Abstract
In cold extracts of senescent leaves of the Lime tree (Tilia cordata), two colorless nonfluorescent chlorophyll catabolites (NCCs) were identified, named Tc-NCC-1 and Tc-NCC-2, as well as a polar yellow chlorophyll catabolite (YCC), named Tc-YCC. The constitution of the two NCCs was determined by spectroscopic means. In addition, a tentative structure was derived for Tc-YCC. The three chlorophyll degradation products exhibited tetrapyrrolic structures, as are typical of NCCs or YCCs, and turned out to be rather polar, due to a glucopyranosyl group at their 82-position. At their 3-positions, the more polar Tc-NCC-1 carried a 1,2-dihydroxyethyl group and the less polar Tc-NCC-2 a vinyl group. Tc-YCC was identified as the product of an oxidation of Tc-NCC-1.
Keywords: Chlorophyll, Senescence, Pigments, Porphyrins
Introduction
Chlorophyll biosynthesis and chlorophyll breakdown are fascinating natural life processes on earth 1, which can be observed from outer space 2. Indeed, an estimated 109 tons of chlorophyll are formed and degraded every year on earth 3 4. Strikingly, chlorophyll breakdown and the appearance of autumnal fall colors have remained a stunning mystery 4–6 until about twenty years ago, when a first colorless tetrapyrrolic chlorophyll catabolite 1a (Hv-NCC-1) was identified in senescent primary leaves of barley 7 8. Nonfluorescent chlorophyll catabolites (NCCs) were subsequently also found in other plants, and they were assumed to represent the final stages of chlorophyll catabolism in senescent leaves 2 6 9 10. Since then, over a dozen NCCs were detected in higher plants, and their structures were analyzed (Scheme and Table 1).
Table.
Structures of Nonfluorescent Chlorophyll Catabolites Found in Senescent Leaves of Higher Plants (the labels R1 to R4 refer to the general constitutional formula of NCCs in the Scheme; atoms numbered according to their original position in chlorophyll a)
| No.a) | R1b) | R2 | R3 | R4 | C(1)c) | Provisional namesd) | Ref. |
|---|---|---|---|---|---|---|---|
| 1a | OH | Me | HOCH2CH(OH) | Me | n | Hv-NCC-1 | [7][8] |
| 1b | OH | Me | HOCH2CH(OH) | Me | epi | So-NCC-2 | [11] [12] |
| 2 | H | Me | CH2 = CH | Me | epi | Cj-NCC-2/So-NCC-5 | [12][13] |
| 3a | OH | Me | CH2 = CH | Me | n | Sw-NCC-58 | [14] |
| 3b | OH | Me | CH2 = CH | Me | epi | Cj-NCC-1/So-NCC-4/Pc-NCC-2/Md-NCC-2 | [12][13][15][16] |
| 4a | b-GlcO | Me | CH2 = CH | Me | n | At-NCC-4 | [17] |
| 4b | b-GlcO | Me | CH2 = CH | Me | epi | Nr-NCC-2/Zm-NCC-2/Pc-NCC-1/Md-NCC-1/Tc-NCC-2 | [16][18][19]e) |
| 5 | b-GlcO | Me | HOCH2CH(OH) | Me | epi | Zm-NCC-1/Tc-NCC-1 | [19]e) |
| 6 | b-(6’-O-Mal) GlcO | Me | CH2 = CH | Me | epi | Nr-NCC-1 | [18] |
| 7 | H | H | CH2 = CH | Me | n | Bn-NCC-4/At-NCC-5 | [17] |
| 8 | H | H | CH2 = CH | HOCH2 | n | At-NCC-3 | [17][20] |
| 9a | OH | H | CH2 = CH | Me | n | Bn-NCC-3/At-NCC-2 | [17][21] |
| 9b | OH | H | CH2 = CH | Me | epi | So-NCC-3 | [12] |
| 10 | OH | H | HOCH2CH(OH) | Me | epi | So-NCC-1 | [12] |
| 11 | MalO | H | CH2 = CH | Me | n | Bn-NCC-1 | [21] [22] |
| 12 | b-GlcO | H | CH2 = CH | Me | n | Bn-NCC-2/At-NCC-1 | [17] [21] |
)Compound number.
)Abbreviations: Mal = malonyl; Glc = glucopyranosyl.
) Configuration at C(1) from correlation with NCCs derived from pFCC (n = ‘normal’) or from epi-pFCC (epi = ‘epimeric’), the absolute configuration at C(1) is still unknown.
) Hv-NCC-1 (1a; from barley, Hordeum vulgare [7] [8]), So-NCCs (1b, 2, 3b, 9b, and 10; from spinach, Spinacia oleracea [11] [12]), Cj-NCCs (2 and 3b; from Katsura tree, Cercidiphyllum japonicum [13] [15]), Sw-NCC-58 (3a; from Peace Lily, Spathiphyllum wallisii [14]), Pc-NCCs (3b and 4b; from Pyrus communis [16]), Md-NCCs (3b and 4b; from Malus domestica [16]), At-NCCs (4a, 7, 8, 9a, and 12; from Arabidopsis thaliana [17] [20]), Nr-NCCs (4b and 6; from tobacco, Nicotiana rustica [18]), Zm- NCCs (4b and 5; from maize, Zea mays [19]), and Bn-NCCs (7, 9a, 11, and 12; from oilseed rape, Brassica napus [21] [22]), and Tc-NCCs (5 and 4b; from Lime tree (Tilia cordata), this work).
) This work.
About ten years ago, two ‘urobilinogenoidic’ chlorophyll catabolites were discovered in extracts of senescent primary leaves of barley 23, i.e., linear tetrapyrroles, which were considered to represent putative products of further breakdown of Hv-NCC-1 (1a) by an oxidative deformylation at ring B. Evidence for another type of further oxidative transformation of NCCs was also provided more recently by the yellow chlorophyll catabolites (YCCs) and pink chlorophyll catabolites (PiCCs) detected in senescent leaves of the Katsura tree (Cercidiphyllum japonicum), which were identified as dehydrogenation products of the tetrapyrrolic NCC 3b (Cj-NCC-1) 24 25. All of these findings were consistent with an essentially ‘linear’ path of chlorophyll breakdown in higher plants (see the Scheme) 26.
Scheme.
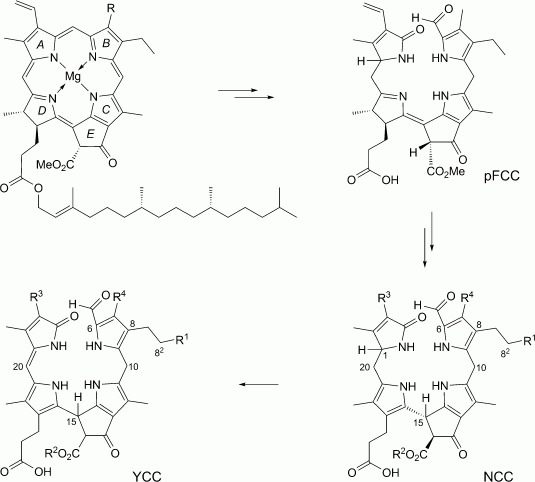
Outline of the Main Path of Chlorophyll Breakdown in Higher Plants, Including Structural Formulae of Chlorophylls a and b, of the Primary Fluorescent Chlorophyll Catabolite (pFCC), of Non-fluorescent Chlorophyll Catabolites (NCCs), and of Yellow Chlorophyll Catabolites (YCCs; with general formulae for NCCs and YCCs, see Table 1 for specific constitutional formulae of individual NCCs). Atom numbering used for chlorophylls according to IUPAC (see, e.g., 1)
However, several recent observations suggested the existence of divergent pathways of chlorophyll breakdown in higher plants. A strikingly contrasting stereo-chemical variant of the ‘urogenobilinoidic’ catabolites from barley was found in naturally de-greened leaves of Norway Maple (Acer platanoides), classified as dioxobilanes, and indicating a different path to these tetrapyrroles 27. Structurally divergent, ‘hypermodified’ blue fluorescent chlorophyll catabolites (FCCs) were observed as remarkably persistent breakdown products in banana (Musa acuminata) fruits 28 and leaves 29, as well as in senescent leaves of the ‘peace Lily’ (Spathiphyllum wallisii), a tropical evergreen 14.
Here, we describe an investigation of chlorophyll breakdown products in senescent leaves of the lime tree (Tilia cordata), a first representative of the genus Tilia (Malvaceae) to be investigated in this respect. Lime trees are well-known deciduous trees native to the forests of the northern hemisphere in Europe, Asia, and Eastern North America and Central America 30. A reference to the importance of Tilia sp. in central Europe can be found in the Middle High German ‘Nibelungenlied', where a lime tree leaf ('linden leaf) covered Siegfried's back during his bath in the blood of a wounded dragon and gave rise to his vulnerable spot1). Beside a special medicinal relevance of lime trees (colds, cough, fever, etc. are often treated with extracts of this plant 31), representatives of these deciduous trees became increasingly important in municipal parks of cities and play a central role as avenue trees 30.
Results and Discussion
In a cold MeOH extract of yellow (senescent) fall leaves of a lime tree (Tilia cor data), two polar colorless and nonfluorescent chlorophyll catabolites (NCCs) and a yellow chlorophyll catabolite (YCC) were provisionally identified by analytical HPLC, on the basis of their characteristic UV-absorbance properties (see Fig. 1 24 32. To inhibit adventitious oxidation of NCCs to yellow catabolites such as Tc-YCC (13; Fig. 2) 24 during isolation and workup, the freshly picked senescent leaves were immediately frozen with liquid N2 and directly analyzed by HPLC. The different HPLC retention times on the stationary ‘reversed’ phase (fR(Tc-NCC-1 (5)) 31.8 min, tR (Tc-YCC (13)) 33.8 min, and tR (Tc-NCC-2 (4b)) 47.8 min) reflected the different polarities of the three catabolites.
Fig 1.
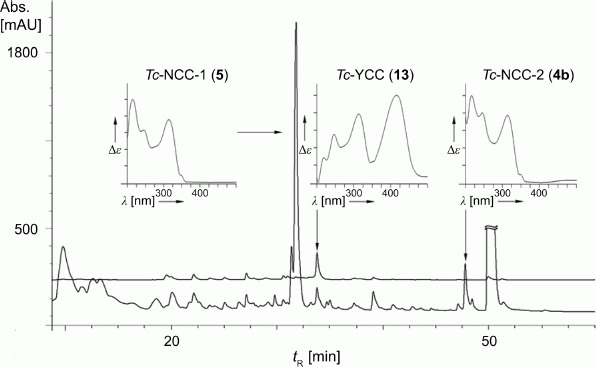
HPLC Analysis of an extract of senescent leaves of Tilia cordata (lower trace: detection at 320 nm, trace above: detection at 420 nm). For details, see the Exper. Part. Insets: Online UV/VIS spectra of Tc-NCC-1 (5; left), Tc-YCC (13; middle), and Tc-NCC-2 (4b; right).
Fig 2.
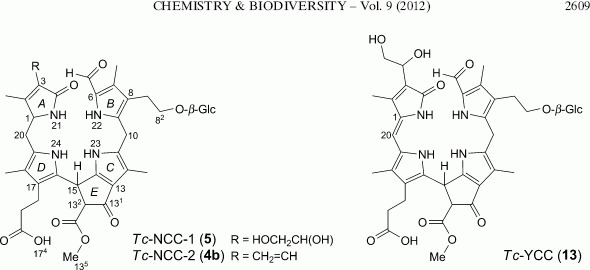
Constitutional formula of Tc-NCC-1 (5), Tc-YCC (13), and Tc-NCC-2 (4b). Numbering of heavy atoms according to their original atom numbering in chlorophylls.
UV/VIS and CD spectra of the NCCs 4b and 5 matched those of the chlorophyll catabolite Hv-NCC-1 (1a). An absorbance maximum near 314 nm indicated the presence of an α-formyl-pyrrole moiety at ring B 7 32. The yellow tetrapyrrolic catabolite Tc-YCC (13) exhibited a characteristic additional maximum at 415 nm, consistent with its color and suggesting (Z)-configuration for its C(20)=C(l) bond 24 25 (see Fig. 1).
For further structural analysis, 100 g (wet weight) of senescent lime tree leaves were extracted according to a three-stage purification procedure based on a cold extraction, followed by separation by MPLC and by preparative HPLC (for details, see the Exper. Part) to give 5.1 mg (6.1 mmol) of Tc-NCC-1 (5), 1 mg (1.2 mmol) of the less polar Tc-NCC-2 (4b), and 0.2 mg of the yellow catabolite Tc-YCC (13) (determined by UV/VIS spectroscopy).
Mass spectrometry was utilized to derive a tentative molecular formula of Tc-NCC-1 (5) as C41H52N4O15 (see Fig. 3, a). ESI-MS in the positive-ion mode showed a signal at m/z 841.49 which corresponded to the pseudo-molecular ion C41H53N4O15+ ([M+H]+; calc. 841.35) of Tc-NCC-1 (5). In the mass spectrum, characteristic fragment-ion peaks at m/z 809.5, 684.4, and 679.3 were also detected, which corresponded to loss (from [M+H] + ) of MeOH, as is typical of the methyl ester functionality, to loss of ring A, and to loss of the sugar moiety (as [C6H10O5]), respectively.
Fig 3.
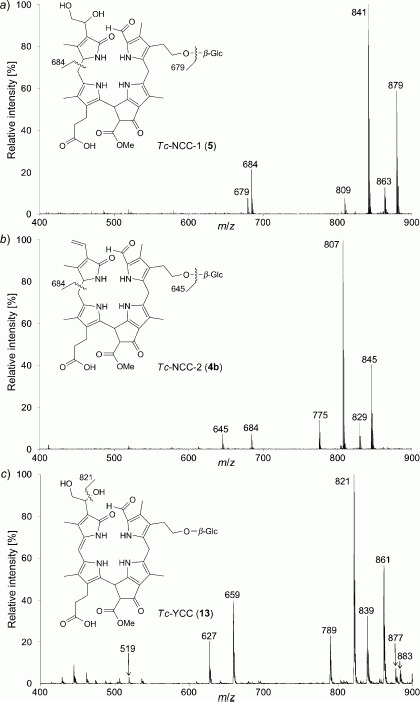
Positive-ion-mode LC/ESI-MS of a) Tc-NCC-1 (5), b) Tc-NCC-2 (4b), and c) Tc-YCC (13) with corresponding constitutional formulae
A 500-MHz 1H-NMR spectrum of a solution of Tc-NCC-1 (5) in CD3OH revealed signals of 44 of the 52 H-atoms: a singlet (at low field) of the formyl H-atom, four singlets (at high field) of the four Me groups attached at the b-pyrrole positions, and a singlet at 3.74 ppm (due to a methyl ester function). In addition, the signals of the H-atoms H-N(21), H-N(24), and H-N(23) were detected between 8.0 and 11.3 ppm, and could be assigned with the help of COSY, ROESY, and HMBC data. However, in contrast to the 1H-NMR spectrum of Tc-NCC-2 (4b), the typical signals for a peripheral vinyl group in the intermediate field range were absent. 1H,13C-Hetero-nuclear NMR correlations (from HSQC and HMBC spectra 33 of Tc-NCC-1 (5) in CD3OH) allowed assignment of the signals of a 1,2-dihydroxyethyl side chain. 1H,1H-Homonuclear correlations from ROESY spectra and 1H,13C-heteronuclear correlations from HMBC spectra 33 indicated C(82) as the site of the attachment of the sugar moiety. The shifts of the 1H and 13C signals for the CH2(82) group were consistent with an O-bridge to the peripheral sugar substituent. The latter was identified as a b-glucopyranoside unit by comparing the 1H and 13C chemical shifts with the spectra of methyl b-d-glucopyranoside 34, as well as by comparing the NMR spectra of Tc-NCC-1 (5) with those of Bn-NCC-2, Nr-NCC-2, and Zm-NCC-1, where a peripheral b-glucopyranosyl group at C(82) had also been found 18 19 22. The signal of H-C(15) appeared as a doublet (J(H,H) = 2.5 Hz, in CD3OH) due to coupling with H-C(132). Therefore, a relative trans-configuration of H-C(132) and H-C(15), which is typical for stable isomers of NCCs 13 32, in Tc-NCC-1 was derived using the Karplus relation 34.
ESI-MS in the positive-ion mode was also used to deduce the tentative molecular formula of Tc-NCC-2 (4b) as C41H50N4O13 (see Fig. 3, b). The peak at m/z 807.37 corresponded to the pseudo-molecular ion C41H51N4O+13 ([M+H]+; calc. 807.34). The basic substitution pattern of the tetrapyrrolic core of Tc-NCC-2 could again be determined by the analysis of characteristic fragment-ion peaks at m/z 775.3, 684.3, and 645.3 due to loss of MeOH, of ring A, and of the sugar moiety (as [C6H10O5]), respectively.
A 600-MHz 1H-NMR-spectrum of 4b (in CD3OD) exhibited signals for 40 of the 50 H-atoms: a singlet (at low field) for the formyl H-atom, four singlets (at high field) for the four Me groups in the b-pyrrole positions, and a singlet at 3.74 ppm (due to the methyl ester function). In addition, the typical signal pattern for a vinyl group was detected around 6 ppm. 1H,13C-Heteronuclear correlations (HSQC and HMBC 33) allowed the assignment of all 1H and 13C signals (see Exper. Part). A sugar moiety was identified as a b-glucopyranosyl group at C(82) from 1H,1H-ROESY correlations, as well as 1H,13C-heternuclear correlations from HMBC spectra 33. Again, the indicated site of attachment for the peripheral glucopyranosyl substituent (C(82)) via a bridging O-atom, O(83), was consistent with the typical downfield shifts of the 1H and 13C signals of CH2(82). The lack of the signal for H-C(132) in the 1H-NMR spectra (in CD3OD) of Tc-NCC-2 (4b) indicated the exchange-labile a-position of the b-keto ester functionality at ring E to have undergone H/D exchange. HPLC Co-injection of Tc-NCC-1 (5) and of the constitutionally identical analog from maize, Zm-NCC-1 19, indicated a common tR of ca. 30 min, suggesting the two NCCs to be identical (see Fig. 4 in the Exper. Part; for details of co-injection experiments, see the Exper. Part).
Fig 4.
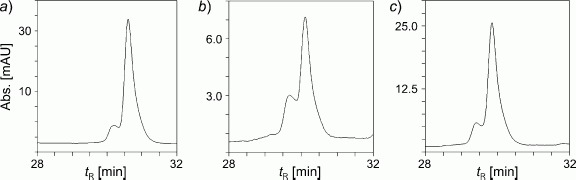
HPLC Analyses of a) Tc-NCC-1, b) ofZm-NCC-1, and c) ofa 1:2 mixture of the two NCCs. In the samples of Tc-NCC-1 and Zm-NCC-1, a second fraction (ca. 10%) occurred in addition to the main fraction (presumably, the minor fraction is the 132-epimer of the main NCCs, due to epimerization at C(132) of the β-keto ester functionality).
The CD spectra of the Tc-NCCs 5 and 4b were consistent with the suggested common configuration of C(15) in naturally occurring NCCs from higher plants 7 26 32. The stereogenic center C(15) has been proposed to result from a nonenzymatic, stereoselective isomerization of fluorescent chlorophyll catabolites 35 36 to the corresponding NCCs 13. The relative configuration at C(15) and C(132), with H-C(15) cis to the COOMe group at C(132) in (the prevailing epimer of) 5 and 4b is a likely result of a nonenzymatic equilibration reaction at the acidic b-keto ester position C(132), which adjusts its configuration to that at C(15). The latter process would occur in the vacuoles of senescent plant cells 13 37, but it also takes place when isolated NCCs are kept in a protic solution.
A tentative molecular formula of Tc-YCC (13) could be deduced as C41H50N4O15 by LC/ESI-MS in the positive-ion mode (see Fig. 3, c), which showed a prominent peak at m/z 839.1 of the pseudo-molecular ion C41H51N4O15+ ([M+H] +; calc. 839.33). The mass spectrum of 13 showed characteristic fragment-ion peaks at m/z 821.1,789.3, and 659.2, due to loss of H2O (from the 1,2-dihydroxyethyl group at ring A), loss of MeOH, and combined loss of the sugar moiety and H2O (in total [C6H12O6]), respectively. The loss of ring A, a typical fragmentation pathway of NCCs (see Fig. 3), could not be observed in the mass spectrum of Tc-YCC (13), compatible with the C=C bond at ring A. For further characterization of 13 as oxidation product of Tc-NCC-1 (5), a nearly colorless spot of analytically pure 5 on a silica-gel TLC plate was irradiated for 10 min with a 365-nm UV lamp 25, whereupon the ‘spot’ acquired a brownish-red color (see Exper. Part). A major yellow product fraction formed, which had a UV/VIS spectrum typical for a YCC with (Z)-configuration of the C(l)=C(20) bond 24 25, and which was shown by analytical HPLC to co-elute with the yellow catabolite Tc-YCC (13).
Conclusions
In the present work, non-green chlorophyll catabolites were analyzed in fresh extracts of naturally de-greened lime tree (Tilia cor data) leaves, a first representative of the genus Tilia to be investigated in this respect. Two colorless nonfluorescent chlorophyll catabolites (NCCs) were identified in the senescent leaves, Tc-NCC-1 (5) and Tc-NCC-2 (4b), and their structures were characterized. The basic build-up of 4b and 5 was found to be the same as known for NCCs from higher plants. The available NMR data revealed the functionalization at C(82) of the Tc-NCCs to imply a common β-glucopyranosyl moiety, as in Bn-NCC-2 (12) from oilseed rape (Brassica napus), in Nr-NCC-2 (4b) from tobacco, and in Zm-NCCs 4b and 5 from senescent maize leaves (Zea mays). Furthermore, the spectroscopic data indicated the tetrapyrrolic cores of the two Tc-NCCs 5 and 4b to have a common relative configuration at the stereogenic centers C(1), C(132), and C(15). The absolute configuration at C(1) was deduced to be the same in the Tc-NCCs 5 and 4b as in the glucosylated Nr-NCCs 4b and 6, and Zm-NCCs 4b and 5. Therefore, the absolute configuration at C(1) was indicated to be opposite to that in Bn-NCC-2 (12). NCCs (and FCCs) fall into two classes with respect to the configuration at C(1) due to the evolution of two types of RCC-reductases (RCCR) in higher plants 36 38–40. Hence, it was of interest to note the structural relationships with respect to the type of peripheral functionalization for the NCCs and of their configuration at C(1). The indicated availability of two closely related NCCs, Tc-NCC-1 and Tc-NCC-2, in senescent lime tree leaves parallels the occurrence of NCCs in other senescent plants, where a range of peripheral groups was observed 32 41. Our studies on the Tc-NCCs provide further support for the view that the main constitutional variations of the chlorophyll catabolites in various higher plants involve enzyme catalyzed, peripheral refunctionalization reactions. The peripheral adaptions with polar functions have been suggested to be of relevance for the transport and for the final deposition of chlorophyll catabolites in the vacuoles 6 10 37 42.
A yellow chlorophyll catabolite Tc-YCC (13) was identified in fresh extracts of lime tree leaves, and it was suggested to be a product of a naturally occurring oxidation reaction of 5 during senescence. Our study, therefore, provides (further) evidence for the notion that NCCs may not generally be the ‘final products’ of chlorophyll in senescent plants 41. All these findings strengthen the view that, while the pathways of chlorophyll catabolism in various higher plants may be closely related, they follow divergent branches, when including functional details. Indeed, the natural formation of the colored and photoactive YCCs such as of 13 would not be consistent with the role of chlorophyll breakdown as a mere ‘detoxification’ process either.
We would like to thank C. Kreutz for acquiring the NMR-spectra, and Hans-Jçrg and Hildegard Patscheider for giving us access to the Hotel Linde lime tree. Financial support by the Bundesministerium fur Wissenschaft und Forschung (BM.W_F, Project SPA/02-88/Recycling the Green to T. M.) and by the Austrian Science Foundation (FWF, Project No. L-472 to B. K.) is gratefully acknowledged.
Experimental Part
General. Commercial solvents (reagent-grade) were redistilled before use for extractions. HPLC-Grade MeOH and Et2O were purchased from Merck (DE-Darmstadt) and Acros Organics (B-Geel). Potassium dihydrogen phosphate,puriss. p.a., potassium phosphate dibasic-anhydrous,puriss. p.a., and ammonium acetate,puriss. p.a., were from Fluka (CH-Buchs).
Five-g and 1-g Sep-Pak-C18 cartridges were from Waters Associates. The pH values were measured with a WTW Sentix 21 electrode connected to a WTW pH535 digital pH-meter.
HPLC: Dionex Summit HPLC system with manual sampler, P680 pump, online degasser and diode array detector, 1-ml or 20-μl injection loop. Data were collected and processed with Chromeleon V6.50.
a) Anal. HPLC: Phenomenex HyperClone ODS 5 μm 250 x 4.6-mm i.d. column at 20° protected with a Phenomenex ODS 4 mm x 3-mm i.d. pre-column was used with a flow rate of 0.5 ml min−1. Solvent A:50 mm aq. potassium phosphate (pH 7.0), solvent B: MeOH; sovent composition (A/B) as function of time (0-75 min): 0-5,80:20; 5-55,80:20 to 30:70; 55-60,30:70 to 0:100; 60-70,0:100;70-75,0:100 to 80:20.
b) Prep. HPLC: Phenomenex HyperClone ODS 5 μm 250 x 21.2-mm i.d. column at 20° protected with a Phenomenex ODS 10 mm x 5-mm i.d. pre-column was used with a flow rate of 5 and 7 ml min−1. Solvent A: 50 mm aq. potassium phosphate (pH 7.0), solvent B: MeOH.
MPLC: Buchi MPLC system equipped with two C-605 pumps, a C-615 pump manager, and a C-630 UV-detector at 254 nm. A 460 x 55-mm i.d. column at 208 filled with Phenomenex Spepra-C18-E, 50 mm, 65A was used with a flow rate of 10 ml min−1. Solvent A: 50 mm aq. potassium phosphate (pH7.0), solvent B: MeOH.
LC/MS: a) HPLC: Dionex Ultimate HPLC system with manual sampler, He degasser, and diode array detector; 20 μl injection loop; Phenomenex HyperClone ODS 5 mm 250 x 4.6-mm i.d. column at 20° protected with a Phenomenex ODS 4 mm x 3-mm i.d. pre-column was used with a flow rate of 0.5 ml min−1. Solvent A: 10 mm ammonium acetate (pH 7.0), solvent B: MeOH; solvent composition (A/B) as function of time (in 75 min): 0-5, 80:20; 5-55, 80:20 to 30:70; 55-60, 30:70 to 0:100; 60-70, 0:100; 70-75, 0:100 to 80:20. Data were collected and processed with Chromeleon V6.50.
b) Mass Spectrometry: Finnigan MAT 95, electrospray ionization (ESI) source, positive-ion mode, 1.4 kV spray voltage. MS and MS/MS: Finnigan LCQ Classic, ESI source, positive-ion mode, 4.5-kV spray voltage (rel. abundance).
Spectroscopy. UV/VIS Spectra: Hitachi U-3000 spectrophotometer; λmax [nm] (log e/rel. E). CD Spectra: JASCO J715; λmax and λ [nm], De. 1H- and 13C-NMR: Varian Unity Inova 500MHz spectrometer; Bruker 600 MHz Avance II+ (d(C1HD2OH) 3.31 ppm, and d(13CD3OD) 49.0 ppm) 43; <5 in ppm, δ in Hz.
Analysis of Chlorophyll Catabolites in Senescent Leaves by Anal. HPLC. Freshly picked lime tree leaves were collected from a lime tree grown near the Hotel Linde (Innsbruck; see illustration for the Table of Contents) and immediately stored in liquid N2. A leaf (with the area of ca. 20 cm2) was grounded in a mortar and extracted with 2 ml of MeOH. The resulting suspension was centrifuged 5 min at 13,000 rpm. The methanolic supernatant was diluted with 50 mm aq. potassium phosphate (pH 7.0) 80:20 (v/v). After centrifugation for 5 min at 13,000 rpm, 1 ml of the extract was injected into the anal. HPLC system, to be analyzed with parallel detection at 320 and 420 nm (see Fig. 1).
Determination of Chlorophyll in Green and Senescent (yellow) Lime Tree Leaves by UV/VIS Spectroscopy, of Nonfluorescent and of Yellow Chlorophyll Catabolites (NCCs and YCC, resp.) in Senescent (yellow) Leaves by Anal. HPLC. Chlorophyll a and b in Green Leaves. A total area of 9 cm2 was cut out of a green lime tree leaf. The leaf was frozen in liquid N2, pulverized in a mortar, and extracted with MeOH. The slurry was filtered through a sintered glass filter, and the residue was grounded in a mortar and extracted with MeOH. The procedure was repeated, until the residue was colorless. The MeOH extracts were combined and diluted with MeOH to 100.00 ml in a volumetric flask. The extracts were analyzed by UV/VIS spectrometry. In green Tilia cordata leaves, 72.09 ± 3.93 mg·cm”2 (80.37 ± 4.38 nmol · cm−2) of chlorophyll a and b were found (n = 4), the data analysis was based on 44.
Chlorophyll a and b in Senescent (Yellow) Leaves. A total area of 99 cm2 was cut out of eleven senescent lime tree leaves. The extraction and the UV/VIS analysis were performed as described above. Yellow Tilia cordata leaves were found to contain 0.55±0.10 μig·cm”2 (0.61 ±0.11 nmol·cm−2) of residual chlorophyll a and b.
NCCs in Senescent (Yellow) Leaves. The quantification of the Tc-NCCs was accomplished by anal. HPLC. An anal. pure sample of Cj-NCC-1 from Cercidiphyllum japonicum was used to prepare a standard soln. (for isolation, see 24; ε320 = 17,000 as described in 15). Senescent Tilia cordata leaves were found to contain 42.2±4.2 μg cm”2 (50.2±5.0 nmol-cm-2) of Tc-NCC-1, 3.7±1.1 u,g-cm-2 (4.6± 1.4nmol-cm-2) of Tc-NCC-2, and 1.6±0.2 ug-cm-2 (1.9±0.3 nmol-cm-2) of Tc-YCC (YCC-content was calculated using e310 = 22,400, see 24). These values indicate, in a green lime tree leaf, a total conversion of chlorophyll a and b to NCCs and YCC of >70.4%.
Collection, Isolation and Structure Elucidation of Tc-NCCs. Senescent Tilia cordata leaves were collected at the main campus of the University of Innsbruck in October 2009 and stored at —80°. Senescent (yellow) leaves (100 g (wet weight)) were frozen in liquid N2, crushed into small pieces, and freeze-dried in vacuo for 3 d, resulting in a dry-weight of ca. 40 g. The dried leaves were pulverized using liquid N2 and a 300-W blender, and extracted with 70 ml of MeOH. The suspension was filtered with suction over a Buchner funnel. The extraction was repeated using another 70 ml of MeOH. The combined 140 ml of MeOH extracts were added portionwise to 350 ml of Et2O to precipitate a raw product that was enriched in chlorophyll catabolites. The raw product was cooled in an ice-bath for 15 min. After filtration with suction the white precipitate was dried in vacuo (dry weight: 1.77 g) and stored at -80° for further purification by MPLC: The crude product was dissolved in 48 ml of aq. potassium phosphate buffer soln. (50 mm; pH 7) using an ultrasonic bath. After filtration with a Sartorius filter two aliquots of the filtrate (24 ml of clear red-brownish solutions) were injected into the MPLC system (flow rate: 10 ml min−1); solvent A: 50 mm aq. potassium phosphate (pH 7.0); solvent B: MeOH; solvent composition (A/B) as function of time (0-120 min): 0-90, 70:30; 90-120, 70:30 to 0:100.
The fractions containing Tc-NCC-1 and Tc-NCC-2 were collected after 50 and 105 min, resp. The collected fractions were concentrated using a rotary evaporator, freeze-dried in vacuo, and stored at - 808 for further purification.
The (more-polar) fraction containing Tc-NCC-1 (5) was dissolved in 0.2 ml MeOH and 2.5 ml of H2O using an ultrasonic bath. After filtration of the suspension through a Sartorius filter the sample was divided in three aliquots and applied to prep. HPLC; injection vol., 1 ml; flow rate, 7 ml min−1; solvent A: 50 mm aq. potassium phosphate (pH 7.0); solvent B: MeOH; solvent composition (A/B) as function of time (0-198 min): 0-180, 90:10 to 65:35; 180-185, 65:35 to 0:100; 185-195, 0:100; 195-198 0:100 to 70:30. Three consecutive prep. HPLC runs were performed, and fractions containing Tc-NCC-1 were collected between 62.5 and 68 min. For de-salting, the aq. soln. was then applied to a pre-conditioned Sepak cartridge, washed with 20 ml of H2O, and eluted with a minimum amount of MeOH. After the sample was dried under high vacuum, 5.1 mg of anal. pure Tc-NCC-1 (5) were obtained.
The (less-polar) Tc-NCC-2 (4b) fraction was likewise dissolved in 0.6 ml of MeOH and 2.4 ml of H2O using an ultrasonic bath. After centrifugation for 3 min at 13,000 rpm, prep. HPLC (injection vol., 1 ml; flow rate, 5 ml min−1) was applied for three aliquots; solvent A: 50 mm aq. potassium phosphate (pH 7.0); solvent B: MeOH; solvent composition (A/B) as function of time (0-180 min): 0-5, 80:20; 5-120,80:20 to 35:65; 120-180: 35:65 to 0:100. Three consecutive prep. HPLC runs were performed, and fractions containing Tc-NCC-2 were collected at 79 min. For de-salting, the aq. soln. was then applied to a pre-conditioned Sepak cartridge, washed with 20 ml of H2O, and eluted with a minimum amount of MeOH. The solvents were removed using a rotary evaporator. To obtain 1.1 mg of an anal. pure Tc-NCC-2, one more prep. HPLC run (the sample was dissolved 0.2 ml of MeOH and 0.6 ml of H2O), followed by Sep-Pak desalting (see above) had to be performed.
Data ofTc-NCC-1 (5). tR 31.8 min (see main text and Fig. 1). UV/VIS (MeOH, c = 7.M0-s M): 245 (4.16), 314 (4.23). CD (MeOH, c = 2.5-10-s M); 226 (18), 258sh (-6), 281 (-17), 315 (3). 1H-NMR (500 MHz, CD3OH, 108): 1.91 (s, Me(181)); 2.04 (s, Me(21)); 2.10 (s, Me(121)); 2.23 (s, Me(71)); 2.25-2.36 (m, CH2(172)); 2.44 (dd, 7=10.1, 14.3, Ha-C(20)); 2.57-2.67 (m, Ha-C(171), CH2(81)); 2.68-2.77 (m, Hb-C(171)); 2.90 (dd, 7=3.8,14.3, Hb-C(20)); 3.16 (t-like, H-C(2')); 3.23-3.29 (m, H-C(4'), H-C(5')); 3.32-3.40 (m, Ha-C(82), H-C(3')); 3.60-3.73 (m, CH2(32), Ha-C(6'), Hb-C(82)); 3.74 (s, Me(135)); 3.78 (br. d, J = 2.5, H-C(132)); 3.84 (d, 7= 11.8, Hb-C(6')); 3.94-4.09 (m, H-C(l), CH2(10)); 4.16 (d, 7=7.8, H-C(l')); 4.56 (t-like, H-C(31)); 4.87 (br. d,J = 2.5, H-C(15)); 8.04 (s, H-N(21)); 9.30 (s, H-C(5)); 9.42 (s, H-N(24)); 11.25 (s, H-N(23)). 13C-NMR (125 MHz, CD3OH, 108; 13C-signal assignment from HSQC and HMBC experiments): 8.2 (C(71); 9.1 (C(181)); 9.1 (C(121)); 12.3 (C(21)); 21.8 (C(171)); 23.7 (C(10)); 24.9 (C(81)); 29.4 (C(20)); 37.1 (C(15)); 39.3 (C(172)); 52.6 (C(135)); 62.4 (C(6')); 62.6 (C(1)); 65.9 (C(32)); 67.9 (C(132)) 68.4 (C(31)); 70.3 (C(82));71.2 (C(4')); 75.1 (C(2')); 77.5 (C(5'));77.9 (C(3')); 104.0 (C(1')); 112.3 (C(12)); 115.0 (C(18)); 120.4 (C(17)); 120.5 (C(8)); 124.4 (C(16)); 124.4 (C(19)); 125.9 (C(13)); 129.0 (C(6)); 131.4 (C(3)); 134.2 (C(11)); 135.3 (C(7)); 139.9 (C(9)); 158.7 (C(2)); 161.5 (C(14)); 171.4 (C(133)); 175.1 (C(4)); 176.8 (C(5)); 181.4 (C(173)); 191.6 (C(131)). ESI-MS: 879.43 (60, ), 863.49 (17, [M + Na] + ), 841.49 (100, [M+H] + ), 809.47 (11, [M + H-MeOH] + ), 684.35 (22, ringA] + ), 679.33 (11, [M + H-QH10Os] + ).
Data ofTc-NCC-2 (4b). tR 47.8 min (see main text and Fig.1). UV/VIS (50 mm aq. potassium phosphate (pH7.0)/MeOH 65:35, c = 2.6-10-s M): 240 (1.0), 312 (0.83). CD (50 mm aq. potassium phosphate (pH7.0)/MeOH 65:35, c = 2.6-10-s M): 225 (26), 262sh (9), 281 (-19), 318 (3). 1H-NMR (600 MHz, CD3OD, 108): 1.92 (s, Me(181)); 1.99 (s, Me(21)); 2.14 (s, Me(121)); 2.21 (s, Me(71)); 2.29-2.38 (m, Ha-C(20), CH2(172)); 2.54-2.68 (m, CH2(81), Ha-C(171)); 2.74–2.81 (m, Hb-C(171)); 2.89 (dd, 7= 4.2,14.2, Hb-C(20)); 3.17 (t-like, H-C(2')); 3.24-3.28 (m, H-C(4'), H-C(5')); 3.33-3.36 (m, Ha-C(82)); 3.42 (t-like, H-C(3')); 3.64 (dd, 7=4.9, 12.0, Ha-C(6')) superimposed by 3.67 (m, Hb-C(82)); 3.74 (s, Me(135)); 3.84 (d,7=12.0, Hb-C(6')); 3.92-4.13 (m, H-C(l), CH2(10)); 4.16 (rf,7=7.8, H-C(l')); 4.88 (br. s, H-C(15)); 5.35 (d-like,J = 11.7, Ha-C(32)); 6.12 (d-like,7= 17.8, Hb-C(32)); 6.46 (dd,7= 11.7,17.8, H-Cp1)); 9.27 (s, H-C(5)). 13C-NMR (125 MHz, CD3OD, 108; 13C-signal assignment from HSQC and HMBC experiments): 8.4 (C(71)); 9.1 (C(181)); 9.2 (C(121)); 12.2 (C(21)); 22.5 (C(171)); 23.8 (C(10)); 24.9 (C(81)); 30.3 (C(20)); 37.0 (C(15)); 40.3 (C(172)); 52.4 (C(135)); 61.9 (C(1)); 62.3 (C(6')); 67.5 (C(132)); 70.3 (C(82)); 71.2 (C(4')); 75.1 (C(2')); 77.6 (C(3')); 77.6 (C(5')); 103.9 (C(1')); 112.1 (C(12)); 115.2 (C(18)); 118.6 (C(32)); 120.1 (C(8)); 120.3 (C(17)); 124.2 (C(16)); 124.4 (C(19)); 125.4 (C(13)); 126.9 (C(31)); 128.1 (C(3)); 128.7 (C(6)); 133.7 (C(11)); 135.4 (C(7)); 139.5 (C(9)); 156.5 (C(2)); 161.8 (C(14)); 171.3 (C(133)); 174.2 (C(4)); 182.7 (C(173)). ESI-MS: 845.32 (42, [M + K] + ), 829.38 (13, [M + Na] + ), 807.37 (100, [M+H] + ), 775.34 (15, [M + H - MeOH] + ), 684.24 (8, [M + H - ring A] + ), 645.29 (7, [M + H-QH10Os] + ).
Co-injection Experiments Using Anal. HPLC. Anal. HPLC was used to identify NCCs from freshly prepared MeOH extracts of senescent leaves of the lime tree and of maize. A yellow lime tree leaf was grounded in a mortar with 0.2 g of sea sand, frozen with liquid N2, and extracted with 2.5 ml of MeOH. The suspension was centrifuged at 13,000 rpm for 5 min. The clear supernatant (200 ml) was diluted with 1.3 ml of phosphate buffer (pH 7) and again centrifuged at 13,000 rpm for 4 min. Fractions of Tc-NCC-1 or of Zm-NCC-1 eluted (both) at a tR of ca. 30 min (see Fig. 4). For co-injection experiments, separated samples of Tc-NCC-1 and of Zm-NCC-1, as well as a 1:2 mixture of Tc-NCC-1 and Zm-NCC-1 were analyzed by anal. HPLC (see Fig. 4).
Formation ofTc-YCC (13) by Oxidation of Tc-NCC-1 (5), and Data ofTc-YCC (13). For further characterization of Tc-YCC (13) as oxidation product of Tc-NCC-1 (5), a MeOH soln. of anal. pure 5 was applied to a silica-gel TLC plate. The nearly colorless ‘spot’ acquired a brownish-red color after 10 min of irradiation with a 365-nm UV lamp (220 V, 50 Hz, SVL, VILBER LOURMAT). HPLC Analysis and a co-injection experiment with isolated Tc-YCC (13) showed 13 to co-elute with the yellow oxidation product of Tc-NCC-1 (5), which had an UV/VIS spectrum typical for YCCs.
Data of 13. tR 33.8 min (see main text and Fig. 1). UV/VIS (online, 50 mm aq. potassium phosphate (pH7)/MeOH 50:50): 247 (0.7), 313 (1.0), 413 (1.3) nm. LC/ESI-MS: 899.20 (5, [M-H + Na + K] + ), 883.20(7, [M-H + 2Na] + ), 877.27 (8, [M + K] + ), 861.27 (57, [M+Na] + ), 839.07 (32, [M+H] + ), 821.13 (100, [M+H-H2O] + ), 789.27 (23, [M+H-H2O-MeOH] + ), 659.20 (40, [M + H-H2O-MeOH]+), 627.27(20, [M + H- H2O-MeOH-C6H10Os] + ), 518.87 (3, [M + H- ring A- ring D] + ). ESI-MS (MS/ MS of the isolated protonated fragment m/z 839.07, positive ion-mode): 821.13 (100, [M+H-H2O] + ), 807.20 (3, [M+H-MeOH] + ), 659.13 (4, [M+H-H2O-C6H10Os] + ). ESI-MS (MS/MS of the isolated fragment at m/z 821.13; positive ion-mode): 789.07 (34, [M + H-H2O-MeOH] + ), 659.13 (100, [M + 5] + ), 627.07 (10, [M+H-H2O-MeOH-C6H10Os] + ).
Verse 902: “When from the wounded dragon / reeking flowed the blood, And therein did bathe him / the valiant knight and good, Fell down between his shoulders / full broad a linden leaf. There may he be smitten; / ‘tis cause to me of mickle grief”, in ‘Project Gutenberg's The Nibelungenlied', translated into rhymed english verse in the metre of the original, translation by George Henry Needler, January, 2005 (e.g., EBook #7321, http://www.gutenberg.org/).
References
- 1.Scheer H , in ‘Chlorophylls’, Ed. H. Scheer, CRC Press, Boca Raton, 1991, p. 3.
- 2.Kräutler B, Matile P. Acc. Chem. Res. 1999;32:35. [Google Scholar]
- 3.Hendry GAF, Houghton JD, Brown SB. New Phytologist. 1987;107:255. doi: 10.1111/j.1469-8137.1987.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 4.Brown SB, Houghton JD, Hendry GAF , in ‘Chlorophylls’, Ed. H. Scheer, CRC-Press, Boca Raton, 1991, pp. 465.
- 5.Matile P. Chimia. 1987;41:376. [Google Scholar]
- 6.Matile P, Hörtensteiner S, Thomas H, Kräutler B. Plant Physiol. 1996;112:1403. doi: 10.1104/pp.112.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kräutler B, Jaun B, Bortlik K, Schellenberg M, Matile P. Angew. Chem., Int. Ed. 1991;30:1315. [Google Scholar]
- 8.Kräutler B, Jaun B, Amrein W, Bortlik K, Schellenberg M, Matile P. Plant Physiol. Biochem. 1992;30:333. [Google Scholar]
- 9.Hörtensteiner S, Kräutler B. Photosynth. Res. 2000;64:137. doi: 10.1023/A:1006456310193. [DOI] [PubMed] [Google Scholar]
- 10.Hinder B, Schellenberg M, Rodoni S, Ginsburg S, Vogt E, Martinoia E, Matile P, Hörtensteiner S. J. Biol. Chem. 1996;271:27233. doi: 10.1074/jbc.271.44.27233. [DOI] [PubMed] [Google Scholar]
- 11.Oberhuber M, Berghold J, Mühlecker W, Hörtensteiner S, Kräutler B. Helv. Chim. Acta. 2001;84:2615. [Google Scholar]
- 12.Berghold J, Breuker K, Oberhuber M, Hörtensteiner S, Kräutler B. Photosynth. Res. 2002;74:109. doi: 10.1023/A:1020991023248. [DOI] [PubMed] [Google Scholar]
- 13.Oberhuber M, Berghold J, Breuker K, Hörtensteiner S, Kräutler B. Proc. Natl. Acad. Sci. U.S.A. 2003;100:6910. doi: 10.1073/pnas.1232207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kräutler B, Banala S, Moser S, Vergeiner C, Müller T, Lütz C, Holzinger A. FEBS Lett. 2010;584:4215. doi: 10.1016/j.febslet.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Curty C, Engel N. Phytochemistry. 1996;42:1531. [Google Scholar]
- 16.Müller T, Ulrich M, Ongania K-H, Kräutler B. Angew. Chem., Int. Ed. 2007;46:8699. doi: 10.1002/anie.200703587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pruzinska A, Tanner G, Aubry S, Anders I, Moser S, Müller T, Ongania K-H, Kräutler B, Youn J-Y, Liljegren SJ, Hörtensteiner S. Plant Physiol. 2005;139:52. doi: 10.1104/pp.105.065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berghold J, Eichmüller C, Hörtensteiner S, Kräutler B. Chem. Biodiversity. 2004;1:657. doi: 10.1002/cbdv.200490057. [DOI] [PubMed] [Google Scholar]
- 19.Berghold J, Müller T, Ulrich M, Hörtensteiner S, Kräutler B. Monatsh. Chem. 2006;137:751. [Google Scholar]
- 20.Müller T, Moser S, Ongania K-H, Pruzinska A, Hörtensteiner S, Kräutler B. ChemBioChem. 2006;7:40. doi: 10.1002/cbic.200500268. [DOI] [PubMed] [Google Scholar]
- 21.Mühlecker W, Kräutler B. Plant Physiol. Biochem. 1996;34:61. [Google Scholar]
- 22.Mühlecker W, Kräutler B, Ginsburg S, Matile P. Helv. Chim. Acta. 1993;76:2976. [Google Scholar]
- 23.Losey FG, Engel N. J. Biol. Chem. 2001;276:8643. doi: 10.1074/jbc.M009288200. [DOI] [PubMed] [Google Scholar]
- 24.Moser S, Ulrich M, Müller T, Kräutler B. Photochem. Photobiol. Sci. 2008;7:1577. doi: 10.1039/b813558d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulrich M, Moser S, Müller T, Kräutler B. Chem. – Eur. J. 2011;17:2330. doi: 10.1002/chem.201003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kräutler B, Hörtensteiner S , in ‘Chlorophylls and Bacteriochlorophylls. Biochemistry, Biophysics, Functions and Applications, Advances in Photosynthesis and Respiration, Vol. 25’, Eds. B. Grimm, R. Porra, W. Rüdiger, H. Scheer, Springer, Dordrecht, 2006, pp. 237.
- 27.Müller T, Rafelsberger M, Vergeiner C, Kräutler B. Angew. Chem., Int. Ed. 2011;50:10724. doi: 10.1002/anie.201103934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moser S, Müller T, Ebert M-O, Jockusch S, Turro NJ, Kräutler B. Angew. Chem., Int. Ed. 2008;47:8954. doi: 10.1002/anie.200803189. ; S. Moser, T. Müller, A. Holzinger, C. Lütz, S. Jockusch, N. J. Turro, B. Kräutler, Proc. Natl. Acad. Sci. U.S.A 2009 106, 15538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banala S, Moser S, Müller T, Kreutz C, Holzinger A, Lütz C, Kräutler B. Angew. Chem., Int. Ed. 2010;49:5174. doi: 10.1002/anie.201000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liesebach H, Sinko Z. Dendrobiology. 2008;59:13. [Google Scholar]
- 31. M. Wenigmann, ‘Phytotherapie – Arzneipflanzen, Wirkstoffe, Anwendung’, Urban & Fischer, München, 1999.
- 32.Kräutler B , in ‘The Porphyrin Handbook, Vol. 13’, Eds. K. M. Kadish, K. M. Smith, R. Guilard, Elsevier Science, Oxford, 2003, pp. 183.
- 33.Kessler H, Gehrke M, Griesinger C. Angew. Chem., Int. Ed. 1988;27:490. [Google Scholar]
- 34.Pretsch E, Bühlmann P, Affolter C , ‘Structure Determination of Organic Compounds’, Springer Verlag, Berlin, 2000.
- 35.Mühlecker W, Ongania K-H, Kräutler B, Matile P, Hörtensteiner S. Angew. Chem., Int. Ed. 1997;36:401. [Google Scholar]
- 36.Mühlecker W, Kräutler B, Moser D, Matile P, Hörtensteiner S. Helv. Chim. Acta. 2000;83:278. [Google Scholar]
- 37.Matile P, Ginsburg S, Schellenberg M, Thomas H. Proc. Natl. Acad. Sci. U.S.A. 1988;85:9529. doi: 10.1073/pnas.85.24.9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodoni S, Vicentini F, Schellenberg M, Matile P, Hörtensteiner S. Plant Physiol. 1997;115:677. doi: 10.1104/pp.115.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hörtensteiner S, Rodoni S, Schellenberg M, Vicentini F, Nandi OI, Qui Y-L, Matile P. Plant Biol. 2000;2:63. [Google Scholar]
- 40.Wüthrich KL, Bovet L, Hunziker PE, Donnison IS, Hörtensteiner S. Plant J. 2000;21:189. doi: 10.1046/j.1365-313x.2000.00667.x. [DOI] [PubMed] [Google Scholar]
- 41.Moser S, Müller T, Oberhuber M, Kräutler B. Eur. J. Org. Chem. 2009:21. doi: 10.1002/ejoc.200800804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hörtensteiner S. Annu. Rev. Plant Biol. 2006;57:55. doi: 10.1146/annurev.arplant.57.032905.105212. [DOI] [PubMed] [Google Scholar]
- 43.Gottlieb HE, Kotlyar V, Nudelman A. J. Org. Chem. 1997;62:7512. doi: 10.1021/jo971176v. [DOI] [PubMed] [Google Scholar]
- 44.Porra RJ, Thompson WA, Kriedemann PE. Biochim. Biophys. Acta. 1989;975:384. [Google Scholar]


