Summary
Exposure of human skin to solar ultraviolet radiation (UVR), a powerful carcinogen [1] comprising ~95% UVA and ~5% UVB at the Earth’s surface, promotes melanin synthesis in epidermal melanocytes [2, 3], which protects skin from DNA damage [4, 5]. UVB causes DNA lesions [6] that lead to transcriptional activation of melanin-producing enzymes, resulting in delayed skin pigmentation within days [7]. In contrast, UVA causes primarily oxidative damage [8] and leads to immediate pigment darkening (IPD) within minutes, via an unknown mechanism [9, 10]. No receptor protein directly mediating phototransduction in skin has been identified. Here we demonstrate that exposure of primary human epidermal melanocytes (HEMs) to UVA causes calcium mobilization and early melanin synthesis. Calcium responses were abolished by treatment with G protein or PLC inhibitors, or by depletion of intracellular calcium stores. We show that the visual photopigment rhodopsin [11] is expressed in HEMs and contributes to UVR phototransduction. Upon UVR exposure, significant melanin production was measured within one hour; cellular melanin continued to increase in a retinal- and calcium-dependent manner up to five-fold after 24 hours. Our findings identify a novel UVA-sensitive signaling pathway in melanocytes that leads to calcium mobilization and melanin synthesis, and may underlie the mechanism of IPD in human skin.
Results and Discussion
UVR evokes retinal-dependent calcium flux in human epidermal melanocytes
To investigate UVR-activated signaling pathways, we designed a system that permits real-time imaging of cultured cells and simultaneous exposure to irradiances comparable to solar UVR. Our light source comprises ~90% UVA (320 – 400 nm) and ~10% UVB (280 – 320 nm) and each 10 mJ/cm2 exposure equates to 10 s of solar UVR exposure on a day with a UV index ~10 (Fig. S1 and Supplemental Information). We tested the effect of physiological UVR doses on intracellular calcium (Ca2+) levels in primary human epidermal melanocytes (HEMs) using the fluorometric Ca2+ indicator Fluo-4 AM. We found that UVR (100 mJ/cm2) evoked rapid Ca2+ transients in HEMs pre-incubated with the 11-cis retinal analogue 9-cis retinal (10 μM) [12], but failed to elicit such responses in the absence of retinal (Fig. 1A, B), suggesting the effect is mediated by an opsin-like photopigment. To characterize the irradiance dependence of Ca2+ responses, we measured the amplitude of transients elicited by increasing UVR doses (20 – 150 mJ/cm2) and found that it increased as a function of stimulus irradiance (Fig. 1C, D).
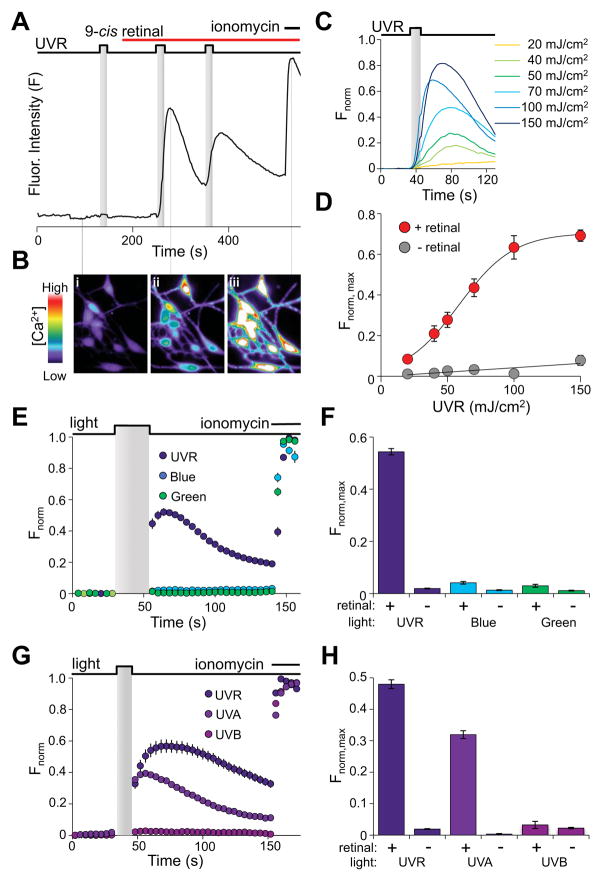
Figure 1. UVR induces retinal-dependent calcium responses in HEMs.
A. Fluorometric Ca2+ imaging trace of a representative HEM stimulated with two 100 mJ/cm2 (10 mW/cm2 for 10 s) UVR pulses shows a measurable increase in fluorescence intensity (F) only after incubation with 9-cis retinal (10 μM). Ionomycin (1 μM) was added for maximal fluorescence intensity.
B. Pseudochrome fluorescence images of HEMs at three time points during the Ca2+ imaging protocol shown in A indicate (i) no Ca2+ increase with UVR stimulation in the absence of 9-cis retinal, (ii) increased Ca2+ in response to UVR applied after incubation with 9-cis retinal, and (iii) maximal Ca2+ increase with ionomycin.
C. Normalized fluorescence intensity (Fnorm) of representative HEMs preincubated with 9-cis retinal (10 μM) and stimulated with 20 – 150 mJ/cm2 of UVR (10 mW/cm2 for 2 – 15 s) as a function of time.
D. Mean amplitudes of Ca2+ responses (Fnorm, max) measured in HEMs exposed to the indicated UVR dose, either with (red) or without (gray) 9-cis retinal represented as a function of dose and fit with a sigmoid function. n ≥ 13 cells for each data point; ± s.e.m.
E. Normalized fluorescence intensity (Fnorm) of HEMs pre-incubated with 9-cis retinal (10 μM) and stimulated with 200 mJ/cm2 (10mW/cm2 for 20 s) UVR (280 – 400 nm), blue light (435 – 460 nm), or green light (500 – 550 nm) as a function of time. Ionomycin (1 μM) was used for normalization. n ≥ 8 cells for each trace; ± s.e.m.
F. Wavelength profile of retinal-dependent Ca2+ responses in HEMs. Peak fluorescence responses (Fnorm, max) of HEMs exposed to 200 mJ/cm2 (20 s of 10 mW/cm2) of UVR (purple), blue light (blue) or green light (green), in the absence (−) or presence (+) of 9-cis retinal (10 μM). Ionomycin (1 μM) was used for normalization. n = 16 – 32 cells, from ≥3 independent experiments; ± s.e.m., P ≤ 0.001 for UVR vs. either blue or green light.
G. Normalized fluorescence intensity (Fnorm) of HEMs pre-incubated with 9-cis retinal (10 μM) and stimulated with 200 mJ/cm2 (20 mW/cm2 for 10 s) UVR (280 – 400 nm; ~90% UVA, ~10% UVB), 180 mJ/cm2 (18 mW/cm2 for 10 s) UVA (320 – 400 nm), or 20 mJ/cm2 (2 mW/cm2 for 10 s) UVB (280 – 320 nm), as a function of time. Ionomycin (1 μM) was used for normalization. n ≥ 8 cells for each condition; ± s.e.m.
H. Mean amplitudes (Fnorm, max) of Ca2+ increases of HEMs exposed to either UVR, UVA or UVB as in G, in the absence (−) or presence (+) of 9-cis retinal (10 μM). Ionomycin (1 μM) was used for normalization. n = 16 – 32 cells from ≥3 independent experiments; ± s.e.m., P < 0.001 for UVR or UVA vs. UVB. See also Fig. S1.
We investigated the spectral sensitivity of Ca2+ transients by stimulating HEMs with 200 mJ/cm2 of UVR (280 – 400 nm), blue light (435 – 460 nm), or green light (500 – 550 nm) (Fig. 1E, F). Only UVR elicited significant Ca2+ transients (normalized peak fluorescence: Fnorm, max = 0.54 ± 0.01, n = 31; P < 0.001). We then exposed HEMs separately to the constituent UVA (320 – 400 nm, 180 mJ/cm2) and UVB (280 – 320 nm, 20 mJ/cm2) doses comprising a 200 mJ/cm2 UVR pulse and found that UVA elicited significantly larger Ca2+ transients than UVB (Fnorm, max = 0.32 ± 0.01, n = 101 for UVA vs. 0.03 ± 0.01, n = 72 for UVB; P < 0.001) (Fig. 1G, H). Thus, retinal-dependent Ca2+ responses are maximally photosensitive to wavelengths between 320 – 400 nm.
A GPCR drives calcium mobilization from intracellular stores in melanocytes
To assess the contributions of extracellular and intracellular Ca2+ to this signaling pathway, we measured UVR-induced responses of HEMs in Ca2+-free extracellular buffer (containing 1.5 mM EGTA) and found they did not significantly differ from those measured in the presence of extracellular Ca2+ (Fig. 2A, B). However, depletion of intracellular Ca2+ stores with thapsigargin (1 μM) [13] abolished UVR-induced transients (Fnorm, max = 0.07 ± 0.03, n = 11 with thapsigargin vs. 0.50 ± 0.02, n = 17 without thapsigargin; P < 0.001) (Fig. 2A, B), indicating that in HEMs, UVR exposure induces Ca2+ mobilization from intracellular stores.
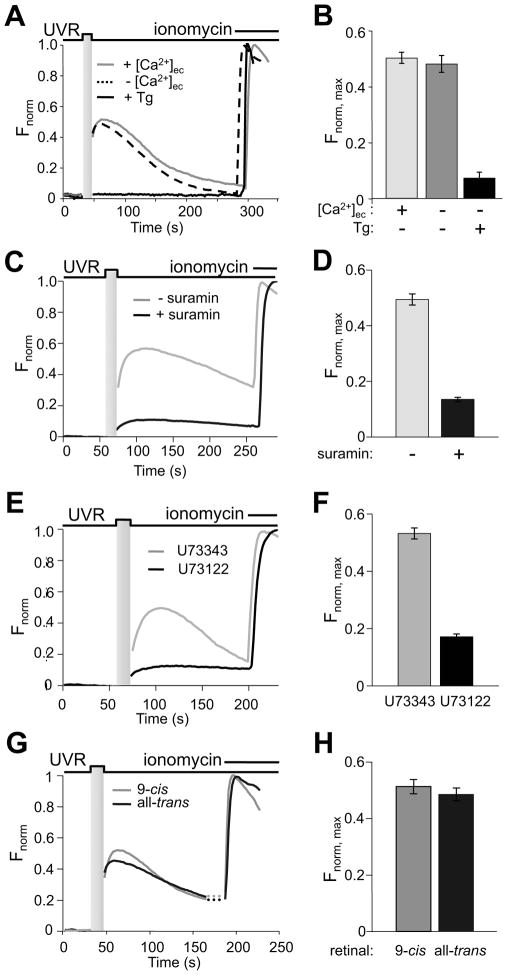
Figure 2. A GPCR mediates retinal-dependent UVR-induced calcium mobilization in HEMs.
A. Dependence of responses on extracellular and intracellular Ca2+. Normalized fluorescence intensity of HEMs pre-incubated with 9-cis retinal (10 μM) and stimulated with UVR (200 mJ/cm2; 20 mW/cm2 for 10 s) with extracellular Ca2+ (1.5 mM, gray), without extracellular Ca2+ (0 mM Ca2+, 1.5 mM EGTA, dashed), or after treatment with thapsigargin (1 μM, black). n = 2 – 6 cells for each condition.
B. Mean peak fluorescence responses (Fnorm, max) of HEMs pre-incubated with 9-cis retinal (10 μM) and stimulated with UVR (200 mJ/cm2; 10 s of 20 mW/cm2) were measured in the presence of extracellular Ca2+ (1.5 mM, light gray), or in the absence of extracellular Ca2+ (0 mM Ca2+, 1.5 mM EGTA, dark gray), or after treatment with thapsigargin (1 μM, black). n = 11 – 17 cells for each condition, from ≥3 independent experiments; ± s.e.m., P < 0.001 for Ca2+ vs. thapsigargin.
C. Effect of G protein inhibition. Normalized fluorescence intensity of HEMs preincubated with 9-cis retinal (10 μM) and stimulated with 250 mJ/cm2 (10 s of 25 mW/cm2) UVR in the absence (gray) or after treatment with 50 μM suramin (black). n = 10 cells for each condition.
D. Suramin treatment (50 μM, black) reduced the mean amplitude of fluorescence responses (Fnorm, max) of HEMs pre-incubated with 9-cis retinal (10 μM) and stimulated with 250 mJ/cm2 (10 s of 25 mW/cm2), compared to control untreated cells (gray) n ≥ 67 cells for each condition, from ≥3 independent experiments; ± s.e.m., P < 0.001.
E. Effects of PLC inhibition. Normalized fluorescence intensity of HEMs pre-incubated with 9-cis retinal (10 μM) and stimulated with 250 mJ/cm2 UVR (25 mW/cm2 for 10 s) and treated with U73343 (9 μM, black) or its inactive analogue U73122 (9 μM, gray). n = 5 – 8 cells for each condition.
F. Mean amplitude of fluorescence responses (Fnorm, max) of HEMs pre-incubated with 9-cis retinal (10 μM) and stimulated with 250 mJ/cm2 (25 mW/cm2 for 10 s) UVR in the presence of U73343 (9 μM, black) or U73122 (9 μM, 5 min, gray). n ≥ 53 cells for each condition, from ≥3 independent experiments; ± s.e.m., P < 0.001.
G. Effects of substituting 9-cis with all-trans retinal on UVR-induced Ca2+ transients. Normalized fluorescence intensity in response to 200 mJ/cm2 (20 mW/cm2 for 10 s) UVR of HEMs pre-incubated with all-trans retinal (10 μM, black) or 9-cis retinal (10 μM, gray). Ionomycin was used for normalization. n = 8 cells for each condition.
H. Mean Peak fluorescence responses (Fnorm, max) of HEMs pre-incubated with all-trans retinal (10 μM, black) or 9-cis retinal (10 μM, gray) and exposed to 200 mJ/cm2 UVR (10 s of 20 mW/cm2). n = 16 cells for each condition, from ≥3 independent experiments; ± s.e.m., P > 0.42.
To test whether Ca2+ mobilization is initiated downstream of G protein-coupled receptor (GPCR) activation, we measured UVR-induced responses in HEMs treated with the G protein inhibitor suramin (50 μM) [14, 15] and found that preincubation significantly reduced Ca2+ responses (Fnorm, max = 0.14 ± 0.01, n = 70 with suramin vs. 0.49 ± 0.02, n = 67 without suramin; P < 0.0001) (Fig. 2C, D). Since G proteins can cause Ca2+ release via phospholipase CβPLCβactivation, we tested the effect of the PLC antagonist U73122 [16] on UVR-induced Ca2+ responses. Treatment of HEMs with U73122 (9 μM), but not its inactive analogue U73343 (9 μM), significantly inhibited UVR-induced Ca2+ transients (Fnorm, max = 0.17 ± 0.01, n = 55 for U73122 vs. 0.53 ± 0.02, n = 53 for U73343; P < 0.001) (Fig. 2E, F) suggesting that UVR-induced Ca2+ responses require PLC activation.
We next evaluated the effects of retinoid substitution. Surprisingly, when we substituted 9-cis with all-trans retinal, UVR-induced Ca2+ transients in HEMs were essentially unchanged (Fig. 2G, H), suggesting that melanocytes may have an intrinsic isomerization mechanism to generate cis from trans retinal.
The retinal dependence of UVR-evoked Ca2+ transients raises the question of an endogenous source in skin. In principle, retinal could be derived from serum retinoids [17] taken up by epidermal cells [18] or from vitamin A (all-trans retinol), which is present at significant concentrations in skin (> 1 nmol/g) [19, 20] and mediates a wide range of cellular processes [21]. Serum retinoids could be converted by the RPE65 isomerase that regenerates cis-retinal in the retina [22, 23] and is expressed in epidermal keratinocytes [24]. Alternatively, vitamin A could be converted to cis-retinal in skin via a pathway similar to the cone visual cycle [25, 26].
The dependence of light-evoked Ca2+ transients on retinal and UVR dose, together with their pharmacological profile, suggests that UVR activates an endogenous opsin receptor in HEMs to initiate Ca2+ mobilization from intracellular stores.
Rhodopsin is expressed in human melanocytes
To identify photopigments that might mediate retinal-dependent Ca2+ signaling in HEMs, we sought to determine opsin expression in these cells. We performed reverse-transcription polymerase chain reaction (RT-PCR) on HEM RNA using degenerate primers corresponding to homologous regions of human opsins (see Supplemental Information) and amplified a ~700 bp transcript corresponding primarily to rhodopsin sequence (Fig. 3A). Subsequent amplifications using rhodopsin-specific primers yielded a ~1 kb transcript corresponding to full-length human rhodopsin (NM_000539) (Fig. 3A). We did not detect expression of any other opsin using primers specific for full-length melanopsin (OPN4) [27, 28], neuropsin (OPN5) [29], or panopsin (OPN3) [30]. We next assessed rhodopsin expression in HEMs by Western blot. Analysis using an anti-rhodopsin antibody revealed a ~37 kDa band in extracts from HEMs and HEK293 cells expressing HA-tagged rhodopsin, but not extracts from untransfected HEK293 cells (Fig. 3B).
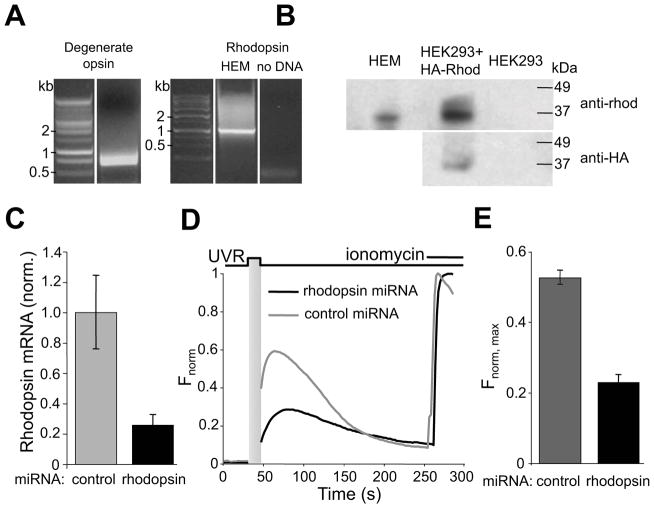
Figure 3. Rhodopsin contributes to UVR-induced calcium mobilization in HEMs.
A. RT-PCR using HEM RNA and either degenerate opsin primers (left) or rhodopsin-specific primers (right) identified a band corresponding to rhodopsin cDNA.
B. Western blot analysis of HEM and HEK293 cell extracts probed with anti-rhodopsin antibody (anti-rhod, top) shows a ~37 kDa band in HEM and HEK293 cells expressing HA-rhodopsin. Anti-HA antibody (anti-HA, bottom) detected a band of similar size in HEK293 cells expressing HA-rhodopsin. Representative of 3 independent experiments.
C. Quantitative PCR analysis of rhodopsin mRNA transcript levels in control and rhodopsin-targeted miRNA-treated HEMs. n = 3; ± s.e.m., P < 0.001.
D. Normalized fluorescence intensity of HEMs pre-incubated with 9-cis retinal (10 μM) and expressing rhodopsin-targeted or control (gray) miRNA. n = 2 – 3 cells for each condition. See also Fig. S2.
E. Mean amplitude of fluorescence responses (Fnorm, max) of HEMs expressing control (gray) or rhodopsin-targeted (black) miRNA, pre-incubated with 9-cis retinal (10 μM) and stimulated with 200 mJ/cm2 (10 s of 20 mW/cm2) UVR. n = 23 – 24 cells for each condition, from ≥3 independent experiments; ± s.e.m., P < 0.002.
To investigate whether rhodopsin contributes to UVR-induced Ca2+ signaling, we reduced endogenous rhodopsin levels using lentivirally transduced microRNAs (miRNAs) and tested the ability of UVR to induce Ca2+ transients in HEMs expressing either rhodopsin-targeted or control miRNA. Treatment with targeted miRNA produced a ~75% reduction in rhodopsin mRNA (mRNA relative to control = 0.21 – 0.33, n = 3; P < 0.001) (Fig. 3C) and markedly reduced Ca2+ transients in response to UVR (Fnorm, max= 0.23 ± 0.02, n = 23 for targeted vs. 0.53 ± 0.02, n = 24 for control miRNA; P < 0.001) (Fig. 3D, E), suggesting that rhodopsin contributes to UVR-induced Ca2+ signaling in HEMs.
These results demonstrate that rhodopsin is expressed in skin [31, 32] and suggest it may contribute to non-visual phototransduction. However, the spectral profile of the light-evoked Ca2+ responses presented here (UVA ≫ UVB > blue ≥ green) is surprisingly different from the spectral sensitivity of rhodopsin (11-cis bound; λmax ~500 nm) or isorhodopsin (9-cis bound; λmax ~478 nm) [33], measured spectrophotometrically [34], electrophysiologically [35] or in a heterologous system [36]. Direct measurement of photopigment absorption in HEMs is not feasible due to the interfering absorptive properties of melanin and low rhodopsin expression levels. Photon absorption by rhodopsin generates the active metarhodopsin II (meta II) intermediate [37], which absorbs maximally at ~380 nm and can regenerate functional pigment upon photon absorption [38]. It is therefore plausible that the cellular environment of HEMs permits stabilization of a meta II-like state that can trigger G protein activation and Ca2+ signaling in response to UVR. Alternatively, the shifted absorption spectrum might result from an endogenous chemical modifier like vitamin A, which increases the sensitivity of rhodopsin to UVR wavelengths [39] or from additional UVR-sensitive molecular components. Recent studies have shown that GPCRs expressed in roundworm neurons [40, 41] and fly larvae [42] mediate retinal-independent UVR-sensing by an unknown mechanism. It is thus conceivable that in skin, rhodopsin, similar to other GPCRs [43], functions as a heterodimer with another opsin or alternate light-sensitive receptor.
UVR causes retinal- and calcium-dependent early melanin synthesis
Opsin-mediated phototransduction in melanocytes might regulate melanogenesis. To test this hypothesis, we irradiated cells with physiological UVR doses (1–5 J/cm2; equivalent to ~20–80 min of UVR exposure on a day with a UV index ~10) and quantified cellular melanin concentration by measuring absorption of purified cellular extracts containing melanin at 405 nm [44]. These exposures did not appreciably alter cellular morphology (Fig. S2A) and resulted in a sustained Ca2+ response (Fig. S2B – E).
To test whether rhodopsin-mediated phototransduction regulates melanogenesis, we measured melanin production in HEMs expressing rhodopsin-targeted or control miRNA and exposed to 4 J/cm2 UVR. We failed to detect significant differences, likely due to the sustained Ca2+ responses caused by residual rhodopsin expression (Fig. 3C and Fig. S2B – C). Instead, we mimicked receptor knockdown by excluding retinal. We compared the melanin concentrations of HEMs at 1, 4, 8 and 24 hours after UVR exposure (4 J/cm2) in the presence or absence of retinal and found that HEMs treated with 9-cis retinal exhibited significantly higher UVR-induced melanin increases compared with cells stimulated in the absence of retinal (relative increase with retinal: 1.51 ± 0.05, n = 9, at 4 h; 2.67 ± 0.22, n = 12, at 8h; 5.03 ± 0.54, n = 12, at 24 h; P ≤ 0.002) (Fig. 4A). Notably, we measured a significant increase in cellular melanin concentration as early as one hour after UVR exposure only in the presence of retinal (1.46 ± 0.10, n = 9 with retinal vs. 1.15 ± 0.08, n = 9 without retinal; P < 0.001) (Fig. 4B). No significant changes in cellular protein concentrations were measured after UVR exposure (two-tailed P > 0.16 for UVR irradiated vs. non-irradiated cells at 1 h, n = 51). Additionally, exposure to increasing irradiances (1 – 5 J/cm2) resulted in proportionally larger retinal-dependent melanin concentrations (Fig. 4C), further evincing a receptor-mediated mechanism.
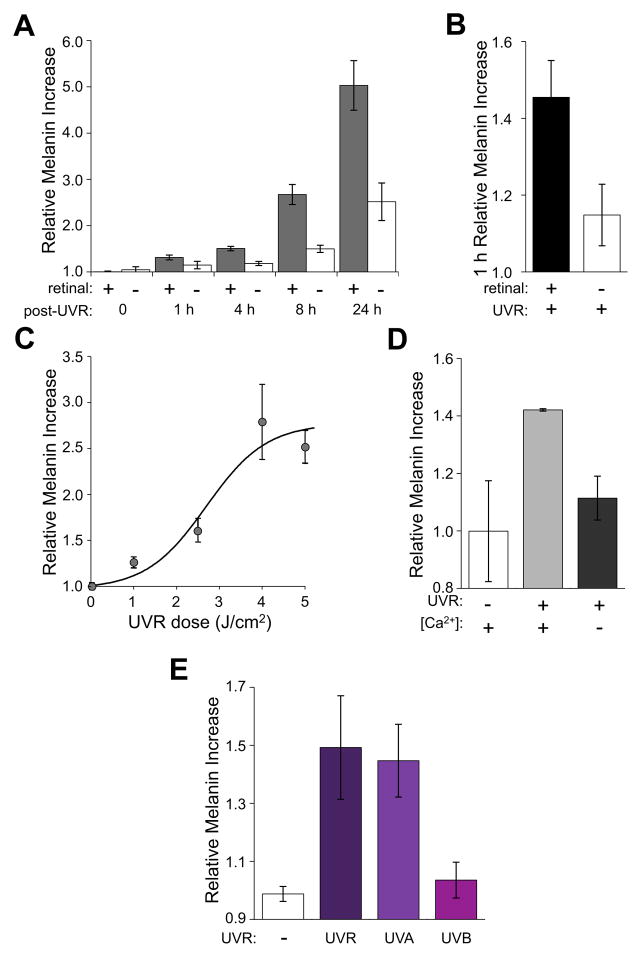
Figure 4. UVA induces retinal-dependent melanin increases in HEMs.
A. Time-dependent changes in intracellular melanin concentration of HEMs preincubated with (gray) or without (white) 9-cis retinal (10 μM) and irradiated with 4 J/cm2 (17.5 mW/cm2 for 228 s) UVR, normalized to melanin concentrations of non-irradiated cells. n = 9 – 30; for each time point; ± s.e.m., P < 0.002 for all time points in the presence of 9-cis retinal vs. untreated.
B. Melanin concentration of HEMs pre-incubated with 9-cis retinal (10 μM, black) quantified 1 h after UVR exposure (4 J/cm2), in parallel with HEMs not treated with retinal (white). n = 9; ± s.e.m., P < 0.001.
C. UVR-dose dependence of intracellular melanin concentration of HEMs pre-incubated with 9-cis retinal (10 μM) and quantified 8 h after exposure to the indicated doses (normalized as in A), and fitted with a sigmoid function. n = 6; ± s.e.m.
D. Intracellular melanin concentration of HEMs preincubated with 9-cis retinal (10 μM) was quantified at 4 h after exposure to 4 J/cm2 (17.5 mW/cm2 for 228 s) either in the presence of Ca2+ (1.5 mM) or in the absence of Ca2+ (0 mM Ca2+, 1.5 mM EGTA, 2 μM thapsigargin and 20 μM BAPTA-AM). n = 2; ± s.e.m., P < 0.04 for Ca2+-free vs. with Ca2+.
E. Melanin concentration of HEMs pre-incubated with 9-cis retinal (10 μM) quantified at 8 h after exposure to UVA (2.15 J/cm2; 15 mW/cm2 for 143 s), UVB (0.215 J/cm2; 1.5 mW/cm2 for 143 s), or the equivalent dose of UVR containing both UVA and UVB (2.5 J/cm2; 17.5 mW/cm2 for 143 s). n = 4 – 6; ± s.e.m., P < 0.02 for UVA vs. UVB and P < 0.043 for UVR vs. UVB. See also Figs. S2 and S3.
We next investigated whether Ca2+ mobilization is required for retinal-dependent early melanin synthesis by measuring the melanin concentration of HEMs stimulated with UVR and incubated under Ca2+-free conditions. Depletion of intracellular stores with thapsigargin (2 μM), combined with intracellular BAPTA (BAPTA-AM, 20 μM) and extracellular EGTA (1.5 mM), abolished sustained UVR-induced Ca2+ transients (Fig. S2E) and markedly reduced retinal-dependent melanin increases at 4 h after exposure to 4 J/cm2 (1.11 ± 0.10, n = 2 for Ca2+-free vs. 1.49 ± 0.01, n = 2 with Ca2+; P < 0.04) (Fig. 4D). Thus, we conclude that in HEMs, UVR-induced Ca2+ release directly contributes to early melanin synthesis within hours of exposure.
Early melanin synthesis is driven by UVA
We reasoned that if retinal-dependent Ca2+ release and early melanin synthesis are part of the same pathway, they should exhibit similar action spectra. To test the wavelength dependence of early melanin synthesis, we exposed HEMs to UVA (2.25 J/cm2), UVB (0.25 J/cm2), or the equivalent dose of UVR (2.5 J/cm2), and measured melanin concentration at 8 h after exposure (Fig. 4E). UVA elicited retinal-dependent melanin increases similar to those measured in response to UVR (1.45 ± 0.13, n = 6 for UVA vs. 1.49 ± 0.18, n = 4 for UVR), while UVB did not significantly alter melanin concentrations under our conditions. The similar spectral sensitivities of early melanogenesis and light-evoked Ca2+ release suggest both events participate in the same phototransduction pathway.
The spectral sensitivity and time scale of the early melanogenesis described here suggests that UVA phototransduction in melanocytes might underlie the elusive mechanism of immediate pigment darkening (IPD). It is widely accepted that UVA causes IPD, but whether UVA exposure results in melanin synthesis [9, 45] or rather in photo-oxidation of existing melanin [46, 47], remains controversial. To distinguish between these possibilities, we exploited the different absorption profiles of oxidized and non-oxidized melanin [48, 49]. We compared the absorption spectra (300 – 500 nm) of our melanin extracts with those of synthetic (non-oxidized) melanin and found that all samples displayed the same characteristic linear absorption profile of synthetic melanin (Fig. S3). We thus conclude that changes in absorption at 405 nm are not due to melanin oxidation or absorption by other cellular components, but instead reflect melanin synthesis.
Melanin synthesis via the UVB/DNA damage pathway occurs >12 h after exposure [7, 50] and requires de novo generation of tyrosinase, the key enzyme required for synthesis. In contrast, the mechanism underling the UVA- and retinal-dependent melanogenesis reported here causes synthesis within 1 – 4 h of exposure. This relatively rapid time course suggests that early synthesis occurs through a novel mechanism, which may use existing tyrosinase that becomes enzymatically active downstream of receptor activation. Such a mechanism could involve phosphorylation of the cytosolic domain of tyrosinase by protein kinase C β [51, 52], a model consistent with the Ca2+-dependence of UVA-induced melanin production in HEMs.
In conclusion, our results demonstrate that human melanocytes use a novel mechanism to sense and respond to ultraviolet light, in which UVA activates endogenous opsin receptors to cause calcium mobilization via a G protein- and PLC-mediated pathway. Our finding that the visual photopigment rhodopsin is expressed in melanocytes and contributes to UVR phototransduction suggests that human opsin receptors function outside the eye. Moreover, in our system, UVA exposure leads to calcium-dependent melanin synthesis on a timescale remarkably faster than that previously reported [50]. This novel UVR phototransduction mechanism has implications for understanding non-ocular opsin signaling pathways and their function in skin physiology and pathology.
Supplementary Material
Highlights.
Exposure of human melanocytes to UV light causes retinal-dependent Ca2+ release
UVA exposure leads to retinal- and Ca2+ -dependent melanin synthesis within one hour
Rhodopsin is expressed in melanocytes and contributes to UV-induced Ca2+ release
Acknowledgments
Primary Support: Grants from Brown University (EO). Additional support: NSERC Post-Graduate Scholarship to NLW; NIH NIAMS Ruth L. Kirschstein Pre-Doctoral Award to JAN. We thank members of the Oancea lab for technical assistance, Drs. C. L. Makino and A. L. Zimmerman for helpful discussions and Drs. D.M. Berson, J.A. Kauer, A.L. Zimmerman, and J. Marshall for critical readings of the manuscript.
Footnotes
Author Contributions: E.O., N.L.W. and J.C. designed the research; E.O. and N.L.W. wrote the paper; E.O and J.W.C designed and built imaging/UVR-stimulation system; N.L.W, J.W.C, J.A.N, and J.M.C collected and analyzed imaging data; N.L.W. and J.M.C. performed melanin quantification assays; J.A.N. performed immunoassays.
References
- 1.U.S. Environmental Agency. Health effects of overexposure to the sun. 2010. [Google Scholar]
- 2.Gilchrest BA, Park HY, Eller MS, Yaar M. Mechanisms of ultraviolet light-induced pigmentation. Photochem Photobiol. 1996;63:1–10. doi: 10.1111/j.1751-1097.1996.tb02988.x. [DOI] [PubMed] [Google Scholar]
- 3.Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–850. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- 4.Pathak M. Functions of melanin and protection by melanin. Melanin: Its Role in Human Photoprotection. 1995:125–134. [Google Scholar]
- 5.Riley P. Melanin. The international journal of biochemistry & cell biology. 1997;29:1235–1239. doi: 10.1016/s1357-2725(97)00013-7. [DOI] [PubMed] [Google Scholar]
- 6.Clingen PH, Arlett CF, Roza L, Mori T, Nikaido O, Green MH. Induction of cyclobutane pyrimidine dimers, pyrimidine(6-4)pyrimidone photoproducts, and Dewar valence isomers by natural sunlight in normal human mononuclear cells. Cancer Res. 1995;55:2245–2248. [PubMed] [Google Scholar]
- 7.Eller MS, Ostrom K, Gilchrest BA. DNA damage enhances melanogenesis. Proc Natl Acad Sci U S A. 1996;93:1087–1092. doi: 10.1073/pnas.93.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrot L, Meunier JR. Skin DNA photodamage and its biological consequences. J Am Acad Dermatol. 2008;58:S139–148. doi: 10.1016/j.jaad.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Pathak MA, Riley FJ, Fitzpatrick TB, Curwen WL. Melanin formation in human skin induced by long-wave ultra-violet and visible light. Nature. 1962;193:148–150. doi: 10.1038/193148a0. [DOI] [PubMed] [Google Scholar]
- 10.Routaboul C, Denis A, Vinche A. Immediate pigment darkening: description, kinetic and biological function. Eur J Dermatol. 1999;9:95–99. [PubMed] [Google Scholar]
- 11.Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan J, Rohrer B, Moiseyev G, Ma J, Crouch R. Isorhodopsin rather than rhodopsin mediates rod function in RPE65 knock-out mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13662. doi: 10.1073/pnas.2234461100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thastrup O, Dawson AP, Scharff O, Foder B, Cullen PJ, Drobak BK, Bjerrum PJ, Christensen SB, Hanley MR. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989;27:17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- 14.Beindl W, Mitterauer T, Hohenegger M, Ijzerman AP, Nanoff C, Freissmuth M. Inhibition of receptor/G protein coupling by suramin analogues. Mol Pharmacol. 1996;50:415–423. [PubMed] [Google Scholar]
- 15.Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- 16.Bleasdale JE, Bundy GL, Bunting S, Fitzpatrick FA, Huff RM, Sun FF, Pike JE. Inhibition of phospholipase C dependent processes by U-73, 122. Adv Prostaglandin Thromboxane Leukot Res. 1989;19:590–593. [PubMed] [Google Scholar]
- 17.Roos TC, Jugert FK, Merk HF, Bickers DR. Retinoid metabolism in the skin. Pharmacol Rev. 1998;50:315–333. [PubMed] [Google Scholar]
- 18.Huang J, Vieira A. Evidence for a specific cell membrane retinol-binding protein transport mechanism in a human keratinocyte line. Int J Mol Med. 2006;17:627–631. [PubMed] [Google Scholar]
- 19.Vahlquist A. Vitamin A in human skin: I. detection and identification of retinoids in normal epidermis. J Invest Dermatol. 1982;79:89–93. doi: 10.1111/1523-1747.ep12500032. [DOI] [PubMed] [Google Scholar]
- 20.Vahlquist A, Lee JB, Michaelsson G, Rollman O. Vitamin A in human skin: II Concentrations of carotene, retinol and dehydroretinol in various components of normal skin. J Invest Dermatol. 1982;79:94–97. doi: 10.1111/1523-1747.ep12500033. [DOI] [PubMed] [Google Scholar]
- 21.Fu PP, Xia Q, Boudreau MD, Howard PC, Tolleson WH, Wamer WG. Physiological role of retinyl palmitate in the skin. Vitam Horm. 2007;75:223–256. doi: 10.1016/S0083-6729(06)75009-9. [DOI] [PubMed] [Google Scholar]
- 22.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinterhuber G, Cauza K, Brugger K, Dingelmaier-Hovorka R, Horvat R, Wolff K, Foedinger D. RPE65 of retinal pigment epithelium, a putative receptor molecule for plasma retinol-binding protein, is expressed in human keratinocytes. J Invest Dermatol. 2004;122:406–413. doi: 10.1046/j.0022-202X.2004.22216.x. [DOI] [PubMed] [Google Scholar]
- 25.Mata NL, Ruiz A, Radu RA, Bui TV, Travis GH. Chicken retinas contain a retinoid isomerase activity that catalyzes the direct conversion of all-trans-retinol to 11-cis-retinol. Biochemistry. 2005;44:11715–11721. doi: 10.1021/bi050942m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mata NL, Radu RA, Clemmons RC, Travis GH. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflugers Arch. 2007;454:849–855. doi: 10.1007/s00424-007-0242-2. [DOI] [PubMed] [Google Scholar]
- 28.Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarttelin EE, Bellingham J, Hankins MW, Foster RG, Lucas RJ. Neuropsin (Opn5): a novel opsin identified in mammalian neural tissue. FEBS Lett. 2003;554:410–416. doi: 10.1016/s0014-5793(03)01212-2. [DOI] [PubMed] [Google Scholar]
- 30.Halford S, Freedman MS, Bellingham J, Inglis SL, Poopalasundaram S, Soni BG, Foster RG, Hunt DM. Characterization of a novel human opsin gene with wide tissue expression and identification of embedded and flanking genes on chromosome 1q43. Genomics. 2001;72:203–208. doi: 10.1006/geno.2001.6469. [DOI] [PubMed] [Google Scholar]
- 31.Tsutsumi M, Ikeyama K, Denda S, Nakanishi J, Fuziwara S, Aoki H, Denda M. Expressions of rod and cone photoreceptor-like proteins in human epidermis. Experimental dermatology. 2009;18:567–570. doi: 10.1111/j.1600-0625.2009.00851.x. [DOI] [PubMed] [Google Scholar]
- 32.Miyashita Y, Moriya T, Kubota T, Yamada K, Asami K. J Investig Dermatol Symp Proc. Vol. 6. Nature Publishing Group; 2001. Expression of opsin molecule in cultured murine melanocyte; pp. 54–57. [DOI] [PubMed] [Google Scholar]
- 33.Makino CL, Groesbeek M, Lugtenburg J, Baylor DA. Spectral tuning in salamander visual pigments studied with dihydroretinal chromophores. Biophys J. 1999;77:1024–1035. doi: 10.1016/S0006-3495(99)76953-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wald G, Brown PK. Human rhodopsin. Science. 1958;127:222–226. doi: 10.1126/science.127.3292.222. [DOI] [PubMed] [Google Scholar]
- 35.Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi W, Osawa S, Dickerson CD, Weiss ER. Rhodopsin mutants discriminate sites important for the activation of rhodopsin kinase and Gt. J Biol Chem. 1995;270:2112–2119. doi: 10.1074/jbc.270.5.2112. [DOI] [PubMed] [Google Scholar]
- 37.Okada T, Ernst OP, Palczewski K, Hofmann KP. Activation of rhodopsin: new insights from structural and biochemical studies. Trends Biochem Sci. 2001;26:318–324. doi: 10.1016/s0968-0004(01)01799-6. [DOI] [PubMed] [Google Scholar]
- 38.Williams TP. Photoreversal of Rhodopsin Bleaching. J Gen Physiol. 1964;47:679–689. doi: 10.1085/jgp.47.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyazono S, Isayama T, Makino CL. Vitamin A as an activator and sensitizing chromophore for rhodopsin. Biophysical Journal Supplement. 2011;100:22a. [Google Scholar]
- 40.Edwards SL, Charlie NK, Milfort MC, Brown BS, Gravlin CN, Knecht JE, Miller KG. A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol. 2008;6:e198. doi: 10.1371/journal.pbio.0060198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Ward A, Gao J, Dong Y, Nishio N, Inada H, Kang L, Yu Y, Ma D, Xu T, et al. C. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog. Nat Neurosci. 2010;13:715–722. doi: 10.1038/nn.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozenfeld R, Devi LA. Exploring a role for heteromerization in GPCR signalling specificity. Biochem J. 2011;433:11–18. doi: 10.1042/BJ20100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oancea E, Vriens J, Brauchi S, Jun J, Splawski I, Clapham DE. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci Signal. 2009;2:ra21. doi: 10.1126/scisignal.2000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parrish JA, Jaenicke KF, Anderson RR. Erythema and melanogenesis action spectra of normal human skin. Photochem Photobiol. 1982;36:187–191. doi: 10.1111/j.1751-1097.1982.tb04362.x. [DOI] [PubMed] [Google Scholar]
- 46.Beitner H. Immediate pigment-darkening reaction. Photodermatol. 1988;5:96–100. [PubMed] [Google Scholar]
- 47.Wolber R, Schlenz K, Wakamatsu K, Smuda C, Nakanishi Y, Hearing VJ, Ito S. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res. 2008;21:487–491. doi: 10.1111/j.1755-148X.2008.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ou-Yang H, Stamatas G, Kollias N. Spectral responses of melanin to ultraviolet A irradiation. J Invest Dermatol. 2004;122:492–496. doi: 10.1046/j.0022-202X.2004.22247.x. [DOI] [PubMed] [Google Scholar]
- 49.Kayatz P, Thumann G, Luther TT, Jordan JF, Bartz-Schmidt KU, Esser PJ, Schraermeyer U. Oxidation causes melanin fluorescence. Invest Ophthalmol Vis Sci. 2001;42:241–246. [PubMed] [Google Scholar]
- 50.Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D’Orazio J, Fung CY, Schanbacher CF, Granter SR, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 51.Park H, Russakovsky V, Ohno S, Gilchrest B. The beta isoform of protein kinase C stimulates human melanogenesis by activating tyrosinase in pigment cells. Journal of Biological Chemistry. 1993;268:11742. [PubMed] [Google Scholar]
- 52.Park H, Perez J, Laursen R, Hara M, Gilchrest B. Protein kinase C- activates tyrosinase by phosphorylating serine residues in its cytoplasmic domain. Journal of Biological Chemistry. 1999;274:16470. doi: 10.1074/jbc.274.23.16470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.