Abstract
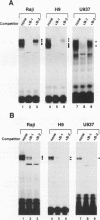
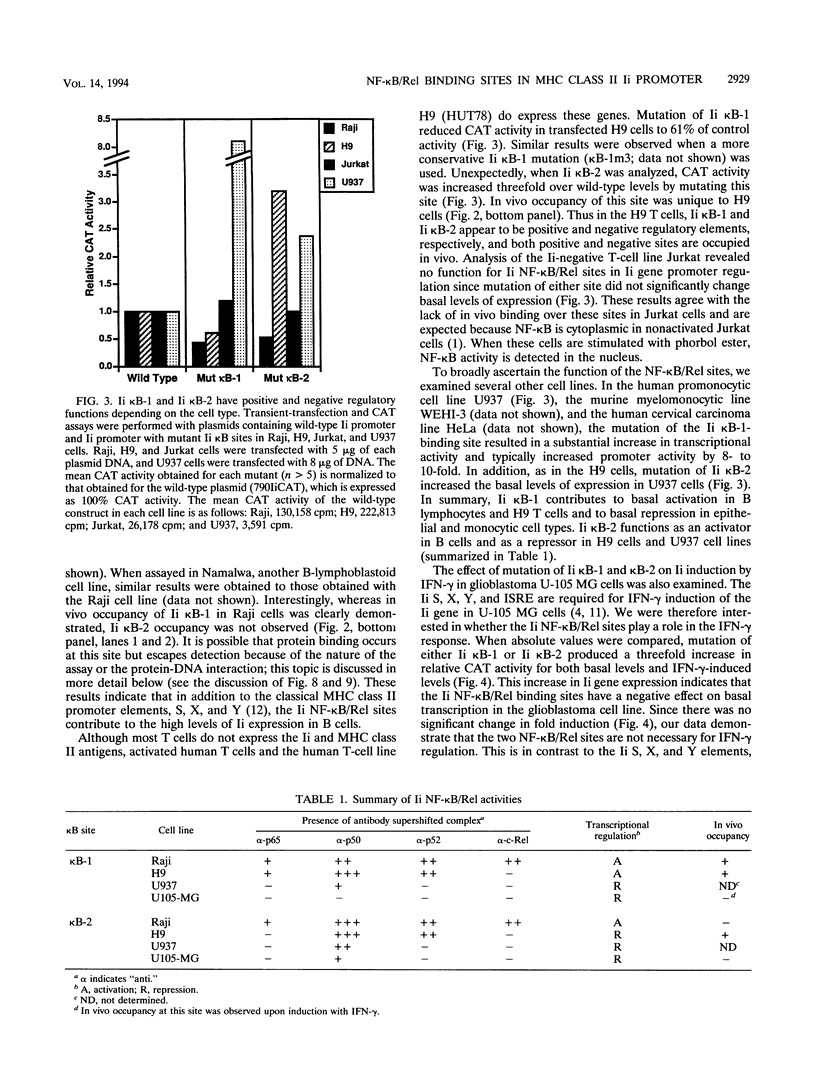
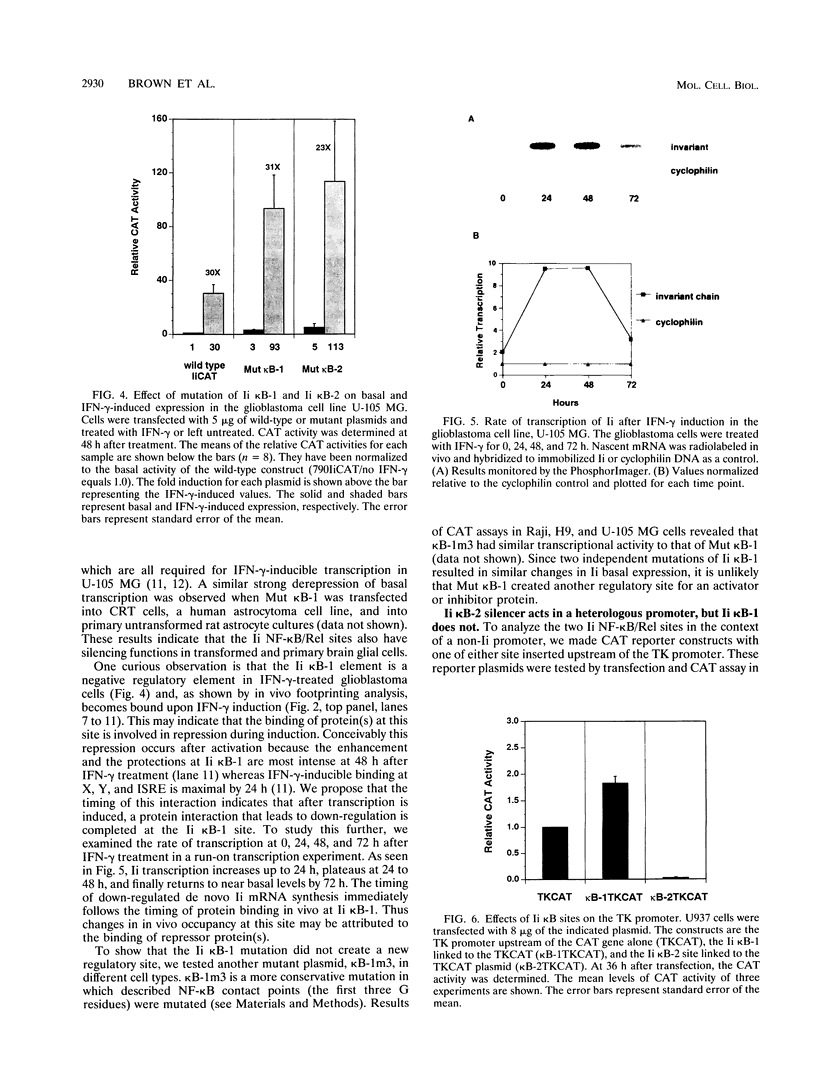
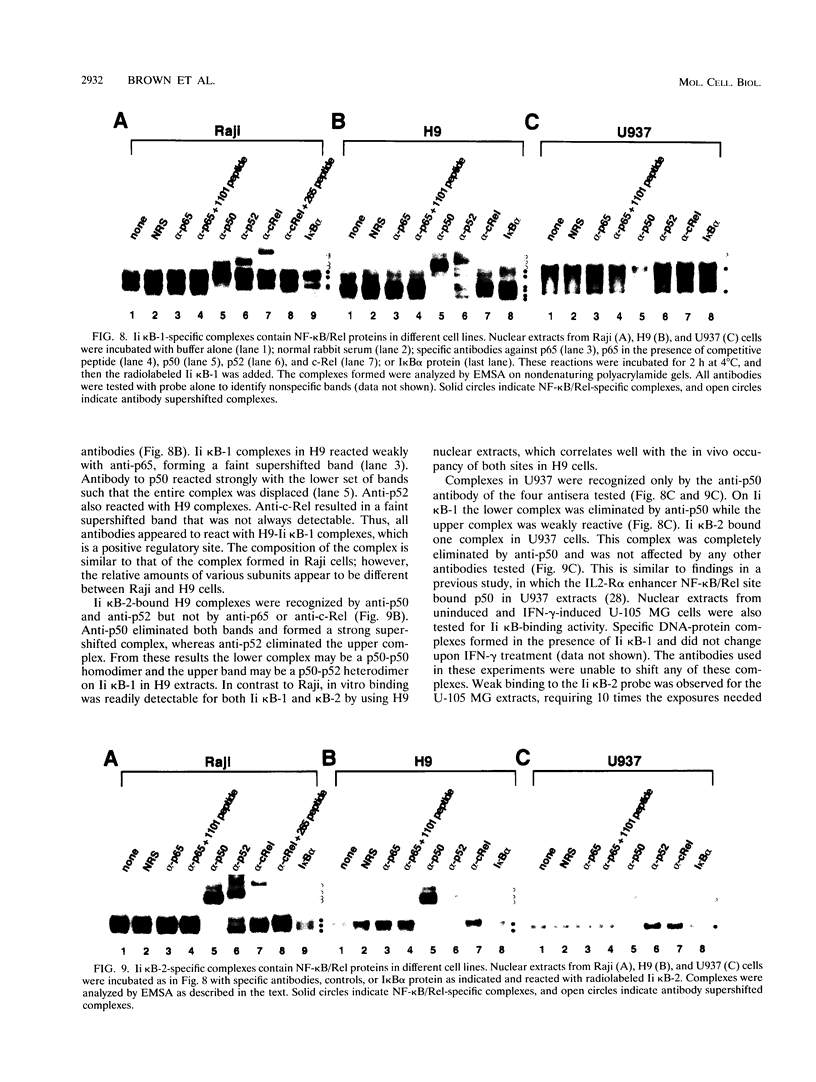
The promoter of the human major histocompatibility complex class II-associated invariant-chain gene (Ii) contains two NF-kappa B/Rel binding sites located at -109 to -118 (Ii kappa B-1) and -163 to -172 (Ii kappa B-2) from the transcription start site. We report here that the differential function of each of these NF-kappa B/Rel sites in several distinct cell types depends on cell-specific binding of NF-kappa B/Rel transcription factors. Ii kappa B-1 is a positive regulatory element in B-cell lines and in the Ii-expressing T-cell line, H9, but acts as a negative regulatory element in myelomonocytic and glia cell lines. In vivo protein-DNA contacts are detectable at Ii kappa B-1 in cell lines in which this site is functional as either a positive or negative regulator. Electrophoretic mobility supershift assays determine that members of the NF-kappa B/Rel family of transcription factors can bind to this site in vitro and that DNA-binding complexes that contain p50, p52, p65, and cRel correlate with positive regulation whereas the presence of p50 correlates with negative regulation. Ii kappa B-2 is a site of positive regulation in B-cell lines and a site of negative regulation in H9 T cells, myelomonocytic, and glial cell lines. In vivo occupancy of this site is observed only in the H9 T-cell line. Again, in vitro supershift studies indicate that the presence of p50, p52, p65, and cRel correlates with positive function whereas the presence of only p50 and p52 correlates with negative function. This differential binding of specific NF-kappa B/Rel subunits is likely to mediate the disparate functions of these two NF-kappa B/Rel binding sites.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Ballard D. W., Dixon E. P., Peffer N. J., Bogerd H., Doerre S., Stein B., Greene W. C. The 65-kDa subunit of human NF-kappa B functions as a potent transcriptional activator and a target for v-Rel-mediated repression. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1875–1879. doi: 10.1073/pnas.89.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr C. L., Saunders G. F. Interferon-gamma-inducible regulation of the human invariant chain gene. J Biol Chem. 1991 Feb 25;266(6):3475–3481. [PubMed] [Google Scholar]
- Becker P. B., Ruppert S., Schütz G. Genomic footprinting reveals cell type-specific DNA binding of ubiquitous factors. Cell. 1987 Nov 6;51(3):435–443. doi: 10.1016/0092-8674(87)90639-8. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Baldwin A. S., Jr The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 1993 Nov;7(11):2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Finco T. S., Nantermet P. V., Baldwin A. S., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993 Jun;13(6):3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C., Mathis D. Regulation of major histocompatibility complex class-II genes: X, Y and other letters of the alphabet. Annu Rev Immunol. 1990;8:681–715. doi: 10.1146/annurev.iy.08.040190.003341. [DOI] [PubMed] [Google Scholar]
- Blank V., Kourilsky P., Israël A. NF-kappa B and related proteins: Rel/dorsal homologies meet ankyrin-like repeats. Trends Biochem Sci. 1992 Apr;17(4):135–140. doi: 10.1016/0968-0004(92)90321-y. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Barr C. L., Ting J. P. Sequences homologous to class II MHC W, X, and Y elements mediate constitutive and IFN-gamma-induced expression of human class II-associated invariant chain gene. J Immunol. 1991 May 1;146(9):3183–3189. [PubMed] [Google Scholar]
- Brown A. M., Wright K. L., Ting J. P. Human major histocompatibility complex class II-associated invariant chain gene promoter. Functional analysis and in vivo protein/DNA interactions of constitutive and IFN-gamma-induced expression. J Biol Chem. 1993 Dec 15;268(35):26328–26333. [PubMed] [Google Scholar]
- Cresswell P. Chemistry and functional role of the invariant chain. Curr Opin Immunol. 1992 Feb;4(1):87–92. doi: 10.1016/0952-7915(92)90131-w. [DOI] [PubMed] [Google Scholar]
- Dey A., Nebert D. W., Ozato K. The AP-1 site and the cAMP- and serum response elements of the c-fos gene are constitutively occupied in vivo. DNA Cell Biol. 1991 Sep;10(7):537–544. doi: 10.1089/dna.1991.10.537. [DOI] [PubMed] [Google Scholar]
- Dey A., Thornton A. M., Lonergan M., Weissman S. M., Chamberlain J. W., Ozato K. Occupancy of upstream regulatory sites in vivo coincides with major histocompatibility complex class I gene expression in mouse tissues. Mol Cell Biol. 1992 Aug;12(8):3590–3599. doi: 10.1128/mcb.12.8.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle C., Ford P. J., Ponath P. D., Spies T., Strominger J. L. Regulation of the class II-associated invariant chain gene in normal and mutant B lymphocytes. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4590–4594. doi: 10.1073/pnas.87.12.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckett C. S., Perkins N. D., Kowalik T. F., Schmid R. M., Huang E. S., Baldwin A. S., Jr, Nabel G. J. Dimerization of NF-KB2 with RelA(p65) regulates DNA binding, transcriptional activation, and inhibition by an I kappa B-alpha (MAD-3). Mol Cell Biol. 1993 Mar;13(3):1315–1322. doi: 10.1128/mcb.13.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eades A. M., Litfin M., Rahmsdorf H. J. The IFN-gamma response of the murine invariant chain gene is mediated by a complex enhancer that includes several MHC class II consensus elements. J Immunol. 1990 Jun 1;144(11):4399–4409. [PubMed] [Google Scholar]
- Franzoso G., Bours V., Park S., Tomita-Yamaguchi M., Kelly K., Siebenlist U. The candidate oncoprotein Bcl-3 is an antagonist of p50/NF-kappa B-mediated inhibition. Nature. 1992 Sep 24;359(6393):339–342. doi: 10.1038/359339a0. [DOI] [PubMed] [Google Scholar]
- Fujita T., Nolan G. P., Ghosh S., Baltimore D. Independent modes of transcriptional activation by the p50 and p65 subunits of NF-kappa B. Genes Dev. 1992 May;6(5):775–787. doi: 10.1101/gad.6.5.775. [DOI] [PubMed] [Google Scholar]
- Glimcher L. H., Kara C. J. Sequences and factors: a guide to MHC class-II transcription. Annu Rev Immunol. 1992;10:13–49. doi: 10.1146/annurev.iy.10.040192.000305. [DOI] [PubMed] [Google Scholar]
- Grilli M., Chiu J. J., Lenardo M. J. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Ip Y. T., Kraut R., Levine M., Rushlow C. A. The dorsal morphogen is a sequence-specific DNA-binding protein that interacts with a long-range repression element in Drosophila. Cell. 1991 Jan 25;64(2):439–446. doi: 10.1016/0092-8674(91)90651-e. [DOI] [PubMed] [Google Scholar]
- Jiang J., Levine M. Binding affinities and cooperative interactions with bHLH activators delimit threshold responses to the dorsal gradient morphogen. Cell. 1993 Mar 12;72(5):741–752. doi: 10.1016/0092-8674(93)90402-c. [DOI] [PubMed] [Google Scholar]
- Kang S. M., Tran A. C., Grilli M., Lenardo M. J. NF-kappa B subunit regulation in nontransformed CD4+ T lymphocytes. Science. 1992 Jun 5;256(5062):1452–1456. doi: 10.1126/science.1604322. [DOI] [PubMed] [Google Scholar]
- Kara C. J., Glimcher L. H. In vivo footprinting of MHC class II genes: bare promoters in the bare lymphocyte syndrome. Science. 1991 May 3;252(5006):709–712. doi: 10.1126/science.1902592. [DOI] [PubMed] [Google Scholar]
- Kaufman P. A., Weinberg J. B., Greene W. C. Nuclear expression of the 50- and 65-kD Rel-related subunits of nuclear factor-kappa B is differentially regulated in human monocytic cells. J Clin Invest. 1992 Jul;90(1):121–129. doi: 10.1172/JCI115824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch N., Harris A. W. Differential expression of the invariant chain in mouse tumor cells: relationship to B lymphoid development. J Immunol. 1984 Jan;132(1):12–15. [PubMed] [Google Scholar]
- Kudo J., Chao L. Y., Narni F., Saunders G. F. Structure of the human gene encoding the invariant gamma-chain of class II histocompatibility antigens. Nucleic Acids Res. 1985 Dec 20;13(24):8827–8841. doi: 10.1093/nar/13.24.8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- König H., Ponta H., Rahmsdorf H. J., Herrlich P. Interference between pathway-specific transcription factors: glucocorticoids antagonize phorbol ester-induced AP-1 activity without altering AP-1 site occupation in vivo. EMBO J. 1992 Jun;11(6):2241–2246. doi: 10.1002/j.1460-2075.1992.tb05283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkovitch J., Darnell J. E., Jr Rapid in vivo footprinting technique identifies proteins bound to the TTR gene in the mouse liver. Genes Dev. 1991 Jan;5(1):83–93. doi: 10.1101/gad.5.1.83. [DOI] [PubMed] [Google Scholar]
- Momburg F., Koch N., Möller P., Moldenhauer G., Butcher G. W., Hämmerling G. J. Differential expression of Ia and Ia-associated invariant chain in mouse tissues after in vivo treatment with IFN-gamma. J Immunol. 1986 Feb 1;136(3):940–948. [PubMed] [Google Scholar]
- Momburg F., Koretz K., Von Herbay A., Möller P. Nonimmune human cells can express MHC class II antigens in the absence of invariant chain--an immunohistological study on normal and chronically inflamed small intestine. Clin Exp Immunol. 1988 Jun;72(3):367–372. [PMC free article] [PubMed] [Google Scholar]
- Mueller P. R., Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989 Nov 10;246(4931):780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- Nolan G. P., Baltimore D. The inhibitory ankyrin and activator Rel proteins. Curr Opin Genet Dev. 1992 Apr;2(2):211–220. doi: 10.1016/s0959-437x(05)80276-x. [DOI] [PubMed] [Google Scholar]
- Perkins N. D., Schmid R. M., Duckett C. S., Leung K., Rice N. R., Nabel G. J. Distinct combinations of NF-kappa B subunits determine the specificity of transcriptional activation. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1529–1533. doi: 10.1073/pnas.89.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessara U., Koch N. Tumor necrosis factor alpha regulates expression of the major histocompatibility complex class II-associated invariant chain by binding of an NF-kappa B-like factor to a promoter element. Mol Cell Biol. 1990 Aug;10(8):4146–4154. doi: 10.1128/mcb.10.8.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares W., Franza B. R., Jr, Herr W. The kappa B enhancer motifs in human immunodeficiency virus type 1 and simian virus 40 recognize different binding activities in human Jurkat and H9 T cells: evidence for NF-kappa B-independent activation of the kappa B motif. J Virol. 1992 Dec;66(12):7490–7498. doi: 10.1128/jvi.66.12.7490-7498.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. W., Gifford A. M., Baltimore D. Silencing of the expression of the immunoglobulin kappa gene in non-B cells. Mol Cell Biol. 1991 Mar;11(3):1431–1437. doi: 10.1128/mcb.11.3.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice N. R., MacKichan M. L., Israël A. The precursor of NF-kappa B p50 has I kappa B-like functions. Cell. 1992 Oct 16;71(2):243–253. doi: 10.1016/0092-8674(92)90353-e. [DOI] [PubMed] [Google Scholar]
- Sasaki A., Levison S. W., Ting J. P. Comparison and quantitation of Ia antigen expression on cultured macroglia and ameboid microglia from Lewis rat cerebral cortex: analyses and implications. J Neuroimmunol. 1989 Nov;25(1):63–74. doi: 10.1016/0165-5728(89)90087-8. [DOI] [PubMed] [Google Scholar]
- Schmitz M. L., Baeuerle P. A. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991 Dec;10(12):3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Stein B., Cogswell P. C., Baldwin A. S., Jr Functional and physical associations between NF-kappa B and C/EBP family members: a Rel domain-bZIP interaction. Mol Cell Biol. 1993 Jul;13(7):3964–3974. doi: 10.1128/mcb.13.7.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B., Rahmsdorf H. J., Steffen A., Litfin M., Herrlich P. UV-induced DNA damage is an intermediate step in UV-induced expression of human immunodeficiency virus type 1, collagenase, c-fos, and metallothionein. Mol Cell Biol. 1989 Nov;9(11):5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viville S., Neefjes J., Lotteau V., Dierich A., Lemeur M., Ploegh H., Benoist C., Mathis D. Mice lacking the MHC class II-associated invariant chain. Cell. 1993 Feb 26;72(4):635–648. doi: 10.1016/0092-8674(93)90081-z. [DOI] [PubMed] [Google Scholar]
- Wright K. L., Ting J. P. In vivo footprint analysis of the HLA-DRA gene promoter: cell-specific interaction at the octamer site and up-regulation of X box binding by interferon gamma. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7601–7605. doi: 10.1073/pnas.89.16.7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan D., Tucker P. W. Transcriptional regulation of the mu-delta heavy chain locus in normal murine B lymphocytes. J Exp Med. 1984 Aug 1;160(2):564–583. doi: 10.1084/jem.160.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Jones P. P. Transcriptional control of the invariant chain gene involves promoter and enhancer elements common to and distinct from major histocompatibility complex class II genes. Mol Cell Biol. 1990 Aug;10(8):3906–3916. doi: 10.1128/mcb.10.8.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Préval C., Lisowska-Grospierre B., Loche M., Griscelli C., Mach B. A trans-acting class II regulatory gene unlinked to the MHC controls expression of HLA class II genes. Nature. 1985 Nov 21;318(6043):291–293. doi: 10.1038/318291a0. [DOI] [PubMed] [Google Scholar]