Abstract
Background
Patients with chronic hepatitis C virus (HCV) infection have high rates of alcohol consumption, which is associated with progression of fibrosis and lower response rates to HCV treatment.
Aims
This prospective cohort study examined the feasibility of a 24-week integrated alcohol and medical treatment to HCV-infected patients.
Methods
Patients were recruited from a hepatology clinic if they had an Alcohol Use Disorders Identification Test score ≥ 4 for women and ≥ 8 for men, suggesting hazardous alcohol consumption. The integrated model included patients receiving medical care and alcohol treatment within the same clinic. Alcohol treatment consisted of six months of group and individual therapy from an addictions specialist and consultation from a study team psychiatrist as needed.
Results
Sixty patients were initially enrolled, and 53 patients participated in treatment. The primary endpoint was the Addiction Severity Index (ASI) alcohol composite scores, which significantly decreased by 0.105 (41.7% reduction) between 0 and 3 months (p<.01) and by 0.128 (50.6% reduction) between 0 and 6 months (p<.01) after adjusting for covariates. Alcohol abstinence was reported by 40% of patients at 3 months and 44% at 6 months. Patients who did not become alcohol abstinent had reductions in their ASI alcohol composite scores from 0.298 at baseline to 0.219 (26.8% reduction) at 6 months (p=.08).
Conclusion
This study demonstrated that an integrated model of alcohol treatment and medical care could be successfully implemented in a hepatology clinic with significant favorable impact on alcohol use and abstinence among patients with chronic HCV.
Keywords: HCV, alcohol-related disorders, delivery of health care
Introduction
More than 170 million people worldwide are infected with hepatitis C virus (HCV), which leads to significant morbidity and mortality through the complications of cirrhosis, portal hypertension and hepatocellular carcinoma (1, 2). Patients with chronic HCV infection have high rates of alcohol consumption, which is associated with progression of fibrosis and the development of hepatocellular carcinoma (3-5). Alcohol use also influences HCV treatment outcomes (6, 7), and the American Association for Study of Liver Diseases recommends patients with HCV infection abstain from alcohol use (8). This same guideline encourages consideration of patients with alcohol abuse through individualized treatment approaches.
Recently, response rates for HCV treatment have significantly improved with the addition of protease inhibitors to the combination of peginterferon-α and ribavirin, and many patients are expected to seek treatment with these medications (9, 10). As patients and their providers consider HCV therapy, the assessment of alcohol intake and abuse is a standard component of the evaluation. Despite evidence that alcohol use damages the health of HCV-infected individuals, few studies have systematically examined effective approaches to address alcohol use in this population. Integrated behavioral health-medical treatment models have demonstrated decreases in alcohol use in primary care settings (11). Additionally, studies have shown that alcohol interventions can be integrated into medical settings (12), effectively delivered by physicians (13), and improve medication and adherence to treatment for co-occurring medical problems (e.g., HIV, diabetes, hypertension) as well as reducing target alcohol symptoms (14, 15). This study examined the feasibility of an integrated model of alcohol abuse treatment along with medical care in patients with co-occurring HCV and problem drinking ranging from hazardous consumption to probable alcohol dependence.
Methods
Trial design
This study was a prospective, open-label, cohort trial to assess the feasibility of an integrated model of alcohol abuse treatment and medical care for patients with HCV and hazardous alcohol consumption. The protocol was approved by the Duke Institutional Review Board and conducted according to Good Clinical Practice guidelines. All patients provided written informed consent before study participation.
Participants
Patients 18 years and older with chronic HCV infection were recruited during clinic visits to the Duke Liver Clinic in Durham, NC. All patients with HCV presenting to clinic completed the Alcohol Use Disorders Identification Test (AUDIT) (16), which is a 10-item self-administered questionnaire designed by the World Health Organization to screen for risky alcohol consumption in primary care settings. A cuff- off of 8 in men has been shown to be optimal in identifying hazardous alcohol consumption with adverse medical consequences (17), and a cut-off of 4 in women has been recommended (18). The AUDIT questions ask about level of alcohol use, alcohol dependence symptoms, and alcohol-related problems. Female patients with an AUDIT score of 4 or greater, and male patients with an AUDIT score of 8 or greater were approached by their HCV medical provider to participate in the study. The AUDIT score for hazardous alcohol consumption and higher, rather than alcohol dependence only, was selected for entry to the study given the negative effects of moderate alcohol consumption on patients with HCV (4, 5). Patients using substances other than alcohol, those with comorbid mental disorders, and patients with HIV-HCV co-infection were also eligible for the study.
Interventions
During the recruitment period, the addictions specialist was present in clinic and was introduced to the patient by their HCV medical provider. The addictions specialist either met the patient that same day or arranged another appointment in clinic to review the study protocol and consent form. Patients who agreed to participate in the study initiated treatment within 1 to 2 weeks. The addictions specialist initially conducted a diagnostic evaluation to determine the individual needs of each patient. Treatment planning followed standards of care for alcohol/substance abuse, mental health, and HCV treatment. Using a multidisciplinary approach, the findings from the evaluation were developed into an individualized treatment program that described goals and actions. A psychiatrist (PM) was available for consultation at the request of the addictions specialist or medical provider, with co-localized visits occurring in the Duke Liver Clinic. For referred patients only, the psychiatrist conducted a comprehensive psychiatric evaluation using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition). Psychiatric treatment was tailored to the individual patient and not standardized, but consisted of prescribing anxiety, depression, or alcohol relapse-prevention medications as appropriate, and seeing the patient two weeks later and then as needed through the end of the study. All study patients were offered a combination of weekly group therapy and bi-weekly individual therapy with the addictions specialist for 6 months. The theoretical orientations underpinning both group and individual therapy were the transtheoretical model of change, motivational interviewing, and cognitive behavioral therapy (19-21). The group therapy sessions were held in a conference room on the same floor as the Duke Liver Clinic and covered both psychoeducational topics and process/interpersonal subjects. Psychoeducational topics included HCV (epidemiology, natural history, transmission risk reduction, and treatment), liver wellness, alcohol use, mental health processes and coping, stress and anxiety management, depression, and grief. Process/interpersonal groups included topics such as desire to use alcohol and recent relapses; struggles with family and other group members; social and interpersonal stigma related to alcohol use, HCV, and mental illness; relapse prevention; and interpersonal effectiveness. The individual therapy sessions were held in rooms within the Duke Liver Clinic, and the focus was maintenance of participation in the treatment process, clarification of alcohol treatment goals, support, cognitive and behavioral approaches to living with HCV (e.g., risk reduction strategies, addressing stigma) and to decreasing alcohol use, as well as treatment of depression and anxiety. The approaches used to address alcohol use are highly relevant to substance use and were applied in individual therapy for patients who additionally used substances. Thus, the emphasis was on alcohol treatment but substance use was also addressed. The addictions specialist entered notes from individual sessions into the electronic medical record, and these were accessible to clinic providers. During the intervention period, the addictions specialist came to clinic at the time of each patient's medical appointment and discussed the case with the medical provider at that time. All patients were discussed weekly at a meeting of the addictions specialist and the principal investigator (AJM); medical providers and the study psychiatrist were contacted as needed.
Outcomes
Patient interviews were conducted at baseline, 3 months, and 6 months of treatment. The patient interviews were conducted in-person in either a private location at the clinic or in the home of the patient by a trained interviewer who was not involved in the treatment intervention. The baseline interview contained demographic items, including race, gender, age, income, education attainment, insurance status, and HIV status. Baseline and follow-up interviews included a number of items and scales. Alcohol and drug use change was assessed using the alcohol (6 items) and drug (13 items) subscales of the Addiction Severity Index – Lite (ASI), which is designed to provide composite scores useful in measuring change over time (22). The ASI is a structured interview that assesses problem areas in alcohol and drug dependent persons, the severity and patterns of drug use, and the severity and patterns of alcohol use. For both alcohol use and substance use, it provides a subjective severity rating and a more objective and standardized composite score ranging from 0 (minimum) to 1 (maximum). In addition, self-reports are obtained for the frequency of substance use during the previous 30 days. The ASI has been used in a variety of clinical research settings as well as addiction treatment programs to assess the severity and consequences of substance use. Reductions in ASI composite scores from baseline have been considered to be reliable and valid measures of improvement in the respective domains(23). Alcohol composite scores of .17 and higher, and drug composite scores of .16 and higher, are predictive of DSM-IV dependence (24). Patients who reported no alcohol use in the past 30 days were considered to be alcohol abstinent at that interview time point.
Statistical analysis
To estimate the overall treatment response (changes in the alcohol abstinence rate and in ASI alcohol and drug composite scores) over the 6-month study period, we modeled abstinence and composite scores at baseline, 3, and 6 months using longitudinal population-averaged models in STATA 11.1 to account for correlations between observations on each subject. Specifically, in estimating treatment effects on alcohol and drug composite scores, we used the linear generalized estimating equations (GEE) method; on alcohol abstinence, because it is binary, we used the logistic population-averaged method instead.
Results
Patient Characteristics
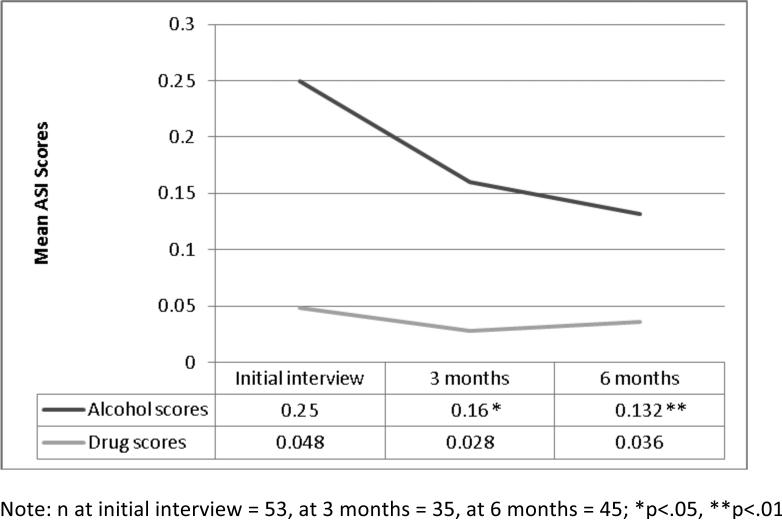
Between February 2009 and March 2010, 60 patients were enrolled in the study. Following the initial medical and addictions specialist evaluation, 7 patients did not participate in the intervention (1 medically unstable, 2 determined to not have chronic HCV infection, 1 spontaneous clearing of HCV, and 3 lost to follow-up after initial interview). The 53 patients who participated in the intervention of individual and group therapy are summarized in Table 1. Mean education was 12.2 years with a standard deviation of 2.4 years. The majority of participants identified as Black or African-American (64.2%), with 30.2% identifying as White, 5.7% identifying as multiracial or another race, and 4.1% identifying as Hispanic in addition to Black, White, or other. The majority of participants (77.0%) had some form of health insurance. No patients were on HCV treatment during the study. All patients reported some degree of alcohol consumption. Most patients (69%) scored above 20 on the AUDIT, indicative of alcohol dependence. At baseline the mean composite scores on alcohol and drug domains were .250 and .048, respectively (Table 1).
Table 1.
Baseline Characteristics
| Variable | % (n) |
|---|---|
| Gender | |
| Male | 60.4 (32) |
| Female | 39.6 (21) |
| Transgender | 0.0 (0) |
| Age (years) | 51.3 (7.0) (mean, SD) |
| 30-65 (range) | |
| HIV-infected | 30.2 (16) |
| Baseline AUDIT scores | |
| <15, risky drinking | 17.3 (9) |
| 15-19, harmful drinking | 13.5 (7) |
| 20+, possible dependence | 69.2 (36) |
| Past 30-day use at baseline | |
| Any illicit drug use | 45.3 (24) |
| Cocaine use | 26.4 (14) |
| Marijuana use only | 9.4 (5) |
Intervention Dosage
The intervention included the offer of weekly group therapy and bi-weekly individual counseling but allowed flexibility based upon patient preferences and logistical challenges such as transportation and work responsibilities. Patients were encouraged to attend both forms of therapy but neither were required to attend both nor mandated to attend a specific proportion of sessions. For group therapy, 32/53 (60.4%) of participants attended at least one group session, with a mean of 9.0 (SD=7.5) group sessions attended, while 47/53 (88.7%) of participants attended at least one individual therapy session, with a mean of 6.8 (SD=3.8) individual sessions attended. The mean number of group plus individual sessions attended was 12.4 (SD=9.4). Interviews were conducted at baseline, 3 months and 6 months; 85% of patients participated in the 6-month interview. The study psychiatrist followed and prescribed medications for depression/anxiety for 12/53 (22.6%) patients. Four patients were additionally prescribed medications approved to treat alcohol dependence. These were acamprosate (n=3) and disulfiram (n=1); no one was prescribed naltrexone. No benzodiazepines were prescribed by either the study psychiatrist or medical providers.
Outcome Analyses
The primary endpoint was change in the ASI scores. As shown in Figure 1, the unadjusted mean ASI alcohol composite score was reduced by 0.090 (36.0%) by the 3-month assessment timepoint (p<0.05) and was reduced by 0.118 (47.2%) at 6 months (p<0.01). The unadjusted mean ASI drug composite score was reduced by 0.020 (42.7%) at 3 months (p=0.07) and by 0.012 (25.7%) at 6 months (p=.18). It may be more useful to consider the adjusted mean ASI composite scores, because they account for differences caused by disproportional distribution of baseline characteristics in later rounds. For example, HIV status significantly relates to ASI drug scores and also our sample size differs at each timepoint of 0, 3, and 6 months. This difference in sample size affects the unadjusted means, but is accounted for in the adjusted means. The adjusted means, as displayed in Table 2, show reductions in alcohol scores by 0.105 (41.7%) at 3 months (p<.01) and 0.128 (50.6%) at 6 months (p<.01), and in drug scores by 0.0228 (47.2%) at 3 months (p<.05) and 0.0150 (30.6%) at 6 months (p=0.15). These p-values indicate statistical significance of time effects from the multivariable regression in Table 3.
Figure 1.
Mean ASI alcohol and drug scores by timepoint
Table 2.
ASI composite scores in participant-time pairs at the beginning, during, and after treatment
| Initial interview | 3 months | Reduction | ||||
|---|---|---|---|---|---|---|
| n = 35 | Mean | SD | Mean | SD | Mean change | % |
| ASI alcohol | 0.252 | 0.052 | 0.147 | 0.052 | 0.105 | 41.7% |
| ASI drug | 0.0483 | 0.0201 | 0.0255 | 0.0201 | 0.0228 | 47.2% |
| Initial interview | 6 months | Reduction | ||||
| n = 45 | Mean | SD | Mean | SD | Mean change | % |
|---|---|---|---|---|---|---|
| ASI alcohol | 0.253 | 0.052 | 0.125 | 0.052 | 0.128 | 50.6% |
| ASI drug | 0.0490 | 0.0202 | 0.0340 | 0.0202 | 0.0150 | 30.6% |
Table 3.
Change in alcohol and drug outcomes between initial interview and 3 months and initial interview and 6 months
| Alcohol abstinence | ASI alcohol scores | ASI drug scores, full sample | ASI drug scores, subsample that reports drug use at any timepoint | |||||
|---|---|---|---|---|---|---|---|---|
| With all covariates | With significant covariates only | With all covariates | With significant covariates only | With all covariates | With significant covariates only | With all covariates | With significant covariates only | |
| Change, Initial interview to 3 months | 1.065** (0.410) | 0.968** (0.371) | -0.105** (0.0283) | -0.104** (0.0284) | -0.0228* (0.0114) | -0.0239* (0.0114) | -0.0266 (0.0158) | -0.0289 (0.0157) |
| Change, Initial interview to 6 months | 1.213** (0.380) | 1.125** (0.343) | -0.128** (0.0258) | -0.127** (0.0258) | -0.0150 (0.0105) | -0.0153 (0.0104) | -0.0258 (0.0139) | -0.0264 (0.0138) |
| Baseline characteristics HIV status: Positive | -0.292 (0.594) | N/A | 0.028 (0.065) | N/A | 0.0329* (0.0157) | 0.0164 (0.0154) | 0.0448* (0.0193) | 0.0234 (0.0188) |
| Income | 0.000 (0.000) | N/A | 0.000 (0.000) | N/A | 0.0000 (0.0000) | N/A | 0.0000 (0.0000) | N/A |
| Age | 0.060 (0.039) | N/A | -0.003 (0.004) | N/A | -0.0019 (0.0010) | N/A | -0.0020 (0.0012) | N/A |
| Gender: Female | -0.505 (0.513) | N/A | -0.044 (0.055) | N/A | -0.0079 (0.0133) | N/A | -0.0081 (0.0168) | N/A |
| Race: Black | -1.369 (1.273) | N/A | 0.004 (0.130) | N/A | 0.0227 (0.0333) | N/A | 0.0407 (0.0355) | N/A |
| Race: White | -1.207 (1.281) | N/A | 0.082 (0.131) | N/A | 0.0128 (0.0335) | N/A | 0.0219 (0.0361) | N/A |
| Observations | 133 | 133 | 133 | 133 | 133 | 133 | 94 | 94 |
| Participants | 1.065** | 0.968** | -0.105** | -0.104** | -0.0228* | -0.0239* | -0.0266 | -0.0289 |
p<0.01
p<0.05
Note: Logistic GEE regression analysis used for Alcohol Abstinence, which is a dichotomous variable in which 1=abstinence. Multiple GEE regression analysis used for ASI Alcohol and Drug Scores, with higher scores indicating greater alcohol and drug use. For Race, “Black” is an indicator variable for Black participants versus White and other races, and “White” is an indicator variable for White participants versus Black and other races.
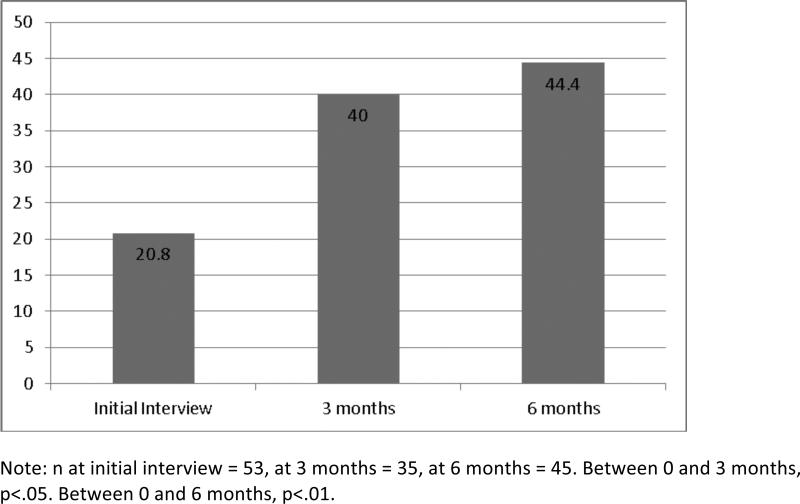
Figure 2 displays the alcohol abstinence rates. At the time of research consent, no patients were alcohol abstinent. However, the act of study enrollment paired with an initial conversation with an addictions specialist was an intervention by itself, and by the time of the initial research interview, 20.8% of patients reported alcohol abstinence. For the majority of patients (n=45), the baseline interview occurred within 1-15 days of enrollment. However, for 15.1% of patients, the baseline interview occurred after more than 15 days of enrollment, therefore making possible substantial study treatment possible before the baseline interview. Specifically, the baseline interview occurred for 4 patients within 28 days, for 3 patients within 40 days, and for 1 patient in 54 days. To account for attrition, we examined abstinence rates for just those patients with interviews at two paired time points. Alcohol abstinence was reported by 40.0% of participants at 3 months and 44.4% at 6 months.
Figure 2.
Percentage reporting alcohol abstinence by timepoint
To understand better what happened with the alcohol use of patients who did not become abstinent, we examined their ASI alcohol composite scores. Their alcohol scores showed improvements, with reductions from 0.298 at baseline to 0.266 (10.7%) at 3 months (p=.31) to 0.219 (26.8%) at 6 months (p=.08). The response to the intervention was also evaluated among patients with probable alcohol dependence, based on baseline AUDIT scores of 20 or greater (n=36). Among the 31 patients with probable alcohol dependence at baseline who provided data at 6 months, 12 (38.7%) reported alcohol abstinence at 6 months. For those patients with probable alcohol dependence who did not become abstinent (n=14 at 3 months; n=19 at 6 months), their ASI alcohol composite scores showed reductions from 0.392 at baseline to 0.286 at 3 months (p=0.09) to 0.262 at 6 months (p=.034).
Table 3 displays the multivariable population-averaged longitudinal regression results for alcohol abstinence and ASI alcohol and drug composite scores. The first two columns of Table 3 report the logistic regression results for alcohol abstinence. In univariable and adjusted analyses for baseline characteristics including gender, age, race, and income, as well as HIV status, improvements in alcohol abstinence rates between 0 and 3 months and between 0 and 6 months were statistically significant. At 3 months, the odds of being alcohol abstinent were 2.900 times those before treatment (log odds ratio=1.065, p<.01), and at 6 months, the odds of being alcohol abstinent were 3.362 times those before treatment (log odds ratio=1.213, p<.01).
The second two columns of Table 3 report the linear regression results for ASI alcohol composite scores. In univariable and adjusted analyses for baseline characteristics, there was a significant decrease (i.e., improvement) in the change scores at both 3 and 6 months. At 3 months, alcohol scores adjusted for covariates decreased by 0.105 (p<.01) and at 6 months, adjusted scores decreased by 0.128 (p<.01).
The third two columns of Table 3 report the linear regression results for ASI drug composite scores for the full sample, which includes participants who never reported drug use. In adjusted analyses for baseline characteristics, there was a significant decrease (i.e., improvement) in the change scores at 3 months (B= -0.0228, p<.05) but not at 6 months (B= -0.0150, p=.16). Among the control covariates included, HIV status was significantly related (p<.05). The coefficient of 0.0329 indicates that, on average, HIV-positive patients reported their ASI drug scores to be higher than HIV-negative patients by 0.0329. We analyzed change in ASI composite drugs scores on the subset of participants (n=40) who reported drug use at any of the three time points (see the fourth two columns of Table 3). Although the findings were not statistically significant, the trends were in the same direction as those for the full sample and the coefficients were slightly larger, suggesting that the lack of statistical significance may be due to lack of power.
Noticeably, except for HIV status for ASI drug scores, the covariates in Table 3 are not significant. We additionally examined total number of group and individual sessions attended, attending any sessions with the study psychiatrist, years of education, and binary insurance status, which were also not significant for any of the outcomes and therefore dropped. We chose to report race, income, gender, and age in the adjusted models because of their known relationships to health. Their lack of significance suggests that the intervention worked equally well regardless of race, income, gender, and age for both alcohol and drug outcomes, and regardless of HIV status for alcohol outcomes.
Discussion
For people infected with HCV, alcohol use has a direct negative impact on the liver, increasing the risk and progression of hepatocellular carcinoma and liver fibrosis, which can lead to liver failure and death. Despite these health implications, studies have shown that adults with HCV are 3 times more likely than uninfected persons to consume more than one alcoholic drink per day (35% vs. 14%) and almost 8 times more likely to consume more than 3 drinks per day (19% vs. 2%) (3). In this study, we developed and manualized an integrated behavioral-medical treatment model for patients with HCV who drink alcohol.
The goal of the treatment program was abstinence, and 44% of patients achieved this objective at the end of the treatment. Natural history studies have demonstrated survival benefits among patients with HCV infection who become abstinent from alcohol even after the development of cirrhosis (25). In addition, ASI alcohol composite scores decreased by nearly half between baseline and 6 months. This reduction in ASI alcohol scores is comparable to changes found following behavioral intervention studies in alcohol-dependent individuals (26, 27). Notably, alcohol scores did not significantly differ by HIV status, race, gender, age, or income, indicating that the treatment model may be equally beneficial for diverse HCV-infected patients.
For patients who did not become abstinent, ASI alcohol scores decreased by 26.8% between baseline and 6 months. Natural histories support benefits to both abstinence and reductions in alcohol use. Poynard and colleagues observed that the median rate of fibrosis progression was highest (0.167 units/year) among patients consuming greater than 50 grams or more of alcohol per day compared with 0.143 units per year among those consuming 1 to 49 grams of alcohol per day and 0.125 units per year among the abstinent patients (4). Others have found a relationship between heavy alcohol consumption and developing cirrhosis faster (5) and increased risk of developing hepatocellular carcinoma (28). Thus, in addition to the 44% of study patients who became abstinent, it is likely that the decreased alcohol use found in other study patients, even when shy of abstinence, will benefit their health.
HCV-infected persons with advancing liver disease can be treated with HCV antiviral therapy, which offers the possibility of being cured. Alcohol use may negatively impact HCV treatment response in terms of degree of fibrosis and intrahepatic HCV replication (6, 29, 30). Treatment guidelines therefore recommend abstinence from alcohol to prevent fibrosis progression and to improve treatment response (8). Cure rates for genotype 1 infection have been approximately 40% (31), but may increase to 69-75% with the addition of two protease inhibitors that have recently received FDA approval (32, 33). However, these medications are given in combination with peginterferon-α and ribavirin, and alcohol use negatively impacts HCV treatment response with this regimen (6, 29, 30). Therefore, alcohol use will remain contraindicated for antiviral therapy according to treatment guidelines (8).
It is interesting that 20.8% of patients reported alcohol abstinence to the interviewer during their baseline interview, even though they reported hazardous to dependence alcohol use to their HCV medical provider at the time of study enrollment. It is possible that patients achieved abstinence because of 1) the brief alcohol intervention that occurred with the medical provider; 2) the initial meeting with the addictions specialist; and 3) the act of making a commitment to participate in alcohol treatment. Studies have found 5- to 15-minute brief alcohol interventions delivered by medical providers to result in significant decreases in alcohol use (34). Although our brief intervention may have led to alcohol abstinence in nearly one-fifth of patients, we do not know if abstinence would have been achieved without making a psychological commitment to an alcohol treatment program, or whether abstinence would have been sustained without further treatment. Future studies should test our 6-month alcohol-HCV treatment model against brief alcohol counseling among HCV-infected patients.
In addition to decreases in ASI alcohol composite scores, we also found decreases in ASI drug composite scores. However, drug composite scores were low at baseline (mean = 0.048), increasing risk of type II error, and the only statistically significant decrease was found for the full sample between baseline and 3 months. Future replications of this integrated intervention may consider augmenting the substance use treatment components for patients affected by substance use in addition to HCV and alcohol use.
Our study had several limitations, including the lack of randomization, the small sample size, the use of a single hepatology clinic, and the lack of an objective measure of alcohol use at initial or outcome timepoints. The study is also limited by the lack of long-term follow-up for treatment outcomes. Patients with HCV may be particularly motivated to quit alcohol use, but it is unknown how long they are able to maintain abstinence based on this treatment. Future research will need to study these interventions in larger numbers among multiple settings and randomize patients to a treatment model or standard care. However, our study was effective in demonstrating the feasibility of incorporating the integrated care model into a hepatology clinic. Integrated behavioral-medical models have demonstrated positive impacts on alcohol use in primary care settings, but have rarely been tested in HCV specialty care settings (11).
In 2004, the National Institute on Drug Abuse convened a panel of experts to review the state of treatment for persons with HCV and co-occurring substance use and psychiatric illness (35). They concluded that early interventions for substance use and psychiatric illness need to be integrated by clinicians into their treatment algorithms and into a variety of health care settings, yet few studies have examined approaches to address alcohol use in HCV-infected patients. Review of the literature found only two integrated treatment studies from a team at the Minneapolis VA (36). In their hepatitis clinic, HCV-infected patients received multi-disciplinary provider consultations and services from a co-located psychiatric clinical nurse specialist (PCNS) who provided cognitive behavioral and motivational therapy. Retrospective chart review indicated that patients seeing the PCNS were more likely to complete an evaluation for and initiate HCV antiviral therapy. Also, of the 47 patients who additionally received one to two brief alcohol counseling sessions by the clinic's medical providers, 36% became alcohol abstinent and 62% decreased their drinking by 50% or more (37).
Like the Minneapolis VA studies, the HCV-alcohol intervention we tested co-located an addictions specialist in the clinic who engaged in multi-disciplinary consults. In contrast, our intervention relied heavily on group therapy, which has been shown to be an effective alcohol treatment format and is less resource-intensive. Our participants received on average more sessions (12.4 versus 4.5). We additionally made explicit efforts to provide education on the relation between liver health and alcohol use, based on the Health Beliefs Model (38), which postulates that treatment participation and adherence are at least partly a function of a person's beliefs about susceptibility to illness, perceived severity of illness, perceived benefits and barriers to treatment, and cues to action. These factors readily apply to persons with HCV who drink alcohol. Relevant content was incorporated into psychoeducation in group and individual therapy sessions.
The integrated HCV-alcohol treatment reported here offers a feasible option to address alcohol use in HCV-infected patients, in a field where there are few rigorously examined options. In future testing in a randomized design, we will know with greater certainty the treatment model's effect size on alcohol use. Nevertheless, this integrated model was associated with substantial reductions in alcohol use for many patients, regardless of gender, age, race, income, and HIV status, thereby offering both providers and patients the opportunity to impact the course of HCV infection.
Acknowledgements
Integrated treatments require the support of all clinic staff. We wish to thank the medical providers of the Duke Liver Clinic, including Manal Abdelmalek, Elizabeth Goacher, Janet Jezsik, Keyur Patel, Dawn Piercy, and Hans Tillmann for their participation in the alcohol screening plan and recruitment of patients. We also thank Matthew Toth, MSW, for providing addictions treatment. The study was funded by a grant from the National Institute of Alcohol Abuse and Alcoholism, 1R21AA017252-01A1.
Footnotes
Publisher's Disclaimer: This is an author-produced PDF of an article accepted for publication in the journal Digestive Diseases and Sciences following peer review. The full article citation is: Proeschold-Bell, R.J., Patkar, A., Naggie, S., Coward, L.J., Mannelli, P., Yao, J., Bixby, P., & Muir, A. (2012). An integrated alcohol abuse and medical treatment model for patients with hepatitis C. Digestive Diseases and Sciences, 57(4), 1083-1091. DOI: 10.1007/s10620-011-1976-4. The final publication is available at: http://www.springerlink.com/content/j4441331643p5722/.
Contributor Information
Rae Jean Proeschold-Bell, Duke Global Health Institute, Center for Health Policy and Inequalities Research, Duke University
Ashwin A. Patkar, Duke Addictions Program and Substance Abuse Consultation Services, Duke University Medical Center.
Susanna Naggie, Duke Clinical Research Institute, Duke School of Medicine, Duke University Medical Center; Division of Infectious Disease, Duke University/Durham VA Medical Center.
Lesleyjill Coward, Department of Gastroenterology, Duke University Medical Center.
Paolo Mannelli, Duke Addictions Program and Substance Abuse Consultation Services, Duke University Medical Center; Department of Psychiatry and Behavioral Sciences, Duke University Medical Center.
Jia Yao, Duke Global Health Institute, Center for Health Policy and Inequalities Research, Duke University.
Patricia Bixby, Department of Medicine, Duke University School of Medicine.
Andrew J. Muir, Duke Clinical Research Institute, Duke School of Medicine, Duke University Medical Center; Department of Gastroenterology, Duke University Medical Center.
References
- 1.Wise M, Bialek S, Finelli L, Bell BP, Sorvillo F. Changing trends in hepatitis C–related mortality in the United States, 1995-2004. Hepatology. 2008;47:1128–1135. doi: 10.1002/hep.22165. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization [May 15th, 2011];Global Alert and Response: Hepatitis C. 2002 from http://www.who.int/csr/disease/hepatitis/whocdscsrlyo2003/en/index8.html.
- 3.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The Prevalence of Hepatitis C Virus Infection in the United States, 1999 through 2002. Ann Intern med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 4.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 5.Wiley TE, McCarthy M, Breidi L, McCarthy M, Layden TJ. Impact of alcohol on the histological and clinical progression of Hepatitis C infection. Hepatology. 1998;28:805–809. doi: 10.1002/hep.510280330. [DOI] [PubMed] [Google Scholar]
- 6.Mochida S, Ohnishi K, Matsuo S, Kakihara K, Fujiwara K. Effect of Alcohol Intake on the Efficacy of Interferon Therapy in Patients with Chronic Hepatitis C as Evaluated by Multivariate Logistic Regression Analysis. Alcohol Clin Exp Res. 1996;20:371A–377A. [PubMed] [Google Scholar]
- 7.Okazaki T, Yoshihara H, Suzuki K, Yamada Y, Tsujimura T, Kawano K, Abe H. Efficacy of Interferon Therapy in Patients with Chronic Hepatitis C: Comparison between Non-Drinkers and Drinkers. Scand J Gastroenterol. 1994;29:1039–1043. doi: 10.3109/00365529409094883. [DOI] [PubMed] [Google Scholar]
- 8.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: An update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, Heathcote EJ, et al. Telaprevir for Previously Treated Chronic HCV Infection. New Eng J Med. 2010;362:1292–1303. doi: 10.1056/NEJMoa0908014. [DOI] [PubMed] [Google Scholar]
- 10.Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, et al. Boceprevir for Untreated Chronic HCV Genotype 1 Infection. New Eng J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisner C, Mertens J, Parthasarathy S, Moore C, Lu Y. Integrating Primary Medical Care With Addiction Treatment. JAMA. 2001;286:1715–1723. doi: 10.1001/jama.286.14.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McManus S, Hipkins J, Haddad P, Guthrie E, Creed F. Implementing an effective intervention for problem drinkers on medical wards. Gen Hosp Psychiat. 2003;25:332–337. doi: 10.1016/s0163-8343(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 13.Reiff-Hekking S, Ockene JK, Hurley TG, Reed GW. Brief physician and nurse practitioner-delivered counseling for high-risk drinking. Results at 12-month follow-up. J Genl Intern Med. 2005;20:7–13. doi: 10.1111/j.1525-1497.2005.21240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming M, Brown R, Brown D. The efficacy of a brief alcohol intervention combined with %CDT feedback in patients being treated for type 2 diabetes and/or hypertension. J Stud Alcohol. 2004;65:631–637. doi: 10.15288/jsa.2004.65.631. [DOI] [PubMed] [Google Scholar]
- 15.Parsons JT, Golub SA, Rosof E, Holder C. Motivational Interviewing and Cognitive-Behavioral Intervention to Improve HIV Medication Adherence Among Hazardous Drinkers: A Randomized Controlled Trial. J Acq Immun Def Synd. 2007;46:443–450. doi: 10.1097/qai.0b013e318158a461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 17.Conigrave KM, Saunders JB, Reznik RB. Predictive capacity of the AUDIT questionnaire for alcohol-related harm. Addiction. 1995;90:1479–1485. doi: 10.1046/j.1360-0443.1995.901114796.x. [DOI] [PubMed] [Google Scholar]
- 18.National Institute of Alchohol Abuse and Alcoholism [May 15th, 2011];Helping patients who drink too much: A clinician's guide. 2005 from http://pubs.niaaa.nih.gov/publications/practitioner/cliniciansguide2005/clinicians_guide.htm.
- 19.Beck AT, Wright FD, Newman CF. Cocaine abuse. In: Freeman A, Dettilio F, editors. Comprehensive Casebook of Cognitive Therapy. Plenum; New York, NY: 1992. pp. 1185–1192. [Google Scholar]
- 20.Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. Guilford Press; New York, NY: 1991. [Google Scholar]
- 21.Prochaska JO, DiClemente CC. Toward a Comprehensive Model of Change. In: Miller WR, Heather N, editors. Treating Addictive Behaviors: Process of Change. Plenum Press; New York, NY: 1986. pp. 3–27. [Google Scholar]
- 22.McGahan P, Griffith J, Parente R, McLellan A. Addiction Severity Index Composite Scores Manual. Department of Veterans Affairs Medical Center; Philadelphia, PA: 1986. [Google Scholar]
- 23.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, et al. The fifth edition of the addiction severity index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 24.Rikoon SH, Cacciola JS, Carise D, Alterman AI, McLellan AT. Predicting DSM-IV dependence diagnoses from Addiction Severity Index composite scores. J Subst Abuse Treat. 2006;31:17–24. doi: 10.1016/j.jsat.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Pessione F, Ramond MJ, Peters L, Pham BN, Batel P, Rueff B, Valla DC. Five-year survival predictive factors in patients with excessive alcohol intake and cirrhosis. Effect of alcoholic hepatitis, smoking and abstinence. Liver Int. 2003;23:45–53. doi: 10.1034/j.1600-0676.2003.01804.x. [DOI] [PubMed] [Google Scholar]
- 26.Pal HR, Yadav D, Mehta S, Mohan I. A comparison of brief intervention versus simple advice for alcohol use disorders in a North India community-based sample followed for 3 months. Alcohol Alcohol. 2007;42:328–332. doi: 10.1093/alcalc/agm009. [DOI] [PubMed] [Google Scholar]
- 27.Patkar AA, Thornton CC, Mannelli P, Hill KP, Gottheil E, Vergare MJ, Weinstein SP. Comparison of Pretreatment Characteristics and Treatment Outcomes for Alcohol-, Cocaine-, and Multisubstance-Dependent Patients. J Addict Dis. 2004;23:93–109. doi: 10.1300/J069v23n01_08. [DOI] [PubMed] [Google Scholar]
- 28.Hassan MM, Hwang L-Y, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, et al. Risk factors for hepatocellular carcinoma: Synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 29.Pessione F, Degos F, Marcellin P, Duchatelle V, Njapoum C, Martinot-Peignoux M, Degott C, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717–1722. doi: 10.1002/hep.510270635. [DOI] [PubMed] [Google Scholar]
- 30.Romero-Gómez M, Grande L, Nogales MC, Fernández M, Chavez M, Castro M. Intrahepatic hepatitis C virus replication is increased in patients with regular alcohol consumption. Digest Liver Dis. 2001;33:698–702. doi: 10.1016/s1590-8658(01)80048-7. [DOI] [PubMed] [Google Scholar]
- 31.McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, McNair L, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. New England Journal of Medicine. 2009;360:1827–1838. doi: 10.1056/NEJMoa0806104. [DOI] [PubMed] [Google Scholar]
- 32.Kwo PY, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, Davis MN, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705–716. doi: 10.1016/S0140-6736(10)60934-8. [DOI] [PubMed] [Google Scholar]
- 33.McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, Nyberg LM, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. New Eng J Med. 2009;361:580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 34.Moyer A, Finney JW, Swearingen CE, Vergun P. Brief interventions for alcohol problems: a meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction. 2002;97:279–292. doi: 10.1046/j.1360-0443.2002.00018.x. [DOI] [PubMed] [Google Scholar]
- 35.Sylvestre D, Loftis J, Hauser P, Genser S, Cesari H, Borek N, Kresina T, et al. Co-occurring hepatitis C, substance use, and psychiatric illness: Treatment issues and developing integrated models of care. J Urban Health. 2004;81:719–734. doi: 10.1093/jurban/jth153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knott A, Dieperink E, Willenbring ML, Heit S, Durfee JM, Wingert M, Johnson JR, et al. Integrated Psychiatric//Medical Care in a Chronic Hepatitis C Clinic: Effect on Antiviral Treatment Evaluation and Outcomes. Am J Gastroenterol. 2006;101:2254–2262. doi: 10.1111/j.1572-0241.2006.00731.x. [DOI] [PubMed] [Google Scholar]
- 37.Dieperink E, Ho SB, Heit S, Durfee JM, Thuras P, Willenbring ML. Significant Reductions in Drinking Following Brief Alcohol Treatment Provided in a Hepatitis C Clinic. Psychosomatics. 2010;51:149–156. doi: 10.1176/appi.psy.51.2.149. [DOI] [PubMed] [Google Scholar]
- 38.Becker M. The Health Belief Model and Personal Health Behavior. Slack; Thorofare, NJ: 1974. [Google Scholar]