Abstract
Emerging infectious diseases (EIDs) pose a significant threat to human health, economic stability, and biodiversity. Despite this, the mechanisms underlying disease emergence are still not fully understood, and control measures rely heavily on mitigating the impact of EIDs after they have emerged. Here, we highlight the emergence of a zoonotic Henipavirus, Nipah virus, to demonstrate the interdisciplinary and macroecological approaches necessary to understand EID emergence. Previous work suggests that Nipah virus emerged due to the interaction of the wildlife reservoir (Pteropus spp. fruit bats) with intensively managed livestock. The emergence of this and other henipaviruses involves interactions among a suite of anthropogenic environmental changes, socioeconomic factors, and changes in demography that overlay and interact with the distribution of these pathogens in their wildlife reservoirs. Here, we demonstrate how ecological niche modeling may be used to investigate the potential role of a changing climate on the future risk for Henipavirus emergence. We show that the distribution of Henipavirus reservoirs, and therefore henipaviruses, will likely change under climate change scenarios, a fundamental precondition for disease emergence in humans. We assess the variation among climate models to estimate where Henipavirus host distribution is most likely to expand, contract, or remain stable, presenting new risks for human health. We conclude that there is substantial potential to use this modeling framework to explore the distribution of wildlife hosts under a changing climate. These approaches may directly inform current and future management and surveillance strategies aiming to improve pathogen detection and, ultimately, reduce emergence risk.
Emerging infectious diseases (EIDs) are a major threat to global public health (1). Here, we define an EID according to Jones et al. (2) and include all infectious diseases (i.e., caused by prions, viruses, bacteria, or eukaryotic pathogens) that have recently (within the past 60 y) (i) expanded their geographic range [e.g., West Nile virus (WNV)], (ii) infected humans for the first time [e.g., severe acute respiratory syndrome (SARS) coronavirus], (iii) evolved into new strains (e.g., triple reassortant influenza A/H1N1), or (iv) increased their pathogenicity (e.g., hantavirus pulmonary syndrome). Diseases that have affected humans historically but have recently increased in incidence or in the size of their outbreaks are also considered emerging. The impact of EIDs varies from those causing relatively few cases and little mortality to those that spread over continental areas or globally (e.g., Chikungunya virus, SARS) and those that cause significant mortality (e.g., drug-resistant tuberculosis, HIV/AIDS). With increasing dependence on international networks of travel and trade for our globalized economy, EIDs that spread through these networks may have a high economic impact (e.g., SARS, highly pathogenic avian influenza (3)]. For these reasons, efforts to understand the causes of EIDs and to predict their future emergence have become part of a global strategy for addressing this public health threat (4). Previous analyses suggest that demographic and anthropogenic environmental changes are the key underlying causes or “drivers” of disease emergence (5–7). These include ecological, political, and socioeconomic drivers, such as climate change, urbanization, international travel and trade, land use change and agricultural intensification, and breakdown of public health measures. The anthropogenic nature of EID drivers suggests that strategies to influence anthropogenic activities directly may minimize emergence or spread. For example, prevention strategies might influence agricultural development (e.g., better sanitation in backyard poultry production), social behavior (e.g., improving hygiene or hunting practices), or demographic changes (e.g., patterns of human travel, trade, and migration). It is therefore useful to identify the underlying causes of EIDs as part of a broad strategy to prevent their emergence.
Interdisciplinary Studies of EID Drivers
Studies of the underlying causes of disease emergence might assist in forecasting or predicting future emergence of novel pathogens (8–11). However, these studies require interdisciplinary efforts (12) and significant time, and are inherently difficult and costly. One reason for this is that drivers of disease emergence usually represent multidecadal temporal shifts in the underlying environmental or demographic state. For example, WNV was first identified in the New World in 1999, in Queens, New York, adjacent to two international airports (13), and analyses suggest that air travel is the most significant risk for future spread (14). It is therefore reasonable to hypothesize that WNV emerged in the Americas due to increasing air travel during the 20th century (15). However, to test this, and to deduce when critical thresholds of travel necessary for successful invasion occurred, would require multidecadal data. These would include data on the expansion of air travel networks and routes, on the capacity of airplanes to transport mosquitoes, on the prevalence of pathogens in vectors at all source countries, and on the capacity of vectors in the target country to carry these pathogens, among others. Similarly, it has been hypothesized that the pathogen responsible for pandemic AIDS (HIV-1) was first introduced into humans through the hunting and butchering of chimpanzees (16). There is substantial molecular evidence for this, including the genetic similarity of simian immunodeficiency virus from chimpanzees (SIVCZ) pointing to initial spillover events during the early 20th century (17). However, to test this hypothesis, and to identify why HIV/AIDS emerged in people as a pandemic in the 20th century despite many thousands of years of primate hunting in the region, would require data on trends in bush-meat hunting, butchering, and consumption in Central and West Africa during the past 150 y. It would also require data on the sociological and demographic changes in the region that lead to expanding human-to-human transmission (18). A range of theories on what would turn an SIV spillover event into stuttering chains of transmission and, ultimately, a pandemic have been proposed, including vaccine production (19), historically high incidence of genitourinary disease (20), and travel and trade (19). Data on each of these would also be required. Thus, understanding disease emergence is inherently a multidisciplinary challenge.
Uncovering the Underlying Drivers of Nipah Virus Emergence
The complex interdisciplinary nature of disease emergence can be highlighted by the emergence of Nipah virus (NiV), a zoonotic paramyxovirus lethal to humans. NiV first emerged in Malaysia in 1998 during an outbreak that caused more than 100 human deaths (21). This paramyxovirus has fruit bat (Pteropus spp.) reservoirs, and the virus was first transmitted to domestic pigs, in which it caused respiratory pathology and allowed transmission to people via droplets. The initial spillover of NiV occurred on a pig farm in which fruit trees were planted next to pigsties as a source of additional revenue and to increase shade, and it is thought that these trees attracted fruit bats. However, given that pigs have been produced in Malaysia for many decades, it has remained unclear until recently why NiV first spilled over to people in the late 1990s. It was proposed that burning of forest fires in Sumatra, linked to anthropogenic deforestation during an El Niño southern oscillation (ENSO) event, forced bat migration from Sumatra to Peninsular Malaysia and introduced the virus into the index farm region (22). Sumatran forest fires have been linked previously to coral die-offs (23), and they cause regular haze events in the dry season in Peninsular Malaysia, which were particularly intense during late 1997. To test this hypothesis, the earliest known cases of human NiV cases were identified and found to have occurred on the index farm months before the ENSO-driven haze events (24). The pattern of infection in fruit bats was examined over a 5-y period, and it was shown that NiV antibodies were widespread, suggesting that the virus was regularly transmitted among bats and that NiV was not newly introduced (24). In addition, satellite telemetry showed regular bat movement between Sumatra and Peninsular Malaysia (25), suggesting that the range distribution of the bat host, and therefore NiV, as we argue below, historically included Peninsular Malaysia.
Significant evidence now exists for an alternative hypothesis that changes to the production of livestock drove the emergence of NiV (24). To test this, multidecadal data on pig and mango production from the Food and Agricultural Organization and the Malaysian Ministry of Agriculture and detailed data from the index farm on pig production before the outbreak were examined. Mathematical modeling of NiV transmission dynamics within the index farm showed that the initial introduction of NiV would have led to a large and rapid epizootic, increased pig mortality, and herd immunity, driving the virus extinct within 1–2 mo. The history of the first five human cases in 1997 is consistent with this evidence. However, human cases continued for over 18 mo, culminating in a full-scale outbreak. The model suggests that NiV must have been reintroduced into the pig population to persist for this period. Such reintroductions are plausible, given that field surveys identified a fruit bat colony within 10 km of the index farm (24). It appears that the initial introduction of NiV created a “priming” effect that allowed a secondary introduction to persist in what was then a partially immune population. As pigs born after the initial event gradually lost their maternal antibodies, they became susceptible and allowed NiV persistence for periods similar to those observed at the index farm. The emergence of NiV in Malaysia was thus the product of two drivers. First, agricultural intensification, in the form of increased commercial pig production and patterns of dual-use agriculture, created a pathway for the repeated transmission of NiV from fruit bat reservoirs to pigs. Second, the initial spillover primed the pig population for persistence of the pathogen on reintroduction, in turn, leading to increased transmission among pigs and to humans. Once infected pigs were sold outside the region, the opportunities for greater human exposure, infection, and disease followed.
This case study illustrates the difficulty in testing complex hypotheses on disease emergence. It required empirical approaches at different scales, from the laboratory to the field, and multidecadal data on hypothesized drivers of emergence, or proxies for unavailable data. The study used mathematical modeling as a framework for these empirical data to be used to test key hypotheses. Importantly, it required commitment of resources to multidisciplinary teams for several years.
This type of case study has value not just in understanding why a specific disease emerged but in providing a pathway to predict and prevent future disease emergence. To push the science of disease emergence forward, novel approaches to data collection, analysis, and collaboration are required. In particular, studies will need to use a causal inference approach to test complex interactions, tipping points, and multiple drivers, and not just simple hypotheses of single causation (26). Although mathematical modeling is often critical to testing complex hypotheses, most approaches involve making basic assumptions about host and pathogen dynamics, how environmental changes affect these, and how host ranges are directly coupled to pathogen occurrence. Studies to elucidate the rules governing these assumptions may significantly improve modeling strategies. Because disease emergence occurs over multiyear or multidecadal periods, archival samples have proven valuable in some studies (27, 28). These would need to be collected systematically and in large enough numbers to give statistical power to identify the presence or absence of diseases, particularly for those found at low prevalence. In addition, samples collected for disease studies often need specific preservation for diagnostic testing. Finally, understanding disease emergence requires collaboration across the biological, physical, and social sciences (29, 30). Drivers of disease emergence are often directly related to anthropogenic change; therefore, studies that analyze how changes in socioeconomic factors alter pathogen dynamics will be particularly useful for understanding past disease risk and predicting future disease risk. This approach has been used successfully to analyze how travel and trade drive the risk of disease spread (14, 31–33), but it has not yet been applied extensively to understand disease emergence.
Climate Change as a Potential Driver of Disease Emergence
The NiV case study demonstrates how understanding the causes of disease emergence requires analysis of long-term historical datasets of host and pathogen dynamics and of the hypothesized anthropogenic drivers. Climate change has been hypothesized as an underlying driver of disease emergence in a number of cases, including directly transmitted pathogens (e.g., hantavirus, Ebola virus, NiV) and vector-borne or water-borne diseases, such as malaria, dengue, and cholera (12, 34–38). Examining linkages between climate change and biological phenomena is difficult, and requires historical time-series data that do not usually exist for emerging diseases. In some cases, these data are easily acquired. For example, colonial studies of malaria cases have been used to test whether emergence of malaria is influenced by climate change (39, 40), climate variability (41), drug resistance (42), or other factors associated with socioeconomic development (43). However, discerning causation from correlation has proven difficult. Models of malaria distribution at larger spatial and temporal scales suggest that, globally, malaria has receded over the past century (44, 45), and this recession is most significantly due to the success of public health interventions rather than climate change (44).
These studies all rely on analyses of historical data on disease occurrence. However, the link between climate change and disease may be better investigated using predictive models that aim to forecast future disease emergence risk under climate change scenarios. This approach has been adopted for vectors of some diseases [e.g., malaria (Anopheles gambiae) (46)] and those with an environmental reservoir [e.g., Bacillus anthracis (47)]. Vectors often have a strong link to climate due to their requirement of water bodies for reproduction, as well as the direct impact of temperature changes on their growth rates, biting rates, and population expansion. However, a recent analysis of one of the most comprehensive databases of EIDs currently available in the literature showed that only 22.7% (76 of 335) of EID events identified from 1940 to 2006 were vector-borne, with 77.3% (259 of 335) being directly transmitted. Thus, applying climate models to directly transmitted pathogens may provide a useful strategy to predict future emergence risk. For directly transmitted pathogens, climate change may affect the distribution of a pathogen’s reservoir host (e.g., mice for hantavirus, frugivorous bats for Ebola virus) or the host’s food source (e.g., grasses for hantavirus reservoirs, fruiting trees for Ebola virus reservoirs). Thus, assuming that pathogen and wildlife host distributions are linked, predictive models may provide an improved understanding of potential climate change impacts on the distribution of directly transmitted EIDs.
Correlative Ecological Niche Modeling as a Tool for Studying Disease Emergence Under Climate Change
Ecological niche modeling (ENM) is a widely used tool to investigate the potential distributions of species under scenarios of environmental change. This technique employs a range of different algorithms [generalized linear model, generalized additive model, genetic algorithm for rule-set production, and MaxEnt; reviewed in (48)] to estimate the relationships between point-locality data, such as museum collection records or field observations of species’ occurrences, and spatial information on factors that constrain species distributions (e.g., climate, vegetation, other biophysical attributes). This correlative method can use the realized niche as represented by occurrence records to help characterize the ecological or fundamental niche of the species (49). The relationships derived can then be used for extrapolating species distributions into different geographic regions or under different climates (50, 51). This technique is now used widely in the fields of ecology, evolutionary biology, conservation biology, agricultural science, and public health (52). However, like any statistical modeling approach, ENM requires careful consideration of inputs (e.g., reliability and representativeness of occurrence records; sampling bias; quality of plausible spatial drivers, such as environmental layers) and model assumptions (e.g., what is being modeled, equilibrium assumptions, implicit inclusion of species interactions, extrapolation beyond the training region) to ensure that results are biologically plausible.
ENM was first used to make ecological predictions of the distribution of wildlife species from occurrence data. This has been applied to some wildlife diseases (53) and is increasingly used to estimate the current distribution of human pathogens [e.g., monkeypox (54)] based on climate, other environmental parameters, and reported human cases of infection (55, 56). These studies show how correlative models may also be used to guide the collection of new information to improve predictions iteratively. Nevertheless, in the case of monkeypox, one unresolved question relates to the role of the as yet unidentified monkeypox virus reservoir (assumed to be wildlife, with one confirmed positive record from a squirrel) and its relationship with human cases of disease.
The predominance of ENM studies using vectors and reservoirs as proxies for pathogen spatial distributions may also be explained by the relative paucity of clinical data on zoonotic infections in people. For example, Chagas disease risk areas were predicted based on the association of bioclimatic habitat for Chagas vectors (Triatoma spp.) and their wildlife host, packrats (Neotoma spp.) (56). Despite the dominance of directly transmitted zoonotic EIDs in the emerging disease literature (2), the geographic distribution of reservoir hosts has rarely been used to model directly transmitted pathogen distributions (57) (Table 1). Host distribution has typically been used indirectly in ENMs. For example, the identities of potential mammal reservoir hosts for Ebola and Marburg viruses in Africa were deduced by intersecting mammal distributions with an ENM of the two pathogens based on occurrence records in humans and nonhuman primates (55, 58). Because the distributions of pathogens are usually strongly linked to the distribution of their hosts, efforts to forecast future disease emergence should include a component that investigates the potential expansion or contraction of host species distributions. The past decade has seen a rapid expansion in the availability of spatial data on species distributions, from global databases such as the Global Biodiversity Information Facility, to those focused on a taxonomic group or geographic region [e.g., VertNet (59)]. Time series analyses linking spatial data on biodiversity and climate have demonstrated realized geographic shifts of up to 60 km per decade in response to climate change from mammals in Yosemite (60) to moths in Borneo (61), among others (62, 63). The magnitude of future range shifts by wildlife in response to changing climates is likely to increase (64), providing new opportunities for pathogens from one species to make contact with another. Although the outcomes of range shifts may be less frequent species interactions in some cases, the wide distribution of humans and livestock suggests that EID risk will, on balance, increase as reservoir hosts make contact with human or livestock populations for the first time. These interactions will be complex, and the risk for disease emergence may be further increased as human populations and livestock are displaced directly in response to climate change (10, 12, 65). Advancing methods to investigate, and perhaps forecast, the magnitude and direction of climate-induced range shifts could help prioritize where preventative public health resources should be allocated. These approaches will naturally be multidisciplinary and will include projections on socioeconomic factors, such as migration, trade routes, and livestock production trends.
Table 1.
Examples of published studies with applications of ENM to pathogen distribution
| Pathogen/disease/species | Pathogen type | Scale | Algorithm | Validation | Time | Ref. |
| Vibrio cholerae | Free-living bacterium | Central California | Mantel | Bootstrap | Current | (97) |
| Yersinia pestis | Vector-borne bacterium | Western Usambara Mountains of Tanzania | GARP | Jackknife | Current | (98) |
| H5N1 avian influenza | Directly transmitted virus | India, Bangladesh, Nepal, and Pakistan | GARP | Actual outbreak locations | Current | (99) |
| Coccidiomycosis | Fungus with environmental spores | Southern California, Arizona, and Sonora | GARP | Available epidemiological data | Current | (100) |
| Bacillus anthracis | Bacterium with environmental spores | United States | GARP | AUC | Current | (101) |
| Triatoma brasiliensis | Vector-borne protozoan | Northeastern Brazil | GARP | Points sample from test data | Current | (102) |
| Campylobacter jejuni | Enteric bacterium | 100 km2 around Cheshire, United Kingdom | GAM, UPGMA | Simulation data from the null model | Current | (103) |
| Range of parasites | Microparasites (e.g., viruses, bacteria, protozoa), macroparasites (helminths), and ectoparasites (arthropods) | North America | Correlations | N/A | Current | (104) |
| Bat-related pathogens | N/A | South America | MaxEnt | Jacknife, ROC, AUC | Current | (105) |
| West Nile encephalitis | Vector-borne virus, Culex pipiens | Illinois, Indiana, and Ohio | GARP | Independent datasets | Current | (106) |
| Chagas, Trypanosoma cruzi | Vector-borne protozoan | South America | NODF | Bootstrap | Current | (107) |
| H5N1 | Directly transmitted virus | West Africa | GARP | Binomial probabilities | Current | (108) |
| Filoviruses | Directly transmitted virus | Africa | GARP | N/A | Current | (55) |
| Chagas, Trypanosoma cruzi | Vector-borne protozoan | Mexico | GARP | None | Current | (56) |
| Leishmaniasis | Vector-borne protozoan | North America | MaxEnt | AUC | Future | (95) |
| Leishmaniasis | Vector-borne protozoan, Lutzomyia | South America | GARP | Bootstrap | Future | (109) |
| Leishmaniasis | Vector-borne protozoan | Spain | Negative binomial regression | Independent dataset | Future | (110) |
| Malaria | Vector-borne protozoan | Africa | GARP | Independent dataset | Future | (46) |
| Dengue | Vector-borne virus | Mexico | GARP | Actual case data | Past | (111) |
Scales of studies varied from state or county levels (e.g., Illinois; Cheshire, United Kingdom) to continental scales (e.g., Africa). Few studies focused on the effects of climate change on the distribution of directly transmitted pathogens, focusing instead on vector-borne or free-living pathogens. A combination of key words was used to search the International Statistical Institute Web of Science: (environmental niche model* OR ecological niche model* OR species distribution model* OR predictive habitat distribution model* OR climate envelope model* and disease* OR pathogen*); nearly 73% of ENM studies referred to vectors or an environmental reservoir (vector* OR environ* reservoir* OR environ*), whereas only 27% of studies referenced a directly transmitted pathogen without vectors or an environmental reservoir [host*NOT (vector* OR environ* reservoir* OR environ*)]. AUC, area under the curve; GAM, Generalized Additive Model; GARP, Genetic Algorithm for Rule-set Production; N/A, Not Applicable; NODF, Nestedness overlap and decreasing fills; ROC, receiver operating characteristic; UPGMA, Unweighted Pair Group Method with Arithmetic Mean.
Case Study: Predicting the Future Potential Distribution of Emerging Henipavirus Reservoirs Due to Climate Change
In this case study, we use ENM to investigate how the distribution of bats known to be reservoirs of lethal emerging viruses (Henipavirus spp.) may shift under modeled future climates, thus altering the risk for disease emergence from this group. Two species of viruses are known from this genus, NiV and Hendra virus (HeV), and other likely members have been identified recently in bats (66). We used the distribution of Henipavirus bat reservoir hosts as a proxy for viral distribution and modeled the bioclimatic range shifts of these hosts under numerous models of potential future climate change. We believe this “proxy” approach is valid because (i) serological studies have reported evidence of circulating henipaviruses in all Pteropus species in which testing has been conducted using validated laboratory assays (including 9 species in 10 countries across a large proportion of the global range of the genus) (25, 67–69); (ii) the species of bats known to harbor henipaviruses often overlap in range, roost together, are highly mobile, and are often migratory (25, 70, 71), suggesting ease of inter- and intraspecific viral mixing; and (iii) henipaviruses occur at high seroprevalence (20–60%), and data on PCR detections and isolations suggest that they are ubiquitous within host species (25, 66, 68, 69, 72). Although this does not prove that host and pathogen distributions are entirely congruent, it is probable that they are very strongly correlated. This approach does not produce a simple proxy for future disease emergence events in humans because socioeconomic and demographic factors influence emergence. Under climate change, some of these factors will themselves shift in magnitude and distribution. However, the presence of the pathogen in a region is a prerequisite for these other factors to drive emergence, and understanding potential shifts in Henipavirus host distributions is therefore critical to understanding future risk under climate change scenarios.
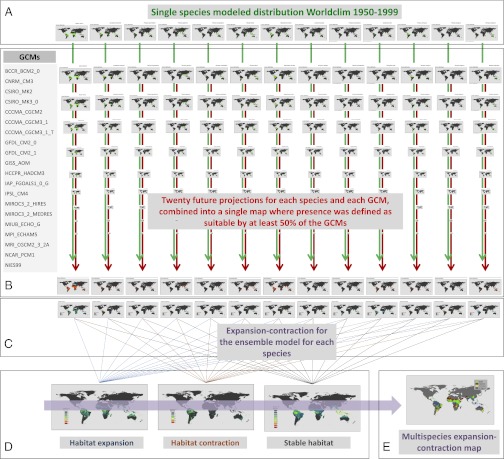
To deal with observational data limitations and uncertainty in current and future climatic layers, we used an ensemble-modeling approach to forecast the impacts of climate change on the geographic distribution of Henipavirus hosts (73, 74) (Fig. 1). We used georeferenced museum specimen data coupled with field-collected global positioning system and satellite telemetry data to model the current and future distribution of 13 species of bats reported in the literature as reservoirs of either NiV or HeV (SI Appendix, Table S1). The full details of the model parameters, methods, calibration, caveats, and justification of our approach are given in SI Appendix. Briefly, for each host species, we obtained known specimen localities from museum sources and filtered these for inconsistencies using International Union for Conservation of Nature range distribution maps to generate an occurrence dataset for bat Henipavirus reservoirs. Nineteen bioclimatic variables at a resolution of 2.5 arcs per minute (∼5 km2) were used to generate current and future bioclimatic niches (SI Appendix, Table S2). To explore future climatic conditions, we used a single midcentury time slice (2050) and the Intergovernmental Panel on Climate Change A2 greenhouse gas emissions scenario, which assumes “business as usual” continued emissions throughout this century (75). The aim of this approach was not to compare the influence of alternative emission scenarios on the future distribution of the bat Henipavirus reservoirs but to demonstrate the utility of ENM for forecasting potential range shifts of the hosts of directly transmitted pathogens.
Fig. 1.
Conceptual model of our methods. (A) Models of a single species’ current bioclimate, based on Worldclim. (B) Projected future suitable bioclimate based on 20 downscaled GCMs, where presence was defined as suitable by at least 50% of the GCMs. (C) Expansion/contraction maps based on the subtraction of the present from the future multi-GCM predictions. (D) All 13 species expansion/contraction maps were combined into three composite maps that show habitat expansion, contraction, and stability. (E) Synthetic map across GCMs and species that shows habitat expansion, contraction, and stability.
All niche models were generated using MaxEnt v3.3.3e because of its established performance with presence-only data and its built-in capacity to deal with multicolinearity in the environmental variables. We created subsets of each species’ locality observations using a spatially structured partitioning procedure (SI Appendix, Fig. S1) to deal with autocorrelation and reported the area under the curve to summarize model performance (SI Appendix, Fig. S2). Each resulting simulation for the current conditions was converted into a presence/absence map, which was then evaluated using an independent field-derived dataset of bat roosts and foraging location, NiV prevalence, and NiV seroprevalence (SI Appendix, Table S3). The presence/absence maps for each species were combined into a final current niche model representing the sum of all 100 iterations. Each one of the final current bioclimatic niches for the bat species were then projected into the midcentury using 20 alternative global circulation models (GCMs) (SI Appendix, Table S4), converted to presence/absence maps using the same threshold rule as the current conditions models, and then combined into a single output representing the sum of the presence/absence maps for each of the 100 simulations for each of the 20 GCMs.
To produce a summary forecast of the potential future distribution of each Henipavirus bat host, we next combined the 20 future projections for each species into a single map, where presence was defined as a pixel that was predicted to contain the species in at least 50% of the 20 GCMs. Contrasting the current modeled distribution and the midcentury projections, we calculated the expansion and contraction in the distribution of suitable bioclimatic habitat (SI Appendix, Figs. S3–S29). A synthesis map integrating the results across all bat host species’ distributions yielded an ensemble estimate of midcentury climate change-induced distributional expansion/contraction for the Henipavirus bat host complex (Fig. 2).
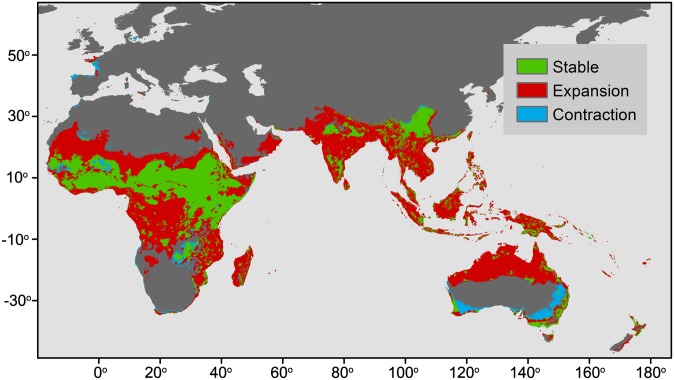
Fig. 2.
Synthetic generalization of the predicted expansion and contraction potential climatic habitat for the midcentury A2 emission scenario based on 20 GCMs and 13 bat species. Because each species was modeled individually, expansion is defined as the presence of at least one species and no change in the other species and contraction was defined as the absence of at least one species and no change in the other species.
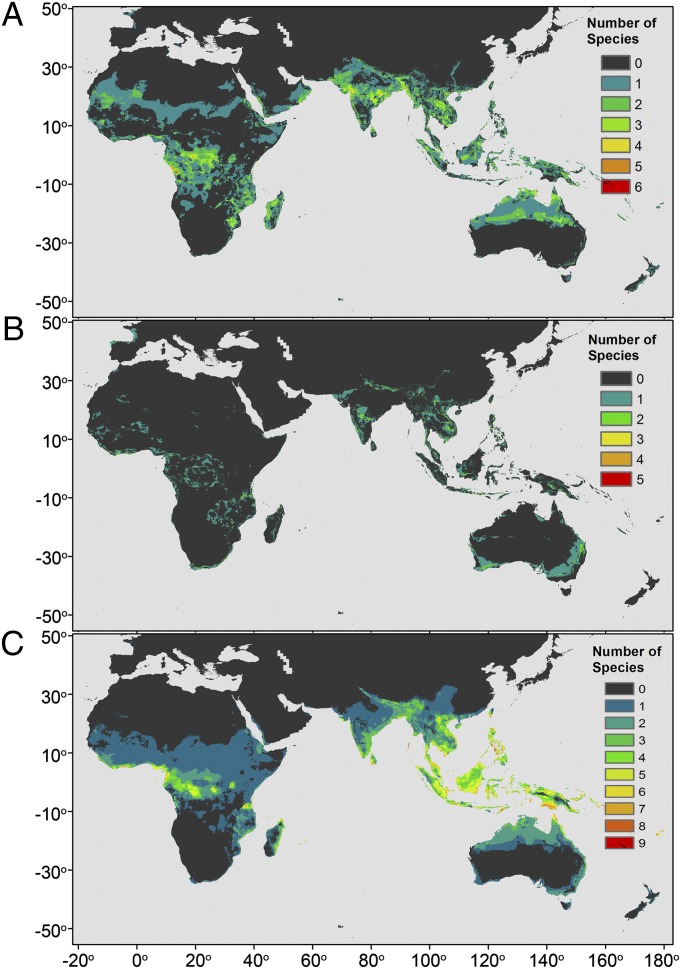
Summing across all models, the ensemble forecast of suitable bioclimatic distribution highlights regions of broad agreement among climate models, describing areas of expansion (Fig. 3A), contraction (Fig. 3B), and stability (Fig. 3C) in Henipavirus host distributions. By midcentury, a significant increase in the geographic area of the potential habitat suitable for Henipavirus bat host species is projected for parts of western Africa, western India, and northern Australia, among other regions. Recently, expansion of the range of Pteropus spp. has been reported in Australia (76), and HeV cases have also recently expanded southward along the eastern coast of Queensland and New South Wales (77), tentatively suggesting that model predictions are consistent with recent observations. The mechanisms by which climate could be linked to the geographic distribution of frugivorous bat Henipavirus hosts are most likely related to their food source. Frugivorous bats, particularly pteropodid bats, are highly mobile, and their local and long-range movements are driven by roost habitat and food resource availability (70, 78–80). Local abundance of fruit bat species in Malaysia, for example, has been shown to fluctuate with fruit availability (81). In Southeast Asia, sporadic fruiting patterns of hardwood tree species are affected by temperature, and cooler La Niña patterns are required for fruiting bursts (82–84). Such associations between fruit bats and the resources on which they depend (and that define their distributions) are implicitly captured in our distribution models. El Niño-related droughts and forest fires, which are increasing in frequency in Southeast Asia, could lead to a reduction in annual rainforest fruit production, and therefore food resource availability for frugivorous species, including Pteropus bats (85). Above all, die-offs of pteropid bats have been reported in association with heat waves in these regions (86), suggesting that these bats will need to track their optimal climate niche to cooler latitudes or altitudes, thus expanding and/or contracting their home ranges.
Fig. 3.
Potential climatic habitat expansion (A), contraction (B), and stability (C) maps for the midcentury A2 emissions scenario based on agreement of 20 GCMs and 13 bat species.
Our host range distribution projections have direct relevance to efforts to control NiV, which is the cause of repeated outbreaks of encephalitis in Bangladesh and India (87, 88), and HeV, which has recently undergone an unprecedented series of spillover events in Australia (89). Expansion or shifts in the range of pteropid bats due to warming temperatures could have an impact on the circulation of Henipavirus and spillover risk in three main ways. First, the current distribution of the hosts (and associated pathogens) may shift geographically, altering emergence potential in the region. Second, decreased local food resources and/or extreme weather events (e.g., heat waves) could place bat populations under physiological stress, leading to immune suppression and prolonged viral shedding, as well as increased viral incidence within populations, as may be the case with HeV in Pteropus scapulatus (90). Second, reduced food production in forest environments may lead bats to seek cultivated fruit, which is consistently available year-round. Preferential feeding on human-cultivated fruit or other plant products, or simply the availability of cultivated fruit in the absence of natural forage, may increase the risk for viral spillover to people or livestock, as was seen with NiV in Bangladesh (transmitted via date palm sap) and Malaysia (transmitted via mangos fed to pigs) (21, 91).
Projections of Henipavirus reservoir range expansion produced in the current study could be used to aid monitoring and prevention efforts in those areas most at risk for future disease introduction. These could include surveillance of bats at the edges of their current distribution; diagnostic laboratory capacity building in regions where new cases of the disease are expected; and enhanced surveillance of hospitals for cases of encephalitis, a primary symptom of Henipavirus infection in people (92, 93).
Strategies for Predicting Future Spread of EIDs
There are many uncertainties associated with ecological forecasting, and previous efforts to project future disease patterns have been criticized as being too speculative to translate into concrete, preventative public health actions (94). The use of ENM inherently involves uncertainties associated with choices of occurrence data, environmental variables, and modeling algorithms. When the future geographic range of a target species is modeled, many additional choices are made, including selection of environmental variables from among a large number of GCMs, each with multiple runs under alternative greenhouse gas emissions scenarios. Most efforts to forecast the future distributions of vectors or reservoirs of infectious disease use only a very small sampling of available simulations of future climates (46, 47, 95, 96). Usually, neither scientists nor managers know if similar results would be produced if the target species were modeled under a different set of data choices. This level of uncertainty often prevents the application of the results of ecological forecast models to public health decision making and risk assessment. Here, we have demonstrated a statistically rigorous ensemble-modeling approach focused on the potential for climate change to shift the host range and on the likely occurrence (or lack thereof) of reservoir species able to support transmission for the particular case study of henipaviruses.
In conclusion, we propose that strategies to deal with EIDs proactively will require, along with continued public health investment, increased focus on (i) identifying the driving mechanisms that underpin the emergence of each new EID and (ii) predictive modeling of how these drivers will promote or shape future EID emergence potential and/or risk. These approaches are inherently multidisciplinary, and they are still in the early stages of their development as disciplines. The former requires extensive ecological studies that examine long-term trends in environmental factors and how changes to these affect disease ecology within reservoir hosts, vectors, and people. The latter requires large datasets on environmental and ecological factors, as well as on pathogen and host distributions. As the discipline of emerging disease ecology develops, the challenge will become one of how to insert these approaches more widely into the toolbox available to agencies to predict and prevent pandemics.
Acknowledgments
This work was supported by a National Institutes of Health/National Science Foundation “Ecology and Evolution of Infectious Diseases” award from the Fogarty International Center (Grant 2R01-TW005869), National Institute of Allergy and Infectious Diseases Grant 1 R01 AI079231, a National Science Foundation Human and Social Dynamics Agents of Change award (BCS 0826779 and BCS 0826840), the Research and Policy for Infectious Disease Dynamics program of the Science and Technology Directorate, the National Institutes of Health National Institute for Allergy and Infectious Diseases (Grant K08AI067549), and the US Department of Homeland Security, as well as by the generous support of the American people through the US Agency for International Development (Grant 1272) “Emerging Pandemic Threats” (PREDICT).
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Fostering Advances in Interdisciplinary Climate Science,” held March 31–April 2, 2011, at the AAAS Auditorium in Washington, DC. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/climate-science.html.
This article is a PNAS Direct Submission. J.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201243109/-/DCSupplemental.
References
- 1.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones KE, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmbhatt M. Avian and Human Pandemic Influenza—Economic and Social Impacts. Geneva, Switzerland: World Bank; 2005. [Google Scholar]
- 4.USAID 2010. Emerging Pandemic Threats: Program overview. Available at http://www.usaid.gov/our_work/global_health/home/News/ai_docs/emerging_threats.pdf. Accessed June 6, 2012.
- 5.Weiss RA, McMichael AJ. Social and environmental risk factors in the emergence of infectious diseases. Nat Med. 2004;10(Suppl):S70–S76. doi: 10.1038/nm1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morse SS. Factors in the emergence of infectious diseases. Emerg Infect Dis. 1995;1:7–15. doi: 10.3201/eid0101.950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smolinski MS, Hamburg MA, Lederberg J. Microbial Threats to Health: Emergence, Detection, and Response. Washington, DC: National Academies Press; 2003. p. 398. [PubMed] [Google Scholar]
- 8.Pedersen AB, Davies TJ. Cross-species pathogen transmission and disease emergence in primates. EcoHealth. 2009;6:496–508. doi: 10.1007/s10393-010-0284-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daszak P. In: Can we predict future trends in disease emergence? Microbial Evolution and Co-Adaptation: A Tribute to the Life and Scientific Legacies of Joshua Lederberg. Relman DA, Hamburg MA, Choffnes ER, Mack A, editors. Washington, DC: Institute of Medicine; 2009. pp. 252–269. [Google Scholar]
- 10.Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife—Threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 11.Daszak P, et al. Collaborative research approaches to the role of wildlife in zoonotic disease emergence. Curr Top Microbiol Immunol. 2007;315:463–475. doi: 10.1007/978-3-540-70962-6_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lafferty KD. The ecology of climate change and infectious diseases. Ecology. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- 13.Lanciotti RS, et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 14.Kilpatrick AM, Gluzberg Y, Burgett J, Daszak P. A quantitative risk assessment of the pathways by which West Nile virus could reach Hawaii. EcoHealth. 2004;1:205–209. [Google Scholar]
- 15.Kilpatrick AM. Globalization, land use, and the invasion of West Nile virus. Science. 2011;334:323–327. doi: 10.1126/science.1201010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn BH, Shaw GM, De Cock KM, Sharp PM. AIDS as a zoonosis: Scientific and public health implications. Science. 2000;287:607–614. doi: 10.1126/science.287.5453.607. [DOI] [PubMed] [Google Scholar]
- 17.Sharp PM, Hahn BH. The evolution of HIV-1 and the origin of AIDS. Philos Trans R Soc Lond B Biol Sci. 2010;365:2487–2494. doi: 10.1098/rstb.2010.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lloyd-Smith JO, et al. Epidemic dynamics at the human-animal interface. Science. 2009;326:1362–1367. doi: 10.1126/science.1177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooper E. The River: A journey to the Source of HIV and AIDS. Boston: Little, Brown; 1999. [Google Scholar]
- 20.de Sousa JD, Müller V, Lemey P, Vandamme AM. High GUD incidence in the early 20 century created a particularly permissive time window for the origin and initial spread of epidemic HIV strains. PLoS One. 2010;5:e9936. doi: 10.1371/journal.pone.0009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chua KB, et al. Nipah virus: A recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- 22.Chua KB, Chua BH, Wang CW. Anthropogenic deforestation, El Niño and the emergence of Nipah virus in Malaysia. Malays J Pathol. 2002;24(1):15–21. [PubMed] [Google Scholar]
- 23.Abram NJ, Gagan MK, McCulloch MT, Chappell J, Hantoro WS. Coral reef death during the 1997 Indian Ocean Dipole linked to Indonesian wildfires. Science. 2003;301:952–955. doi: 10.1126/science.1083841. [DOI] [PubMed] [Google Scholar]
- 24.Pulliam JRC, et al. Henipavirus Ecology Research Group (HERG) Agricultural intensification, priming for persistence and the emergence of Nipah virus: A lethal bat-borne zoonosis. J R Soc Interface. 2012;9(66):89–101. doi: 10.1098/rsif.2011.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epstein J, et al. Pteropus vampyrus, a hunted migratory species with a multinational home-range and a need for regional management. J Appl Ecol. 2009;46:991–1002. [Google Scholar]
- 26.Plowright RK, Sokolow SH, Gorman ME, Daszak P, Foley JE. Causal inference in disease ecology: Investigating ecological drivers of disease emergence. Front Ecol Environ. 2008;6:420–429. [Google Scholar]
- 27.Cheng TL, Rovito SM, Wake DB, Vredenburg VT. Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis. Proc Natl Acad Sci USA. 2011;108:9502–9507. doi: 10.1073/pnas.1105538108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daszak P, et al. Amphibian population declines at savannah river site are linked to climate, not chytridiomycosis. Ecology. 2005;86:3232–3237. [Google Scholar]
- 29.Wilcox BA, et al. EcoHealth: A transdisciplinary imperative for a sustainable future. EcoHealth. 2004;1:3–5. [Google Scholar]
- 30.Zinsstag J, Schelling E, Waltner-Toews D, Tanner M. From “one medicine” to “one health” and systemic approaches to health and well-being. Prev Vet Med. 2011;101(3–4):148–156. doi: 10.1016/j.prevetmed.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosseini P, Sokolow SH, Vandegrift KJ, Kilpatrick AM, Daszak P. Predictive power of air travel and socio-economic data for early pandemic spread. PLoS One. 2010;5:e12763. doi: 10.1371/journal.pone.0012763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilpatrick AM, et al. Predicting the global spread of H5N1 avian influenza. Proc Natl Acad Sci USA. 2006;103:19368–19373. doi: 10.1073/pnas.0609227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hufnagel L, Brockmann D, Geisel T. Forecast and control of epidemics in a globalized world. Proc Natl Acad Sci USA. 2004;101:15124–15129. doi: 10.1073/pnas.0308344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers DJ, Randolph SE. Climate change and vector-borne diseases. Adv Parasitol. 2006;62:345–381. doi: 10.1016/S0065-308X(05)62010-6. [DOI] [PubMed] [Google Scholar]
- 35.Patz JA, Olson SH, Uejio CK, Gibbs HK. Disease emergence from global climate and land use change. Med Clin North Am. 2008;92:1473–1491, xii. doi: 10.1016/j.mcna.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Gubler DJ, et al. Climate variability and change in the United States: Potential impacts on vector- and rodent-borne diseases. Environ Health Perspect. 2001;109(Suppl 2):223–233. doi: 10.1289/ehp.109-1240669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolivras KN. Changes in dengue risk potential in Hawaii, USA, due to climate variability and change. Clim Res. 2010;42(1):1–11. [Google Scholar]
- 38.Mills JN, Gage KL, Khan AS. Potential influence of climate change on vector-borne and zoonotic diseases: A review and proposed research plan. Environ Health Perspect. 2010;118:1507–1514. doi: 10.1289/ehp.0901389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Epstein PR. Global warming and vector-borne disease. Lancet. 1998;351:1737. doi: 10.1016/S0140-6736(05)77777-1. author reply 1738. [DOI] [PubMed] [Google Scholar]
- 40.Githeko AK, Ototo EN, Guiyun Y. Progress towards understanding the ecology and epidemiology of malaria in the western Kenya highlands: Opportunities and challenges for control under climate change risk. Acta Trop. 2012;121(1):19–25. doi: 10.1016/j.actatropica.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou GF, Minakawa N, Githeko AK, Yan GY. Association between climate variability and malaria epidemics in the East African highlands. Proc Natl Acad Sci USA. 2004;101:2375–2380. doi: 10.1073/pnas.0308714100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kant R. Global malaria burden and achieving universal coverage of interventions: A glimpse on progress and impact. Curr Sci. 2011;101:286–292. [Google Scholar]
- 43.Beguin A, et al. The opposing effects of climate change and socio-economic development on the global distribution of malaria. Global Environmental Change-Human and Policy Dimensions. 2011;21:1209–1214. [Google Scholar]
- 44.Gething PW, et al. Climate change and the global malaria recession. Nature. 2010;465:342–345. doi: 10.1038/nature09098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gething PW, et al. Modelling the global constraints of temperature on transmission of Plasmodium falciparum and P. vivax. Parasit Vectors. 2011;4:92–102. doi: 10.1186/1756-3305-4-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peterson AT. Shifting suitability for malaria vectors across Africa with warming climates. BMC Infect Dis. 2009;9:59–64. doi: 10.1186/1471-2334-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joyner TA, et al. Modeling the potential distribution of Bacillus anthracis under multiple climate change scenarios for Kazakhstan. PLoS One. 2010;5:e9596. doi: 10.1371/journal.pone.0009596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elith J, et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography. 2006;29(2):129–151. [Google Scholar]
- 49.Kearney M, Porter W. Mechanistic niche modelling: Combining physiological and spatial data to predict species’ ranges. Ecol Lett. 2009;12:334–350. doi: 10.1111/j.1461-0248.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 50.Kearney M. Habitat, environment and niche: What are we modelling? Oikos. 2006;115(1):186–191. [Google Scholar]
- 51.Elith J, Leathwick J. Species distribution models: Ecological explanation and prediction across space and time. Annu Rev Ecol Evol Syst. 2009;40:677–697. [Google Scholar]
- 52.Franklin J. Moving beyond static species distribution models in support of conservation biogeography. Divers Distrib. 2010;16:321–330. [Google Scholar]
- 53.Murray KA, et al. Assessing spatial patterns of disease risk to biodiversity: Implications for the management of the amphibian pathogen, Batrachochytrium dendrobatidis. J Appl Ecol. 2011;48:163–173. [Google Scholar]
- 54.Ellis CK, et al. Ecology and geography of human monkeypox case occurrences across Africa. J Wildl Dis. 2012;48:335–347. doi: 10.7589/0090-3558-48.2.335. [DOI] [PubMed] [Google Scholar]
- 55.Peterson AT, Carroll DS, Mills JN, Johnson KM. Potential mammalian filovirus reservoirs. Emerg Infect Dis. 2004;10:2073–2081. doi: 10.3201/eid1012.040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peterson AT, Sánchez-Cordero V, Beard CB, Ramsey JM. Ecologic niche modeling and potential reservoirs for Chagas disease, Mexico. Emerg Infect Dis. 2002;8:662–667. doi: 10.3201/eid0807.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patz JA, et al. Millennium Ecosystem Assessment. Condition and Trends Working Group (2005) Human health: Ecosystem regulation of infectious diseases. Ecosystems and Human Well-Being: Current State and Trends. Findings of the Condition and Trends Working Group (Island Press, Washington DC), Vol 1, pp 391–415.
- 58.Peterson AT, Bauer JT, Mills JN. Ecologic and geographic distribution of filovirus disease. Emerg Infect Dis. 2004;10:40–47. doi: 10.3201/eid1001.030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Constable H, Guralnick R, Wieczorek J, Spencer C, Peterson AT. VertNet Steering Committee VertNet: A new model for biodiversity data sharing. PLoS Biol. 2010;8:e1000309. doi: 10.1371/journal.pbio.1000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moritz C, et al. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science. 2008;322:261–264. doi: 10.1126/science.1163428. [DOI] [PubMed] [Google Scholar]
- 61.Chen IC, et al. Elevation increases in moth assemblages over 42 years on a tropical mountain. Proc Natl Acad Sci USA. 2009;106:1479–1483. doi: 10.1073/pnas.0809320106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hitch AT, Leberg PL. Breeding distributions of north American bird species moving north as a result of climate change. Conserv Biol. 2007;21:534–539. doi: 10.1111/j.1523-1739.2006.00609.x. [DOI] [PubMed] [Google Scholar]
- 63.Pöyry J, Luoto M, Heikkinen RK, Kuussaari M, Saarinen K. Species traits explain recent range shifts of Finnish butterflies. Glob Change Biol. 2009;15:732–743. [Google Scholar]
- 64.Loarie SR, et al. The velocity of climate change. Nature. 2009;462:1052–1055. doi: 10.1038/nature08649. [DOI] [PubMed] [Google Scholar]
- 65.Perch-Nielsen S, Bättig M, Imboden D. Exploring the link between climate change and migration. Clim Change. 2008;91:375–393. [Google Scholar]
- 66.Drexler JF, et al. Bats host major mammalian paramyxoviruses. Nat Commun. 2012;3:796. doi: 10.1038/ncomms1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Epstein JH, Field HE, Luby S, Pulliam JRC, Daszak P. Nipah virus: Impact, origins, and causes of emergence. Curr Infect Dis Rep. 2006;8(1):59–65. doi: 10.1007/s11908-006-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayman DTS, et al. 2008. Evidence of Henipavirus infection in West African fruit bats. PLoS One 3:e2739.
- 69.Field HE, Mackenzie JS, Daszak P. Henipaviruses: Emerging paramyxoviruses associated with fruit bats. Curr Top Microbiol Immunol. 2007;315:133–159. doi: 10.1007/978-3-540-70962-6_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tidemann CR, Vardon MJ, Loughland RA, Brocklehurst PJ. Dry season camps of flying foxes (Pteropus spp.) in Kakadu World Heritage Area, north Australia. J Zool. 1999;247:155–163. [Google Scholar]
- 71.Vardon MJ, et al. Seasonal habitat use by flying-foxes, Pteropus alecto and P. scapulatus (Megachiroptera), in monsoonal Australia. J Zool. 2001;253:523–535. [Google Scholar]
- 72.Epstein JH, et al. Henipavirus infection in fruit bats (Pteropus giganteus), India. Emerg Infect Dis. 2008;14:1309–1311. doi: 10.3201/eid1408.071492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Araújo MB, New M. Ensemble forecasting of species distributions. Trends Ecol Evol. 2007;22(1):42–47. doi: 10.1016/j.tree.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 74.Buisson L, Thuiller W, Casajus N, Lek S, Grenouillet G. Uncertainty in ensemble forecasting of species distribution. Glob Change Biol. 2010;16:1145–1157. [Google Scholar]
- 75.Nakicenovic N, Swart R. Emissions Scenarios. Special Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: IPCC; 2000. [Google Scholar]
- 76.van der Ree R, McDonnell MJ, Temby I, Nelson J, Whittingham E. The establishment and dynamics of a recently established urban camp of flying foxes (Pteropus poliocephalus) outside their geographic range. J Zool. 2006;268:177–185. [Google Scholar]
- 77.Field H, McLaughlin A, Fitzpatrick B. Investigating the ‘why’ and ‘where’ of Hendra virus infection in horses. EcoHealth. 2011;7(Suppl):S86–S87. [Google Scholar]
- 78.Palmer C, Price O, Bach C. Foraging ecology of the black flying fox (Pteropus alecto) in the seasonal tropics of the Northern Territory, Australia. Wildl Res. 2000;27(2):169–178. [Google Scholar]
- 79.Richter HV, Cumming GS. Food availability and annual migration of the straw-colored fruit bat (Eidolon helvum) J Zool. 2006;268:35–44. [Google Scholar]
- 80.Eby P, Richards G, Collins L, Parry-Jones K. The distribution, abundance and vulnerability to population reduction of a nomadic nectarivore, the grey-headed flying-fox Pteropus poliocephalus in New South Wales, during a period of resource concentration. Aust Zool. 1999;31:240–253. [Google Scholar]
- 81.Hodgkison R, Balding ST, Zubaid A, Kunz TH. Temporal variation in the relative abundance of fruit bats (Megachiroptera: Pteropodidae) in relation to the availability of food in a lowland Malaysian rain forest. Biotropica. 2004;36:522–533. [Google Scholar]
- 82.Numata S, Yasuda M, Okuda T, Kachi N, Noor NSM. Temporal and spatial patterns of mass flowerings on the Malay Peninsula. Am J Bot. 2003;90:1025–1031. doi: 10.3732/ajb.90.7.1025. [DOI] [PubMed] [Google Scholar]
- 83.Wich SA, Van Schaik CP. The impact of El Nino on mast fruiting in Sumatra and elsewhere in Malesia. J Trop Ecol. 2000;16:563–577. [Google Scholar]
- 84.Yasuda M, et al. The mechanism of general flowering in Dipterocarpaceae in the Malay Peninsula. J Trop Ecol. 1999;15:437–449. [Google Scholar]
- 85.Fredriksson GM, Danielsen LS, Swenson JE. Impacts of El Nino related drought and forest fires on sun bear fruit resources in lowland dipterocarp forest of East Borneo. Biodivers Conserv. 2007;16:1823–1838. [Google Scholar]
- 86.Welbergen JA, Klose SM, Markus N, Eby P. Climate change and the effects of temperature extremes on Australian flying-foxes. Proc Biol Sci. 2008;275:419–425. doi: 10.1098/rspb.2007.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luby SP, et al. Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001-2007. Emerg Infect Dis. 2009;15:1229–1235. doi: 10.3201/eid1508.081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chadha MS, et al. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg Infect Dis. 2006;12:235–240. doi: 10.3201/eid1202.051247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Queensland Government Department of Primary Industries and Fisheries 2011. Hendra virus case confirmed on the Gold Coast (Queensland Government, Department of Agriculture, Fisheries and Forestry, Brisbane, QLD, Australia). Available at http://www.daff.qld.gov.au/30_20823.htm. Accessed August 14, 2012.
- 90.Plowright RK, et al. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus) Proc Biol Sci. 2008;275:861–869. doi: 10.1098/rspb.2007.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luby SP, et al. Foodborne transmission of Nipah virus, Bangladesh. Emerg Infect Dis. 2006;12:1888–1894. doi: 10.3201/eid1212.060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Epstein J, et al. Integrating Nipah virus ecology in pteropodid bats into a comprehensive surveillance program in Bangladesh. EcoHealth. 2011;7(Suppl):S38–S38. [Google Scholar]
- 93.Homaira N, et al. Nipah virus outbreak with person-to-person transmission in a district of Bangladesh, 2007. Epidemiol Infect. 2010;138:1630–1636. doi: 10.1017/S0950268810000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosenthal J. Climate change and the geographic distribution of infectious diseases. EcoHealth. 2009;6:489–495. doi: 10.1007/s10393-010-0314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.González C, et al. Climate change and risk of leishmaniasis in north america: predictions from ecological niche models of vector and reservoir species. PLoS Negl Trop Dis. 2010;4:e585. doi: 10.1371/journal.pntd.0000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holt AC, Salkeld DJ, Fritz CL, Tucker JR, Gong P. Spatial analysis of plague in California: Niche modeling predictions of the current distribution and potential response to climate change. Int J Health Geogr. 2009;8:38–51. doi: 10.1186/1476-072X-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Keymer DP, Lam LH, Boehm AB. Biogeographic patterns in genomic diversity among a large collection of Vibrio cholerae isolates. Appl Environ Microbiol. 2009;75:1658–1666. doi: 10.1128/AEM.01304-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Neerinckx S, et al. Predicting potential risk areas of human plague for the Western Usambara Mountains, Lushoto District, Tanzania. Am J Trop Med Hyg. 2010;82:492–500. doi: 10.4269/ajtmh.2010.09-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Adhikari D, Chettri A, Barik SK. Modelling the ecology and distribution of highly pathogenic avian influenza (H5N1) in the Indian subcontinent. Curr Sci. 2009;97:73–78. [Google Scholar]
- 100.Baptista-Rosas RC, Hinojosa A, Riquelme M. 2007. Ecological niche modeling of Coccidioides spp. in Western North American deserts. Coccidioidomycosis: Sixth International Symposium, Annals of the New York Academy of Sciences, eds Clemons KV, Laniado Laborin R, Stevens DA, Vol 1111, pp 35–46.
- 101.Blackburn JK, McNyset KM, Curtis A, Hugh-Jones ME. Modeling the geographic distribution of Bacillus anthracis, the causative agent of anthrax disease, for the contiguous United States using predictive ecological [corrected] niche modeling. Am J Trop Med Hyg. 2007;77:1103–1110. [PubMed] [Google Scholar]
- 102.Costa J, Peterson AT, Beard CB. Ecologic niche modeling and differentiation of populations of Triatoma brasiliensis neiva, 1911, the most important Chagas’ disease vector in northeastern Brazil (Hemiptera, Reduviidae, Triatominae) Am J Trop Med Hyg. 2002;67:516–520. doi: 10.4269/ajtmh.2002.67.516. [DOI] [PubMed] [Google Scholar]
- 103.French N, et al. Spatial epidemiology and natural population structure of Campylobacter jejuni colonizing a farmland ecosystem. Environ Microbiol. 2005;7:1116–1126. doi: 10.1111/j.1462-2920.2005.00782.x. [DOI] [PubMed] [Google Scholar]
- 104.Harris NC, Dunn RR. Using host associations to predict spatial patterns in the species richness of the parasites of North American carnivores. Ecol Lett. 2010;13:1411–1418. doi: 10.1111/j.1461-0248.2010.01527.x. [DOI] [PubMed] [Google Scholar]
- 105.Moratelli R, de Andreazzi CS, de Oliveira JA, Cordeiro JLP. Current and potential distribution of Myotis simus (Chiroptera, Vespertilionidae) Mammalia. 2011;75:227–234. [Google Scholar]
- 106.Peterson AT, Robbins A, Restifo R, Howell J, Nasci R. Predictable ecology and geography of West Nile virus transmission in the central United States. J Vector Ecol. 2008;33:342–352. doi: 10.3376/1081-1710-33.2.342. [DOI] [PubMed] [Google Scholar]
- 107.Rabinovich JE, et al. Ecological patterns of blood-feeding by kissing-bugs (Hemiptera: Reduviidae: Triatominae) Mem Inst Oswaldo Cruz. 2011;106:479–494. doi: 10.1590/s0074-02762011000400016. [DOI] [PubMed] [Google Scholar]
- 108.Williams RAJ, Fasina FO, Peterson AT. Predictable ecology and geography of avian influenza (H5N1) transmission in Nigeria and West Africa. Trans R Soc Trop Med Hyg. 2008;102:471–479. doi: 10.1016/j.trstmh.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 109.Peterson AT, Shaw J. Lutzomyia vectors for cutaneous leishmaniasis in Southern Brazil: Ecological niche models, predicted geographic distributions, and climate change effects. Int J Parasitol. 2003;33:919–931. doi: 10.1016/s0020-7519(03)00094-8. [DOI] [PubMed] [Google Scholar]
- 110.Gálvez R, Descalzo MA, Guerrero I, Miró G, Molina R. Mapping the current distribution and predicted spread of the leishmaniosis sand fly vector in the madrid region (Spain) based on environmental variables and expected climate change. Vector Borne Zoonotic Dis. 2011;11:799–806. doi: 10.1089/vbz.2010.0109. [DOI] [PubMed] [Google Scholar]
- 111.Peterson AT, Martínez-Campos C, Nakazawa Y, Martínez-Meyer E. Time-specific ecological niche modeling predicts spatial dynamics of vector insects and human dengue cases. Trans R Soc Trop Med Hyg. 2005;99:647–655. doi: 10.1016/j.trstmh.2005.02.004. [DOI] [PubMed] [Google Scholar]