., Wang T. S. Cell cycle-dependent phosphorylation of human DNA polymerase alpha. J Biol Chem. 1991 Apr 25;266(12):7893–7903. [PubMed] [Google Scholar]
- Nasheuer H. P., von Winkler D., Schneider C., Dornreiter I., Gilbert I., Fanning E. Purification and functional characterization of bovine RP-A in an in vitro SV40 DNA replication system. Chromosoma. 1992;102(1 Suppl):S52–S59. doi: 10.1007/BF02451786. [DOI] [PubMed] [Google Scholar]
- O'Reilly D. R., Miller L. K. Expression and complex formation of simian virus 40 large T antigen and mouse p53 in insect cells. J Virol. 1988 Sep;62(9):3109–3119. doi: 10.1128/jvi.62.9.3109-3119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
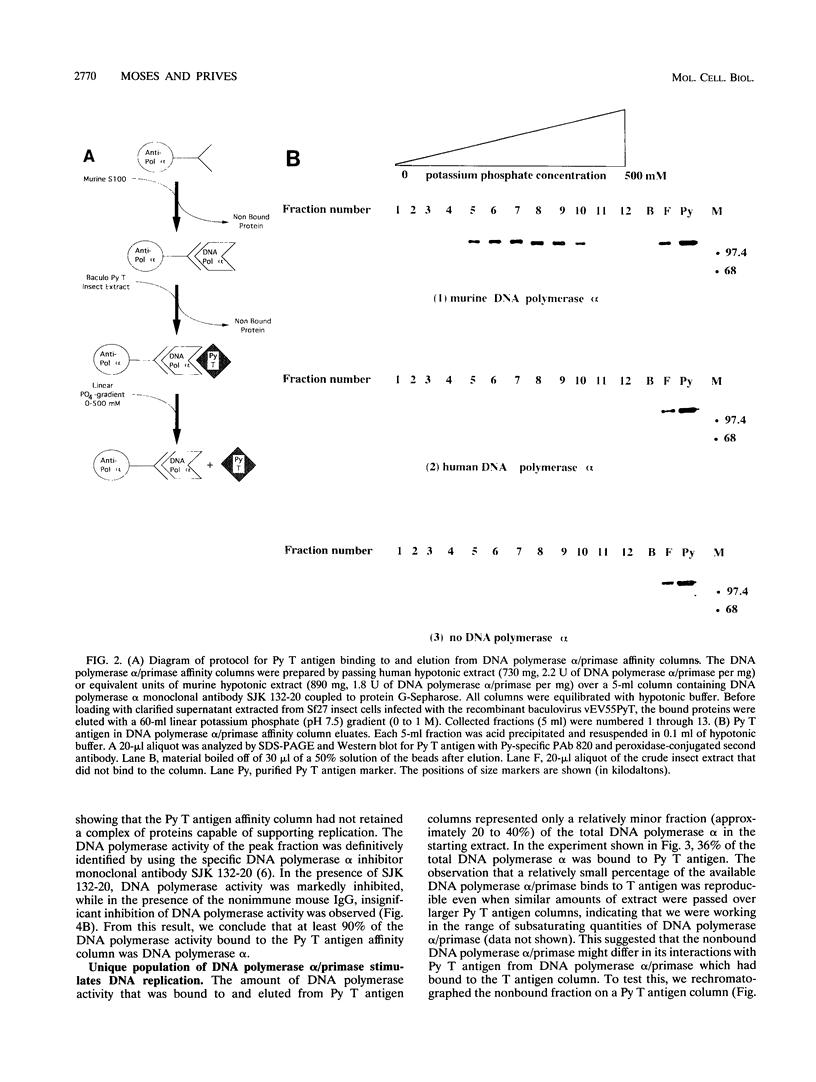
- Pallas D. C., Schley C., Mahoney M., Harlow E., Schaffhausen B. S., Roberts T. M. Polyomavirus small t antigen: overproduction in bacteria, purification, and utilization for monoclonal and polyclonal antibody production. J Virol. 1986 Dec;60(3):1075–1084. doi: 10.1128/jvi.60.3.1075-1084.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz B. J., Hassell J. A. Polyomavirus and simian virus 40 large T antigens bind to common DNA sequences. J Virol. 1984 Mar;49(3):925–937. doi: 10.1128/jvi.49.3.925-937.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C. The replication functions of SV40 T antigen are regulated by phosphorylation. Cell. 1990 Jun 1;61(5):735–738. doi: 10.1016/0092-8674(90)90179-i. [DOI] [PubMed] [Google Scholar]
- Rice W. C., Lorimer H. E., Prives C., Miller L. K. Expression of polyomavirus large T antigen by using a baculovirus vector. J Virol. 1987 May;61(5):1712–1716. doi: 10.1128/jvi.61.5.1712-1716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller A., Prives C. Simian virus 40 and polyomavirus large tumor antigens have different requirements for high-affinity sequence-specific DNA binding. J Virol. 1985 May;54(2):532–545. doi: 10.1128/jvi.54.2.532-545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
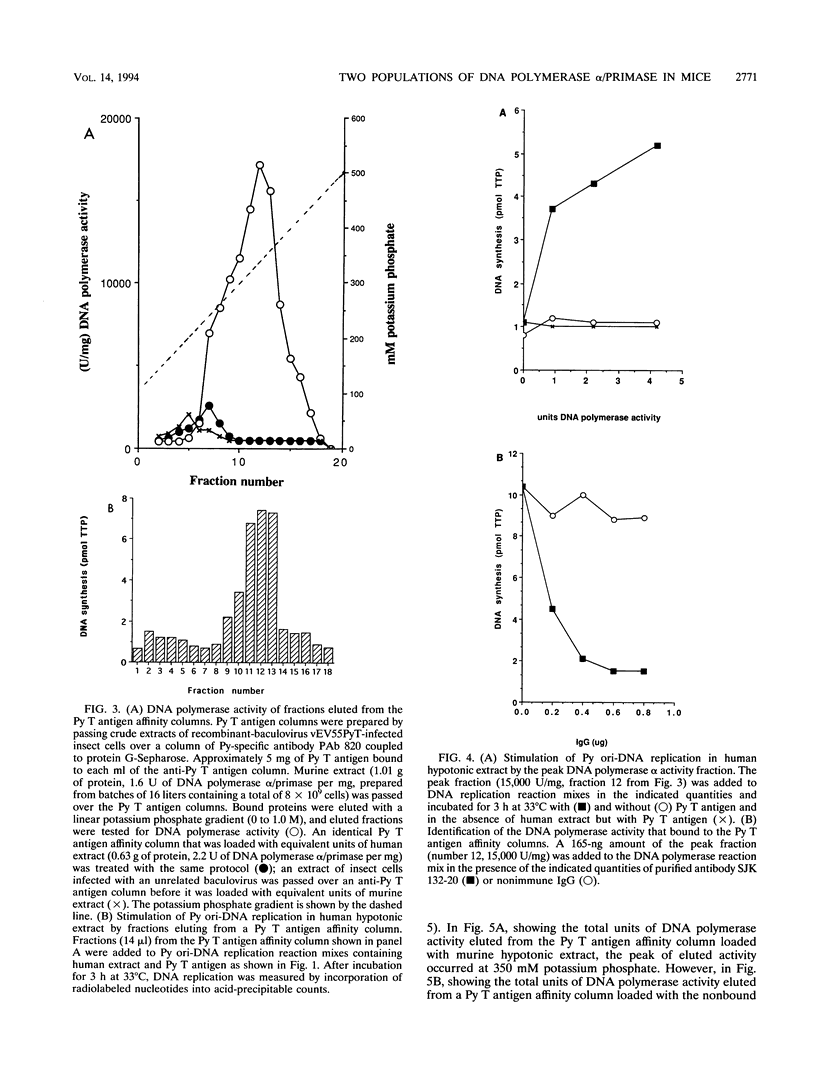
- Seki M., Enomoto T., Eki T., Miyajima A., Murakami Y., Hanaoka F., Ui M. DNA helicase and nucleoside-5'-triphosphatase activities of polyoma virus large tumor antigen. Biochemistry. 1990 Jan 30;29(4):1003–1009. doi: 10.1021/bi00456a024. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Tjian R. T-antigen-DNA polymerase alpha complex implicated in simian virus 40 DNA replication. Mol Cell Biol. 1986 Nov;6(11):4077–4087. doi: 10.1128/mcb.6.11.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H., Dröge P., Knippers R. DNA helicase activity of SV40 large tumor antigen. EMBO J. 1986 Aug;5(8):1939–1944. doi: 10.1002/j.1460-2075.1986.tb04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Enomoto T., Masutani C., Hanaoka F., Yamada M., Ui M. DNA primase-DNA polymerase alpha assembly from mouse FM3A cells. Purification of constituting enzymes, reconstitution, and analysis of RNA priming as coupled to DNA synthesis. J Biol Chem. 1989 Jun 15;264(17):10065–10071. [PubMed] [Google Scholar]
- Tanaka S., Hu S. Z., Wang T. S., Korn D. Preparation and preliminary characterization of monoclonal antibodies against human DNA polymerase alpha. J Biol Chem. 1982 Jul 25;257(14):8386–8390. [PubMed] [Google Scholar]
- Tsurimoto T., Melendy T., Stillman B. Sequential initiation of lagging and leading strand synthesis by two different polymerase complexes at the SV40 DNA replication origin. Nature. 1990 Aug 9;346(6284):534–539. doi: 10.1038/346534a0. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Stillman B. Replication factors required for SV40 DNA replication in vitro. I. DNA structure-specific recognition of a primer-template junction by eukaryotic DNA polymerases and their accessory proteins. J Biol Chem. 1991 Jan 25;266(3):1950–1960. [PubMed] [Google Scholar]
- Tsurimoto T., Stillman B. Replication factors required for SV40 DNA replication in vitro. II. Switching of DNA polymerase alpha and delta during initiation of leading and lagging strand synthesis. J Biol Chem. 1991 Jan 25;266(3):1961–1968. [PubMed] [Google Scholar]
- Wang E. H., Friedman P. N., Prives C. The murine p53 protein blocks replication of SV40 DNA in vitro by inhibiting the initiation functions of SV40 large T antigen. Cell. 1989 May 5;57(3):379–392. doi: 10.1016/0092-8674(89)90913-6. [DOI] [PubMed] [Google Scholar]
- Wang E. H., Prives C. ATP induces the assembly of polyoma large tumor antigen into hexamers. Virology. 1991 Sep;184(1):399–403. doi: 10.1016/0042-6822(91)90858-9. [DOI] [PubMed] [Google Scholar]
- Wang E. H., Prives C. DNA helicase and duplex DNA fragment unwinding activities of polyoma and simian virus 40 large T antigen display similarities and differences. J Biol Chem. 1991 Jul 5;266(19):12668–12675. [PubMed] [Google Scholar]
- Wang T. S. Eukaryotic DNA polymerases. Annu Rev Biochem. 1991;60:513–552. doi: 10.1146/annurev.bi.60.070191.002501. [DOI] [PubMed] [Google Scholar]
- Weinberg D. H., Collins K. L., Simancek P., Russo A., Wold M. S., Virshup D. M., Kelly T. J. Reconstitution of simian virus 40 DNA replication with purified proteins. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8692–8696. doi: 10.1073/pnas.87.22.8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., Kelly T. Purification and characterization of replication protein A, a cellular protein required for in vitro replication of simian virus 40 DNA. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2523–2527. doi: 10.1073/pnas.85.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., Li J. J., Kelly T. J. Initiation of simian virus 40 DNA replication in vitro: large-tumor-antigen- and origin-dependent unwinding of the template. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3643–3647. doi: 10.1073/pnas.84.11.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]