Abstract
Despite the importance of regaining independent ambulation after stroke, the amount of daily walking completed during in-patient rehabilitation is low. The purpose of this study is to determine if (1) walking-related heart rate responses reached the minimum intensity necessary for therapeutic aerobic exercise (40%–60% heart rate reserve) or (2) heart rate responses during bouts of walking revealed excessive workload that may limit walking (>80% heart rate reserve). Eight individuals with subacute stroke attending in-patient rehabilitation were recruited. Participants wore heart rate monitors and accelerometers during a typical rehabilitation day. Walking-related changes in heart rate and walking bout duration were determined. Patients did not meet the minimum cumulative requirements of walking intensity (>40% heart rate reserve) and duration (>10 minutes continuously) necessary for cardiorespiratory benefit. Only one patient exceeded 80% heart rate reserve. The absence of significant increases in heart rate associated with walking reveals that patients chose to walk at speeds well below a level that has meaningful cardiorespiratory health benefits. Additionally, cardiorespiratory workload is unlikely to limit participation in walking. Measurement of heart rate and walking during in-patient rehabilitation may be a useful approach to encourage patients to increase the overall physical activity and to help facilitate recovery.
1. Background
Regaining independent ambulation is important to those with stroke [1, 2] and is the most frequently reported rehabilitation goal [3, 4]. Therefore, walking should be an integral part of in-patient rehabilitation. However, accelerometer-based monitoring of walking activity has revealed that the amount of daily walking completed by individuals with stroke during in-patient rehabilitation is low [5, 6]. Importantly, the majority of walking bouts are of short duration (<1 minute) [5–7] and typically involve walking to essential activities (e.g., washroom, dining area, or therapy) [5].
While activity monitors provide insight into total daily activity [5–10], they do not inform the possible determinants or consequences of this activity. Aerobic capacity is reduced in the early months following stroke [11–13]. Furthermore, poststroke gait is inefficient, and there are increased aerobic demands on those with stroke when walking compared to healthy controls, even when walking at the same speed [14]. Therefore, individuals with stroke are closer to their maximal aerobic threshold when walking than healthy controls. This potentially limits the intensity (i.e., speed) and total duration of walking activity during daily life.
Walking can be a valuable means to improve aerobic capacity [15]. Aerobic exercise can help to improve aerobic capacity following stroke and improve recovery from stroke [16, 17]. Furthermore, there is evidence that increased amount of rehabilitation early after stroke improves recovery [18, 19]. However, limited resources within rehabilitation hospitals may impede the ability to provide formalized or structured aerobic training. Unstructured and unsupervised activities, such as daily walking, provide an opportunity to benefit aerobic fitness afterstroke. Presently, it is not known if in-patients engage in episodes of walking that have aerobic benefit outside of therapy.
This study aims to answer two questions: (1) does unsupervised, unstructured daily walking activity provide aerobic benefit to individuals after stroke and (2) is daily walking activity limited after stroke due to increased energy demands of walking? This initial study was specifically focused on a sample of stroke patients who were able to walk independently and who resided within a rehabilitation hospital. We view this subacute phase to be particularly important for enhancing aerobic training after stroke. To address the first objective, we determined if individuals with stroke engaged in walking bouts that were at least 10 minutes long at an intensity of 40%–80% of heart rate reserve (HRR), totaling at least 20 minutes per day [15]. To address the second question, we determined the duration of walking activity that reached or exceeded the aerobic threshold of 80% of HRR [20]. The latter, if it occurred, would reflect that the challenge of everyday walking may pose a potential barrier to being more active.
2. Methods
2.1. Patients
We included individuals who were attending in-patient rehabilitation following stroke and who were able to walk independently without supervision (with or without use of a walking aid). We excluded patients who used heart-rate (HR) altering medication (e.g., beta-blockers) as HR response would be variable depending on when medication was taken. Eight individuals volunteered to participate and provided informed consent. The study was approved by the institution's research ethics board, and study procedures were in accordance with institutional guidelines. Characteristics of the eight volunteers are presented in Table 1. Participants underwent clinical assessment of gait, functional balance, and motor impairment. Spatiotemporal characteristics of gait were collected using a pressure-sensitive mat (GAITRite, CIR Systems Inc., Havertown, PA, USA). Participants walked across the mat three times at their preferred speed and the location and timing of each footstep were sampled at 30 Hz. We then calculated walking speed, cadence, and temporal symmetry [21]. Motor impairment was assessed using the Chedoke-McMaster Stroke Assessment (CMSA) [22]. Functional balance was assessed using the Berg Balance Scale (BBS) [23].
Table 1.
Demographic and clinical characteristics of patients.
| Participant | Gender | Age (years) | Time after stroke (days) | CMSA | BBS (score) |
Resting HR (beats/min) |
Gait speed (m/s) |
Cadence (steps/min) |
Temporal symmetry (ratio) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Leg | Foot | |||||||||
| A* | M | 59 | 13 | 4 | 4 | 51 | 64 | 0.54 | 72.8 | 1.26 |
| B* | F | 31 | 28 | 3 | 4 | 32 | 72 | 0.28 | 67.2 | 1.15 |
| C* | M | 76 | 14 | 5 | 6 | 51 | 59 | 1.11 | 103.5 | 1.08 |
| D | M | 57 | 25 | 4 | 4 | 38 | 51 | 0.83 | 93.9 | 1.10 |
| E* | M | 38 | 68 | 5 | 5 | 41 | 64 | 1.10 | 100.6 | 0.98 |
| F | M | 54 | 46 | 5 | 5 | 49 | 55 | 0.86 | 98 | 1.03 |
| G* | F | 63 | 30 | 4 | 3 | 44 | 82 | 0.53 | 81.5 | 1.29 |
| H* | F | 47 | 32 | 3 | 4 | 33 | 51 | 0.31 | 60.0 | 1.20 |
|
| ||||||||||
| Mean | 53.1 | 32 | 4.1 | 4.4 | 42.3 | 62.3 | 0.70 | 88.2 | 1.15 | |
| Standard deviation | 14.2 | 17.9 | 0.8 | 0.9 | 7.7 | 10.7 | 0.33 | 14.4 | 0.96 | |
*Denotes use of an assistive device (e.g., single-point cane/rollator) throughout data collection.
BBS: Berg balance scale; CMSA: Chedoke-McMaster stroke assessment; F: female; HR: heart rate; M: male.
2.2. Ambulatory and Heart Rate Data Acquisition
The ABLE system [5] (Figure 1) was used to collect ambulatory data. The ABLE system is comprised of two triaxial accelerometers (SparkFun Electronics, Boulder, CO, USA) worn bilaterally around the ankles, which transmit data wirelessly to a personal digital assistant (PDA) (Hewlett Packard, Palo Alto, CA, USA) worn around the waist. The accelerometers were placed just proximal to the lateral malleoli using custom ankle sleeves, and the PDA was secured to the participant's waist using a polyester belt and pouch. Data from each accelerometer unit were recorded on the PDA at 50 Hz. HR data were acquired using a commercially available HR monitoring system (Polar Electro, Kempele, Finland). Participants wore a chest strap and a wristwatch. Heart beats were recorded by the chest strap and transmitted wirelessly to the wristwatch, which logged HR data at 0.2 Hz. The two collection systems were synchronized by initiating data collection in tandem.
Figure 1.
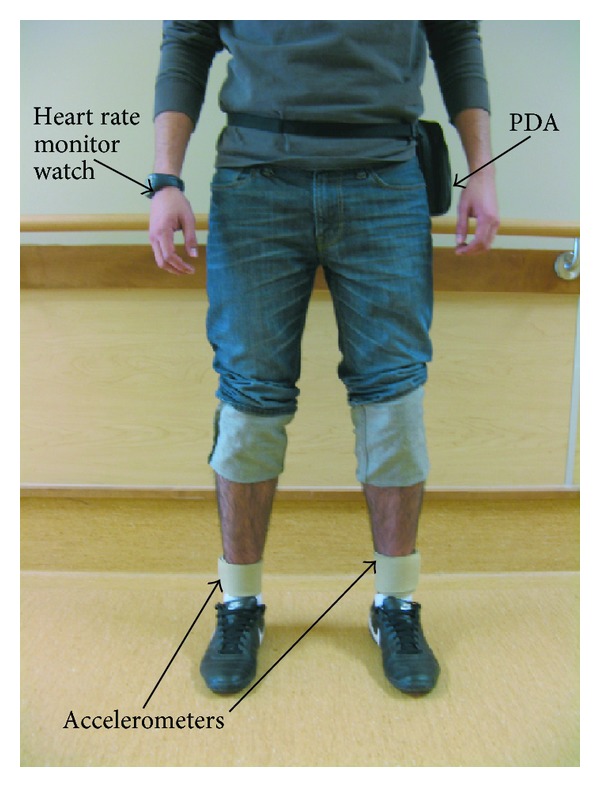
Placement of the ABLE system on a patient. Highlighted in the figure is the personal digital assistant (PDA) data logger worn around the waist of the patient, bilateral placement of accelerometer straps worn superior to the lateral malleolus, and heart rate wrist watch data logger.
Patients were fitted with the ABLE and HR monitoring systems in the morning after routine activities were completed (e.g., bathing). The investigator checked every one to two hours to ensure that there was no discomfort and that all devices remained operational. Data collection continued for approximately eight hours, between approximately 9 am and 5 pm.
Periods of walking activity were identified and delimited to “bouts” of walking in our analysis. A bout of walking consisted of at least 10 consecutive steps; shorter bouts would not likely have yielded a measurable HR response. Individual bouts of walking were differentiated by a pause of at least 5 seconds prior to the next bout of walking [5].
2.3. Physiological Change Detection
The Karvonen formula [20] was used to determine the cardiovascular intensity (i.e., % of HRR) during bouts of walking. Using HR collected from the HR monitor (HRobserved), the % of HRR was determined for each bout of walking by using the following modified Karvonen formula:
| (1) |
HRmax was the estimated maximum HR and was determined by subtracting the participant's age from 220 [15]. While the patient remained seated, resting HR (HRrest) was determined by recording the lowest HR measured within the initial 10-minute period of collection. For each identified bout of walking, HR response (HRobserved) for that specific bout was determined by averaging the three highest consecutive HR measures. This approach was taken in order to acquire a sustained HR response over 15 s (e.g., three HR measurement points), as opposed to using a single-point HR, which has the potential to be influenced by transient mutant HR responses.
3. Results
Mean data collection duration was 8.4 hours (standard deviation: 0.8 hours). Six of the eight participants required a walking aid during both daily walking and clinical data collection (Table 1). At the time of collection, participant B had begun independently ambulating only recently with a walking aid after having been limited to a wheelchair since the onset of her stroke.
3.1. Characteristics of Daily Walking
Walking characteristics are outlined in Table 2. The mean number and duration of bouts throughout the collection period were 62.6 bouts (standard deviation: 21.4 bouts) and 57.1 s (standard deviation: 31.6 s), respectively. Overall, 80.8% of all walking bouts were less than 1 min in duration, only 1.8% of all bouts were greater than 5 minutes, and only two walking bouts were greater than 10 minutes. Average step count was 3,708 steps (standard deviation: 1,452). Participant B demonstrated the lowest number of walking bouts with 33 (1,774 steps), while participant E demonstrated the greatest number of walking bouts with 91 (4,778 steps). The single longest bout duration was 13 minutes by participant F, which was performed during structured therapy.
Table 2.
Summary of walking measures for each patient collected throughout the day.
| Participant | Total collection time (hours) |
Total walking time (minutes) |
Number of walking bouts | Mean bout duration (s) | Total step count | Mean cadence (steps/minute) |
|---|---|---|---|---|---|---|
| A | 7.06 | 33.3 | 46 | 43.4 | 2643 | 73.2 |
| B | 8.85 | 24.6 | 33 | 57.6 | 1774 | 79.2 |
| C | 7.86 | 31.6 | 55 | 34.5 | 3743 | 85.8 |
| D | 8.65 | 31.7 | 79 | 31.8 | 2201 | 78.5 |
| E | 8.91 | 59.3 | 91 | 45.8 | 4778 | 87.2 |
| F | 7.87 | 58.4 | 60 | 131.3 | 5621 | 103.6 |
| G | 8.41 | 78.3 | 89 | 52.4 | 5377 | 78 |
| H | 9.69 | 74.7 | 48 | 60.2 | 3532 | 61.6 |
|
| ||||||
| Mean | 8.4 | 49 | 62.6 | 57.1 | 3708 | 80.8 |
| Standard deviation | 0.8 | 21.2 | 21.3 | 31.6 | 1452 | 12.1 |
3.2. Did Patients Meet Recommended Physiological Intensities and Durations for Aerobic Exercise during Daily Walking?
Overall, none of the participants fulfilled both requirements of duration (bouts ≥10 minutes long for a total of 20 minutes per day) and intensity (% of HRR ≥ 40%) for aerobic benefit (Figure 2). This was most profoundly limited by the duration of the bouts of walking as detailed in the preceding section. With respect to amplitude only 3.1% of all bouts detected, occurring in only two participants, were found to be above 40% HRR. The overall average intensity for bouts of walking was 19.4% HRR. Of the 8 participants tested, only participants B and G exhibited bouts of walking that exceeded the minimum 40% HRR. Participant B had the greatest number of bouts over 40% HRR, which occurred in 31 bouts (93% of their total bouts); participant G exhibited 3 bouts (3.3% of their total bouts) during which HRR exceeded 40%. The remaining participants did not present walking intensities above 40% HRR in any bout.
Figure 2.
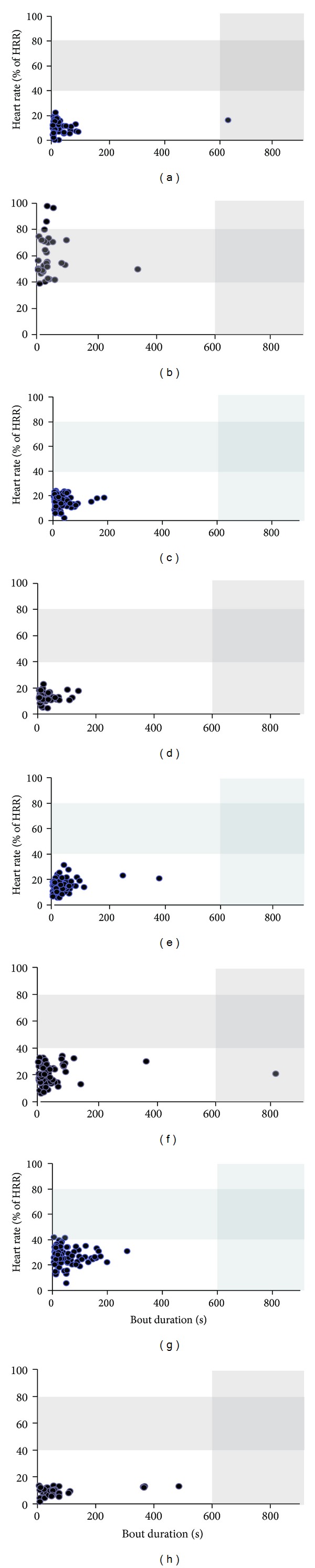
Mean heart rate response, expressed as a percentage of heart rate reserve (HRR), versus duration of walking bout throughout the collection period for all participants. Each point represents a single bout of walking performed during the collection period. The shaded areas indicate the recommended intensity (40%–80% maximum heart rate) and duration (10 minutes of continuous walking) of walking.
3.3. Did Patients Exceed Recommended Intensities during Daily Walking?
Participant B was the only participant found to exceed the 80% HRR threshold for any bout of walking (Figure 2). This participant presented 3 bouts (9% of her total bouts) above 80% HRR which ranged from 85.8% to 97.5% HRR. These bouts were not long in duration (<1 minute) and were not performed at high cadences (70–74 steps/minute). These bouts were performed while the participant walked with a rollator on a pedestrian pathway outside the hospital under the supervision of a physiotherapist.
4. Discussion
The present investigation sought to determine the extent to which individuals with stroke met or exceeded the recommended cardiovascular intensities of everyday walking activity during in-patient rehabilitation. Together, the quantity and intensity of everyday walking have the potential to positively or negatively influence poststroke recovery. Such information provides insight into the potential value of everyday walking and contributes to the understanding of adaptations to current rehabilitative practices that can be made to maximize time spent in therapeutically beneficial activities.
4.1. Participants Did Not Meet Recommended Duration and Intensity of Walking Activity
In agreement with previous work [5, 6, 24] the total amount of spontaneous walking activity was low. As our prior study has also indicated [5], durations of walking throughout an in-patient's day primarily consist of short bouts (e.g., less than one minute). Although there were no data available on healthy individuals for comparison, it is possible that short durations of walking activity observed are not just specific to patients with stroke, but more broadly reflect common walking patterns for inside environments. However, among all eight participants, only one spontaneous walking bout was longer than 10 minutes in duration; one other walking bout was longer than 10 minutes, but this occurred during scheduled physiotherapy. In terms of step counts, it is recommended that individuals with disability take at least 6,000 steps per day, of which 3,000 should be engaged in moderate-to-vigorous physical activity [25]. No participant in the current study attained 6,000 steps in one day.
The majority of walking was at <40% HRR, and no participant completed 3,000 steps at a moderate-vigorous level. The overall mean of 19.4% HRR for everyday walking (including periods of structured therapy) was similar to the 24.2% (SD 21.2) HRR of walking found in patients with stroke performing standing and walking tasks exclusively during physical therapy [26]. HRR provides a more informative measure of intensity than cadence or walking speed as it is linked to the patients' aerobic capacity. While the present and previous studies were based on single day “snapshot” of activity, the low duration and intensity of activity for in-patients residing in a rehabilitation facility are striking. The absence of therapeutically beneficial walking activity may be attributed to factors such as short durations, low walking speeds, and the purpose for which patients walked. It is likely that low walking duration and low intensity levels may well be associated with more conventional walking activities, such as activities of everyday living (e.g., going to meals). Subsequent measurement will need to be extended to longer periods of time. However, the modest HR responses confirmed other work that monitored HR through the day [27] leading to the view that such moderate within-day HR responses may be “typical” of patients' experiences.
4.2. Excessive Walking-Related Cardiovascular Load Does Not Limit Walking Duration
We sought to determine if HR response might be a limiter to walking duration and a potential barrier to activity. As noted the occurrence of HR response greater than 80% HRR was rare and occurred in only one participant (B). This participant had only begun to start walking independently one day prior to data collection after having been mostly wheelchair bound for one month following her stroke. Therefore, this individual was likely extremely deconditioned as a result of limited mobility and, consequently, reached the threshold of her aerobic capacity easily during daily walking. Limited aerobic capacity potentially limited total walking time for this participant; she had the lowest total walking duration and fewest total steps among all participants. However, reduced balance control and increased motor impairment may also have limited total daily walking. Participant B also had the lowest BBS score, CMSA scores, and self-selected walking speed. Increased motor impairment may have increased the energy demands of walking and caused participant B to reach the threshold of aerobic capacity more easily than other participants [14, 28]. Alternatively, reduced walking duration may have been a strategy to prevent a fall given impaired balance control [24].
Among the remaining seven participants, there was no evidence that reduced aerobic capacity limited total daily walking activity. No other participant attained >80% HRR while walking during the day. Furthermore, long-duration walking bouts (e.g., 5 minutes or longer) were not associated with increased HR response. Therefore, aerobic capacity did not limit frequency of long-duration walking bouts. The barriers to increasing total spontaneous walking activity following stroke remain to be determined. While HR was typically low, it is possible that patients' perceived fatigue caused them to limit walking speed and time. We did not record perceived exertion or fatigue in the current study; this should be considered in future work. In hospital, patients are likely to be reluctant to walk outside, particularly if balance control is impaired or if the weather is poor. Reduced balance control may play a role, and patients may limit physical activity due to fear of falling [29, 30]. Long-duration walking bouts may be influenced by the size of the unit or the length of the corridors within the hospital, although the current unit features an approximately 50 m long hallway where patients can walk unencumbered. Such distances would provide a much greater opportunity for someone to walk indoors than would be possible in many indoor living settings in the community. However, patients who were transferred from in-patient care to the community were found to increase bout durations in the community, amounting to an extra 30 minutes of activity per day [6]. Such an increase may be partially attributed to opportunity and willingness to walk outside and participate in community activities [6] or may reflect the improved functional capacity at the time after discharge from in-patient rehabilitation. Finally, patients may not be aware of their limited walking activity, and interventions may be required to increase spontaneous walking activity during in-patient rehabilitation. Additional work is required to determine the factors contributing to reduced daily walking activity during in-patient stroke rehabilitation.
4.3. Clinical Significance
Profiling the relationship between ambulatory activity and HR response can have important health-related implications to poststroke rehabilitation. The absence of activity that may benefit cardiorespiratory health and fitness during supervised and unsupervised periods of the day highlights a larger problem associated with conventional in-patient care. Feasibility studies have demonstrated that structured equipment-based exercise programs can be implemented safely and without negative effects on conventional therapy [17]. Patients receiving aerobic training in addition to standard care have significant improvements in indices of neuromuscular control and functional ambulation [17]. However, these exercise programs require therapist supervision and other resources such as space and equipment. These results can be viewed as a missed or lost opportunity for supplementary rehabilitation practice. The benefit of measuring both walking and HR, as in the present study, is to help clinicians consider periods of unstructured activity. Emphasizing additional therapeutically relevant activities throughout the day may be one method to better address “down” time frequently experienced by patients [31, 32]. However, it can be argued that simply requesting patients to engage in additional walking activities outside of therapy may not occur or may occur at inadequate intensities or durations to provide meaningful improvements in cardiovascular health. By using heart rate and activity monitors to identify the absence of walking bouts required for therapeutic benefits, clinicians would be able to better guide treatment decisions regarding walking exercise programs. Future studies will need to examine intensity and duration of patient activity across multiple days to develop a better understanding of patient activity levels and barriers to increased activity. In addition, the potential use of bout duration and its associated physiological response as an outcome measure for clinical practice requires further study [6].
5. Summary/Conclusions
This preliminary investigation between walking activity and task-related HR responses provides insight into the physiological demands associated with daily walking on patients residing in a rehabilitation hospital. These results indicate that daily walking (performed indoors) likely does not provide cardiorespiratory benefit within this group. Consequently, to achieve aerobic benefits from daily walking patients should be encouraged to increase the quantity of walking, and additional emphasis needs to be placed on increasing the intensity of walking if possible. Among those patients for whom the recommended walking intensity is not yet possible aerobic training should be formally included into structured therapy or performed using equipment that poses no risk of falling (e.g., recumbent stepper). Ideally, encouraging adaptations to daily walking activity, to increase both duration and intensity, may help promote and facilitate recovery after stroke and reduce the risk of subsequent vascular events.
Acknowledgments
This study was supported by contributions made by the Natural Sciences and Engineering Research Council of Canada (NSERC), Canadian Institutes of Health Research and Collaborative Health Research and Development Program (no.323492-06), and the Heart & Stroke Foundation Centre for Stroke Recovery. The authors also acknowledge support of the Toronto Rehabilitation Institute, which receives funding under the Provincial Rehabilitation Research Program from the Ontario Ministry of Health and Long-Term Care. D. brooks holds a Canada Research Chair in Rehabilitation (tier 2).
References
- 1.Pound P, Gompertz P, Ebrahim S. A patient-centred study of the consequences of stroke. Clinical Rehabilitation. 1998;12(4):338–347. doi: 10.1191/026921598677661555. [DOI] [PubMed] [Google Scholar]
- 2.Lord SE, McPherson K, McNaughton HK, Rochester L, Weatherall M. Community ambulation after stroke: how important and obtainable is it and what measures appear predictive? Archives of Physical Medicine and Rehabilitation. 2004;85(2):234–239. doi: 10.1016/j.apmr.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Bohannon RW, Andrews AW, Smith MB. Rehabilitation goals of patients with hemiplegia. International Journal of Rehabilitation Research. 1988;11(2):181–183. [Google Scholar]
- 4.Hesse S. Recovery of gait and other motor functions after stroke: novel physical and pharmacological treatment strategies. Restorative Neurology and Neuroscience. 2004;22(3-4):359–369. [PubMed] [Google Scholar]
- 5.Prajapati SK, Gage WH, Brooks D, Black SE, McIlroy WE. A novel approach to ambulatory monitoring: investigation into the quantity and control of everyday walking in patients with subacute stroke. Neurorehabilitation and Neural Repair. 2011;25(1):6–14. doi: 10.1177/1545968310374189. [DOI] [PubMed] [Google Scholar]
- 6.Manns PJ, Baldwin E. Ambulatory activity of stroke survivors measurement options for dose, intensity, and variability of activity. Stroke. 2009;40(3):864–867. doi: 10.1161/STROKEAHA.108.531590. [DOI] [PubMed] [Google Scholar]
- 7.Michael K, Macko RF. Ambulatory activity intensity profiles, fitness, and fatigue in chronic stroke. Topics in Stroke Rehabilitation. 2007;14(2):5–12. doi: 10.1310/tsr1402-5. [DOI] [PubMed] [Google Scholar]
- 8.Lincoln NB, Willis D, Philips SA, Juby LC, Berman P. Comparison of rehabilitation practice on hospital wards for stroke patients. Stroke. 1996;27(1):18–23. doi: 10.1161/01.str.27.1.18. [DOI] [PubMed] [Google Scholar]
- 9.Shaughnessy M, Michael KM, Sorkin JD, Macko RF. Steps after stroke: capturing ambulatory recovery. Stroke. 2005;36(6):1305–1307. doi: 10.1161/01.STR.0000166202.00669.d2. [DOI] [PubMed] [Google Scholar]
- 10.Haeuber E, Shaughnessy M, Forrester LW, Coleman KL, Macko RF. Accelerometer monitoring of home- and community-based ambulatory activity after stroke. Archives of Physical Medicine and Rehabilitation. 2004;85(12):1997–2001. doi: 10.1016/j.apmr.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 11.Duncan PW, Studenski S, Richards L, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34(9):2173–2180. doi: 10.1161/01.STR.0000083699.95351.F2. [DOI] [PubMed] [Google Scholar]
- 12.Kelly JO, Kilbreath SL, Davis GM, Zeman B, Raymond J. Cardiorespiratory fitness and walking ability in subacute stroke patients. Archives of Physical Medicine and Rehabilitation. 2003;84(12):1780–1785. doi: 10.1016/s0003-9993(03)00376-9. [DOI] [PubMed] [Google Scholar]
- 13.MacKay-Lyons MJ, Makrides L. Exercise capacity early after stroke. Archives of Physical Medicine and Rehabilitation. 2002;83(12):1697–1702. doi: 10.1053/apmr.2002.36395. [DOI] [PubMed] [Google Scholar]
- 14.Zamparo P, Francescato MP, De Luca G, Lovati L, di Prampero PE. The energy cost of level walking in patients with hemiplegia. Scandinavian Journal of Medicine & Science in Sports. 1995;5(6):348–352. doi: 10.1111/j.1600-0838.1995.tb00057.x. [DOI] [PubMed] [Google Scholar]
- 15.Whaley M, Brubaker PH, Otto RM. ACSM'S Guidelines For Exercise Testing and Prescription. 7th edition. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 16.Ivey FM, Macko RF, Ryan AS, Hafer-Macko CE. Cardiovascular health and fitness after stroke. Topics in Stroke Rehabilitation. 2005;12(1):1–16. doi: 10.1310/GEEU-YRUY-VJ72-LEAR. [DOI] [PubMed] [Google Scholar]
- 17.Tang A, Sibley KM, Brooks D, Thomas S, McIlroy WE. Early exercise intervention after stroke: influence on aerobic and functional capacity: a pilot study. Journal of Aging and Physical Activity. 2004;12:318–319. [Google Scholar]
- 18.Kwakkel G, Wagenaar RC, Twisk JWR, Lankhorst GJ, Koetsier JC. Intensity of leg and arm training after primary middle-cerebral-artery stroke: a randomised trial. The Lancet. 1999;354(9174):191–196. doi: 10.1016/S0140-6736(98)09477-X. [DOI] [PubMed] [Google Scholar]
- 19.Kwakkel G, Wagenaar RC, Koelman TW, Lankhorst GJ, Koetsier JC. Effects of intensity of rehabilitation after stroke: a research synthesis. Stroke. 1997;28(8):1550–1556. doi: 10.1161/01.str.28.8.1550. [DOI] [PubMed] [Google Scholar]
- 20.Karvonen M, Kentala K, Mustala O. The effects of training on heart rate: a longitudinal study. Annales Medicinae Experimentalis et Biologiae Fenniae. 1957;35:307–315. [PubMed] [Google Scholar]
- 21.Patterson KK, Parafianowicz I, Danells CJ, et al. Gait asymmetry in community-ambulating stroke survivors. Archives of Physical Medicine and Rehabilitation. 2008;89(2):304–310. doi: 10.1016/j.apmr.2007.08.142. [DOI] [PubMed] [Google Scholar]
- 22.Gowland C, Stratford P, Ward M, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24(1):58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 23.Berg K, Wood-Dauphinee S, Williams JI, Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiotherapy Canada. 1989;41(6):304–311. [Google Scholar]
- 24.Michael KM, Allen JK, MacKo RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Archives of Physical Medicine and Rehabilitation. 2005;86(8):1552–1556. doi: 10.1016/j.apmr.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 25.Tudor-Locke C, Craig CL, Aoyagi Y, et al. How many steps/day are enough? For older adults and special populations. International Journal of Behavioral Nutrition and Physical Activity. 2011;8, article 80 doi: 10.1186/1479-5868-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuys S, Brauer S, Ada L. Routine physiotherapy does not induce a cardiorespiratory training effect post-stroke, regardless of walking ability. Physiotherapy Research International. 2006;11(4):219–227. doi: 10.1002/pri.344. [DOI] [PubMed] [Google Scholar]
- 27.Gage WH, Zabjek KF, Sibley KM, Tang A, Brooks D, McIlroy WE. Ambulatory monitoring of activity levels of individuals in the sub-acute stage following stroke: a case series. Journal of NeuroEngineering and Rehabilitation. 2007;4, article 41 doi: 10.1186/1743-0003-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernardi M, Macaluso A, Sproviero E, et al. Cost of walking and locomotor impairment. Journal of Electromyography and Kinesiology. 1999;9(2):149–157. doi: 10.1016/s1050-6411(98)00046-7. [DOI] [PubMed] [Google Scholar]
- 29.Mackintosh SFH, Hill K, Dodd KJ, Goldie P, Culham E. Falls and injury prevention should be part of every stroke rehabilitation plan. Clinical Rehabilitation. 2005;19(4):441–451. doi: 10.1191/0269215505cr796oa. [DOI] [PubMed] [Google Scholar]
- 30.Yardley L, Smith H. A prospective study of the relationship between feared consequences of falling and avoidance of activity in community-living older people. Gerontologist. 2002;42(1):17–23. doi: 10.1093/geront/42.1.17. [DOI] [PubMed] [Google Scholar]
- 31.Bernhardt J, Dewey H, Thrift A, Donnan G. Inactive and alone: physical activity within the first 14 days of acute stroke unit care. Stroke. 2004;35(4):1005–1009. doi: 10.1161/01.STR.0000120727.40792.40. [DOI] [PubMed] [Google Scholar]
- 32.Tudor-Locke C, Williams JE, Reis JP, Pluto D. Utility of pedometers for assessing physical activity: convergent validity. Sports Medicine. 2002;32(12):795–808. doi: 10.2165/00007256-200232120-00004. [DOI] [PubMed] [Google Scholar]


