Abstract
The nucleus of the solitary tract (NTS) is the principal integrating relay in the processing of visceral sensory and gustatory information. In the present study, patch-clamp electrophysiological experiments were conducted using rat horizontal brainstem sections. Pre-synaptic and somatic/dendritic nicotinic acetylcholine receptors (nAChRs) expressed in neurons of the caudal NTS (cNTS) were found to be randomly distributed between pre-synaptic and somatic/dendritic sites (χ2 =0.72, df =3, p>0.87, n=200). Pre-synaptic nAChRs were detected by their facilitating effects on glutamatergic neurotransmission of a sub-population of cNTS neurons (categorized as “effect-positive”) upon brief picospritzer applications of 0.1-0.5 mM nicotine. These effects were resistant to inhibition by 20 nM methyllycaconitine (MLA) and 4 μM dihydro-β-erythroidine (DHβE), and were replicated by brief picospritzer applications of 0.2-1 mM cytisine. Picospritzer applications of 0.2 mM RJR-2403, a potent agonist of α4β2 nAChRs, did not facilitate synaptic release of glutamate in effect-positive cNTS neurons. The population of somatic/dendritic nAChRs has been found to be heterogeneous and included nAChRs that were activated by RJR-2403 and/or cytisine; or insensitive to cytisine; or inhibited by MLA. The presented results are consistent with the expression of β4-containing (i.e., β4*) nAChRs, likely α3β4*, in presynaptic terminals of effect-positive cNTS neurons. Somatic/dendritic nAChRs appear to involve both α7 and non-α7 subunits. Heterogeneity in the subunit composition of pre-synaptic and somatic/dendritic nAChRs may underlie diverse roles that these receptors play in regulation of behavioral and visceral reflexes, and may reflect specific targeting by endogenous nicotinic agents and nicotine.
Keywords: nicotine, RJR-2403, cytisine, brainstem, solitary tract, pre-synaptic
Introduction
The nucleus of the solitary tract (NTS) is a functionally and anatomically heterogeneous group of neurons that acts as a key integrating relay in the processing of visceral sensory and gustatory information (Lawrence and Jarrott, 1996;Contreras et al., 1982;Hamilton and Norgren, 1984). The caudal portion of NTS (cNTS) receives baroreceptor and chemoreceptor afferents from the heart and blood vessels via cranial nerves IX and X to control cardiac output. The NTS also receives visceral afferents from various regions of the gastrointestinal tract (Sun et al., 2005;Zittel et al., 1994;Wang et al., 1999), second-order neurons of the dorsal horn, and other components of the central autonomic system, such as respiratory afferents (Boscan et al., 2002). The rostral portion of NTS (rNTS) receives mostly gustatory information directly from the oral cavity via cranial nerves VII, IX and X (Ashworth-Preece et al., 1998;Lawrence and Jarrott, 1996;Torvik A., 1956).
The presence of choline acetyltransferase (ChAT) and acetylcholinesterase (AChE) immunostaining in the NTS suggests that acetylcholine (ACh) is involved in modulation of visceral sensory and gustatory information (Armstrong et al., 1988;Ueno et al., 1993;Helke et al., 1983;Barry et al., 1993). In the rNTS region, the location of cholinergic neurons is consistent with the distribution of preganglionic parasympathetic neurons labeled by horseradish peroxidase applied to the chorda tympani branch of the VIIth nerve and the lingual-tonsillar branch of the IXth nerve (Contreras et al., 1980). Furthermore, both low- and high-affinity nicotinic binding sites have been demonstrated in the NTS of cats (Maley and Seybold, 1993); and electrophysiological recordings from the NTS in brainstem slices revealed a functionally heterogeneous population of nicotinic (nACh) and muscarinic (mACh) receptors (Uteshev and Smith, 2006;Ernsberger et al., 1988;Shihara et al., 1999). For the most part, nicotinic and muscarinic receptors were expressed by different cells (Shihara et al., 1999;Uteshev and Smith, 2006).
Although the main neurotransmitter released from the solitary tract appears to be glutamate (Shihara et al., 1999;Li and Smith, 1997;Wang and Bradley, 1995), some solitary tract afferents may be cholinergic, providing extrinsic cholinergic inputs to the NTS. Moreover, glutamate has been demonstrated to be the main neurotransmitter of baroreceptor afferents terminating in the NTS (Talman et al., 1980;Reis et al., 1981). However, microinjections of nicotine into the NTS elicited hypotension and bradycardia similar to that elicited by activation of baroreceptors (Kubo and Misu, 1981;Talman and Lewis, 1991). Local cholinergic interneurons may also provide cholinergic inputs within the NTS region (Kobayashi et al., 1978). Therefore, both glutamatergic and cholinergic systems seem to be involved in processing visceral sensory and gustatory information by the NTS. Additional extrinsic cholinergic inputs may arrive to the NTS from the adjacent dorsal motor nucleus of the vagus (Maley, 1996;Farkas et al., 1997) or nucleus ambiguus (Farkas et al., 1997).
Materials and Methods
Electrophysiological patch-clamp experiments have been conducted in the Department of Anatomy and Neurobiology at the University of Tennessee Health Science Center in Memphis, TN and in the Department of Pharmacology at the Southern Illinois University School of Medicine in Springfield, IL. The experimental results obtained in both institutions have resulted in identical conclusions.
Animals
Sprague-Dawley male or female rats (P18-25, ∼100 g) were used in experiments. Animal care was in accordance with the Guide for the Care and Use of Laboratory Animals (NIH 865-23, Bethesda, MD) and was approved by the Animal Care and Use Committee of the University of Tennessee and Southern Illinois University.
Electrophysiology
Rat brains were rapidly removed following decapitation and placed for 1 min in icecold oxygenated sucrose-based solution of the following composition (in mM): sucrose 250, KCl 2.5, NaH2PO4 1.23, MgCl2 5, CaCl2 0.5, NaHCO3 26, glucose 10 (pH 7.4), when bubbled with carbogen (95% O2 and 5% CO2). The brainstem was then transferred to the brain slicer chamber (Vibratom-3000 (UT, Memphis) or Vibratom-1000 (SIU, Springfield), Vibratome, St. Louis, MO) and two-three horizontal brainstem slices (250 μm thick) were cut. Slices were transferred to a storage chamber, where they were perfused at 30°C for 30 min in an oxygenated artificial cerebrospinal fluid (ACSF) solution of the following composition (in mM): NaCl 125, KCl 2.5, NaH2PO4 1.23, MgCl2 1, CaCl2 2, NaHCO3 26, glucose 10 (pH 7.4). In experiments conducted at SIU Springfield, IL, the ACSF contained 35 mM of NaHCO3 to maintain pH 7.4. Slices were then perfused with an identical oxygenated ACSF at 24°C for up to 10 h.
For patch-clamp experiments, slices were transferred to the recording chamber and perfused with an oxygenated ACSF at a rate of 1.5 ml/min using a Dynamax peristaltic pump (Rainin Instrument CO, Emeryville, CA, USA) at UT, Memphis; or 1 ml/min using a 2232 Microperpex S peristaltic pump (LK.B, Upsalla, Sweden) at SIU, Springfield. Typically, cNTS neurons selected for patch-clamp recordings were smaller than 25 μm in diameter. Whole-cell recordings were conducted at 24°C. The intracellular electrode solution contained (in mM): K-gluconate 125, KCl 1, MgCl2 2, EGTA 1, and K-HEPES 10 (pH 7.3). In some experiments, the intracellular solution did not contain MgCl2 and EGTA. The electrophysiological data were recorded using: a HEKA-9/2 patch-clamp amplifier (HEKA Elektronik, Lambrecht, Germany) at UT Memphis; or MultiClamp-700B patch-clamp amplifier at SIU Springfield (Molecular Devices, Sunyvale, CA, USA). The seal resistance was > 2 GΩ; the access resistance was between 10 and 30 MΩ, and typically, was not compensated. Patches with access resistances higher than 30 MΩ were corrected by applying an additional negative suction or discarded. Data were sampled at 10 kHz or 20 kHz and filtered at 3.33 kHz or 6.67 kHz, respectively. Occasionally, low frequency electrical noise (60 Hz) was filtered out during off-line analysis.
Syringe pumps Pump-33 (HARVARD Apparatus, Holliston, MA, USA) at UT Memphis; or Genie Plus (Kent Scientific Corporation, Torrington, CT, USA) at SIU Springfield were used to add experimental drugs to the ACSF just before they entered the recording chamber. The final drug concentrations in the bath were arithmetically calculated based on the known concentrations of stock solutions and adjustable rates of all pumps. Peristaltic and syringe pumps used in this study provide exceptional stability of fluid flow and are routinely calibrated. The high degree of stability of flows generated by pumps translates into a high stability of final drug concentrations that are easily calculated. Solutions are mixed in a ∼0.3 ml reservoir, ∼10 s before entering the first “entrance” chamber (∼ 1 ml), where additional mixing occurs. After the “entrance” chamber, solutions enter the main recording chamber (∼ 2 ml), where the final mixing and application takes place. This 3-step process guarantees a complete mixture of solutions.
A picospritzer (Parker Hannifin Instrumentation, Cleveland, OH, USA) was used for agonist applications via a pipette (4-7 MΩ) identical to that used for patch-clamp recordings. The bath solution always contained 0.1-0.5 µM tetrodotoxin (TTX) to block Na+ channels. The absence of sodium action potentials was routinely tested in the current-clamp mode by applying 10-50 pA depolarizing steps from the resting potential of ∼ -60 mV.
In some experiments, an iontophoretic pump (Union-40, Kation Scientific, Minneapolis, MN, USA) was used to apply nicotine or RJR-2403. The retention and ejection currents were set around -20 nA and +180 nA, respectively. The output current was monitored during experiments via a built-in current monitor. Iontophoretic pipettes that did not provide specified current amplitudes were replaced. Patch-clamp pipettes with resistances 20-60 MΩ (if filled with the K-gluconate-based internal solution) were used for iontophoretic applications of nicotine (100 mM) and RJR-2403 (50 mM). Application pipettes for picospritzer and iontophoretic applications were mounted on two Sutter 285-3 manipulators allowing applications of at least two nicotinic agents to the same neuron during the course of a single experiment. In experiments with iontophoretic applications of nicotine, 4 μM DHβE was also added to the ACSF. In all tests involving iontophoresis, antagonists (i.e., TTX, MLA and DHβE) were added directly to the ACSF therefore, syringe pumps were not used.
Biocytin labeling
In some experiments, the recording pipette contained 0.2% biocytin (Sigma Chemical Co., St. Louis, MO) and the neurons were filled by diffusion during patch recording. Slices were fixed in 4% buffered paraformaldehyde for at least 24 hours. After fixation, slices were rinsed in phosphate-buffered saline for 15 min and then incubated for 4 hours with a Vectastain ABC kit (Vector Laboratories, Burlingame, CA) at room temperature. The avidin/biotin was diluted 1:100 in PBS containing 0.5% Triton-X100 (Sigma). The slices were rinsed for 15 minutes and reacted with 3,3′diaminobenzidine (DAB) from a DAB substrate kit (Vector). After rinsing, the sections were mounted on gelatin-coated slides, dried overnight, and coverslipped using 50% glycerol. Tissue shrinkage was minimal, as the sections were not dehydrated in alcohols nor cleared in xylenes. Nineteen cNTS neurons were successfully filled to allow characterization and 3D-reconstruction using the NeuroLucida system (MicroBrightField, Inc., Williston, VT). Neuronal somal area, form factor and the number of primary branches have been measured. Form factor (ff) measures the roundness of somata, where 1.0 indicates a circle and 0.0 indicates a line (ff = 4 π area/perimeter2, where π ≈ 3.1416).
Data analysis
The liquid junction potential (VLJ ∼13 mV, UT Memphis; and ∼12.9 mV, SIU Springfield) was determined using a method described in detail previously (Neher 1992). The data presented are nominal, i.e., have not been corrected for VLJ. Data are presented as means ±SD. Statistical analysis was performed using Mathematica 2.2.3 software (Wolfram Research, Inc., USA). In brief, let xi and yi be the observed and expected numbers of Type i cNTS neurons, respectively. Then, the value is distributed according to the chi-square distribution, . The p-value (the probability that neurons are randomly distributed among Types I-IV) is then calculated as an integral: , representing the area under the tail χ2 < λ < ∞ of the density function f (λ,k).
Results
Horizontal brainstem sections containing the cNTS region (Figure 1A) were used in patch-clamp experiments to investigate pre-synaptic and somatic/dendritic responses of cNTS neurons (Figure 1B) to brief exposures to nicotine. Sodium ion channels were blocked by 0.1-0.5 μM TTX constantly present in the ACSF. The absence of sodium action potentials was routinely tested in current-clamp by applying 10-50 pA depolarizing current steps (not shown). A total of 232 cNTS neurons were investigated in this study.
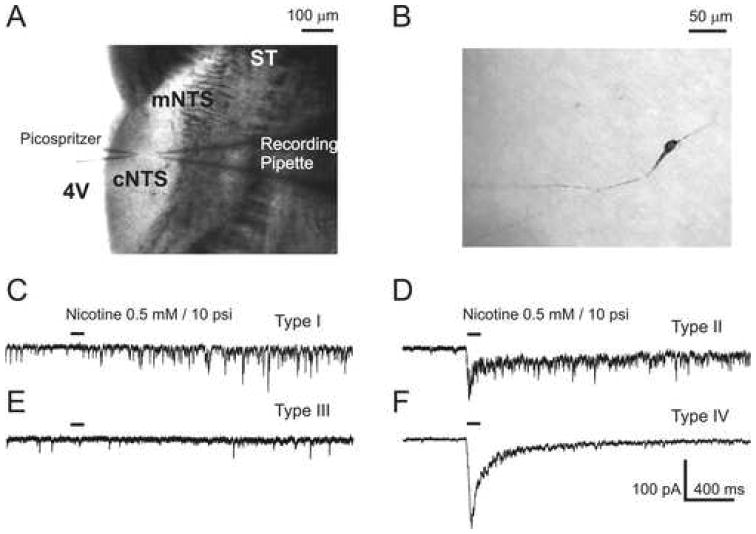
Figure 1. Location and neuronal types of cNTS neurons.
A) An infrared image of the recording and application picospritzer pipettes during a patch-clamp experiment. Horizontal brainstem section (∼250 μm thickness) were used in all experiments. The approximate location of cNTS region relative to the solitary tract (ST), the fourth ventricle (4V) and the medial NTS region is indicated. B) An example of a Type II cNTS neuron labeled with biocytin. Morphological analysis gave the following parameters: somal area, 199.8 μm2; form factor, 0.35; number of branches, 2.0 C-F) Typical responses (C, D, F)), or lack thereof (E), of cNTS neurons to 0.5 mM nicotine applied via a picospritzer (100 ms duration, ∼10 psi pressure, ∼10 μm application distance). Four clearly distinct types of cNTS neuron have been identified (see text for details).
The effect of mEPSC facilitation
Brief picospritzer applications of nicotine (0.1-0.5 mM, 50-400 ms duration, 10 psi pressure, 10 μm application distance) to cNTS neurons held at the membrane voltage of -60 mV in voltage-clamp were found to activate pre-synaptic and somatic/dendritic nAChRs. The activation of pre-synaptic nAChRs was detected by facilitation of miniature excitatory post-synaptic currents (mEPSCs) (Figures 1C-D). Usually, this facilitation lasted for less than a minute, but it could be repeated as often as every minute. However, the mEPSC facilitation has been found to be most reliable when at least three minutes between applications were reserved to allow for a complete recovery from desensitization and/or residual inhibition of pre-synaptic nAChRs by nicotine (Papke et al., 2000).
The activation of somatic/dendritic nAChRs by nicotine generated whole-cell currents (Figures 1D and 1F). Based on responses of 200 cNTS neurons to nicotine, four neuronal types have been defined: Type I – only pre-synaptic, no somatic/dendritic responses (17%, n=34, Figure 1C); Type II – pre-synaptic and somatic/dendritic responses (13%, n=25, Figure 1D); Type III – no pre-synaptic, no somatic/dendritic responses (34%, n=69, Figure 1E); and Type IV – no pre-synaptic, only somatic/dendritic responses (36%, n=72, Figure 1F). Pre-synaptic and somatic/dendritic effects of nicotine were found to be randomly distributed among cNTS neurons tested (χ2 = 0.72, df = 3, p > 0.87, n=200). In addition, 5 Type I and 14 Type II neurons were successfully reconstructed from biocytin fillings and their morphology was analyzed using the NeuroLucida system (Methods). The differences among mean values of the main morphological parameters of Type I and Type II neurons were found to be statistically insignificant. The mean somal areas were: 154.8±39.8 μm2 (n=5, Type I) and 130.2±124.4 μm2 (n=12, Type II), p>0.67; the mean form factors (ff) were: 0.7±0.16 (n=5, Type I) and 0.58±0.14 (n=14, Type II), p>0.15; the mean numbers of primary branches were: 2.8±0.8 (n=5, Type I) and 2.6±1.2 (n=14, Type II), p>0.73. When Type I-II neurons were analyzed together, the mean values were found to be: somal area, 137.4±105.7 μm2 (n=17); form factor, 0.62±0.15 (n=19); and number of branches, 2.6±1.2 (n=19). The morphologies of Type III-IV cNTS neurons have not been analyzed in the present study.
In some experiments, a leak of nicotine out of picospritzer application pipettes may have desensitized some pre- and/or postsynaptic nAChRs, causing an underestimation of the number of Type I and II neurons. This possibility, however, has been addressed in Iontophoretic agonist application.
Somatic/dendritic α7 nAChRs
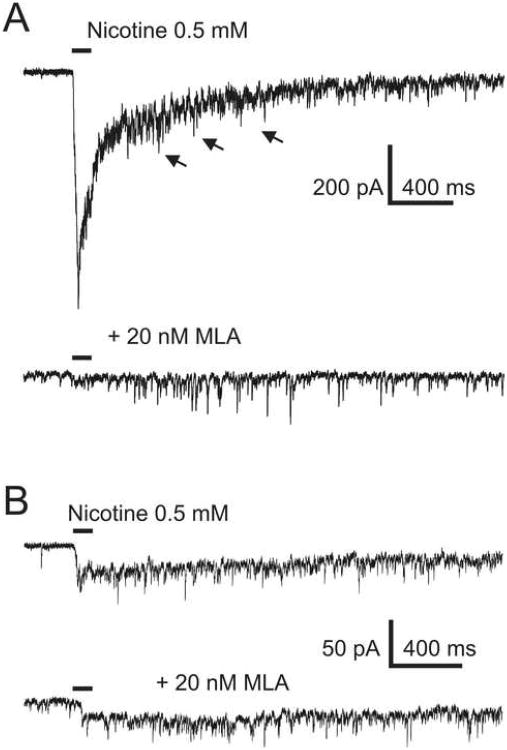
Type II currents were detected in 25 out of 200 cNTS neurons that were used for statistical analysis. In 7 of those 25 experiments, 20 nM MLA (methyllycaconitine, a selective antagonist of α7 nAChRs) was applied to the bath to test for the presence of α7 nAChRs. Somatic/dendritic nAChR-mediated responses were blocked in 5 of those 7 experiments (Figure 2A); in the remaining 2 experiments, the block by 20 nM MLA was incomplete (Figure 2B). These results indicate the expression of both α7 and non-α7 somatic/dendritic nAChRs in cNTS neurons. To eliminate α7 nAChR-mediated somatic/dendritic responses, 20 nM MLA was constantly present in the ACSF in the majority of experiments in this study.
Figure 2. Pre-synaptic nAChRs are non-α7.
A) In some Type II cNTS neurons, somatic/dendritic responses to nicotine (top trace) were completely blocked by 20 nM MLA, a selective α7 nAChR antagonist. Pre-synaptic effects of nicotine were insensitive to MLA (bottom trace). B) In 2 Type II cNTS neurons, somatic/dendritic responses to nicotine were not completely blocked by 20 nM MLA and the MLA-insensitive current component was slower than that mediated by α7 nAChRs in the absence of MLA (compare with A)). In these 2 experiments, the background frequency of mEPSCs was insignificantly low.
Non-α7 nAChRs
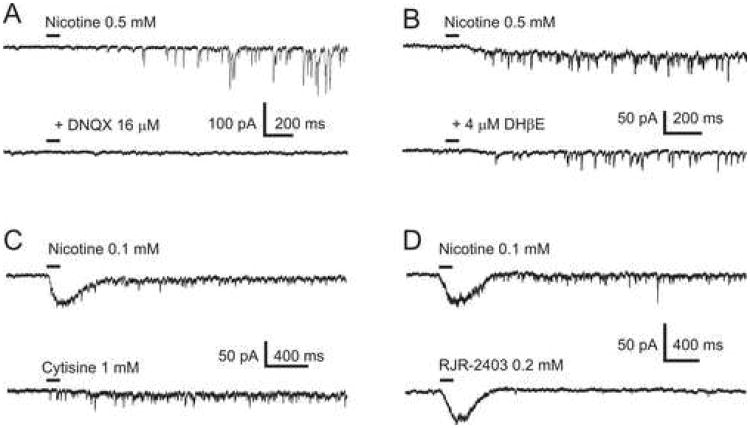
Miniature EPSCs were blocked by 16 μM DNQX (6,7-Dinitroquinoxaline-2,3-dione, a selective AMPA receptor blocker) applied to the bath, indicating that they were mediated by AMPA receptors (n=4, Figure 3A). The facilitation of mEPSCs by nicotine was resistant to 4 μM DHPE (dihydro-β-erythroidine, a putative selective antagonist of β2-containing (i.e., β2*) nAChRs, n=9, Figure 3B). In 5 of 9 experiments, 4 μM DHβE was added to the bath directly i.e., without using a syringe pump (Method). Cytisine, a nicotine-like alkaloid and a potent agonist of rat β4* nAChRs (Luetje and Patrick, 1991), produced similar effects of facilitation of mEPSCs (n=23, Figures 3C). Cytisine is also known to activate the rat α7 nAChR subtype (Stokes et al., 2004). In the presence of 20 nM MLA, applications of cytisine produced somatic/dendritic responses in numerous experiments (n=18, Figures 6, bottom traces). However, in 11 experiments, 1 mM cytisine failed to generate somatic/dendritic responses (Figure 3C, bottom trace). In 4 of those 11 experiments, applications of 0.1 mM nicotine to the same cNTS neurons did produce somatic/dendritic responses (Figure 3C, top trace). Therefore, some Type II cNTS neurons may express non-α7-non-β4 somatic/dendritic nAChRs, possibly β2*.
Figure 3. Pharmacological properties of nAChRs expressed in Type I and Type II cNTS neurons.
A) Miniature EPSCs were blocked by 16 μM DNQX indicating the involvement of AMPA receptors. B) Pre-synaptic effects were resistant to 4 μM DHβE applied to the bath indicating that presynaptic nAChRs are unlikely to be β2*. C) Pre-synaptic effects of 0.1 mM nicotine were reproduced by 1 mM cytisine applied to the same cNTS neuron. In some Type II neurons, 1 mM cytisine did not evoke somatic/dendritic responses (bottom trace). D) Picospritzer applications of RJR-2403 (0.2 mM) did not facilitate mEPSCs in any of the effect-positive neurons tested (n=11). The same neurons, however, exhibited facilitation of mEPSCs when 0.1 mM nicotine was applied (top trace) and hence, those neurons were defined as effect-positive.
Applications of RJR-2403 (0.2 mM; 10 psi), a potent agonist of α4β2 nAChRs (Papke et al., 2000) did not facilitate mEPSCs in effect-positive cNTS neurons (n=11; Figures 3D, bottom trace). The exact same pool of neurons exhibited facilitation of mEPSCs when nicotine (n=6; Figure 3D, top trace) or cytisine (n=5; not shown) were applied instead of RJR-2403 via a separate picospritzer pipette (and hence, these neurons were defined as effect-positive). These results indicate that a contribution of β2* nAChRs to the mEPSC facilitation by nicotine in effect-positive cNTS neurons is unlikely.
All pre-synaptic and somatic/dendritic nicotine-evoked effects were completely blocked by 10 μM mecamylamine, a broad spectrum non-selective blocker of nAChRs (n=9, not shown) confirming the involvement of nAChRs in the observed effects.
Quantal analysis
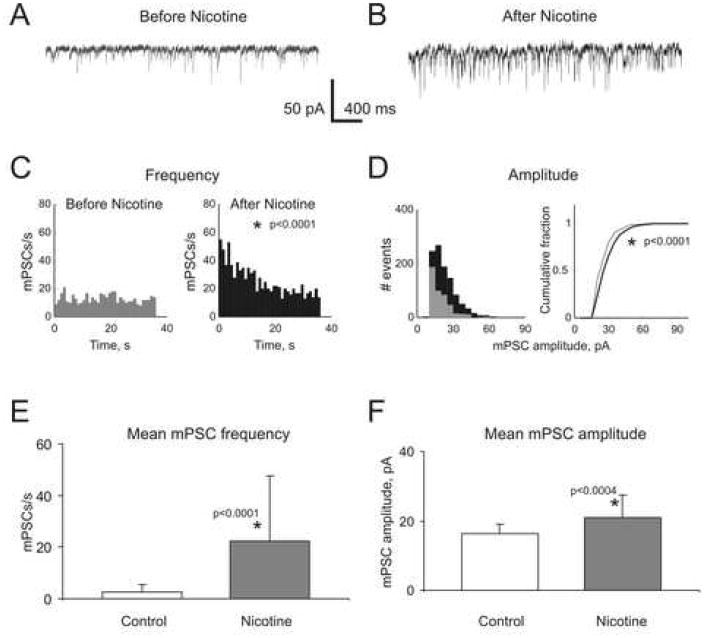
Thirty two effect-positive cNTS neurons (both Type I and II) recorded for at least 30 s before and after picospritzer applications of nicotine were used for a thorough statistical analysis. The constant presence of 0.2-0.5 μM TTX in these experiments eliminated sodium action potentials. The absence of sodium action potentials was routinely tested in current-clamp by applying 10-50 pA current steps (not shown). Therefore, the observed facilitation of mEPSC frequency by nicotine was likely produced by pre-synaptically located nAChRs. Surprisingly, however, in 68.8% of effect-positive cNTS neurons, statistically significant increases in mEPSC frequency (p < 0.0002, n=32, Figure 4A-C) were accompanied by statistically significant increases in mEPSC amplitudes (p < 0.003, n=22, Figure 4D). In the remaining 31.2% of effect-positive cNTS neurons, nicotine-mediated changes in the mean mEPSC amplitudes were not statistically significant (p > 0.4, n=10, not shown). When all 32 neurons were analyzed together, increases in both mEPSC frequency and amplitude were still statistically significant (p < 0.0001 and p < 0.0004, respectively, Figure 4E-F).
Figure 4. Quantal analysis.
A-B) Current traces from an effect-positive NTS neuron before and after nicotine application under control conditions. In 68.8% of analyzed effect-positive neurons, the increase in mEPSC frequency was accompanied by a statistically significant increase in the mean mEPSC amplitude (p=0.003±0.006, n=22). In the remaining 31.2% of neurons, nicotine-mediated changes in the mean mEPSC amplitudes were not statistically significant (p=0.40±0.23, n=10). C-D) Frequency and amplitude histograms built using quantal events recorded from the same neuron as those shown in A-B) before (gray) and after (black) nicotine application. E-F) Mean frequency and amplitude histograms built using data obtained from all 32 tested neurons. Increases in both mEPSC frequency and amplitudes were found to be statistically significant (p<0.0001 and p<0.0004, respectively).
Nicotine-mediated increases in the mEPSC frequency in effect-positive cNTS neurons were always transient and decayed to the background frequency observed before the application of nicotine (Figure 4C, right histogram). The decay time constants varied from neuron to neuron between 1.2 s and 81.2 s with the mean value of 10.8±15.6 s (n=32).
Iontophoretic agonist application
A leak of nicotine from a picospritzer pipette may have desensitized some pre- and/or postsynaptic nAChRs, for instance, high potency α4β2, and thus, potentially may have led to an underestimation of the number of effect-positive cNTS neurons and the types of nAChRs involved in mediating the effect. Therefore, in 8 experiments with effect-positive cNTS neurons, iontophoretic applications of nicotine (100 mM, n=4) or RJR-2403 (50 mM, n=4) were conducted in combination with picospritzer applications of 0.2 mM cytisine to determine whether the results are dependent on the method of agonist application. Cytisine applications were used to define cNTS neurons as effect-positive or -negative. In these experiments, 0.1-0.2 μM TTX and 20 nM MLA were always present in the ACSF. The absence of action potentials was tested in current-clamp by applying 10-50 pA current steps. In experiments with iontophoretic applications of nicotine, 4 μM DHβE was also added to the ACSF. In all tests involving iontophoresis, antagonists (i.e., TTX, MLA and DHβE) were added to the ACSF directly, i.e., syringe pumps were not used.
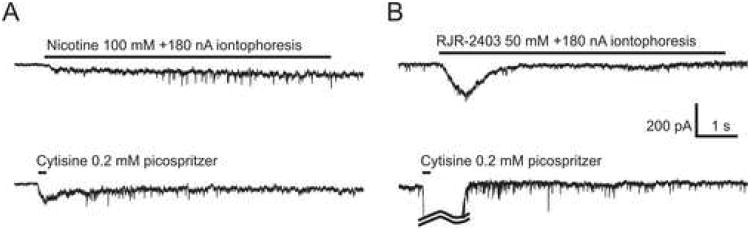
Iontophoretic applications of nicotine and picospritzer applications of cytisine facilitated mEPSCs in four effect-positive cNTS neurons (n=4, Figure 5A, top and bottom traces, respectively). In contrast, iontophoretic applications of RJR-2403 failed to facilitate mEPSCs in a different group of four effect-positive cNTS neurons (Figure 5B, top trace) that exhibited mEPSC facilitation to picospritzer applications of cytisine (n=4, Figure 5B, bottom trace).
Figure 5. Iontophoretic agonist application.
In some experiments, iontophoresis was employed instead of a picospritzer to eliminate a possibility of a leak of nicotine or RJR-2403 from application pipettes. A) Iontophoretic applications of nicotine (100 mM, +180 nA) facilitated the mEPSC frequency (top trace) in a neuron that also exhibited a facilitation of mEPSC frequency upon picospritzer applications of 0.2 mM cytisine (bottom trace). B) In a different experiment, iontophoretic applications of RJR-2403 (50 mM, +180 nA) did not facilitate the mEPSC frequency (top trace) in a neuron that exhibited such a facilitation upon picospritzer applications of 0.2 mM cytisine (bottom trace). The somatic/dendritic response to cytisine was truncated (curved lines).
In general, even in experiments where only picospritzer applications were used to deliver nicotinic agonists, the tips of application pipettes were only barely (<20 μm) inserted in the brain slice and in several experiments, where neurons were located just under the surface of the slice (<10 μm), applications were made with the tip positioned just above the investigated neuron (<10 μm) and completely outside of the brain slice. Moreover, in all experiments, application pipettes were always positioned behind the investigated neuron, relative to the perfusion flow. Therefore, in the event of a leak of nicotine from the application pipette, nicotine would have had to diffuse against perfusion flow to be able to desensitize the majority of pre-synaptic nAChRs that contributed to the effect. All of the above considerations suggest that a leak of nicotine from picospritzer application pipettes would have produced only a negligible influence, if any, on the presented results.
Discussion
The presented study reports that nicotine facilitates synaptic release of glutamate in a subpopulation (i.e., “effect-positive”) of cNTS neurons in the brainstem. Nicotinic AChRs that mediate these effects of nicotine are most likely located on pre-synaptic glutamatergic terminals and not somata of neighboring cNTS neurons, because experiments were conducted in the constant presence of 0.1-0.5 μM TTX to block sodium action potentials. The absence of action potentials was routinely tested in current-clamp by applying 10-50 pA current steps. The presented experimental results are consistent with the pre-synaptic expression of β4* nAChRs, likely α3β4*, in effect-positive cNTS neurons. This conclusion is based on the observations that the facilitation of mEPSCs by nicotine: 1) is not blocked by 4 μM DHβE (Figure 3B); 2) is not triggered by RJR-2403, a potent agonist of α4β2 nAChRs, applied either via a picospritzer (0.2 mM, Figures 3D) or iontophoresis (50 mM, +180 nA, Figure 5B); and 3) can be replicated by picospritzer applications of cytisine (0.2 mM, Figures 3C and 5A-B).
Applications of DHβE (4 μM) to the bath would be expected to block α3β2 (IC50 = 0.41), α4β2 (IC50 = 0.37) and α4β4 (IC50 = 0.19) receptors (Harvey et al., 1996). DHβE is slightly less effective for blocking α2β2 (IC50 =1.3 μM) and α2β4 (IC50 =2.3 μM) nAChRs (Harvey et al., 1996), but at 4 μM, DHβE would still be expected to significantly inhibit both of those receptor subtypes. In the experiments presented in this study, 4 μM DHβE failed to block mEPSC facilitation. Moreover, the α2 nAChR subunit has not been detected in the NTS area of the brainstem (Wada et al., 1989). Therefore, pre-synaptic effects of nicotine in cNTS neurons are unlikely to be mediated by α3β2, α4β2, α4β4, α2β2 and α2β4 nAChRs. On the other hand, the IC50 of DHβE for α3β4 is 23.1 μM (Harvey et al., 1996). Therefore, 4 μM DHβE would be expected to have only a minor inhibitory effect on α3β4 nAChR which is consistent with the presented observations (Figure 3B).
Cytisine is known to be at least 100-fold more effective in activation of β4* nAChRs than β2* nAChRs (Luetje and Patrick, 1991;Papke and Heinemann, 1994;Fenster et al., 1999). In the present study, brief applications of 0.2-1 mM cytisine via a picospritzer replicated pre-synaptic effects of nicotine (Figures 3C and 5A-B). In contrast, 0.2 mM RJR-2403 applied via a picospritzer (Figure 3D, bottom trace) or iontophoretically (Figure 5B, top trace) failed to elicit mEPSC facilitation. RJR-2403 is a relatively potent and effective agonist of a number of β2* and β4* nAChRs (Papke et al., 2000): α4β2 (EC50 =16 μM), α3β2 (EC50 =150 μM) and α4β4 (EC50 =50 μM), but not α3β4 (EC50 =347 μM) or α3β2α5 (EC50 =360 μM) (Papke, 2002;Papke et al., 2000). Moreover, RJR-2403 has a significantly lower efficacy for α3β4 than for other non-α7 nAChRs (Papke et al., 2000). Therefore, α4β2, α3β2 and α4β4 nAChRs may be safely excluded from the list of potential contributors to the observed facilitation of mEPSCs in cNTS neurons. In addition, α3β2α5 may be excluded because it is poorly activated by cytisine (Nelson et al., 2001). Taken together, these results are consistent with the pre-synaptic expression of β4* nAChRs, most likely α3β4*, that mediate facilitation of glutamatergic mEPSCs in a sub-population of cNTS neurons in the brainstem. The expression of somatic/dendritic nAChRs appears to be more heterogeneous and involves both α7 and non-α7 nAChRs (Figures 2, 3 and 5). However, in the present study somatic/dendritic effects of nicotine have not been studied in detail.
Four clearly distinct types of cNTS neuron have been identified on the bases of both pre-synaptic and somatic/dendritic responses to nicotine (Figure 1C-F). Statistical analysis conducted on the basis of the χ2 test statistic (Methods) determined that nAChR expression was distributed randomly between pre-synaptic and somatic/dendritic sites (χ2 = 0.72, df = 3, p > 0.87, n=200). Type I-II neurons were generally found to be small (somal area, 137.4±105.7 μm2, n=17), elongated (form factor, 0.62±0.15, n=19) with only 2-3 primary branches (number of branches, 2.6±1.2, n=19). Differences in the main morphological parameters of Type I-II neurons were found to be insignificant. However, Type I-II neurons were significantly smaller (p<0.017), more elongated (p<0.003) and had fewer branches (p<0.034) than neurons from the rostral NTS region analyzed previously using identical techniques (somal area, 204.3±106.9 μm2, form factor, 0.72±0.12, and number of branches, 3.15±0.93, (n=74); data were re-analyzed from (Uteshev and Smith, 2006)).
In 68.8% of effect-positive cNTS neurons, the increase in mEPSC frequency by nicotine was accompanied by an increase in mEPSC amplitudes despite the presence of 0.5 μM TTX. In the remaining 31.2% of effect-positive neurons, nicotine increased only the frequency of mEPSCs. This finding may indicate that two different mechanisms of facilitation of synaptic release of glutamate exist in cNTS neurons, or that two different populations of effect-positive neurons exist in the cNTS; or the presence of TTX-insensitive voltage-gated sodium channels in a sub-population of neurons that synapse with effect-positive cNTS neurons. A thorough comparison of the pharmacology, kinetics and, perhaps, calcium dependence of mEPSC facilitation may be necessary to resolve this uncertainty. Ultimately, understanding the effects of nicotine in cNTS may require a detailed investigation of mechanisms of potentiation of mEPSC amplitudes. Several general possibilities have been discussed in detail elsewhere and must be considered in future studies including multi-vesicular release (Gordon and Bains, 2005) and direct modulation of AMPA receptors by nicotine (Neff et al., 1998).
Although, β2* and α7 are the most common types of nAChRs in the CNS, mounting evidence supports the idea that functional β4* nAChRs are equally abundant, or even predominant in selected brain regions, and that their involvement in regulation of neuronal functions and neurotransmission may have important physiological consequences (Mulle et al., 1991;Fenster et al., 1997;Takeda et al., 2003;Dhar et al., 2000).
The observed diversity of nAChRs in the cNTS may be physiologically significant. The pharmacological properties of β2*, β4* and α7 nAChRs are quite different (Fenster et al., 1997;Luetje and Patrick, 1991;Gotti et al., 1997;Briggs and McKenna, 1998;Papke et al., 2000). For example, nicotine has been shown to cause significant residual inhibition in α4β2 and α7, but not α3β4 nAChRs (Papke et al., 2000). Micromolar concentrations of choline, a selective endogenous agonist of α7 nAChRs (Alkondon et al., 1997;Papke et al., 1996), have been shown to activate α7 nAChRs or reduce the responsiveness of α7 nAChRs to ACh (Uteshev et al., 2003;Alkondon and Albuquerque, 2006). Choline however, has been reported to inhibit β4* nAChRs expressed in Xenopus oocytes (Zwart and Vijverberg, 2000;Gonzalez-Rubio et al., 2006). Therefore, the effects of choline on pre-synaptic and somatic/dendritic nAChRs would be expected to be opposite. Also different are the biophysical properties of β2*, β4* and α7 nAChRs (Cachelin and Jaggi, 1991;Fenster et al., 1997;Gerzanich et al., 1994;Bertrand et al., 1992). For instance, the desensitization kinetic of α7 nAChRs is considerably faster than that of β2* and β4* nAChRs (Fenster et al., 1997). Therefore, pre-synaptic and somatic/dendritic nAChRs in cNTS neurons would be expected to respond to identical nicotinic stimuli differently.
Differences in pharmacological properties of pre-synaptic and somatic/dendritic nAChRs presents an opportunity to pharmacologically separate and individually investigate pre-synaptic and somatic/dendritic effects of nicotine in a brain region involved in regulation of cardiovascular and other visceral reflexes that is susceptible to nicotine toxicity (Mendelowitz, 1999). Differences in the subunit composition of nAChRs expressed in cNTS neurons pre-synaptically and somatic/dendritically may underlie different roles that these receptors play in regulation of behavioral and visceral reflexes and may reflect a specific targeting by endogenous nicotinic agents and nicotine.
Acknowledgments
Portions of these results were presented at the annual meetings of the Association for Chemoreception Sciences (Sarasota FL, April 2006) and the Society for Neuroscience (Atlanta, GA October 2006). We thank Drs. Andon Placzek and Carl Faingold for valuable criticism. This study was supported by the NIH grant DC000066 to D.V.S. and funds provided by the Department of Pharmacology of the Southern Illinois University School of Medicine to V.V.U.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alkondon M, Albuquerque EX. Subtype-specific inhibition of nicotinic acetylcholine receptors by choline: a regulatory pathway. J Pharmacol Exp Ther. 2006;318:268–275. doi: 10.1124/jpet.106.103135. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Rotler A, Hersh LB, Pickel VM. Localization of choline acetyltransferase in perikarya and dendrites within the nuclei of the solitary tracts. J Neurosci Res. 1988;20:279–290. doi: 10.1002/jnr.490200302. [DOI] [PubMed] [Google Scholar]
- Ashworth-Preece MA, Jarrott B, Lawrence AJ. Nicotinic acetylcholine receptor mediated modulation of evoked excitatory amino acid release in the nucleus tractus solitarius of the rat: evidence from in vivo microdialysis. Brain Res. 1998;806:287–291. doi: 10.1016/s0006-8993(98)00773-2. [DOI] [PubMed] [Google Scholar]
- Barry MA, Halsell CB, Whitehead MC. Organization of the nucleus of the solitary tract in the hamster: acetylcholinesterase, NADH dehydrogenase, and cytochrome oxidase histochemistry. Microsc Res Tech. 1993;26:231–244. doi: 10.1002/jemt.1070260306. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Devillers-Thiery A, Revah F, Galzi JL, Hussy N, Mulle C, Bertrand S, Ballivet M, Changeux JP. Unconventional pharmacology of a neuronal nicotinic receptor mutated in the channel domain. Proc Natl Acad Sci U S A. 1992;89:1261–1265. doi: 10.1073/pnas.89.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscan P, Pickering AE, Paton JF. The nucleus of the solitary tract: an integrating station for nociceptive and cardiorespiratory afferents. Exp Physiol. 2002;87:259–266. doi: 10.1113/eph8702353. [DOI] [PubMed] [Google Scholar]
- Briggs CA, McKenna DG. Activation and inhibition of the human alpha7 nicotinic acetylcholine receptor by agonists. Neuropharmacology. 1998;37:1095–1102. doi: 10.1016/s0028-3908(98)00110-5. [DOI] [PubMed] [Google Scholar]
- Cachelin AB, Jaggi R. Beta subunits determine the time course of desensitization in rat alpha 3 neuronal nicotinic acetylcholine receptors. Pflugers Arch. 1991;419:579–582. doi: 10.1007/BF00370298. [DOI] [PubMed] [Google Scholar]
- Contreras RJ, Beckstead RM, Norgren R. The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: an autoradiographic study in the rat. J Auton Nerv Syst. 1982;6:303–322. doi: 10.1016/0165-1838(82)90003-0. [DOI] [PubMed] [Google Scholar]
- Contreras RJ, Gomez MM, Norgren R. Central origins of cranial nerve parasympathetic neurons in the rat. J Comp Neurol. 1980;190:373–394. doi: 10.1002/cne.901900211. [DOI] [PubMed] [Google Scholar]
- Dhar S, Nagy F, McIntosh JM, Sapru HN. Receptor subtypes mediating depressor responses to microinjections of nicotine into medial NTS of the rat. Am J Physiol Regul Integr Comp Physiol. 2000;279:R132–R140. doi: 10.1152/ajpregu.2000.279.1.R132. [DOI] [PubMed] [Google Scholar]
- Ernsberger P, Arneric SP, Arango V, Reis DJ. Quantitative distribution of muscarinic receptors and choline acetyltransferase in rat medulla: examination of transmitter-receptor mismatch. Brain Res. 1988;452:336–344. doi: 10.1016/0006-8993(88)90038-8. [DOI] [PubMed] [Google Scholar]
- Farkas E, Jansen AS, Loewy AD. Periaqueductal gray matter projection to vagal preganglionic neurons and the nucleus tractus solitarius. Brain Res. 1997;764:257–261. doi: 10.1016/s0006-8993(97)00592-1. [DOI] [PubMed] [Google Scholar]
- Fenster CP, Rains MF, Noerager B, Quick MW, Lester RA. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J Neurosci. 1997;17:5747–5759. doi: 10.1523/JNEUROSCI.17-15-05747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RA. Upregulation of surface alpha4beta2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J Neurosci. 1999;19:4804–14. doi: 10.1523/JNEUROSCI.19-12-04804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzanich V, Anand R, Lindstrom J. Homomers of alpha 8 and alpha 7 subunits of nicotinic receptors exhibit similar channel but contrasting binding site properties. Mol Pharmacol. 1994;45:212–220. [PubMed] [Google Scholar]
- Gonzalez-Rubio JM, Rojo J, Tapia L, Maneu V, Mulet J, Valor LM, Criado M, Sala F, Garcia AG, Gandia L. Activation and blockade by choline of bovine alpha7 and alpha3beta4 nicotinic receptors expressed in oocytes. Eur J Pharmacol. 2006;535:53–60. doi: 10.1016/j.ejphar.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Bains JS. Noradrenaline triggers multivesicular release at glutamatergic synapses in the hypothalamus. J Neurosci. 2005;25:11385–11395. doi: 10.1523/JNEUROSCI.2378-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Fornasari D, Clementi F. Human neuronal nicotinic receptors. Prog Neurobiol. 1997;53:199–237. doi: 10.1016/s0301-0082(97)00034-8. [DOI] [PubMed] [Google Scholar]
- Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol. 1984;222:560–577. doi: 10.1002/cne.902220408. [DOI] [PubMed] [Google Scholar]
- Harvey SC, Maddox FN, Luetje CW. Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits. J Neurochem. 1996;67:1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- Helke CJ, Handelmann GE, Jacobowitz DM. Choline acetyltransferase activity in the nucleus tractus solitarius: regulation by the afferent vagus nerve. Brain Res Bull. 1983;10:433–436. doi: 10.1016/0361-9230(83)90139-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi RM, Palkovits M, Hruska RE, Rothschild R, Yamamura HI. Regional distribution of muscarinic cholinergic receptors in rat brain. Brain Res. 1978;154:13–23. doi: 10.1016/0006-8993(78)91047-8. [DOI] [PubMed] [Google Scholar]
- Kubo T, Misu Y. Changes in arterial blood pressure after microinjections of nicotine into the dorsal area of the medulla oblongata of the rat. Neuropharmacology. 1981;20:521–524. doi: 10.1016/0028-3908(81)90188-x. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Jarrott B. Neurochemical modulation of cardiovascular control in the nucleus tractus solitarius. Prog Neurobiol. 1996;48:21–53. doi: 10.1016/0301-0082(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Li CS, Smith DV. Glutamate receptor antagonists block gustatory afferent input to the nucleus of the solitary tract. J Neurophysiol. 1997;77:1514–1525. doi: 10.1152/jn.1997.77.3.1514. [DOI] [PubMed] [Google Scholar]
- Luetje CW, Patrick J. Both alpha- and beta-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J Neurosci. 1991;11:837–845. doi: 10.1523/JNEUROSCI.11-03-00837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley BE. Immunohistochemical localization of neuropeptides and neurotransmitters in the nucleus solitarius. Chem Senses. 1996;21:367–376. doi: 10.1093/chemse/21.3.367. [DOI] [PubMed] [Google Scholar]
- Maley BE, Seybold VS. Distribution of [3H]quinuclidinyl benzilate, [3H]nicotine, and [125I]alpha-bungarotoxin binding sites in the nucleus tractus solitarii of the cat. J Comp Neurol. 1993;327:194–204. doi: 10.1002/cne.903270203. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D. Advances in Parasympathetic Control of Heart Rate and Cardiac Function. News Physiol Sci. 1999;14:155–161. doi: 10.1152/physiologyonline.1999.14.4.155. [DOI] [PubMed] [Google Scholar]
- Mulle C, Vidal C, Benoit P, Changeux JP. Existence of different subtypes of nicotinic acetylcholine receptors in the rat habenulo-interpeduncular system. J Neurosci. 1991;11:2588–2597. doi: 10.1523/JNEUROSCI.11-08-02588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff RA, Humphrey J, Mihalevich M, Mendelowitz D. Nicotine enhances presynaptic and postsynaptic glutamatergic neurotransmission to activate cardiac parasympathetic neurons. Circ Res. 1998;83:1241–1247. doi: 10.1161/01.res.83.12.1241. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Wang F, Kuryatov A, Choi CH, Gerzanich V, Lindstrom J. Functional properties of human nicotinic AChRs expressed by IMR-32 neuroblastoma cells resemble those of alpha3beta4 AChRs expressed in permanently transfected HEK cells. J Gen Physiol. 2001;118:563–582. doi: 10.1085/jgp.118.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL. Enhanced inhibition of a mutant neuronal nicotinic acetylcholine receptor by agonists: protection of function by (E)-N-methyl-4-(3-pyridinyl)-3-butene-1-amine (TC-2403) J Pharmacol Exp Ther. 2002;301:765–73. doi: 10.1124/jpet.301.2.765. [DOI] [PubMed] [Google Scholar]
- Papke RL, Bencherif M, Lippiello P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the alpha 7 subtype. Neurosci Lett. 1996;213:201–204. doi: 10.1016/0304-3940(96)12889-5. [DOI] [PubMed] [Google Scholar]
- Papke RL, Heinemann SF. Partial agonist properties of cytisine on neuronal nicotinic receptors containing the beta 2 subunit. Mol Pharmacol. 1994;45:142–149. [PubMed] [Google Scholar]
- Papke RL, Webster JC, Lippiello PM, Bencherif M, Francis MM. The activation and inhibition of human nAChR by RJR-2403 indicate a selectivity for the α4β2 receptor subtype. J Neurochem. 2000;75:204–216. doi: 10.1046/j.1471-4159.2000.0750204.x. [DOI] [PubMed] [Google Scholar]
- Reis DJ, Granata AR, Perrone MH, Talman WT. Evidence that glutamic acid is the neurotransmitter of baroreceptor afferent terminating in the nucleus tractus solitarius (NTS) J Auton Nerv Syst. 1981;3:321–334. doi: 10.1016/0165-1838(81)90073-4. [DOI] [PubMed] [Google Scholar]
- Shihara M, Hori N, Hirooka Y, Eshima K, Akaike N, Takeshita A. Cholinergic systems in the nucleus of the solitary tract of rats. Am J Physiol. 1999;276:R1141–R1148. doi: 10.1152/ajpregu.1999.276.4.R1141. [DOI] [PubMed] [Google Scholar]
- Stokes C, Papke JK, Horenstein NA, Kem WR, McCormack TJ, Papke RL. The structural basis for GTS-21 selectivity between human and rat nicotinic alpha7 receptors. Mol Pharmacol. 2004;66:14–24. doi: 10.1124/mol.66.1.14. [DOI] [PubMed] [Google Scholar]
- Sun Y, Qin C, Foreman RD, Chen JD. Intestinal electric stimulation modulates neuronal activity in the nucleus of the solitary tract in rats. Neurosci Lett. 2005;385:64–69. doi: 10.1016/j.neulet.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Takeda D, Nakatsuka T, Papke R, Gu JG. Modulation of inhibitory synaptic activity by a non-alpha4beta2, non-alpha7 subtype of nicotinic receptors in the substantia gelatinosa of adult rat spinal cord. Pain. 2003;101:13–23. doi: 10.1016/s0304-3959(02)00074-x. [DOI] [PubMed] [Google Scholar]
- Talman WT, Lewis SJ. Altered cardiovascular responses to glutamate and acetylcholine microinjected into the nucleus tractus solitarii of the SHR. Clin Exp Hypertens A. 1991;13:661–668. doi: 10.3109/10641969109042069. [DOI] [PubMed] [Google Scholar]
- Talman WT, Perrone MH, Reis DJ. Evidence for L-glutamate as the neurotransmitter of baroreceptor afferent nerve fibers. Science. 1980;209:813–815. doi: 10.1126/science.6105709. [DOI] [PubMed] [Google Scholar]
- Torvik A. Afferent connections to the sensory trigeminal nuclei, the nucleus of the solitary tract and adjacent structures; an experimental study in the rat. J Comp Neurol. 1956;106:51–141. doi: 10.1002/cne.901060104. [DOI] [PubMed] [Google Scholar]
- Ueno S, Kakehata S, Akaike N. Nicotinic acetylcholine receptor in dissociated rat nucleus tractus solitarii neurons. Neurosci Lett. 1993;149:15–18. doi: 10.1016/0304-3940(93)90336-j. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Meyer EM, Papke RL. Regulation of neuronal function by choline and 4OH-GTS-21 through alpha 7 nicotinic receptors. Journal of Neurophysiology. 2003;89:1797–1806. doi: 10.1152/jn.00943.2002. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Smith DV. Cholinergic modulation of neurons in the gustatory region of the nucleus of the solitary tract. Brain Res. 2006 doi: 10.1016/j.brainres.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha2, alpha3, alpha4, and beta2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wang L, Bradley RM. In vitro study of afferent synaptic transmission in the rostral gustatory zone of the rat nucleus of the solitary tract. Brain Res. 1995;702:188–198. doi: 10.1016/0006-8993(95)01062-6. [DOI] [PubMed] [Google Scholar]
- Wang L, Cardin S, Martinez V, Tache Y, Lloyd KC. Duodenal loading with glucose induces fos expression in rat brain: selective blockade by devazepide. Am J Physiol. 1999;277:R667–R674. doi: 10.1152/ajpregu.1999.277.3.R667. [DOI] [PubMed] [Google Scholar]
- Zittel TT, De Giorgio R, Sternini C, Raybould HE. Fos protein expression in the nucleus of the solitary tract in response to intestinal nutrients in awake rats. Brain Res. 1994;663:266–270. doi: 10.1016/0006-8993(94)91272-6. [DOI] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HP. Potentiation and inhibition of neuronal alpha4beta4 nicotinic acetylcholine receptors by choline. Eur J Pharmacol. 2000;393:209–214. doi: 10.1016/s0014-2999(00)00002-9. [DOI] [PubMed] [Google Scholar]