Abstract
Velaglucerase alfa is a glucocerebrosidase produced by gene activation technology in a human fibroblast cell line (HT-1080), and is indicated as an enzyme replacement therapy (ERT) for the treatment of Gaucher disease type 1 (GD1). This multicenter, open-label, 12-month study examined the safety and efficacy of velaglucerase alfa in patients with GD1 previously receiving imiglucerase. Eligible patients, ≥2 years old and clinically stable on imiglucerase therapy, were switched to velaglucerase alfa at a dose equal to their prior imiglucerase dose. Infusion durations were 1 hour every other week. Forty patients received velaglucerase alfa (18 male, 22 female; four previously splenectomized; age range 9–71 years). Velaglucerase alfa was generally well tolerated with most adverse events (AEs) of mild or moderate severity. The three most frequently reported AEs were headache (12 of 40 patients), arthralgia (nine of 40 patients), and nasopharyngitis (eight of 40 patients). No patients developed antibodies to velaglucerase alfa. There was one serious AE considered treatment-related: a Grade 2 anaphylactoid reaction within 30 minutes of the first infusion. The patient withdrew; this was the only AE-related withdrawal. Hemoglobin concentrations, platelet counts, and spleen and liver volumes remained stable through 12 months. In conclusion, adult and pediatric patients with GD1, previously treated with imiglucerase, successfully transitioned to velaglucerase alfa, which was generally well tolerated and demonstrated efficacy over 12-months’ treatment consistent with that observed in the velaglucerase alfa Phase 3 clinical trial program.
Keywords: red cells, anemia-clinical, enzyme disorders, type 1 Gaucher disease, velaglucerase alfa, imiglucerase
Introduction
Gaucher disease type 1 (GD1) is an autosomal recessive disorder, in which defective function of the lysosomal enzyme acid β-glucosidase (EC 4.2.1.45, GCase) leads to progressive accumulation of glucosylceramide within the macrophages of various tissues and organs (typically liver, spleen, and bone marrow). As a consequence, clinical signs of hepatosplenomegaly, anemia, thrombocytopenia, and skeletal involvement develop [1]. For the past 15 years, the standard of care for patients with symptomatic GD1 disease has been enzyme replacement therapy (ERT) with the recombinant GCase, imiglucerase (Cerezyme®, Genzyme Corporation, Cambridge, MA, USA), a human GCase with a point mutation compared to the native sequence that is secreted from overexpression in transduced Chinese hamster ovary (CHO) cells [2].
Velaglucerase alfa (VPRIV®, Shire Human Genetic Therapies, Inc. [Shire HGT], Lexington, MA, USA) is a replacement enzyme produced by gene activation in a human fibroblast cell line (HT-1080). This approach leads to overexpression of the endogenous GCase-encoding gene, GBA1, and hence, a GCase that has the same amino acid sequence as that of wild-type human GCase [3]. Velaglucerase alfa was approved in 2010 by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for long-term ERT for treatment of pediatric and adult patients with GD1.
The safety and efficacy of velaglucerase alfa in GD1 patients naïve to ERT has been studied in three separate Phase 3 clinical trials (www.clinicaltrials.gov identifiers, NCT00430625, NCT00553631, NCT00391625) [4]. Here, the results of switching GD1 patients from imiglucerase to velaglucerase alfa infusions are presented, to identify its continuing efficacy and safety in a population previously treated with imiglucerase.
Methods
Overview and ethics
This study, registered as NCT00478647 (www.clinicaltrials.gov), was conducted between July 2007 and June 2009 at 15 centers in Israel, Poland, Spain, the UK, and the USA. The study was sponsored by Shire HGT. Access to the primary clinical trial data was available to all authors. The study was conducted in compliance with the principles of the Declaration of Helsinki, local country regulations, and the International Conference on Harmonization Good Clinical Practice E6 guidelines. The independent ethics committee or internal review board at each study institution approved the protocol. The participants or their parent(s) or legal guardian(s) provided written informed consent or assent in minors before commencing any procedures.
Participants
Eligible participants were males or females at age ≥2 years with diagnosed GD1 (deficient GCase activity in leukocytes with additional genotype analysis), who had received uninterrupted treatment with imiglucerase for a minimum of 30 consecutive months prior to commencing the study. The previous imiglucerase dose had to be between ~15 and 60 U/kg every other week (based on recorded values for the patient’s weight), and the same dose was used for infusions in the 6 months before enrollment.
The main exclusion criteria were: 1) both hemoglobin concentration ≤10 g/dL and platelet count ≤80 × 109/L as assessed by the site’s local laboratory; 2) unstable hemoglobin concentration (exceeding a range of ±1 g/dL of the screening value) or platelet count (exceeding ±20% of the screening value) during the 6 months before screening as assessed by the site’s local laboratory; 3) diagnosed with (or were suspected of having) type 2 or 3 GD; 4) sensitized to imiglucerase as evidenced by an anaphylactic reaction; 5) on intermittent treatment with imiglucerase or had received miglustat in the 6 months prior to study entry; or 6) diagnosed with radiologically-confirmed active, clinically significant splenic infarction or worsening bone necrosis in the 12 months prior to screening. Other exclusion criteria included treatment with any investigational drug or device within 30 days prior to study entry; positive test for HIV, hepatitis B or C; non–GD-related anemia at screening; or any significant comorbidity that could affect study data.
Pregnant or lactating women were excluded. Women of child-bearing potential and men were required to use a medically acceptable method of contraception throughout study participation, and men were to report pregnancy of a partner.
Trial design
This 12-month, multicenter, Phase 2/3, open-label study was designed to evaluate the safety of intravenous velaglucerase alfa in patients with GD1 previously treated with imiglucerase. In a screening phase, patients who provided written informed consent were evaluated for study eligibility by analysis of hemoglobin concentration and platelet count by the site’s local laboratory. Patients who met eligibility criteria had baseline safety and efficacy evaluations within 14 days of the screening visit. Eligible participants were assigned to receive open-label velaglucerase alfa at a dose equal to that of their previously prescribed imiglucerase dose. The protocol specified that velaglucerase alfa was to be administered as a continuous 60-minute intravenous infusion, irrespective of the previous infusion time used for imiglucerase, and was given every other week for 51 weeks for a total of 26 infusions. There was no wash-out period, and patients received their first dose of velaglucerase alfa 14–30 days after their last imiglucerase dose.
The first three infusions for each patient were administered at the clinical site, after which patients who had not experienced a drug-related serious adverse event (AE) or an infusion-related AE were eligible to receive subsequent infusions at home by qualified medical personnel, per the discretion and direction of the investigator.
Patients were monitored throughout the treatment period for changes in clinical parameters and the investigators had the option of increasing the patient’s dose by 15 U/kg if a clinically significant deterioration occurred in two or more of the following four criteria over two consecutive evaluations: 1) a decrease from baseline in hemoglobin concentration of >1 g/dL; 2) a decrease from baseline in platelet count of >20%; 3) an increase in liver volume as indicated by organ palpation and confirmed to be >15% relative to baseline as measured by magnetic resonance imaging (MRI); or 4) an increase in spleen volume as indicated by organ palpation and confirmed to be >15% relative to baseline as measured by MRI. If the clinical parameters did not return to baseline values within 3 months, the investigator had the option of increasing the dose by additional increments of 15 U/kg. Since the study design did not allow a dose greater than 60 U/kg, no dose increase was offered to patients already receiving 60 U/kg. If the patient’s disease failed to respond at a maximum dose of 60 U/kg, the patient could be withdrawn, based on the investigator’s clinical judgment.
Patients who completed this study could elect to enroll in a subsequent long-term open-label clinical trial, with continuous velaglucerase alfa treatment across the two studies. Patients who did not elect to enroll in the long-term extension, or who did not complete the study, were monitored for a further 30 days after their final infusion for AEs.
Safety assessments
Safety was evaluated every other week (from first infusion through 30 days after the last infusion) through assessment of AEs and measurement of vital signs. Treatment-emergent AEs, defined as AEs that occurred on or after the time of the first infusion, were coded using the MedDRA coding dictionary version 9.0 and summarized by system organ class and preferred term. Other safety assessments included physical examination and electrocardiogram at baseline and Weeks 13, 25, 37, and 51, and clinical laboratory assessments (safety hematology, coagulation, serum chemistry, and urinalysis) at baseline and Weeks 13, 25, 37, 51, and 53.
Shire HGT’s Bioanalytics department assessed serum anti-imiglucerase antibodies at baseline, and anti-velaglucerase alfa antibodies at baseline and Weeks 7, 13, 19, 25, 31, 37, 45, and 51. Samples were screened for the presence of anti-drug antibodies using an electrochemiluminescence assay; samples screened positive were further analyzed using an enzymatic activity neutralizing antibody assay. These methods were described previously by Séllos-Moura et al [5].
Efficacy
Blood samples were collected to determine hemoglobin concentration and platelet counts (screening, baseline, Weeks 7, 13, 19, 25, 31, 37, 45, 51, and 53) at a centralized laboratory. Plasma chitotriosidase activity and CC chemokine ligand 18 (CCL18) levels (baseline, Weeks 13, 25, 37, 51, and 53) were analyzed by J.M.F.G. Aerts (Academic Medical Center, Amsterdam, The Netherlands), using the method of Aguilera et al [6,7] and a modification of the method of Boot et al [8], respectively. CCL18 was assessed by a solid-phase, two-site time-resolved fluoroimmunometric assay (DELFIA) using a mouse anti-human CCL18 monoclonal capture antibody and biotinylated goat anti-human CCL18 polyclonal detection antibody [8].
Abdominal MRIs for quantification of liver and spleen volumes were performed at the trial sites at baseline, Week 25, and Week 51. Images were collected and sent to a single independent reviewer at Biomedical Systems (St Louis, MO, USA) who remained blinded to the study dose and the order in which the images were taken.
Statistical analysis
The sample size of 40 patients was selected to comply with an FDA recommendation and was not based on statistical considerations.
Data were analyzed using SAS® software (version 9.1) by the Biometrics department at Shire HGT. The primary objective was to evaluate the safety of every other week dosing of velaglucerase alfa in patients with GD1 who were previously treated with imiglucerase. The number and proportion of patients experiencing treatment-emergent AEs were tabulated overall and by dose group. Dose groups were determined by taking an average across all infusions for a particular patient to account for minor dosing interval variance. Dose groups consisted of 15 U/kg (≤22.5 U/kg), 30 U/kg (>22.5 U/kg but ≤37.5 U/kg), 45 U/kg (>37.5 U/kg but ≤52.5 U/kg), and 60 U/kg (>52.5 U/kg) velaglucerase alfa. No formal statistical tests were performed on safety results.
The four secondary efficacy endpoints were the absolute change from baseline to 12 months in hemoglobin concentration and the percentage change from baseline to 12 months in platelet count, and spleen and liver volumes normalized by body weight. The pre-specified hypotheses were that the mean changes from baseline (that is, at the end of imiglucerase treatment) to 12 months remained within the pre-specified clinically significant cutoffs: within 1 g/dL for hemoglobin concentration, within 20% for platelet count, and within 15% for spleen and liver volumes. A two-sided 90% confidence interval (CI) for the change from baseline for each parameter was used to test the pre-specified hypotheses. If the two-sided 90% CI fell within the corresponding pre-specified clinically meaningful range, the parameter was considered no different from baseline to the end of 12 months of velaglucerase alfa exposure.
All analyses were performed on an intent-to-treat (ITT) basis, defined as all patients who received at least one full or partial velaglucerase alfa infusion. For hemoglobin concentrations and platelet counts, baseline was defined as the average of the screening and baseline values. If only one of these values was available, it was used as baseline. Where values were missing for the four secondary efficacy endpoints, a pre-specified imputation strategy was applied. After applying last observation carried forward for post-baseline measurements, if data were still missing (including at baseline), the median value in the corresponding age group (2–17 years; ≥18 years) was used. Sensitivity analyses were performed on each of the four secondary efficacy endpoints excluding patients with either a missing baseline or Month 12 value.
Data were summarized separately at 3-month follow-up time points. Mean within-patient absolute changes and within-patient percentage changes from baseline were presented for hemoglobin concentrations and platelet counts, and mean within-patient percentage changes were presented for plasma biomarkers and organ volumes normalized to body weight. Corresponding 90% CI were provided to assist interpretation.
The evaluations of changes from baseline to Month 12 in plasma chitotriosidase activity and CCL18 levels were tertiary objectives and were considered exploratory. Patients deficient in chitotriosidase were excluded from the analyses of chitotriosidase activity.
Results
Patient characteristics and disposition
Of the 41 participants enrolled in the study, 40 (98%) received at least one full or partial dose of study drug and were included in the ITT analysis, and 38 patients (93%) completed the study (on-line supporting information Fig. A). One participant withdrew from the study prior to receiving study drug and two participants discontinued: one because of an anaphylactoid reaction during the first infusion with velaglucerase alfa (patient number 33); and one at Week 31 for personal reasons (patient number 14). All 38 patients who completed the study have enrolled in the study extension phase (not reported here).
The summary of patient characteristics at baseline is shown in Table I, with individual patient data in Table A of the on-line supporting information. Approximately a quarter of the patients were younger than 18 years. The median period of prior imiglucerase use was 67 months; a protocol exception was allowed for one patient (number 31), enabling him to participate after having had only 22 consecutive months of previous treatment with imiglucerase. Although all patients had stable hemoglobin concentration and platelet counts, not all patients were at normal levels at baseline; eight patients, for example, had platelet counts <100 × 109/L.
TABLE I.
Patient Characteristics at Baseline
| ITT population (n = 40) | |
|---|---|
| Age, years, median (range) | 37 (9–71); 22.5% <18 |
| Gender, n (%) | 18 (45) male/22 (55) female |
| Clinical parameters, median (range) | |
| Hemoglobin concentration, g/dL | 13.8 (10.4–16.5) |
| Platelet count, × 109/L | 162 (29–399) |
| Spleen volume, MNa | 2.5 (1.0–16.0) |
| Liver volume, MNb | 0.8 (0.6–1.6) |
| Biomarkers, median (range) | |
| Chitotriosidase activity, nmol/mL/h (n = 39)c,d | |
| Wild-type (n = 26) | 3072 (404–30,785) |
| Heterozygotes (n = 13) | 2651 (54–6929) |
| CCL18 level, ng/mL | 325 (49–1582) |
| Prior imiglucerase use, months, median (range) | 67.0 (22.0–191.5) |
| Anti-imiglucerase antibody-positive prior to receiving velaglucerase alfa, n (%) | 3 (8) |
ITT, intent-to-treat; MN, multiples of normal.
In 36 patients with spleen intact; four patients had undergone a splenectomy prior to enrollment. A normal spleen volume is 0.2% of body weight.
A normal liver volume is 2.5% of body weight.
One patient had two copies of the 24 bp duplication and was therefore deficient in chitotriosidase activity.
Subjects heterozygous for the chitotriosidase gene typically have lower levels of chitotriosidase activity than subjects with the wild-type genotype; thus data for these subgroups are given separately.
The patients were well distributed across the range of prior imiglucerase doses: 14 patients received imiglucerase at a dose ≤22.5 U/kg, 12 patients received imiglucerase at a dose >22.5 to ≤37.5 U/kg, seven patients received a dose >37.5 to ≤52.5 U/kg, and seven patients received a dose >52.5 U/kg. The velaglucerase alfa doses received are shown in Table A of the on-line supporting information. No analysis of patient characteristics among the four dose groups was performed, although the patients previously receiving the highest imiglucerase doses appeared to be younger than other patients. No dose adjustments were made during the study, and no patient was withdrawn because the investigator wished to increase the dose above 60 U/kg.
No patients were administered pre-infusion medication during the course of the study, although one patient was administered a pre-infusion medication (dexchlorpheniramine maleate) while on imiglucerase before her participation in this study.
During the study, 25 of 40 eligible patients (63%) received home infusions at least once, 10 patients (71%) in the 15 U/kg group, six (50%) in the 30 U/kg group, five (71%) in the 45 U/kg group, and four (57%) in the 60 U/kg group.
Safety
Velaglucerase alfa was generally well tolerated, with most AEs of mild (14 of 40 patients) or moderate (15 of 40 patients) severity (Table II). The three most frequently reported AEs were headache (12 of 40 patients), arthralgia (nine of 40 patients), and nasopharyngitis (eight of 40 patients). Seven severe AEs were reported in five patients, none of which were deemed related to velaglucerase alfa. There were no life-threatening AEs.
TABLE II.
Safety Summary
| Patients, n (%) | |||||
|---|---|---|---|---|---|
|
| |||||
| Total (n = 40) | Velaglucerase alfa dose group
|
||||
| 15 U/kg (n = 14) | 30 U/kg (n = 12) | 45 U/kg (n = 7)a | 60 U/kg (n = 7) | ||
| Experienced ≥1 treatment-emergent AEb | 34 (85) | 11 (79) | 11 (92) | 6 (86) | 6 (86) |
| Experienced ≥1 drug-related AE | 11 (28) | 5 (36) | 3 (25) | 2 (29) | 1 (14) |
| Experienced ≥1 infusion-related AEc | 9 (23) | 5 (36) | 2 (17) | 1 (14) | 1 (14) |
| Experienced ≥1 severe (grade 3) AE | 5 (13) | 0 | 2 (17) | 1 (14) | 2 (29) |
| Possibly/probably treatment-related | 0 | 0 | 0 | 0 | 0 |
| Experienced ≥1 serious AE | 4 (10) | 0 | 1 (8) | 3 (43) | 0 |
| Possibly/probably treatment-related | 1 (3) | 0 | 0 | 1 (14) | 0 |
| Experienced ≥1 life-threatening AE | 0 | 0 | 0 | 0 | 0 |
| Discontinued due to an AE | 1 (3) | 0 | 0 | 1 (14) | 0 |
| Deaths | 0 | 0 | 0 | 0 | 0 |
| Developed anti-velaglucerase alfa antibodies | 0 | 0 | 0 | 0 | 0 |
AE, adverse event.
One patient who received 50 U/kg of imiglucerase was categorized in the 45 U/kg velaglucerase alfa group. This patient was to receive a prescribed dose of 50 U/kg (4000 U/82 kg) of velaglucerase alfa; however, because the patient’s first infusion was discontinued after 30 minutes due to an anaphylactoid reaction she only received 12.7 U/kg but was categorized into the 45 U/kg group for analysis purposes.
A treatment-emergent AE was defined as an AE that occurred on or after the day of the first infusion until 30 days after the patient’s last infusion.
An infusion-related AE was defined as an AE that (1) began either during or within 12 hours after the start of the infusion, and (2) was judged as possibly or probably related to study drug.
Five serious AEs were reported in four patients. One patient (patient number 33) experienced a Grade 2 anaphylactoid reaction beginning approximately 30 minutes after the start of her first infusion of velaglucerase alfa, deemed probably related to velaglucerase alfa. The patient had not received pre-infusion medication while on imiglucerase, and was negative for anti-imiglucerase antibodies. The patient had no known food or drug allergies, and tested negative for anti-imiglucerase and anti-velaglucerase alfa antibodies prior to administration of study drug. During the infusion, the patient reported itching, and erythematous hives and swelling on the left side of the neck and cheek area were observed. The infusion was stopped as per the protocol, and normal saline was infused. The patient then complained of an unusual swelling or closing sensation in her throat, along with chills, stomach discomfort, and feeling anxious. Diphenhydramine and hydrocortisone were administered, and the itching, redness, and throat discomfort resolved after approximately 1 hour; however, the patient’s left cheek remained swollen in the submandibular area. The patient was instructed by the supervising physician to take diphenhydramine and to return to the center the next day for follow-up. Diphenhydramine was electively discontinued by the patient, who recovered without sequelae. The patient was tested for anti-velaglucerase alfa antibodies prior to, 24 hours after, and approximately 2 weeks after receiving velaglucerase alfa, and tested negative for anti-velaglucerase alfa antibodies at all time-points. The AE led to study discontinuation; no other patients discontinued due to an AE.
The other reported serious AEs were swelling of the face and urticaria (patient number 19), arthralgia, and allergic reaction to alteplase, all of which were considered unrelated to study drug, and resolved without sequelae, with the patients continuing in the study and their doses of velaglucerase alfa unchanged.
Immunogenicity
Since the patients had previously been treated with imiglucerase, baseline samples from all patients were tested for the presence of anti-imiglucerase antibodies. Among 41 patients enrolled in the study, 38 baseline samples tested negative for anti-imiglucerase antibodies and three baseline samples tested positive. Using calibration curves generated using a mouse monoclonal antibody that binds to imiglucerase and velaglucerase alfa with similar affinities, the anti-imiglucerase antibody concentrations in these three baseline samples were determined to be 191,100 ng/mL IgG for patient number 16, 363 ng/mL IgG for patient number 25, and 5860 ng/mL IgG for patient number 35. These baseline samples were also tested for the presence of antibodies that can bind velaglucerase alfa. As expected, all the samples that tested negative for imiglucerase also tested negative for velaglucerase alfa. Among the three baseline samples that tested positive for imiglucerase, one sample (patient number 25) tested negative for velaglucerase alfa and two samples tested positive, with antibody concentrations determined to be 48 ng/mL and 28 ng/mL, for patient 16 and patient 35, respectively.
After the start of the velaglucerase alfa dosing, samples collected from Weeks 7, 13, 19, 25, 31, 37, 45, and 51 were tested for the presence of anti-velaglucerase alfa antibodies. Samples from all patients who tested negative at baseline continued to test negative through the duration of the study. Samples from patient number 16 tested positive, with low antibody concentrations (60 ng/mL on average across all time points), similar to that at baseline. This was interpreted as residual anti-imiglucerase antibodies that had weak cross reactivity to velaglucerase alfa, since the concentration was similar before and after dosing with velaglucerase alfa, and the concentrations were much lower than that of anti-imiglucerase antibodies (191,100 ng/mL). Samples from patient number 35 tested positive in five out of eight time point samples, with low and decreasing antibody concentrations (15 ng/mL on average among the five samples that tested positive), and became negative toward the end of the study. Again, this was interpreted as residue anti-imiglucerase antibodies that had weak cross reactivity to velaglucerase alfa, which decreased through the duration of the study and finally became negative.
Clinical parameters
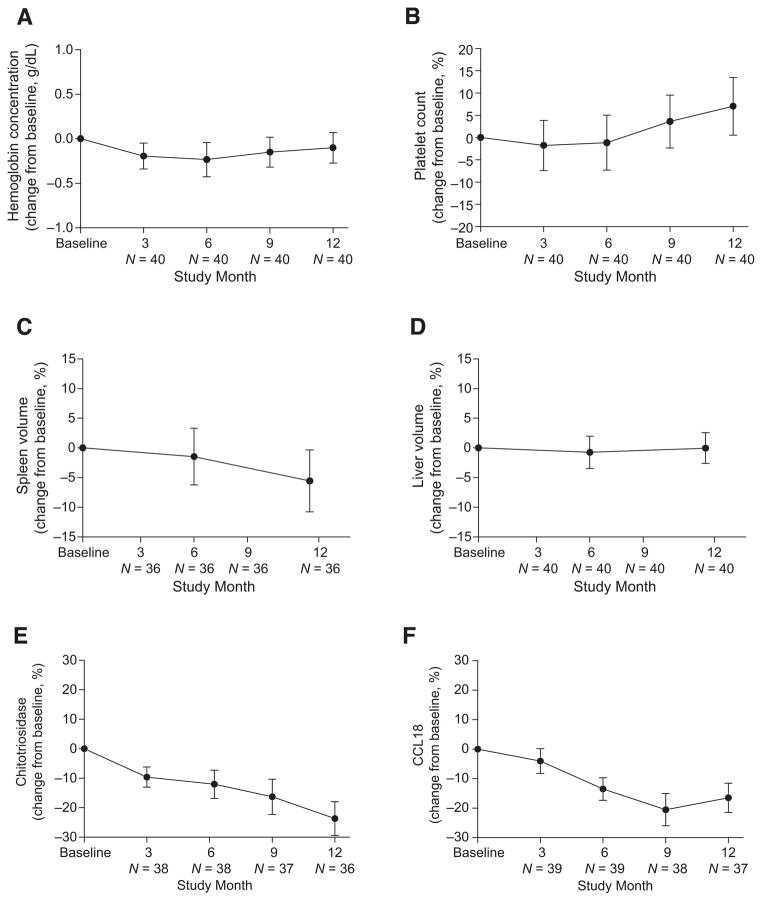
Clinical parameters (hemoglobin concentrations, platelet counts, and spleen and liver volumes) were sustained through 12-months of velaglucerase alfa treatment, as demonstrated by pre-specified efficacy criteria for clinically significant change. Individual patient data are displayed in Table A of the on-line supporting information. For hemoglobin concentrations, the mean change from baseline was −0.1 g/dL (90% CI −0.3, 0.1), which was within the pre-defined efficacy criterion of ±1 g/dL (Fig. 1). For platelet counts, the mean percentage change from baseline was +7.0%, (90% CI 0.5, 13.5), and was within the predefined efficacy criterion of ±20% (Fig. 1). For the 36 patients with intact spleens, the mean percentage change from baseline in spleen volume was −5.6% (90% CI −10.8, −0.4), and was within the pre-defined efficacy criterion of ±15% (Fig. 1). For liver volume, the mean percentage change from baseline was 0.0%, (90% CI −2.6, 2.6), and was within the pre-defined efficacy criterion of ±15% (Fig. 1). For each parameter, similar results were seen across the four dose groups (data not shown). Sensitivity analyses excluding patients with missing values showed no difference in the results (data not shown).
Figure 1.
Change in hematological parameters, organ volume, and biomarkers relative to baseline during 12 months’ treatment with velaglucerase alfa. Mean change from baseline (90% CI) through 12 months’ treatment with velaglucerase alfa for hemoglobin concentrations (indicated as absolute change; A) and platelet counts (indicated as percentage change; B). Mean percentage change from baseline (90% CI) through 12 months’ treatment with velaglucerase alfa for spleen volume (C) and liver volume (D). Mean percentage change from baseline (90% CI) through 12 months’ treatment with velaglucerase alfa for chitotriosidase activity (E) and CCL18 levels (F). One patient deficient in chitotriosidase was excluded from the analysis of chitotriosidase activity.
Both plasma biomarkers (chitotriosidase activity and CCL18 levels) were at least stable with a potential trend toward reduction over the 12-month treatment period (Fig. 1).
Discussion
Velaglucerase alfa has been previously shown to be generally well tolerated in patients with GD1 who were naïve to ERT [4]. In comparison with treatment-naïve patients, transitioning patients between therapeutic proteins raises additional theoretical concerns regarding safety; hence this clinical trial was undertaken to rigorously assess the safety of switching from imiglucerase to velaglucerase alfa infusions. The results demonstrate that patients can be safely transitioned to intravenous velaglucerase alfa from imiglucerase at the same number of units (15–60 U/kg) every other week.
Because all patients had received imiglucerase infusions regularly for at least 22 months, they may represent a selected group able to tolerate ERT. This is difficult to assess, as the study design did not capture AEs the patients experienced while on imiglucerase before enrollment in the trial; however, published clinical trial data indicate that ERT with imiglucerase has been generally well tolerated [9–11]. In addition, the AE profile of velaglucerase alfa was similar to imiglucerase [12,13].
An important aspect of safety with all therapeutic proteins, including imiglucerase, is the development of immune responses. Development of anti-GCase antibodies (primarily non-neutralizing IgG antibodies) has been reported in ~15% of patients receiving long-term imiglucerase, ~50% of whom reported symptoms of hypersensitivity [14]. To date, long-term experience (>48 months) with velaglucerase alfa is available for only 10 adult patients enrolled in a Phase 1/2 extension study, none of whom developed an antibody response to velaglucerase alfa, and thus general conclusions about hypersensitivity rates cannot be accurately assessed until data are available for more patients [4].
In the current 12-month trial, which enrolled patients who had previously received imiglucerase, no patients developed de novo antibodies to velaglucerase alfa. Among the 40 patients dosed with velaglucerase alfa, 37 patients tested negative for the presence of anti-velaglucerase alfa antibodies at all sampling time points throughout the study. For one of the three patients whose baseline samples contained anti-imiglucerase antibodies, all time point samples also tested negative for the presence of anti-velaglucerase alfa antibodies. These data suggest the imiglucerase epitopes recognized by anti-drug antibodies developed in this patient appeared to be unique to imiglucerase. For the remaining two of the three patients whose baseline samples contained anti-imiglucerase antibodies, some of the time points samples tested positive for anti-velaglucerase alfa antibodies, though with much lower apparent concentrations (based on calibration curves) than for anti-imiglucerase. Since the apparent anti-velaglucerase alfa concentrations in these two patients were either similar before and after the dosing with velaglucerase alfa started, or gradually declined, we believe these were residual anti-imiglucerase antibodies that cross reacted to velaglucerase alfa. It would appear that the antibody affinities to these potentially shared epitopes were not similar, since using highly sensitive, equivalent methods to assay anti-velaglucerase alfa and anti-imiglucerase antibodies, the apparent antibody concentration for anti-imiglucerase and for anti-velaglucerase alfa were very different (for patient number 16, 191,100 ng/mL for anti-imiglucerase and 48 ng/mL for anti-velaglucerase alfa; and for patient number 35, 5860 ng/mL for anti-imiglucerase and 28 ng/mL for anti-velaglucerase alfa). As specified in the protocol, after baseline, the presence of anti-imiglucerase antibodies was not tested for and it is unknown whether the three patients that tested positive for anti-imiglucerase at baseline continued to be positive for anti-imiglucerase during the remaining period of time. Although long-term data are not available for imiglucerase, immunosurveillance in patients receiving long-term alglucerase (placenta-purified glucocerebrosidase with modified mannose residues) suggests that it would be unusual for a patient to be persistently IgG positive at high titer, since most antibody-positive patients have been reported to become tolerant by 24 months of continuous therapy [15]. Negligible cross-reactivity was unexpected for a polyclonal IgG mixture, given the similar (but not identical) sequences of the proteins [16]. Further research into possible differences in immunogenicity between the two enzymes could provide interesting insights into the differences that underlie these immunologic reactions, but it should be noted that seroconversion is rarely associated with disease recrudescence and only then with directly inactivating antibodies [17,18].
During the 12-month velaglucerase alfa study period, all patients maintained stable hemoglobin concentrations and platelet counts and stable key outcome measures of disease, with no requirements for increases in velaglucerase alfa dose. Each clinical efficacy parameter evaluated (hemoglobin concentration, platelet count, and spleen and liver volumes) showed no clinically or statistically significant differences on average between the start of the trial (that is, at the end of imiglucerase therapy) and after 12 months of velaglucerase alfa therapy. Given that patients were stable at baseline, improvement in clinical outcomes would not be predicted within 12 months, and the study was neither designed nor powered to detect improvements in clinical outcomes. Nevertheless, the tertiary outcomes of Gaucher-related biomarkers, platelet counts and spleen volume, suggested trends toward further improvement.
Review of the baseline clinical characteristics highlights that although patients were stable on treatment, some had not achieved recommended therapeutic targets for Gaucher disease [19]. It could be speculated that some patients enrolled in the current trial were a selected group of “poor responders” to imiglucerase therapy, since patients achieving treatment goals and not experiencing intolerable AEs with imiglucerase may have little motivation to enroll in clinical trials. However, it is known that only a minority of patients with GD1 receiving long-term imiglucerase therapy achieve all therapeutic targets [20], and the broad range of patients enrolled in this multicenter trial was generally representative of typical patients with GD1, supporting the generalizability of the current results.
The need for treatment options for patients with GD became evident during the global imiglucerase shortage in 2009–2010 [21], and which has continued into 2012. In 2009 velaglucerase alfa provided an alternative to imiglucerase. In the USA, patients received velaglucerase alfa via a specifically designed treatment protocol [22], which may provide further clinical trial data on the safety of transitioning from imiglucerase to velaglucerase alfa. In addition, recent guidance issued by the European Working Group on Gaucher Disease concluded that, where applicable, administration of alternative treatments such as velaglucerase alfa should continue in patients who have made a successful switch from imiglucerase [23], and potential real-world analyses of the long-term effects of switching would provide further information for clinicians considering velaglucerase alfa as a therapeutic option.
Conclusions
The results of this 12-month, multicenter trial demonstrate that adult and pediatric patients with GD1 can be safely transitioned from intravenous imiglucerase to the same number of units of velaglucerase alfa, while sustaining clinical parameters. In addition, velaglucerase alfa showed a favorable adverse event profile among enzyme replacement therapies, and no patients developed antibodies to velaglucerase alfa.
Supplementary Material
Acknowledgments
The authors would like to acknowledge fellow investigator and pediatrician Dr Paul Fernhoff from Emory University, Decatur, GA, USA, who passed away in September 2011; this paper is dedicated to his memory. This study was sponsored by Shire HGT and, in part, with funds provided by the National Center for Research Resources, 5 M01 RR-01271 (Dr Harmatz). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The authors would like to acknowledge Yune Kunes PhD, of Shire HGT for her immunological expertise in the preparation of this paper and Candida Fratazzi MD, formerly of Shire HGT for her assistance in developing the clinical protocol. Geraldine Thompson and Dave Cornick of UBC Scientific Solutions, medical writers supported by funding from Shire HGT provided drafts and editorial assistance to the authors during preparation of this manuscript.
Footnotes
Disclosures
The authors declare the following potential competing interests: Dr Zimran receives consulting fees from Protalix Biotherapeutics; has options in Protalix Biotherapeutics and sits on its Scientific Advisory Board; receives support from Genzyme for participation in the International Collaborative Gaucher Group (ICGG) Gaucher Registry; and receives honoraria from Shire HGT, Actelion, and Pfizer. He served as consultant to Shire HGT during the course of the trial (until October 2011). Dr Pastores is the recipient of research grants/support from Actelion, Amicus, Biomarin, Genzyme, Protalix, and Shire HGT, pharmaceutical/biotechnology companies engaged in drug development programs for the lysosomal storage disorders. Dr Hughes has received consulting fees, and travel and research grants and honoraria for speaking from Shire HGT, Genzyme, Protalix, and Amicus. Dr Mardach has no competing interests to declare. Dr Eng has received clinical trial research support and speaker support from Shire HGT. Dr Smith has received research grants from Genzyme, Shire HGT, and BioMarin, and consultant fees from Shire HGT and BioMarin. Dr Charrow has received consulting fees and honoraria from Genzyme, and consultant fees from Protalix/Pfizer. Dr Elstein received consulting fees and honoraria from Shire HGT. Dr Harmatz has provided consulting support to Shire HGT; received speaker’s honorarium and travel support from Shire HGT; participates in advisory boards (HOS) for Shire; provides consulting support to Biomarin; participated on advisory boards and has received research support and travel and speaker’s honorariums from Biomarin; and has received speaker’s honorarium from Genzyme. Dr Fernhoff received educational grants and research support from Shire HGT. Dr Rhead has received clinical trial support from Transkaryotic Therapies, Genzyme, Shire and Hyperion, and speaker fees from Ucyclyd. Dr Longo has received grant support from Shire HGT, Genzyme, Protalix/Pfizer, BioMarin, Amicus and Hyperion and consulting fees from BioMarin and Shire HGT. Dr Giraldo has received consulting fees from Shire HGT, Genzyme, and Actelion. Mr Zahrieh and Dr Crombez are employees of Shire HGT. Dr Grabowski has received consulting fees from Shire HGT, Genzyme, Pfizer, and Amicus Therapeutics. The Division of Human Genetics at Cincinnati Children’s Hospital Medical Center, for which Dr Grabowski serves as the Director, receives grants-in-aid for the conduct of basic and clinical research from Shire HGT and Genzyme, including the current study, as well as preclinical biopharmaceutical and small molecule studies that are unrelated to the studies presented here. Dr Grabowski does not hold stock or have stock options in any biopharmaceutical commercial concern.
References
- 1.Grabowski GA, Petsko GA, Kolodny EH. The Online Metabolic and Molecular Bases of Inherited Diseases. [Accessed 8 May 2012];Chapter 146: Gaucher Disease. Available at: http://dx.doi.org/10.1036/ommbid.176.
- 2.Tsuji S, Choudary PV, Martin BM, et al. Nucleotide sequence of cDNA containing the complete coding sequence for human lysosomal glucocerebrosidase. J Biol Chem. 1986;261:50–53. [PubMed] [Google Scholar]
- 3.Zimran A, Loveday K, Fratazzi C, et al. A pharmacokinetic analysis of a novel enzyme replacement therapy with Gene-Activated human glucocerebrosidase (GA-GCB) in patients with type 1 Gaucher disease. Blood Cells Mol Dis. 2007;39:115–118. doi: 10.1016/j.bcmd.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Zimran A, Altarescu G, Philips M, et al. Phase 1/2 and extension study of velaglucerase alfa replacement therapy in adults with type 1 Gaucher disease: 48-month experience. Blood. 2010;115:4651–4656. doi: 10.1182/blood-2010-02-268649. [DOI] [PubMed] [Google Scholar]
- 5.Séllos-Moura M, Barzegar S, Pan L, et al. Development of a panel of highly sensitive, equivalent assays for detection of antibody responses to velaglucerase alfa or imiglucerase enzyme replacement therapy in patients with Gaucher disease. J Immunol Methods. 2011;373:45–53. doi: 10.1016/j.jim.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Aguilera B, Ghauharali-van der Vlugt K, Helmond MT, et al. Transglycosidase activity of chitotriosidase: improved enzymatic assay for the human macrophage chitinase. J Biol Chem. 2003;278:40911–40916. doi: 10.1074/jbc.M301804200. [DOI] [PubMed] [Google Scholar]
- 7.Schoonhoven A, Rudensky B, Elstein D, et al. Monitoring of Gaucher patients with a novel chitotriosidase assay. Clin Chim Acta. 2007;381:136–139. doi: 10.1016/j.cca.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 8.Boot RG, Verhoek M, de Fost M, et al. Marked elevation of the chemokine CCL18/PARC in Gaucher disease: a novel surrogate marker for assessing therapeutic intervention. Blood. 2004;103:33–39. doi: 10.1182/blood-2003-05-1612. [DOI] [PubMed] [Google Scholar]
- 9.Zimran A, Elstein D, Levy-Lahad E, et al. Replacement therapy with imiglucerase for type 1 Gaucher’s disease. Lancet. 1995;345:1479–1480. doi: 10.1016/s0140-6736(95)91038-7. [DOI] [PubMed] [Google Scholar]
- 10.Kishnani PS, DiRocco M, Kaplan P, et al. A randomized trial comparing the efficacy and safety of imiglucerase (Cerezyme) infusions every 4 weeks versus every 2 weeks in the maintenance therapy of adult patients with Gaucher disease type 1. Mol Genet Metab. 2009;96:164–170. doi: 10.1016/j.ymgme.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Grabowski GA, Barton NW, Pastores G, et al. Enzyme therapy in type 1 Gaucher disease: comparative efficacy of mannose-terminated glucocerebrosidase from natural and recombinant sources. Ann Intern Med. 1995;122:33–39. doi: 10.7326/0003-4819-122-1-199501010-00005. [DOI] [PubMed] [Google Scholar]
- 12.Shire Human Genetic Therapies Inc. [Accessed 3 January 2012];VPRIV (velaglucerase alfa for injection) prescribing information. 2010 Available at: http://www.drugs.com/pro/vpriv.html.
- 13.Genzyme Corporation. [Accessed 3 January 2012];CerezymeR (imiglucerase for injection) prescribing information. Available at: http://www.cerezyme.com/~/media/Files/CerezymeUS/pdf/cerezyme_pi.pdf.
- 14.Starzyk K, Richards S, Yee J, et al. The long-term international safety experience of imiglucerase therapy for Gaucher disease. Mol Genet Metab. 2007;90:157–163. doi: 10.1016/j.ymgme.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg M, Kingma W, Fitzpatrick MA, et al. Immunosurveillance of alglucerase enzyme therapy for Gaucher patients: induction of humoral tolerance in seroconverted patients after repeat administration. Blood. 1999;93:2081–2088. [PubMed] [Google Scholar]
- 16.Brumshtein B, Salinas P, Peterson B, et al. Characterization of gene-activated human acid-beta-glucosidase: crystal structure, glycan composition, and internalization into macrophages. Glycobiology. 2010;20:24–32. doi: 10.1093/glycob/cwp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brady RO, Murray GJ, Oliver KL, et al. Management of neutralizing antibody to Ceredase in a patient with type 3 Gaucher disease. Pediatrics. 1997;100:E11. doi: 10.1542/peds.100.6.e11. [DOI] [PubMed] [Google Scholar]
- 18.Zhao H, Bailey LA, Grabowski GA. Enzyme therapy of gaucher disease: clinical and biochemical changes during production of and tolerization for neutralizing antibodies. Blood Cells Mol Dis. 2003;30:90–96. doi: 10.1016/s1079-9796(03)00012-3. [DOI] [PubMed] [Google Scholar]
- 19.Pastores GM, Weinreb NJ, Aerts H, et al. Therapeutic goals in the treatment of Gaucher disease. Semin Hematol. 2004;41:4–14. doi: 10.1053/j.seminhematol.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Weinreb N, Taylor J, Cox T, et al. A benchmark analysis of the achievement of therapeutic goals for type 1 Gaucher disease patients treated with imiglucerase. Am J Hematol. 2008;83:890–895. doi: 10.1002/ajh.21280. [DOI] [PubMed] [Google Scholar]
- 21.Hollak CE, vom Dahl S, Aerts JM, et al. Force majeure: therapeutic measures in response to restricted supply of imiglucerase (Cerezyme) for patients with Gaucher disease. Blood Cells Mol Dis. 2010;44:41–47. doi: 10.1016/j.bcmd.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Shire Human Genetic Therapies Inc. [Accessed 23 September 2010];Treatment protocol of velaglucerase alfa for patients with type 1 Gaucher disease. 2009 Available at: http://clinicaltrials.gov/ct2/show/NCT00954460.
- 23.Hollak CE, Aerts JM, Belmatoug N, et al. Guidelines for the restart of imiglucerase in patients with Gaucher disease: recommendations from the European Working Group on Gaucher disease. Blood Cells Mol Dis. 2010;44:86–87. doi: 10.1016/j.bcmd.2009.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.