Abstract
Plastic bronchitis (PB) is a poorly understood disease that can complicate any underlying pulmonary disease. However, it appears to most often occur in patients with surgically palliated congenital heart disease, particularly after the Fontan procedure. Few data exist about the prevalence and etiology of PB in this population. In an effort to establish data about prevalence, we conducted a retrospective study of an existing Fontan surgery database (n = 654) comprised of data, including sex, age at date of surgery, alive/dead status, New York Heart Association classification at last follow-up, right-ventricular end-diastolic pressure and pulmonary artery pressure before Fontan surgery, and the presence of a Fontan fenestration. An initial medical record review of 173 patients in the database who were followed at the University of Michigan identified seven patients with PB resulting in an estimated prevalence of 4 %. Subsequently, 14 % of 211 surveyed patients reported that they presently expectorate mucus or fibrin plugs (casts). Demographic and clinical variables did not differ between patients with or without possible PB. Collectively, these findings suggest that Fontan patients presently with PB may range from 4 to 14 %, indicating potential under-diagnosis of the disease. There were no remarkable physical or hemodynamic indicators that differentiated patients with or without possible PB. These data also highlight the need for more elaborate, prospective studies to improve our understanding of PB pathogenesis so that more definitive diagnostic criteria for this devastating disease can be established and its prevalence more accurately determined.
Keywords: Congenital heart disease, Hypoplastic left heart syndrome, Fontan procedure adverse effects
Introduction
Plastic bronchitis (PB) is an uncommon condition that has been reported most often in children [2, 12, 16, 20]. However, because PB has not been extensively studied, its prevalence is unknown and its cause is poorly understood. It is characterized by the formation of large, cohesive, rubbery plugs of mucus and fibrin in the tracheobronchial airways. Patients may present with cough, dyspnea, fever, wheezing, and persistent pulmonary obstruction, which can lead to death [3, 19, 20]. The diagnosis of PB, however, hinges on visualization of an airway cast by either bronchoscopy or after expectoration. It has been our experience that cast formation and expectoration can vary over time (months) and that respiratory symptoms, such as cough and wheezing, can be present with or without obvious cast expectoration.
The primary source of information on PB originates from published case reports. A recent review highlighted the broad range of conditions in which PB can occur in children, including sickle cell disease, viral infection, and smoke inhalation [8]. In addition, PB has been reported in patients with asthma and cystic fibrosis and, more recently, in children with H1N1 influenza [5, 7, 8]. Despite the variety of conditions that underlie PB, it seems to most often happen in children with congenital heart disease (CHD), particularly in patients with single-ventricle physiologies that have been surgically palliated with Fontan surgery [1, 5, 8, 18].
The pathophysiology leading to PB in Fontan patients remains unclear. Presently, PB is considered a rare (≤1 %) pulmonary complication of the Fontan circuit [10, 11]. It has been proposed that Fontan circulation, which can lead to a number of complications, contributes to the development of PB secondary to disrupted hemodynamics. These alterations can be caused by increased pulmonary vascular pressure, valve dysfunction, or intractable arrhythmias [6, 8], all of which can contribute to injury of the alveolar–capillary barrier [11]. Lymphatic dysfunction may also be a contributing factor to the etiology of PB [15]. This may be propagated by increased pulmonary pressures or occur secondary to injury of the lymphatic system during surgery. This hypothesis is supported by evidence that thoracic duct ligation can remedy PB [17]. However, this approach is not consistently curative [15]. Collectively, these observations suggest that both the vascular and lymphatic systems may contribute to the pathogenesis of PB [8]. However, because knowledge of PB primarily stems from case reports and reviews, it is presently unknown whether specific individual demographic and historical factors predispose Fontan patients to develop PB.
The University of Michigan has performed more than 600 Fontan surgeries since 1992. A database of patient demographic and hemodynamic variables was retrospectively compiled [13, 14]. Given our poor understanding of the prevalence and pathogenesis of PB, it has been difficult to direct future research toward more definitive diagnostic criteria and treatment. This study aimed to improve our knowledge regarding PB by studying a large cohort of patients in the University of Michigan Congenital Heart Surgery database. The study goals were to estimate the prevalence of PB and to determine if differences in individual patient factors exist between patients who develop PB and those who do not.
Methods
The study was a retrospective medical record review of the 654 patients who underwent Fontan surgery at the University of Michigan C. S. Mott Children’s Hospital between 1992 and 2007 and a survey of the surviving patients [13, 14]. The study was approved by the University of Michigan Health System’s (UMHS) Institutional Review Board (HUM 00026149 and HUM 00031493).
Medical Record Review
The entire database (n = 654) of Fontan procedures, including Fontan revisions, was initially interrogated for possible cases of PB by cross-referencing it with International Classification of Diseases (ICD)-9 and procedure codes 466, 33.22, and 33.23 for PB, flexible bronchoscopy, and rigid bronchoscopy, respectively (Fig. 1a). To test the validity of the query logic, 100 Fontan cases that lacked these ICD-9 codes were randomly selected from the database. The medical records of these patients were reviewed for diagnostic evidence of PB.
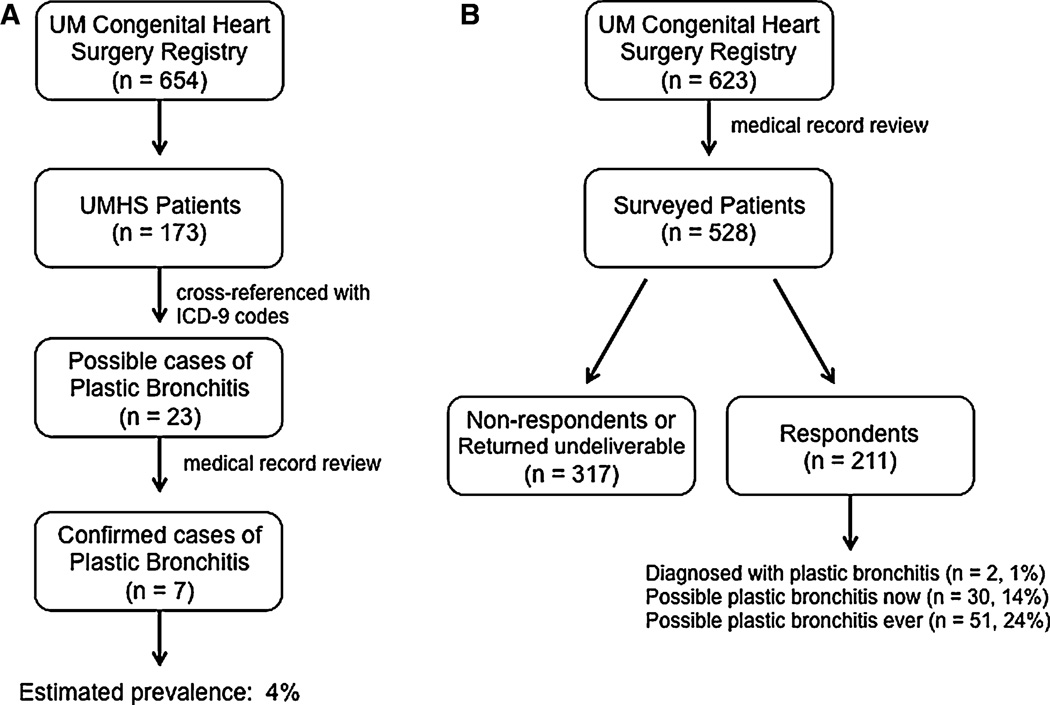
Fig. 1.
a Schematic representation of the medical record review phase of the study. Of the 654 patients in the database, 26.5 % of them were followed-up at UMHS. Cross-referencing these records with ICD-9 and procedure codes 466, 33.22, and 33.23 for PB, flexible bronchoscopy, and rigid bronchoscopy, respectively, resulted in the identified 23 probable cases. Subsequent medical record review confirmed seven cases. b Schematic representation of the mail-survey portion of the study. After the application of exclusion criteria (see text), the survey was mailed to 528 patients. Of the 211 survey respondents, 1 % reported having been diagnosed with PB, whereas 14 and 24 %, respectively, reported having possible PB now or ever
For the cases identified by ICD-9 codes and the 100 control cases, patients were categorized as having PB if there was documentation in the medical record of the diagnosis, cast production, or removal of casts by bronchoscopy. Hospital readmissions, clinic visit records, and pathology reports were also reviewed to aid in the identification of patients with PB. The utility of this strategy was limited because is it was only able to identify PB cases in patients who were followed-up by the UMHS and included older (adolescent and adult) patients who underwent Fontan revision surgery. To evaluate the approximately 75 % of our Fontan patients who are followed outside UMHS, and to better evaluate only patients after their primary Fontan palliation, we conducted a survey to query patients who had undergone a primary Fontan surgery at the University of Michigan about airway mucus or fibrin plug (cast) expectoration.
PB Survey
In advance of the survey, we reviewed the medical record of patients in the University of Michigan Congenital Heart Surgery database who had undergone primary Fontan surgery to confirm alive/dead status and acquire mailing addresses (Fig. 1b). A survey packet was sent to patients or the parent/guardian of patients in the database. The packet included a cover letter describing the survey. Within the cover letter was the following description of PB: “PB is the formation and expectoration of mucus or fibrous plugs called ‘casts’ that form in the lungs and cause coughing or difficulty breathing.” Patients were excluded if they were >60 months at the date of operation (DOO), dead, confirmed as having PB by medical record review, or if they had a non-United States or P.O. Box mailing address. All surveys were sent by way of the United States Postal Service (USPS) with an enclosed prepaid return envelope. Approximately 30 days after the survey mailing, a reminder card was sent. Survey responses were recorded as they were received. If the survey was not returned after 90 days, the patient was categorized as a nonresponder. Questionnaires or reminder cards that were returned by the USPS as “no longer at this address,” “undeliverable,” “address unknown” or “unable to forward” were categorized as not deliverable (ND). No other means (e.g., phone) of contact were used, and no additional contact attempts were made. The questionnaire included four items to assess whether the patient had ever or was currently producing casts or had been diagnosed with PB (Table 1).
Table 1.
Questions asked of survey recipients (yes or no)
| The patient regularly produces and coughs up mucus or fibrous plugs (casts) |
| The patient has never produced or coughed up mucus or fibrous plugs (casts) |
| The patient has produced mucus or fibrous plugs (casts) in the past but presently is not producing them |
| The patient has been diagnosed with PB (now or anytime in the past) |
Patients who answered “yes” to question no. 1 were categorized as PB now (PB now); patients who answered “yes” to questions nos. 3 or 4 were categorized as PB ever (PB ever)
University of Michigan Congenital Heart Surgery Database
The following information was extracted from the congenital heart surgery database for analysis: sex; age at DOO; New York Heart Association (NYHA) classification at last follow-up; right-ventricular end-diastolic pressure (RVEDP) and pulmonary artery pressure before Fontan surgery; whether the Fontan had been fenestrated at the time of surgery (early); and whether it was present at the most recent (late) follow-up. All patients in the database had a single-ventricle variant with the following associated diagnoses: hypoplastic left heart syndrome (HLHS), dextrocardia, truncus arteriosus, double-inlet left ventricle (DILV), pulmonary atresia (PA), tetralogy of Fallot (TOF), single ventricle (SV), complete atrioventricular septal defect (CAVSD), Ebstein’s anomaly, pulmonary atresia with an intact ventricular septum (PA/IVS), tricuspid atresia (TA), transposition of the great arteries (TGA), and heterotaxy.
Data and Statistical Analysis
Descriptive data were generated from the ICD-9 cross-referencing scheme and the medical record review of the 654 patients in the Fontan cohort. Because both UMHS and non-UMHS patients were surveyed, we used the data generated from the survey respondents for our primary statistical analysis. Patients were classified as possibly having PB now if they answered “yes” to question no. 1; as possibly having PB ever if they answered “yes” to question no. 3; and as having PB if they answered “yes” to question no. 4 (Table 1). Patients were further categorized as UMHS or non-UMHS and as respondents or ND. An assessment of response bias was made based on the whether or not surveyed patients responded. The two groups were compared using unpaired Student t test and a chi-square test for continuous variables and categorical data, respectively. All statistical analyses were performed using SPSS (PASW Statistics 18, Armonk, NY). In all cases, p ≤ 0.05 was considered statistically significant. Figures were constructed using GraphPad Prism (GraphPad Software, La Jolla, CA).
Results
From the 654 Fontan cases in the congenital heart surgery database from 1992 to 2007, the ICD-9 cross-referencing logic identified 23 possible cases of PB in patients followed-up by UMHS (n = 173; Fig. 1a). All 23 possible cases of PB were in patients who were age <60 months at DOO. Review of the medical records of these patients resulted in the confirmation of seven cases of PB. The medical record review of the 100 randomly selected patients identified one additional case of PB. The seven cases of PB identified by the medical record review logic represent an estimated prevalence of PB within this cohort of 4 % (Fig. 1a). The mean (±SEM) time from DOO to onset of PB in these patients was 26.6 ± 13.7 months.
A total of 528 patients were sent the questionnaire (Fig. 1b; Table 1). The demographics of the surveyed patients are listed in Table 2. The overall response rate to the questionnaire was 40 %. There were 213 nonresponders, and 104 surveys were returned as ND. Of the respondents, none of the patients who answered “no” to question no. 2 responded “yes” to either question nos. 3 or 4. Based on the variables in the database, there was no apparent response bias with the exception that patients with HLHS were more likely to respond to the survey (p = 0.016); this likely reflects the CHD patient population followed by the UMHS [13].
Table 2.
Demographics of surveyed Fontan patients (n = 528)
| Male (%) | 333 (63.1) |
| Age (mean ± SEM) at DOO (months) | 28.5 ± 0.6 |
| Followed-up at UMHS (%) | 122 (23) |
| Underlying CHD (%)a | |
| HLHS | 274 (51.9) |
| TA | 81 (15.3) |
| TGA | 77 (14.6) |
| PA | 66 (12.5) |
| DILV | 62 (11.7) |
| PA/IVS | 42 (8.0) |
| CAVSD | 31 (5.9) |
| Heterotaxy | 15 (2.8) |
| Ebstein’s anomaly | 8 (1.5) |
| SV | 7 (1.3) |
| Dextrocardia | 2 (0.4) |
| TA | 2 (0.4) |
| TOF | 1 (0.2) |
The addition of percentages exceeds 100 because some patients had more than one condition
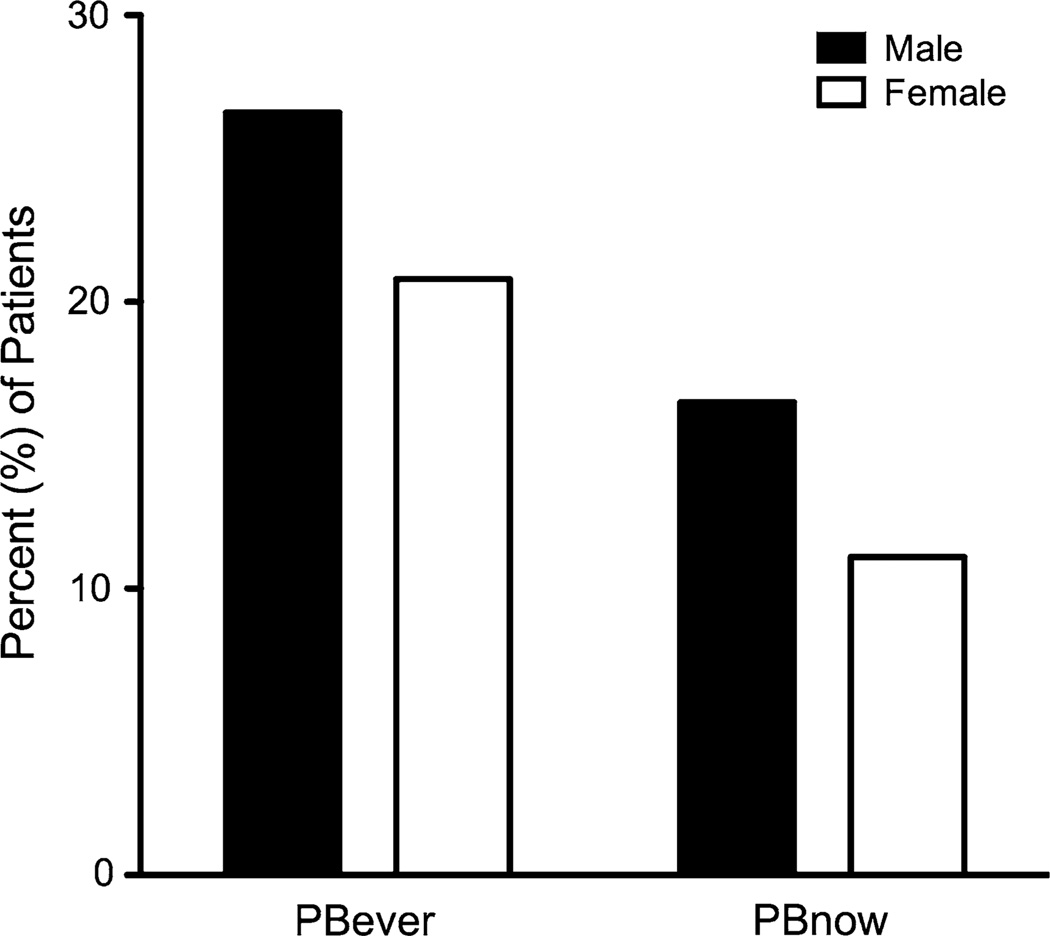
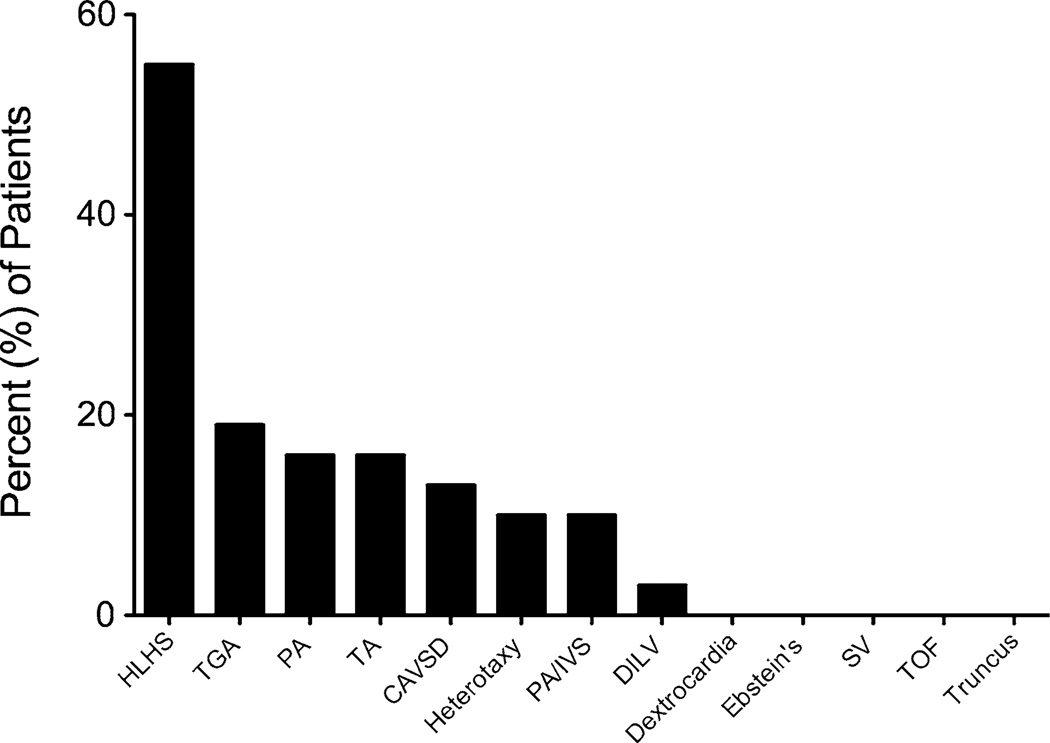
The percent of surveyed patients who responded that they have expectorated a cast or plug now or ever was higher, 14 and 24 %, respectively, than determined by medical record review and by those who answered that they have been diagnosed with PB (1 %) (Fig. 1b). The male-to-female ratio of patients that reported possible PB ever was 2.5:1 (n = 51) compared with 1.8:1 for patients without cast or plug expectoration (n = 159; p = 0.35). The male-to-female ratio of patients who reported having cast or plug expectoration now was similar to that of having cast or plug expectoration ever, but the percentages of patients were lower, thus illustrating the sporadic pattern of cast production that often occurs in patients who have PB (Fig. 2). Compared with patients without cast or plug expectoration, patients who responded to the survey as having cast or plug expectoration now tended to be older (mean ± SEM 31.1 ± 2.8 vs. 26.9 ± 0.9 months; p = 0.097) at the DOO. The distribution of types of CHD in patients with possible PB is shown in Fig. 3. Neither underlying CHD nor any other evaluated parameter, including NYHA classification, PA and RVEDP, or early or late fenestration, differentiated patients with possible PB from those without it. In our cohort, most patients had a fenestration at the DOO [14]. Notably, known mortality in patients with medical record-confirmed PB was higher than that of the entire Fontan database (38 vs. 9 %, p = 0.03); however, the cause of death could not be directly attributed to PB.
Fig. 2.
There was no difference in the number of male (26.6 %) and female (20.8 %) survey respondents who reported having possible PB ever (PB ever; p = 0.355) or having possible PB now (PB now; male 16.5 % vs. female 11.1 %, p = 0.290)
Fig. 3.
Distribution of underlying CHD among surveyed primary-Fontan patients who reported having possible PB now (n = 31). Percentages do not total 100 because some patients had more than one condition. The surplus of patients with HLHS reflects the specialty of the University of Michigan’s Congenital Heart Program
Discussion
In this first retrospective review of a large cohort of patients with Fontan physiology, we used two different strategies to estimate the prevalence of PB in post-Fontan patients. From this, we estimated that the prevalence of patients with possible active PB may be somewhere between 4 and 14 % based on results of a review of medical records and a self-report survey. In addition, we also showed that cases of PB were not associated with any specific differentiating clinical features present at the DOO or at the most recent follow-up visit. We recognize, however, that data from a voluntary response survey of patients asked about plug or cast expectoration are likely not indicative of the actual overall prevalence of the disease in part because of the absence of definitive diagnostic criteria for PB. Nevertheless, our findings raise the possibility that PB may be more common than currently recognized.
Most often, PB is considered a complication of Fontan surgery that can manifest months to years after surgery and is associated with a poor prognosis [4, 9, 10, 18]. What remains perplexing is why some Fontan patients develop PB and others do not. This suggests that there may be physiologic factors contributing to the pathogenesis of PB. We assessed a broad range of variables in our analysis, including fenestration, which may be indicative of high pulmonary pressure and the development of PB, none of which were associated with PB [10]. Our measurements were obtained either before Fontan surgery or at most recent follow-up visit, the occurrence of which varied across the cohort. It is therefore possible that physiologic variables, such as worsening hemodynamic parameters over time and/or the existence of other noncardiac anomalies or genetic abnormalities, which we did not assess, could contribute to the development of PB. These associations may exist because PB is not typically a perioperative or early postoperative complication of Fontan surgery as illustrated by the mean onset of nearly 27 months from the DOO in patients with confirmed PB.
We acknowledge that use of a retrospective analysis and survey to characterize prevalence has limitations. The random selection and review of 100 medical records resulted in the identification of one case of PB missed by our query logic. This suggests that there may have been other cases missed by this approach. However, given that the estimated prevalence of PB in the University of Michigan’s Fontan population was found to be higher than expected based on its presumed rareness (≤1 %), missed cases would have further increased this surprising result. Alternatively, we acknowledge that nonrespondents and ND surveys could have swayed the prevalence lower if these patients were indeed negative for PB. The difficulty in assessing the prevalence of PB is further complicated by the disease’s diagnostic criterion, i.e., direct visualization of an airway cast. As such, it is possible that some patients with respiratory symptoms, such as cough and/or periodic wheezing and oxygen desaturation, may either never form casts of sufficient size to expectorate or form and expectorate small inconsequential casts that go unnoticed. These patients may be spuriously diagnosed with asthma, chronic bronchitis, or pneumonia when in fact they actually have subclinical PB. Another potentially confounding variable is that surveyed patients who have mucus plugging and expectoration may have self-reported as having possible PB because our survey included the term “mucus plugs.” From a clinical perspective, the presence of mucus plugs would not be characterized as PB. In the absence of definitive diagnostic criteria and given the recently discovered information (postsurvey distribution) that PB airway casts from children with CHD are most often comprised of fibrin, the use of this nonspecific terminology may have resulted in patients inaccurately reporting themselves or their child as having PB [12]. If this occurred, it explains, at least in part, the much higher prevalence of PB in surveyed patients compared with that determined by medical record review. Nevertheless, and despite the absence of definitive diagnostic criteria, our findings are important because they introduce the possibility that PB, in particular PB that has not been formally diagnosed and may be subclinical in severity, could be more prevalent than currently realized. They also highlight the need for continued efforts to improve understanding of the natural history and pathogenesis of the illness. In doing so, diagnostic criteria and prevalence can be more accurately defined. This should include continued studies of detailed characterization of cast composition and content, which may also provide insight into disease etiology.
In summary, our findings illustrate that Fontan-associated PB may be more common than previously recognized. Based on our analysis of an existing database of Fontan patients, there were no remarkable clinical indicator(s) or historical factors, either physical (e.g., sex, underlying CHD) or hemodynamic that differentiate patients who develop PB from those that do not. Our results also highlight the need for more elaborate, prospective studies that can include additional risk factors to determine PB pathogenesis so that more definitive diagnostic criteria and effective therapy can be established.
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant No. R15 HD065594 (KAS). One of the objectives of the R15 funding mechanism is to expose students to research. We therefore acknowledge the work of the four student coauthors (M. K., A. L., E. K., and E. G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health.
Contributor Information
Regine L. Caruthers, Department of Pharmacy, University of Michigan Hospitals and Health Centers, Ann Arbor, MI, USA
Mollie Kempa, Department of Pharmacy, University of Michigan Hospitals and Health Centers, Ann Arbor, MI, USA.
Angela Loo, Department of Clinical, Social and Administrative Sciences, College of Pharmacy, University of Michigan, Ann Arbor, MI, USA.
Erin Gulbransen, Department of Clinical, Social and Administrative Sciences, College of Pharmacy, University of Michigan, Ann Arbor, MI, USA.
Elizabeth Kelly, Department of Clinical, Social and Administrative Sciences, College of Pharmacy, University of Michigan, Ann Arbor, MI, USA.
Steven R. Erickson, Department of Clinical, Social and Administrative Sciences, College of Pharmacy, University of Michigan, Ann Arbor, MI, USA
Jennifer C. Hirsch, Department of Cardiac Surgery, Pediatric Surgery, School of Medicine, University of Michigan, Ann Arbor, MI, USA
Kurt R. Schumacher, Department of Pediatrics, Pediatric Cardiology, School of Medicine, University of Michigan, Ann Arbor, MI, USA
Kathleen A. Stringer, Email: stringek@umich.edu, Department of Clinical, Social and Administrative Sciences, College of Pharmacy, University of Michigan, Ann Arbor, MI, USA.
References
- 1.Angelos P, MacArthur C. Pediatric plastic bronchitis: a case report and literature review. Int J Pediatr Otorhinolaryngol Extra. 2010;5:66–69. [Google Scholar]
- 2.Brogan TV, Finn LS, Pyskaty DJ, Jr, Redding GJ, Ricker D, Inglis A, et al. Plastic bronchitis in children: a case series and review of the medical literature. Pediatr Pulmonol. 2002;34:482–487. doi: 10.1002/ppul.10179. [DOI] [PubMed] [Google Scholar]
- 3.Cajaiba MM, Borralho P, Reyes-Mugica M. The potentially lethal nature of bronchial casts: plastic bronchitis. Int J Surg Pathol. 2008;16:230–232. doi: 10.1177/1066896907307234. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhari M, Stumper O. Plastic bronchitis after Fontan operation: treatment with stent fenestration of the Fontan circuit. Heart. 2004;90:801. doi: 10.1136/hrt.2003.029041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costello JM, Steinhorn D, McColley S, Gerber ME, Kumar SP. Treatment of plastic bronchitis in a Fontan patient with tissue plasminogen activator: a case report and review of the literature. Pediatrics. 2002;109:e67. doi: 10.1542/peds.109.4.e67. [DOI] [PubMed] [Google Scholar]
- 6.Davies RR, Chen JM, Mosca RS. The Fontan procedure: evolution in technique; attendant imperfections and transplantation for “failure”. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2011;14:55–66. doi: 10.1053/j.pcsu.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Deng J, Zheng Y, Li C, Ma Z, Wang H, Rubin BK. Plastic bronchitis in three children associated with 2009 influenza A(H1N1) virus infection. Chest. 2010;138:1486–1488. doi: 10.1378/chest.10-0548. [DOI] [PubMed] [Google Scholar]
- 8.Do P, Randhawa I, Chin T, Parsapour K, Nussbaum E. Successful management of plastic bronchitis in a child post Fontan: case report and literature review. Lung. 2012 Mar 20; doi: 10.1007/s00408-012-9384-x. [DOI] [PubMed] [Google Scholar]
- 9.Fredenburg TB, Johnson TR, Cohen MD. The Fontan procedure: anatomy, complications, and manifestations of failure. Radiographics. 2011;31:453–463. doi: 10.1148/rg.312105027. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg DJ, Dodds K, Rychik J. Rare problems associated with the Fontan circulation. Cardiol Young. 2010;20(Suppl 3):113–119. doi: 10.1017/S1047951110001162. [DOI] [PubMed] [Google Scholar]
- 11.Healy F, Hanna BD, Zinman R. Pulmonary complications of congenital heart disease. Paediatr Respir Rev. 2012;13:10–15. doi: 10.1016/j.prrv.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Heath L, Ling S, Racz J, Mane G, Schmidt L, Myers JL, et al. Prospective, longitudinal study of plastic bronchitis cast pathology and responsiveness to tissue plasminogen activator. Pediatr Cardiol. 2011;32:1182–1189. doi: 10.1007/s00246-011-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch JC, Ohye RG, Devaney EJ, Goldberg CS, Bove EL. The lateral tunnel Fontan procedure for hypoplastic left heart syndrome: results of 100 consecutive patients. Pediatr Cardiol. 2007;28:426–432. doi: 10.1007/s00246-007-9002-5. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch JC, Goldberg C, Bove EL, Salehian S, Lee T, Ohye RG, et al. Fontan operation in the current era: a 15-year single institution experience. Ann Surg. 2008;248:402–410. doi: 10.1097/SLA.0b013e3181858286. [DOI] [PubMed] [Google Scholar]
- 15.Languepin J, Scheinmann P, Mahut B, Le Bourgeois M, Jaubert F, Brunelle F, et al. Bronchial casts in children with cardiopathies: the role of pulmonary lymphatic abnormalities. Pediatr Pulmonol. 1999;28:329–336. doi: 10.1002/(sici)1099-0496(199911)28:5<329::aid-ppul4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 16.Seear M, Hui H, Magee F, Bohn D, Cutz E. Bronchial casts in children: a proposed classification based on nine cases and a review of the literature. Am J Respir Crit Care Med. 1997;155:364–370. doi: 10.1164/ajrccm.155.1.9001337. [DOI] [PubMed] [Google Scholar]
- 17.Shah SS, Drinkwater DC, Christian KG. Plastic bronchitis: Is thoracic duct ligation a real surgical option? Ann Thorac Surg. 2006;81:2281–2283. doi: 10.1016/j.athoracsur.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Tzifa A, Robards M, Simpson JM. Plastic bronchitis: a serious complication of the Fontan operation. Int J Cardiol. 2005;101:513–514. doi: 10.1016/j.ijcard.2004.03.085. [DOI] [PubMed] [Google Scholar]
- 19.Wakeham MK, Van Bergen AH, Torero LE, Akhter J. Long-term treatment of plastic bronchitis with aerosolized tissue plasminogen activator in a Fontan patient. Pediatr Crit Care Med. 2005;6:76–78. doi: 10.1097/01.PCC.0000149320.06424.1D. [DOI] [PubMed] [Google Scholar]
- 20.Werkhaven J, Holinger LD. Bronchial casts in children. Ann Otol Rhinol Laryngol. 1987;96:86–92. doi: 10.1177/000348948709600121. [DOI] [PubMed] [Google Scholar]