Abstract
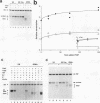
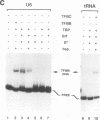
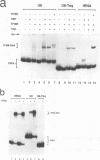
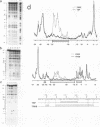
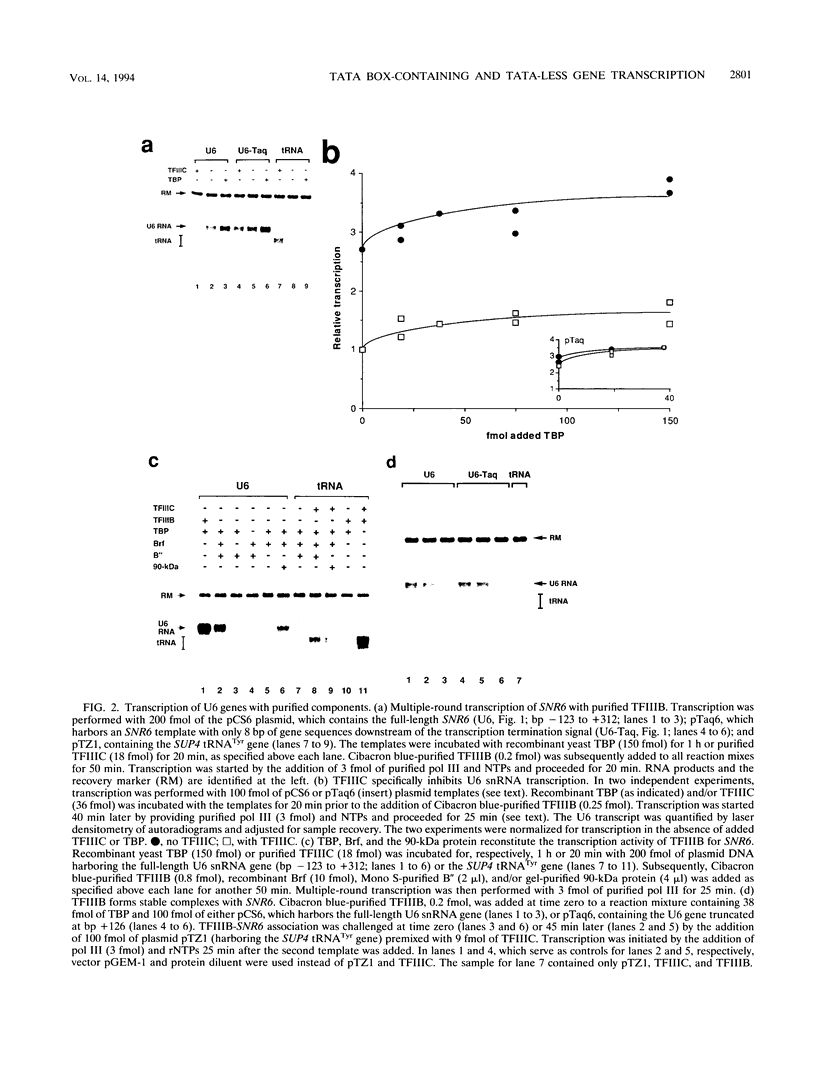
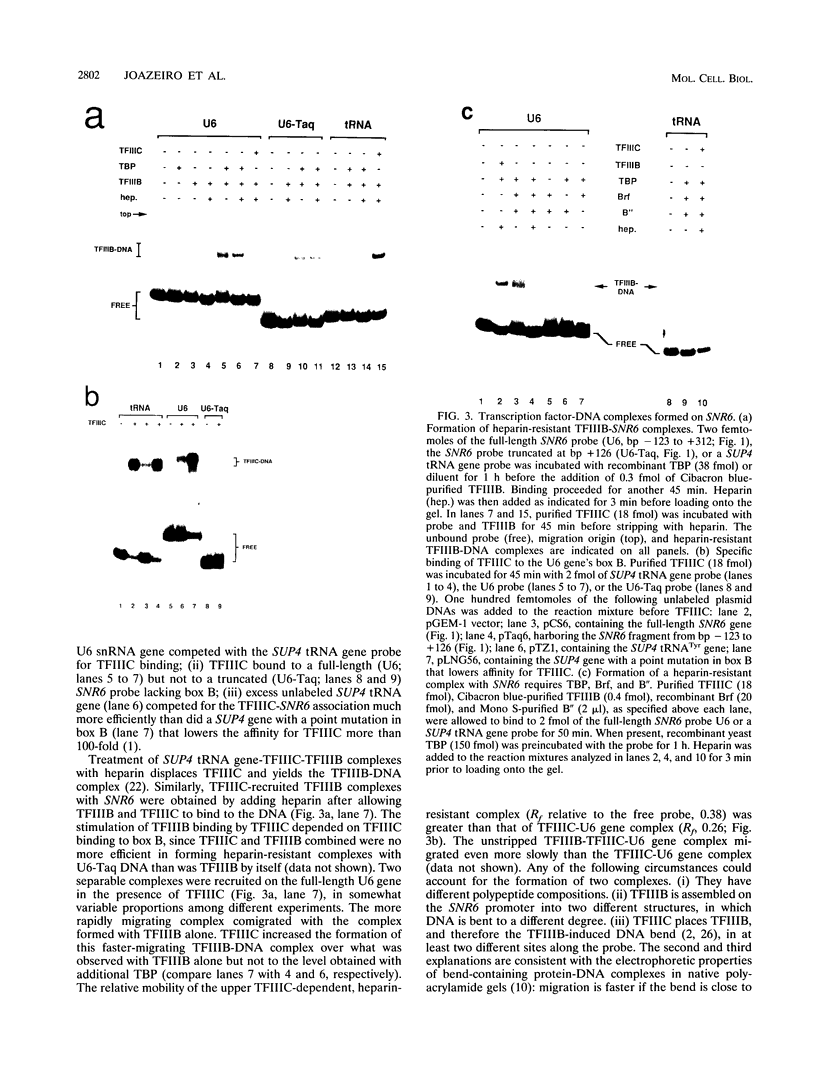
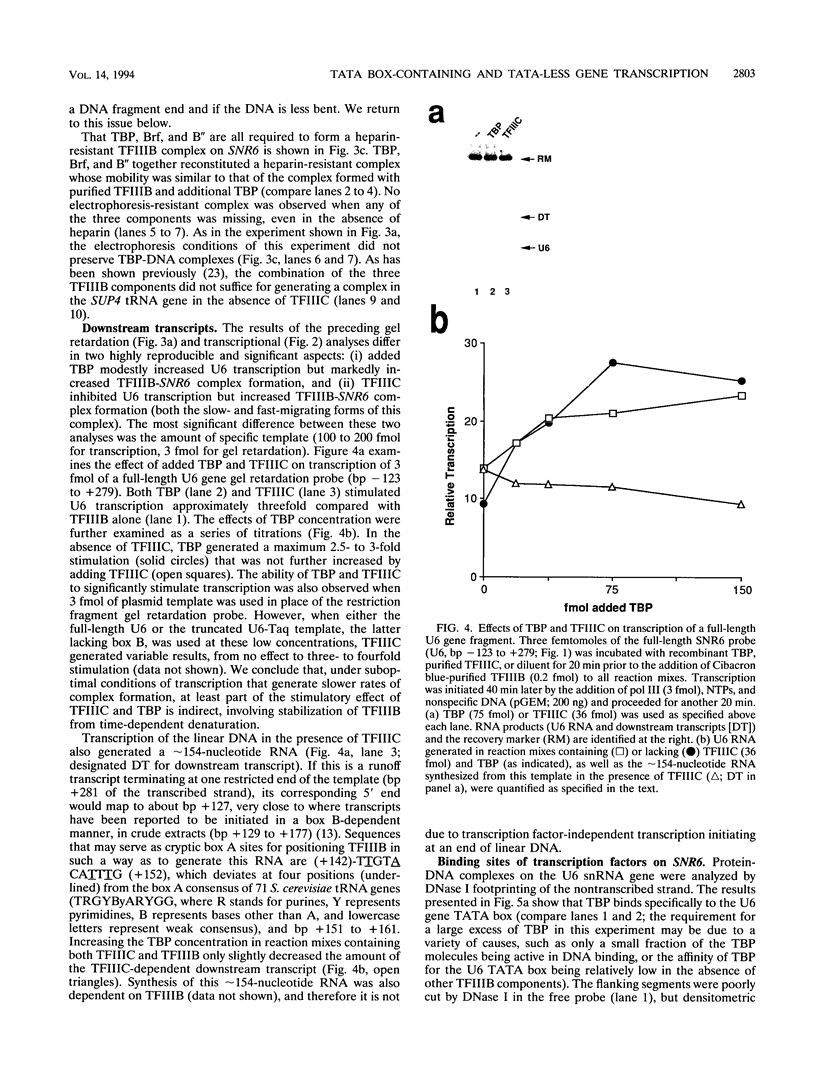
Specific transcription by RNA polymerase III requires recognition of the promoter-bound transcription factor IIIB (TFIIIB), of which the TATA-binding protein (TBP) is a subunit. The recruitment of TFIIIB to TATA-less genes is mediated by protein-protein interactions with transcription factor IIIC (TFIIIC) bound to the box A and box B elements. Here we examine interactions involved in the recruitment of TFIIIB to the TATA element-containing yeast U6 small nuclear RNA gene SNR6. TFIIIC is not required for the formation of TFIIIB-SNR6 gene complexes with purified components. The same three components of TFIIIB that are necessary for TFIIIC-dependent transcription of tRNA genes (recombinant TBP and Brf and the denaturing-gel-purified 90-kDa subunit) are required and sufficient for TATA box-directed U6 transcription. Despite its TFIIIC-independent, DNA sequence-dependent assembly, the TFIIIB-SNR6 complex shares important features with tDNA- and 5S rDNA-TFIIIB complexes, such as extent and location of footprint, stability, and resistance to heparin. These properties are clearly distinct from those of a TBP-SNR6 complex. In the SNR6 gene, box B, the primary binding site for TFIIIC, is suboptimally spaced relative to box A. At limiting TBP concentrations and on bare DNA, TFIIIC stimulates the formation of TFIIIB complexes with SNR6 but contributes poorly, at best, to the formation of properly placed complexes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R. E., Gabrielsen O., Hall B. D. Effects of tRNATyr point mutations on the binding of yeast RNA polymerase III transcription factor C. J Biol Chem. 1986 Apr 25;261(12):5275–5282. [PubMed] [Google Scholar]
- Braun B. R., Kassavetis G. A., Geiduschek E. P. Bending of the Saccharomyces cerevisiae 5S rRNA gene in transcription factor complexes. J Biol Chem. 1992 Nov 5;267(31):22562–22569. [PubMed] [Google Scholar]
- Braun B. R., Riggs D. L., Kassavetis G. A., Geiduschek E. P. Multiple states of protein-DNA interaction in the assembly of transcription complexes on Saccharomyces cerevisiae 5S ribosomal RNA genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2530–2534. doi: 10.1073/pnas.86.8.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brow D. A., Guthrie C. Spliceosomal RNA U6 is remarkably conserved from yeast to mammals. Nature. 1988 Jul 21;334(6179):213–218. doi: 10.1038/334213a0. [DOI] [PubMed] [Google Scholar]
- Brow D. A., Guthrie C. Transcription of a yeast U6 snRNA gene requires a polymerase III promoter element in a novel position. Genes Dev. 1990 Aug;4(8):1345–1356. doi: 10.1101/gad.4.8.1345. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Burnol A. F., Margottin F., Huet J., Almouzni G., Prioleau M. N., Méchali M., Sentenac A. TFIIIC relieves repression of U6 snRNA transcription by chromatin. Nature. 1993 Apr 1;362(6419):475–477. doi: 10.1038/362475a0. [DOI] [PubMed] [Google Scholar]
- Challice J. M., Segall J. Transcription of the 5 S rRNA gene of Saccharomyces cerevisiae requires a promoter element at +1 and a 14-base pair internal control region. J Biol Chem. 1989 Nov 25;264(33):20060–20067. [PubMed] [Google Scholar]
- Colbert T., Hahn S. A yeast TFIIB-related factor involved in RNA polymerase III transcription. Genes Dev. 1992 Oct;6(10):1940–1949. doi: 10.1101/gad.6.10.1940. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Gartenberg M. R., Shrader T. E. DNA bending in protein-DNA complexes. Methods Enzymol. 1991;208:118–146. doi: 10.1016/0076-6879(91)08011-6. [DOI] [PubMed] [Google Scholar]
- Dieci G., Duimio L., Coda-Zabetta F., Sprague K. U., Ottonello S. A novel RNA polymerase III transcription factor fraction that is not required for template commitment. J Biol Chem. 1993 May 25;268(15):11199–11207. [PubMed] [Google Scholar]
- Emanuel P. A., Gilmour D. S. Transcription factor TFIID recognizes DNA sequences downstream of the TATA element in the Hsp70 heat shock gene. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8449–8453. doi: 10.1073/pnas.90.18.8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenlauer J. B., Kaiser M. W., Gerlach V. L., Brow D. A. Architecture of a yeast U6 RNA gene promoter. Mol Cell Biol. 1993 May;13(5):3015–3026. doi: 10.1128/mcb.13.5.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen O. S., Sentenac A. RNA polymerase III (C) and its transcription factors. Trends Biochem Sci. 1991 Nov;16(11):412–416. doi: 10.1016/0968-0004(91)90166-s. [DOI] [PubMed] [Google Scholar]
- Garrity P. A., Wold B. J. Effects of different DNA polymerases in ligation-mediated PCR: enhanced genomic sequencing and in vivo footprinting. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1021–1025. doi: 10.1073/pnas.89.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S., Buratowski S., Sharp P. A., Guarente L. Yeast TATA-binding protein TFIID binds to TATA elements with both consensus and nonconsensus DNA sequences. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5718–5722. doi: 10.1073/pnas.86.15.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopes B. C., LeBlanc J. F., Hawley D. K. Kinetic analysis of yeast TFIID-TATA box complex formation suggests a multi-step pathway. J Biol Chem. 1992 Jun 5;267(16):11539–11547. [PubMed] [Google Scholar]
- Horikoshi M., Wang C. K., Fujii H., Cromlish J. A., Weil P. A., Roeder R. G. Purification of a yeast TATA box-binding protein that exhibits human transcription factor IID activity. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4843–4847. doi: 10.1073/pnas.86.13.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassavetis G. A., Bartholomew B., Blanco J. A., Johnson T. E., Geiduschek E. P. Two essential components of the Saccharomyces cerevisiae transcription factor TFIIIB: transcription and DNA-binding properties. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7308–7312. doi: 10.1073/pnas.88.16.7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassavetis G. A., Blanco J. A., Johnson T. E., Geiduschek E. P. Formation of open and elongating transcription complexes by RNA polymerase III. J Mol Biol. 1992 Jul 5;226(1):47–58. doi: 10.1016/0022-2836(92)90123-2. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Braun B. R., Nguyen L. H., Geiduschek E. P. S. cerevisiae TFIIIB is the transcription initiation factor proper of RNA polymerase III, while TFIIIA and TFIIIC are assembly factors. Cell. 1990 Jan 26;60(2):235–245. doi: 10.1016/0092-8674(90)90739-2. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Joazeiro C. A., Pisano M., Geiduschek E. P., Colbert T., Hahn S., Blanco J. A. The role of the TATA-binding protein in the assembly and function of the multisubunit yeast RNA polymerase III transcription factor, TFIIIB. Cell. 1992 Dec 11;71(6):1055–1064. doi: 10.1016/0092-8674(92)90399-w. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Riggs D. L., Negri R., Nguyen L. H., Geiduschek E. P. Transcription factor IIIB generates extended DNA interactions in RNA polymerase III transcription complexes on tRNA genes. Mol Cell Biol. 1989 Jun;9(6):2551–2566. doi: 10.1128/mcb.9.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. K., Horikoshi M., Roeder R. G. Interaction of TFIID in the minor groove of the TATA element. Cell. 1991 Dec 20;67(6):1241–1250. doi: 10.1016/0092-8674(91)90300-n. [DOI] [PubMed] [Google Scholar]
- Lieberman P. M., Schmidt M. C., Kao C. C., Berk A. J. Two distinct domains in the yeast transcription factor IID and evidence for a TATA box-induced conformational change. Mol Cell Biol. 1991 Jan;11(1):63–74. doi: 10.1128/mcb.11.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo S. M., Tanaka M., Sullivan M. L., Hernandez N. A TBP complex essential for transcription from TATA-less but not TATA-containing RNA polymerase III promoters is part of the TFIIIB fraction. Cell. 1992 Dec 11;71(6):1029–1040. doi: 10.1016/0092-8674(92)90397-u. [DOI] [PubMed] [Google Scholar]
- Léveillard T., Kassavetis G. A., Geiduschek E. P. Repression and redirection of Saccharomyces cerevisiae tRNA synthesis from upstream of the transcriptional start site. J Biol Chem. 1993 Feb 15;268(5):3594–3603. [PubMed] [Google Scholar]
- Léveillard T., Kassavetis G. A., Geiduschek E. P. Saccharomyces cerevisiae transcription factors IIIB and IIIC bend the DNA of a tRNA(Gln) gene. J Biol Chem. 1991 Mar 15;266(8):5162–5168. [PubMed] [Google Scholar]
- Margottin F., Dujardin G., Gérard M., Egly J. M., Huet J., Sentenac A. Participation of the TATA factor in transcription of the yeast U6 gene by RNA polymerase C. Science. 1991 Jan 25;251(4992):424–426. doi: 10.1126/science.1989075. [DOI] [PubMed] [Google Scholar]
- Moenne A., Camier S., Anderson G., Margottin F., Beggs J., Sentenac A. The U6 gene of Saccharomyces cerevisiae is transcribed by RNA polymerase C (III) in vivo and in vitro. EMBO J. 1990 Jan;9(1):271–277. doi: 10.1002/j.1460-2075.1990.tb08105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell B. A., Gilmour D. S. Contribution of sequences downstream of the TATA element to a protein-DNA complex containing the TATA-binding protein. Mol Cell Biol. 1993 Apr;13(4):2593–2603. doi: 10.1128/mcb.13.4.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W. Three in one and one in three: it all depends on TBP. Cell. 1993 Jan 15;72(1):7–10. doi: 10.1016/0092-8674(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Schmidt M. C., Zhou Q., Berk A. J. Sp1 activates transcription without enhancing DNA-binding activity of the TATA box factor. Mol Cell Biol. 1989 Aug;9(8):3299–3307. doi: 10.1128/mcb.9.8.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr D. B., Hawley D. K. TFIID binds in the minor groove of the TATA box. Cell. 1991 Dec 20;67(6):1231–1240. doi: 10.1016/0092-8674(91)90299-e. [DOI] [PubMed] [Google Scholar]