Summary
Background
Development of antigen-specific preventive strategies is a challenging goal in IgE-mediated allergy. We have recently shown in proof-of-concept experiments that allergy can be successfully prevented by induction of durable tolerance via molecular chimerism. Transplantation of syngeneic hematopoietic stem cells genetically modified to express the clinically relevant grass pollen allergen Phl p 5 into myeloablated recipients led to high levels of chimerism (i.e. macrochimerism) and completely abrogated Phl p 5-specific immunity despite repeated immunizations with Phl p 5.
Objective
It was unclear, however, whether microchimerism (drastically lower levels of chimerism) would be sufficient as well which would allow development of minimally toxic tolerance protocols.
Methods
Bone marrow cells were transduced with recombinant viruses integrating Phl p 5 to be expressed in a membrane-anchored fashion. The syngeneic modified cells were transplanted into non-myeloablated recipients that were subsequently immunized repeatedly with Phl p 5 and Bet v 1 (control). Molecular chimerism was monitored using flow cytometry and PCR. T cell, B-cell and effector-cell tolerance were assessed by allergen-specific proliferation assays, isotype levels in sera and RBL assays.
Results
Here we demonstrate that transplantation of Phl p 5-expressing bone marrow cells into recipients having received non-myeloablative irradiation resulted in chimerism persisting for the length of follow-up. Chimerism levels, however, declined from transient macrochimerism levels to persistent levels of microchimerism (followed for 11 months). Notably, these chimerism levels were sufficient to induce B-cell tolerance as no Phl p 5-specific IgE and other high affinity isotypes were detectable in sera of chimeric mice. Furthermore, T-cell and effector-cell tolerance were achieved.
Conclusions and Clinical Relevance
Low levels of persistent molecular chimerism are sufficient to induce long-term tolerance in IgE-mediated allergy. These results suggest that it will be possible to develop minimally toxic conditioning regimens sufficient for low level engraftment of genetically modified bone marrow.
Keywords: allergen Phl p 5, B-cell tolerance, membrane-expression, molecular chimerism, T-cell tolerance
Introduction
IgE-mediated allergy is a hypersensitivity disease and a growing problem in developed countries. Development of antigen-specific therapeutic and preventive strategies implies fundamental knowledge of antigens causing allergy (i.e. allergens). In the last few decades a variety of allergens have been cloned, characterized and three-dimensional structures have been decoded [1, 2]. Currently, the only antigen-specific, disease-modulating and long-lasting treatment can be provided by allergen-specific immunotherapy (SIT). An obvious risk of SIT using crude allergen-containing extracts is that of provoking a systemic allergic reaction which may lead to fatalities [3, 4]. Therefore, prevention would be a desirable goal especially for individuals with high risk. It remains an unmet challenge to develop tolerance strategies that avoid the occurrence of pathogenic immunological responses towards allergens. Although some prophylactic approaches such as mucosal tolerance have been explored [5] additional strategies are necessary to avoid T cell priming and antibody production (mainly IgE).
Molecular chimerism is a strategy to induce tolerance by transplantation of autologous (or syngeneic in experimental models) hematopoietic stem cells (HSC) genetically modified to express the disease-causing antigen [6, 7]. This approach has been demonstrated in predominantly Th 1-dependent models of organ transplantation as well as in selected autoimmune disease models [8, 9]. Induction of T cell tolerance has been demonstrated in several studies in molecular chimerism suggesting central T cell tolerance and peripheral regulation [10-14]. Nevertheless, only few studies in molecular chimerism exist regarding humoral responses towards the introduced antigens [15, 16]. In a proof-of-concept study we showed for the first time that molecular chimerism is a promising tool for prevention of IgE-mediated allergy [17]. However, intense preconditioning of recipients [i.e. myeloablative total body irradiation (TBI)] is a major hurdle for clinical application.
The degree of conditioning (e.g. the dose of irradiation) directly correlates with the ensuing chimerism levels. However, the minimum level of chimerism necessary to induce tolerance still remains unclear. Donor bone marrow transplantation (BMT) into a conditioned host establishing mixed cellular chimerism (denoting a state of macrochimerism with a donor fraction > 1% < 100%) reliably leads to immunological tolerance towards a donor allograft in several settings including clinical pilot trials [18-23]. In contrast, the spontaneous persistence of ‘passenger’ leukocytes after organ transplantation (i.e. microchimerism), seems to have no causal role in graft acceptance [24, 25]. Moreover, in experimental models microchimerism is not sufficient for tolerance induction towards MHC-mismatched grafts [26, 27]. Contrarily, microchimerism can lead to the tolerization of certain antigens (i.e. viral antigens) [28, 29].
In the study presented herein we investigated if both, B cell and T cell tolerance towards a clinically relevant major allergen can be achieved with a reduced-intensity BMT protocol which results only in low molecular chimerism.
Materials and methods
Animals
Female BALB/c mice were purchased from Charles River Laboratories (Sulzfeld, Germany). All mice were housed under specific pathogen-free conditions and were used between 6 and 12 weeks of age. All experiments were approved by the local review board of the Medical University of Vienna, and were performed in accordance with national and international guidelines of laboratory animal care.
Retroviral constructs and production of retroviruses
To generate membrane-anchored Phl p 5, full length Phl p 5 was fused to a signal sequence and a transmembrane domain (TMD) (both pDisplay; Invitrogen, Carlsbad, CA, USA) by overlapping PCR technique as described in Baranyi et al. [17] As control vector, the membrane-anchored GFP was fused to a signal sequence and a transmembrane domain by overlapping PCR technique as described above. Primer sequences are as follows: leader peptide: sense: 5′-GGCGCCATGGAGACAGACACACTCCTG-3′, antisense: 5′-CTTGCTCACGTCACCAGT-3′ eGFP: sense: 5′-ACTGGTGACGTGAGCAAG-3′, antisense: 5′-GCCCACAGCTCTAGATCC-3′MD: sense: 5′-GGATCTAGAGCTGTGGGC-3′, antisense: 5′-CCGGCCTCGAGCTAACGTGGCTTCTTCTG-3′.
The PCR product was cloned into the retroviral vector pMMP NcoI and Xho I sites resulting in pMMP-GFP-TM. The start codon was inserted with the Nco I site, the stop codon was inserted with the Xho I site. For virus production plasmids pMMP-Phl p 5-TM or pMMP-eGFP-TM, VSV-G protein and pMLV, encoding for viral proteins gag and pol, were co-transfected using the calcium phosphate method into 293 T cells resulting in VSV-Phl p 5-TM or VSV-GFP-TM viruses [30]. Viral supernatants were concentrated by ultracentrifugation (16 500 rpm 2 h).
Retroviral transduction of bone marrow cells
BALB/c donor mice were injected i.p. with 5-fluorouracil (150 mg/kg) 7 days before BM isolation. Mice were killed and BM was harvested from tibiae, femurs, humeri and pelvis [31]. BM cells were cultured and transduced with VSV-Phl p 5-TM or transduced with VSV-GFP-TM as described in [17] with a multiplicity of infection of 5 [32].
Bone marrow transplantation
One day before BMT, recipients received 6 Gy of TBI and a depleting dose of anti-CD8 (2.43; 0.5 mg/mouse) and anti-CD4 (GK1.5; 0.5 mg/mouse) monoclonal antibodies (mAb). On the day of reconstitution mice were transplanted with 4 × 106 transduced BM cells i.v. After BMT mice received anti-CD40L mAb (MR1; 0.5 mg/mouse). Anti-CD4, anti-CD8 and anti-CD40L were used as they were shown to enhance engraftment of transduced BM. All mAb used in vivo were purchased from BioXCell (West Lebanon, NH, USA).
Recombinant allergens and immunization of mice
Purified recombinant (r) timothy grass pollen and birch pollen allergens (rPhl p 5, rBet v 1) were obtained from Biomay (Vienna, Austria). All groups of mice were immunized s.c. with 5 μg rPhl p 5 and 5 μg rBet v 1 adsorbed to aluminium hydroxide (Alu-Gel-S; Serva, Ingelheim, Germany) as described previously [33].
Flow cytometric analysis
Non-specific Fcγ receptor binding was blocked with mAb against mouse FcγII/III receptor (CD16/CD32). Phl p 5 polyclonal antiserum against full length rPhl p 5 was purified from rabbit serum (Charles River) by a protein G column (Pierce, Rockford, IL, USA) according to the manufacturer’s instructions. Polyclonal anti-Phl p 5 IgG was biotinylated and developed by counterstaining with phycoerythrin streptavidin. To detect Phl p 5+-expressing cells among various leukocyte lineages white blood cells were stained with FITC-conjugated antibodies against CD4, CD8, B220, Mac-1 and isotype controls (all antibodies from Pharmingen, San Diego, CA, USA) and analysed using flow cytometry. Propidium iodide staining was used to exclude dead cells. Two-colour flow cytometric analysis was used to determine the percentage of Phl p 5-expressing cells of particular lineages. The percentage of Phl p 5+ cells (i.e. molecular chimerism) was calculated by subtracting control staining from quadrants containing Phl p 5+ and Phl p 5 negative cells expressing a particular lineage marker, and by dividing the net percentage of Phl p 5+ cells by the total net percentage of Phl p 5+ plus Phl p 5 negative cells of that lineage as described in [17]. A Cytomics FC500 flow cytometer (Coulter Werfen, Austria) was used for acquisition and the CXP software (Coulter Werfen, Austria) was used for analysis of flow cytometric data.
Isolation of genomic DNA and detection of Phl p 5-specific products
Genomic DNA of splenocytes was isolated as described in [29]. A 250-bp Phl p 5-specific product was amplified and sequenced (for confirmation) using primers: sense Phl p 5 fw: 5′-CTGCAGGTCATCGAGAAGGT-3′, antisense Phl p 5 rev: 5′-TTTCAGTGCGGTCTCAAAGA-3′, β-actin specific primers: sense β-actin fw: 5′-TGGAAATCCTGTGGCATCCATGAAAC-3′. antisense β-actin rev. 5′-TAAAACGCAGCTCAGTTACAGTCCG-3′. PCR products were separated on a 5% Acrylamidgel (Bio-Rad, Hercule, CA, USA) in 1xTBE and visualized by EthBr.
ELISA
To measure allergen-specific antibodies in the sera of immunized mice ELISAs were performed as described previously [34]. Plates were coated with rPhl p 5 (5 μg/mL), sera were diluted 1 : 20 for IgE, 1 : 100 for IgM, IgA, IgG2a and IgG3, respectively, and 1 : 500 for IgG1. Bound antibodies were detected with monoclonal rat anti-mouse IgM, IgG1, IgE, IgA, IgG2a and IgG3 antibodies (Pharmingen, San Diego, CA, USA) diluted 1 : 1000 and a HRP-coupled goat anti-rat antiserum (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK) diluted 1 : 2000. The substrate for HRP was ABTS [60 mm/L citric acid, 77 mm/L Na2HPO4 × 2H2O, 1.7 mm/L ABTS (Sigma, St. Lois, MO, USA), 3 mm/L H2O2].
Lymphocyte proliferation assay
Spleens were removed under aseptic conditions (week 44), single-cell suspensions were generated and erythrocytes were lysed. Suspended splenocytes were plated into 96-well round-bottom plates at a concentration of 2 × 105 cells per well in triplicates and stimulated with concanavalin A (Con A; 0.5 μg/well, Sigma), rPhl p 5 (2 μg/well) and rBet v 1 (2 μg/well). On day 5, cultures were pulsed with 0.5 μCi per well [3H]thymidine (Amersham Biosciences) and harvested approximately 16 h thereafter. The proliferative response was measured using scintillation counting. The stimulation index (SI) was calculated as the ratio of the mean proliferation after allergen stimulation and medium control values.
Rat basophil leukaemia (RBL) cell degranulation assay
RBL-2H3 cell subline was cultured as described previously, in RPMI 1640 medium (Biochrome AG, Berlin, Germany) containing 10% fetal calf serum. Aliquots of 4 × 104 cells were plated in 96-well tissue culture plates (Greiner Bio-One, Stuttgart, Germany), loaded with 1 : 50 diluted mouse sera and incubated for 2 h at 37°C and 5% CO2. Supernatants were removed and the cell layer was washed with 2 × Tyrode’s buffer (137 mm NaCl, 2.7 mm KCL, 0.5 mm MgCl2, 1.8 mm CaCl2, 0.4 mm NaH2PO4, 5.6 mm D-glucose, 12 mm NaHCO3, 10 mm HEPES and 0.1% w/v BSA, pH 7.2). Preloaded cells were stimulated with optimal concentrations of rPhl p 5 or rBet v 1 (i.e. 0.03 μg per well) for 30 min at 37°C. The supernatants were analysed for β-hexosaminidase activity by incubation with the substrate 80 μm 4-methylumbelliferyl-N-acetyl-β-d-glucosamide (Sigma-Aldrich, Vienna, Austria) in citrate buffer (0.1 m, pH4.5) for 1 h at 37°C. The reaction was stopped by addition of 100 μL glycine buffer (0.2 m glycine, 0.2 m NaCl, pH 10.7) and the fluorescence was measured at λex : 360/λem : 465 nm using a fluorescence microplate reader (Wallac; Perkin Elmer, Vienna, Austria). Results are reported as percentage of total β-hexosaminidase released after addition of 1% Triton X-100. Determinations were done in triplicates and are displayed as mean value + SD.
Statistical analysis
The reported P-values are results of two-sided Student’s t-tests. P-values < 0.05 were considered statistically significant. Error bars indicate standard deviations (SD).
Results
Transplantation of Phl p 5-transduced BM cells in non-myeloablatively conditioned syngeneic recipients
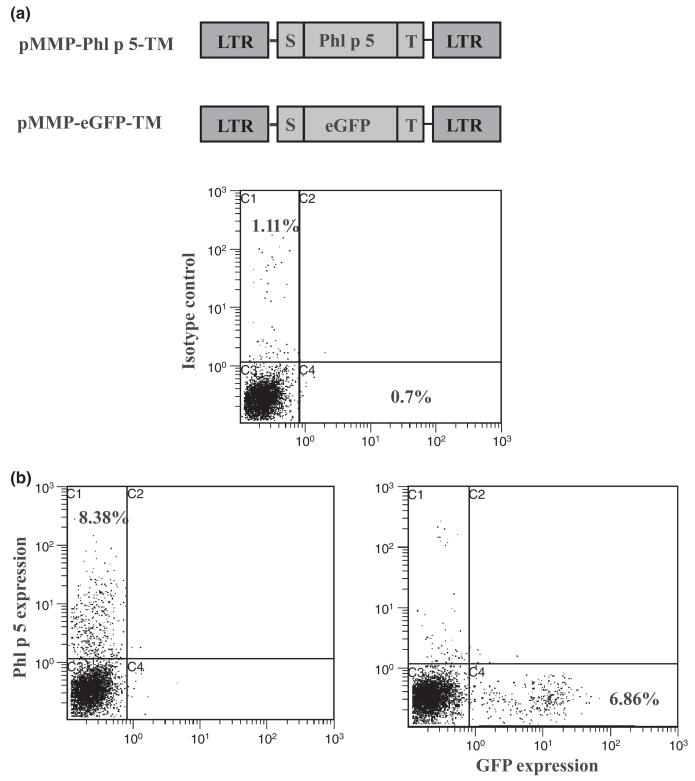
A previously established murine model was used to determine whether long-term tolerance in IgE-mediated allergy can be induced via molecular chimerism under non-myeloablative host conditioning [17]. The molecularly well-defined, clinically relevant major timothy grass pollen allergen Phl p 5 was retrovirally transduced into BM cells (BMC) to be expressed on the cell surface. BMC of BALB/c mice (treated with 5-FU 7 days earlier) were transduced with recombinant virus VSV-Phl p 5-TM in vitro. As control, the reporter gene GFP was fused to a signal peptide and a transmembrane domain and cloned into the retroviral vector pMMP and retroviruses VSV-GFP-TM were produced as described in [17, 35] (Fig. 1a). Transduction of VSV-Phl p 5-TM and VSV-GFP-TM showed an efficiency of about 7% Phl p 5- and 6% GFP-expression, respectively, on the surface of BMC as determined using flow cytometry (Fig. 1b).
Fig. 1.
Expression levels of membrane-anchored Phl p 5 and GFP in retrovirally transduced BM. (a) Schematic representation of pMMP-Phl p 5-TM retroviral construct and pMMP-eGFP-TM retroviral construct. Full length Phl p 5 and eGFP were fused to a leader peptide (S) and a transmembrane domain (T) and ligated into retroviral vector pMMP. LTR-long terminal repeats. (b) Expression levels of membrane-anchored Phl p 5 (left dot blot) and membrane-anchored GFP as control (right dot blot) in BM cells before BMT demonstrated in flow cytometric analysis. The upper dot blot represents untransduced cells.
BMT of Phl p 5- transduced cells leads to low level chimerism in an irradiation reducing protocol
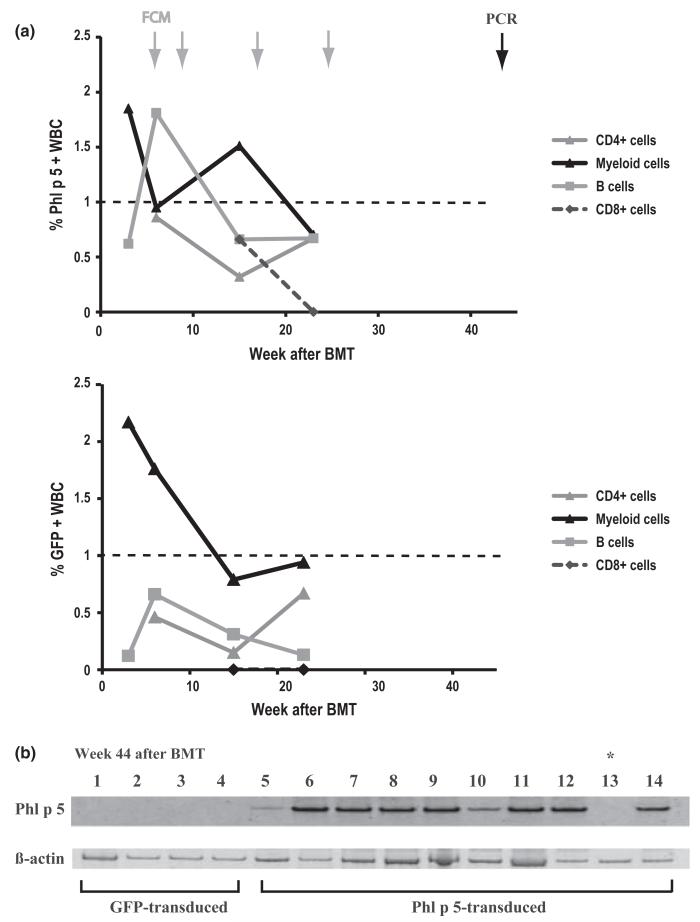
BALB/c recipients, known to be a high IgE responder strain [analogous to the susceptible (atopic) human phenotype] [36] were preconditioned with non-myeloablative TBI of 6 Gy in addition to T cell-depleting antibodies and anti-CD40L mAb which were shown to enhance BM engraftment [37]. Levels of molecular Phl p 5-chimerism were determined using flow cytometry (FCM) at multiple time points (weeks 3, 6, 15 and 23) after BMT (Fig.2a). Although mean levels of Phl p 5-positive cells within the myeloid line were clearly macrochimeric at week 15 (1.51 ± 0.44%), blood cells expressing Phl p 5 declined over time to less than 1% in most (7 of 10) mice, the cut-off level commonly defined as indicating microchimerism [29]. Mean levels of Phl p 5 positive cells at week 23 post BMT were only 0.7 ± 0.9% in myeloid subtypes. Similarly, within the B cells chimerism was 0.67 ± 0.5% at week 23 (vs. 1.81 ± 1.11% 6 weeks after BMT) and within CD4+ T cells 0.67 ± 0.29% (n = 10). These levels of expression reached the detection limit in FCM. Indeed, by FCM none of the chimeric mice showed any Phl p 5+ CD8 cells at week 23. Moreover, GFP-levels were comparable to Phl p 5-levels in the peripheral blood detected using flow cytometry. Therefore, macrochimerism was no longer detectable at 23 weeks post BMT and hence FCM analysis was not performed thereafter (Fig. 2a).
Fig. 2.
Phl p 5-chimerism is detectable in flow cytometry at early and PCR at late time points. (a) Phl p 5-specific expression within lineages of WBC as indicated was detected by flow cytometry at early time points. Data represent the mean% of Phl p 5 chimerism in recipients of Phl p 5-transduced BM (n = 10). Arrows in grey indicate time points of FCM analysis, arrow in black indicates PCR analysis at 44 weeks post-BMT. Note: Phl p 5-expressing CD4+ and CD8+ cells became detectable only 6–15 weeks after BMT due to delayed recovery of T cells after administration of T-cell-depleting antibodies (b) Upper gel: Phl p 5-specific PCR-products (lane 1–4) shown in genomic DNA of recipients transplanted with GFP-transduced BM (GFP-transduced) and recipients of Phl p 5-transduced BM (Phl p 5-transduced; lane 5–14). In chromosomal DNA of mouse 9 (lane 13) no Phl p 5-specific product was detectable (*). Lower gel: Lane 1–14 show β-actin specific PCR-products in genomic DNA of splenocytes of recipients of GFP-transduced BM (lane 1–4) and Phl p 5-transduced BM (lane 5–14).
To distinguish between the possibilities that molecular chimerism either persisted at lower levels or disappeared (i.e. were of transient nature), Phl p 5-specific PCR was performed at the end of follow-up (week 44; Fig. 2b). Chromosomal DNA of splenocytes, described to develop similar chimerism levels as BM, was isolated at the time when mice were killed [17]. In recipients transplanted with Phl p 5-transduced BMC (n = 10), Phl p 5-specific products were detectable in 9/10 mice suggesting that Phl p 5-expressing cells were still available and chimerism was persistent. In chromosomal DNA of splenocytes of one Phl p 5-BM transplanted mouse (Fig. 2b, marked with *) no Phl p 5-specific PCR product could be detected suggesting that chimerism in this mouse might have been lost. Thus, almost all recipients of Phl p 5-transduced BM developed persistent microchimerism.
B cell tolerance in low level Phl p 5-chimeric mice
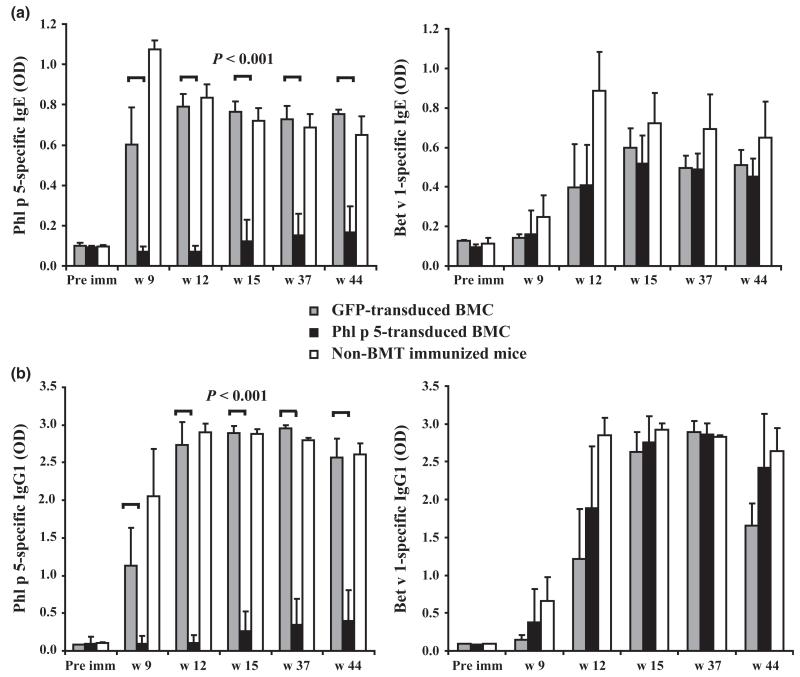
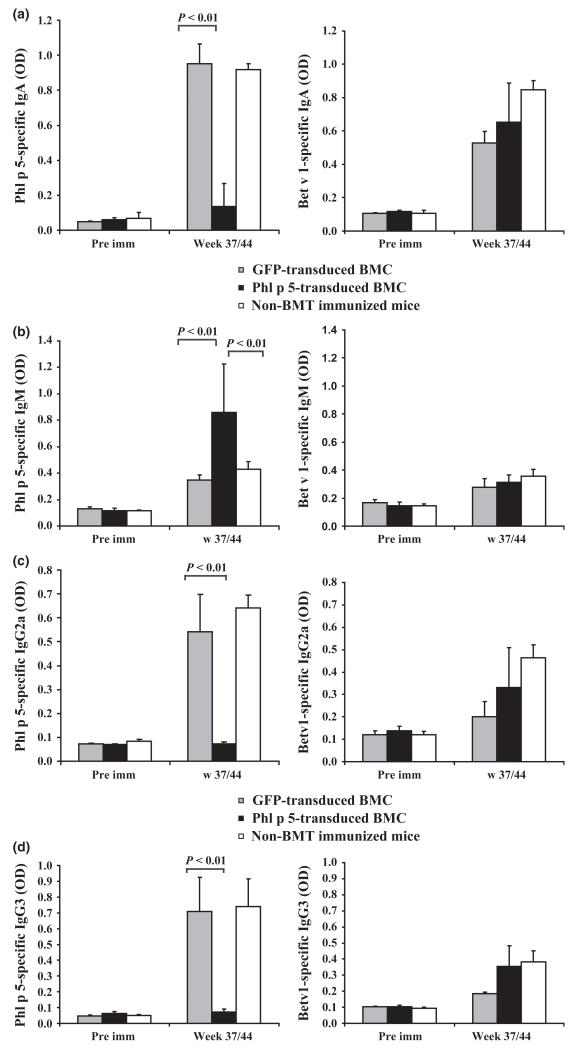
Recipients of syngeneic Phl p 5-transduced BMC (n = 10) were immunized with recombinant (r) Phl p 5 and the unrelated major birch pollen allergen Bet v 1 at weeks 6, 9 and 12 after BMT [17]. To assess if low level chimerism is sufficient to induce humoral tolerance, sera of chimeric mice were tested for Phl p 5-specific antibodies by ELISA (Figs 3 and 4). Eight of 10 recipients developed no detectable amounts of Phl p 5-specific IgE, IgG1 or IgA, while recipients of GFP-transduced BM and naïve controls developed high levels (Figs 3a and 4a). Interestingly, in chromosomal DNA of one mouse no Phl p 5-specific product was detectable. This mouse developed low levels of Phl p 5-specific IgE and IgG1 suggesting that loss of chimerism in this mouse had led to loss of tolerance (Fig. 2b*). In contrast, mice of all groups developed high levels of Bet v 1-specific IgE, IgG1 and IgA (Figs 3a and b, 4a right panels). Interestingly recipients of Phl p 5-tranduced BMC developed high amounts of Phl p 5-specific IgM, a phenomenon also observed in recipients of Phl p 5-transduced BMC after myeloablative conditioning [17]. Bet v 1-specific IgM levels were similar in all groups of mice (Fig. 4b right panel). None of the 10 mice receiving Phl p 5-transduced BMC developed detectable Phl p 5-specific IgG2a or IgG3 (Figs 4c and d left panels) at late time points but high levels of Bet v 1-specific IgG antibody levels (Figs 4c and d right panels). These results demonstrate that low-level persistent Phl p 5 molecular chimerism leads to long-lasting humoral tolerance.
Fig. 3.
Lack of Phl p 5-specific IgE and IgG1 in Phl p 5-chimeric mice. Allergen-specific IgE and IgG1 levels in sera of recipients of Phl p 5-transduced BM (n = 10), sera of recipients of GFP-transduced BM (n = 4) and sera of non-transplanted immunized mice (n = 5), were demonstrated before (pre imm), and after BMT (week 9,12,15,37 and 44) and analysed by ELISA. Mean antibody levels (+SD) are shown for each group. (a) Left panel: Phl p 5-specific IgE levels. Right panel: Bet v 1-specific IgE levels. (b) Left panel: Phl p 5-specific IgG1 levels. Right panel: Bet v 1-specific IgG1 levels. P-values for Phl p 5-specific IgE and IgG1 in sera of recipients of Phl p 5-transduced BM vs. recipients of GFP-transduced BM are demonstrated.
Fig. 4.
Phl p 5-chimeric mice do not develop Phl p 5-specific IgA, IgG2a and IgG3- but IgM. Allergen-specific IgA, IgG2a, IgG3 and IgM levels in sera of recipients of GFP-transduced BM (n = 4), recipients of Phl p 5-transduced BM (n = 10) and non-BMT sensitized mice (n = 10) were analysed by ELISA at late time points (weeks 37 or 44, pooled data) compared to pre immune (pre imm) sera. Mean antibody levels (+SD) are shown for each group. (a) Left panel: Phl p 5-specific IgA levels. Right panel: Bet v 1-specific IgA levels. (b) Left panel: Phl p 5-specific IgM levels. Right panel: Bet v 1-specific IgM levels. P-values for Phl p 5-specific IgA and IgM in sera of recipients of Phl p 5-transduced BM vs. recipients of GFP-transduced BM are demonstrated and recipients of Phl p 5-transduced BM vs. non-transplanted immunized mice. (c) Left panel: Phl p 5-specific IgG2a levels. Right panel: Bet v 1-specific IgG2a levels. (d) Left panel: Phl p 5-specific IgG3 levels. Right panel: Bet v 1-specific IgG3 levels. P-values for Phl p 5-specific IgG2a and IgG3 in sera of recipients of Phl p 5-transduced BM vs. recipients of GFP-transduced BM are demonstrated.
Tolerance at the T cell level in low chimeric recipients
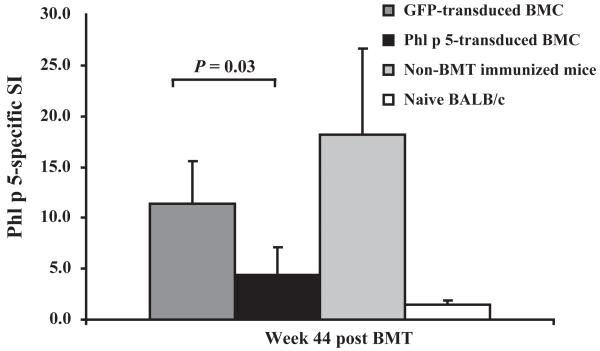
To assess if recipients of Phl p 5-transduced BM showed T cell unresponsiveness towards Phl p 5, splenocytes of these mice were isolated at the end of follow-up (week 44) and stimulated with rPhl p 5 in in vitro proliferation assays. Phl p 5-chimeric mice (n = 10) showed a significantly reduced proliferation rate upon Phl p 5 stimulation compared to control groups (Fig. 5). These results demonstrate that Phl p 5-chimeric mice are tolerant at the T cell level.
Fig. 5.
T cell non-responsiveness of splenocytes of Phl p 5-chimeric mice. Splenocytes of recipients of GFP-transduced BM (n = 4), Phl p 5-transduced BM (n = 10), non-transplanted sensitized mice (n = 5) and naïve mice (n = 3) were stimulated with rPhl p 5. Proliferation rates are shown for splenocytes of each group after H3-labelled thymidine incorporation and demonstrated by Phl p 5-specific stimulation indices (SI). P-values for recipients of Phl p 5-transduced vs. recipients of GFP-transduced BM are demonstrated.
Unresponsiveness at the effector-cell level in sera of Phl p 5-chimeric mice
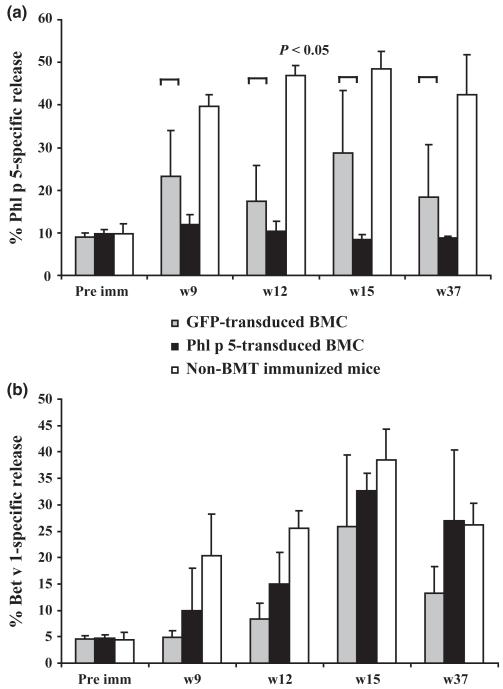
The RBL cell degranulation assay allows evaluation of activation and mediator release of effector cells in vitro [38]. To determine if Phl p 5-chimeric mice are tolerant at the effector-cell level, RBL cells were loaded with sera of recipients of Phl p 5-transduced BMCs, or sera of control groups and stimulated with rPhl p 5 or rBet v 1. Phl p 5-specific release of β-hexosaminidase was induced when cells were loaded with sera of control groups (Fig. 6a), whereas sera of Phl p 5-chimeric mice did not induce Phl p 5-specific release above the spontaneous release similar as when cells were loaded with pre-immune sera. Bet v 1-specific release was detectable in sera of all groups of mice (Fig. 6b). Thus, recipients of Phl p 5-transduced BMC showed tolerance towards Phl p 5 at the effector-cell level.
Fig. 6.
Complete lack of Phl p 5-specific effector cell degranulation in Phl p 5-chimeric mice. RBL cells were loaded with sera of Phl p 5-chimeric mice (n = 10) collected at time points indicated, sera of recipients of GFP-transduced mice (n = 4) and sera of non-BMT immunized mice and the corresponding preimmune sera (pre imm). Subsequently cells were challenged with r allergens. (a) Phl p 5-specific release of β-hexosaminidase. (b) Bet v 1-specific release of β-hexosaminidase. The mean percentages of allergen-specific β-hexosaminidase release (+ SD) are shown for each group. P-values for recipients of Phl p 5-transduced vs. recipients of GFP-transduced BM are demonstrated.
Discussion
Here it is shown that low levels of molecular chimerism following non-myeloablative recipient conditioning are sufficient for tolerance induction in IgE-mediated allergy. These results may be considered as an important step for advancing allergen-specific gene therapy towards clinical application in allergy. In preclinical studies stable long-lasting macrochimerism is required for robust tolerance towards alloantigens but the minimum level of chimerism has not been defined. However, about 2% of myeloid chimerism at late time points post BMT is sufficient to induce tolerance in some murine protocols [39, 40]. In molecular chimerism protocols levels of 1–5% of persistent leukocyte chimerism were required to maintain tolerance towards skin grafts in an MHC I-congeneic and an experimental autoimmune encephalitis (EAE) model [9, 14, 26]. Therefore, macrochimerism seems to be indispensable for induction of long-term tolerance in cellular and molecular chimerism protocols. Yet it was found that microchimerism was sufficient for maintaining peripheral tolerance towards a viral antigen [29], but not MHC antigens [26]. In the study herein we show for the first time that long-lasting microchimerism (less than 1%) is sufficient to avoid immune responses in IgE-mediated allergy.
Transient mixed macrochimerism seems to be sufficient also for operational tolerance in clinical pilot trials. Following combined kidney and BMT taken from an HLA-mismatched donor, macro- and microchimerism became undetectable after some weeks [20, 22]. Mechanistically, it was suggested recently that regulatory T cells may play an early role during the lymphopenic period in tolerance induction, although deletion or anergy mechanisms might be responsible for long-term tolerance in clinical studies [41]. Similarly, in mice, regulation contributes to tolerance induction early after BMT, whereas clonal deletion of donor-reactive T cells is the main mechanism maintaining tolerance long-term [40, 42]. These data are encouraging in that transient chimerism might be sufficient (with further less toxic preconditioning protocols) for tolerance induction also for IgE-mediated allergy.
Molecular chimerism is a gene-therapeutic approach integrating transgenes into HSC with viral vectors. To date, gene-therapeutic trials in infants suffering e.g. from SCID–X1 (X-linked severe combined immune deficiency) were successful after transfer of modified viral transgene-integrated autologous HSCs. Nevertheless, the risk of severe immunological side effects by insertional mutagenesis in some patients provoked by gene-transfer vectors can still not be excluded. Therefore, ongoing research is focusing on improving safety and efficiency of different gene-transfer systems [43]. An alternative to the gene-therapeutic approach integrating transgenes would be coupling of allergens to hematopoietic cells. In fact, in a recently published study prevention of food allergy was demonstrated by fixing proteins from whole peanut extracts to syngeneic splenocytes before transfer into recipient mice. After several oral challenges with these extracts mice were at least partially tolerant (no specific IgE but IgG1) towards these extracts short term indicating that prevention of allergy using allergen-modified stem cells may be a feasible strategy [44].
In our approach we show that non-myeloablative recipient conditioning leads to robust B-cell tolerance by persistent low level chimerism avoiding the production of any high affinity Phl p 5-specific isotypes, mainly Phl p 5-specific IgE. In addition, no anaphylactic activity was triggered by this approach as determined in commonly accepted basophil in vitro assays testing for allergenic activity. Interestingly, Phl p 5-specific IgM was produced in detectable amounts but no class switch recombination seems to occur possibly due to the lack of T cell help [45]. In mice, IgG1 and IgE antibodies are both generated during T cell-dependent B cell responses mediated by Th2 lymphocytes, IgG2a and IgG3, in contrast, by Th1 lymphocytes [46-48]. Here we demonstrate tolerance at the B-cell level regarding Phl p 5-specific IgE and IgG1 as well as complete avoidance of Phl p 5-specific IgG2a and IgG3 production, suggesting that both Th1 and Th2 cells were tolerized.
The allergen Phl p 5 is a highly immunogenic and allergenic protein even when expressed in mammalian cells and therefore a very stringent model for induction of tolerance via molecular chimerism [7]. This approach can be extended to a variety of different allergens. In fact we have data suggesting that tolerance can also be induced to the unrelated allergen Bet v 1 with this approach (Gattringer et al., submitted). Also tolerance induction against several clinical allergens should be possible because it has been demonstrated that functional or hypoallergenic hybrid allergens/allergen derivatives can be constructed by fusion of the DNAs coding for several different allergens [49-51]. It should therefore be possible to transduce stem cells with DNA constructs coding for several allergens or immobilize hybrid molecules consisting of several allergens on their surfaces for tolerance induction. Furthermore, intracellular expression of allergens or the use of hypoallergenic derivatives allows to target selectively either T cells alone or both T and B cells and thus risks of allergic sensitization or anaphylaxis can be potentially minimized.
Our findings show a robust tolerance protocol that permanently prevents IgE-mediated allergy and may advance molecular chimerism strategies towards clinical application for the prevention of allergy.
Acknowledgements
This work was supported by the Austrian Science Fund (FWF, F2310 to T. W. and FWF, F46 to R. V. and FWF, P21989 to U. B.), and in part by the Christian Doppler Association and a research grant from Biomay.
Footnotes
The authors declare that they have no conflicts of interest.
References
- 1.Valenta R, Ferreira F, Focke-Tejkl M, et al. From allergen genes to allergy vaccines. Annu Rev Immunol. 2010;28:211–41. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- 2.Valenta R. The future of antigen-specific immunotherapy of allergy. Nat Rev Immunol. 2002;2:446–53. doi: 10.1038/nri824. [DOI] [PubMed] [Google Scholar]
- 3.Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 4.Frew AJ. Allergen immunotherapy. J Allergy Clin Immunol. 2010;125:S306–13. doi: 10.1016/j.jaci.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 5.Burks AW, Laubach S, Jones SM. Oral tolerance, food allergy, and immunotherapy: Implications for future treatment. J Allergy Clin Immunol. 2008;121:1344–50. doi: 10.1016/j.jaci.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 6.Baranyi U, Pilat N, Gattringer M, Wekerle T. A chimerism-based approach to induce tolerance in IgE-mediated allergy. Crit Rev Immunol. 2009;29:379–97. doi: 10.1615/critrevimmunol.v29.i5.20. [DOI] [PubMed] [Google Scholar]
- 7.Baranyi U, Gattringer M, Boehm A, et al. Expression of a major plant allergen as membrane-anchored and secreted protein in human cells with preserved T cell and B cell epitopes. Int Arch Allergy Immunol. 2011;156:259–66. doi: 10.1159/000323733. [DOI] [PubMed] [Google Scholar]
- 8.Bagley J, Iacomini J. Gene therapy progress and prospects: gene therapy in organ transplantation. Gene Ther. 2003;10:605–11. doi: 10.1038/sj.gt.3302020. [DOI] [PubMed] [Google Scholar]
- 9.Alderuccio F, Chan J, Scott DW, Toh BH. Gene therapy and bone marrow stem-cell transfer to treat autoimmune disease. Trends Mol Med. 2009;15:344–51. doi: 10.1016/j.molmed.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Kang ES, Iacomini J. Induction of central deletional T cell tolerance by gene therapy. J Immunol. 2002;169:1930–5. doi: 10.4049/jimmunol.169.4.1930. [DOI] [PubMed] [Google Scholar]
- 11.Bagley J, Tian C, Sachs DH, Iacomini J. Induction of T-cell tolerance to an MHC class I alloantigen by gene therapy. Blood. 2002;99:4394–9. doi: 10.1182/blood.v99.12.4394. [DOI] [PubMed] [Google Scholar]
- 12.Tian C, Bagley J, Forman D, Iacomini J. Induction of central tolerance by mature T cells. J Immunol. 2004;173:7217–22. doi: 10.4049/jimmunol.173.12.7217. [DOI] [PubMed] [Google Scholar]
- 13.Forman D, Kang ES, Tian C, Paez-Cortez J, Iacomini J. Induction of alloreactive CD4 T cell tolerance in molecular chimeras: a possible role for regulatory T cells. J Immunol. 2006;176:3410–6. doi: 10.4049/jimmunol.176.6.3410. [DOI] [PubMed] [Google Scholar]
- 14.Chan J, Ban EJ, Chun KH, et al. Transplantation of bone marrow transduced to express self-antigen establishes deletional tolerance and permanently remits autoimmune disease. J Immunol. 2008;181:7571–80. doi: 10.4049/jimmunol.181.11.7571. [DOI] [PubMed] [Google Scholar]
- 15.Bracy JL, Iacomini J. Induction of B-cell tolerance by retroviral gene therapy. Blood. 2000;96:3008–15. [PubMed] [Google Scholar]
- 16.Fischer-Lougheed JY, Tarantal AF, Shulkin I, et al. Gene therapy to inhibit xenoantibody production using lentiviral vectors in non-human primates. Gene Ther. 2007;14:49–57. doi: 10.1038/sj.gt.3302818. [DOI] [PubMed] [Google Scholar]
- 17.Baranyi U, Linhart B, Pilat N, et al. Tolerization of a type I allergic immune response through transplantation of genetically modified hematopoietic stem cells. J Immunol. 2008;180:8168–75. doi: 10.4049/jimmunol.180.12.8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wekerle T, Sykes M. Mixed chimerism and transplantation tolerance. Annu Rev Med. 2001;52:353–70. doi: 10.1146/annurev.med.52.1.353. [DOI] [PubMed] [Google Scholar]
- 19.Pilat N, Wekerle T. Transplantation tolerance through mixed chimerism. Nat Rev Nephrol. 2010;6:594–605. doi: 10.1038/nrneph.2010.110. [DOI] [PubMed] [Google Scholar]
- 20.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–61. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fudaba Y, Spitzer TR, Shaffer J, et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. Am J Transplant. 2006;6:2121–33. doi: 10.1111/j.1600-6143.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 22.LoCascio SA, Morokata T, Chittenden M, et al. Mixed chimerism, lymphocyte recovery, and evidence for early donor-specific unresponsiveness in patients receiving combined kidney and bone marrow transplantation to induce tolerance. Transplantation. 2010;90:1607–15. doi: 10.1097/TP.0b013e3181ffbaff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fehr T, Sykes M. Clinical experience with mixed chimerism to induce transplantation tolerance. Transpl Int. 2008;21:1118–35. doi: 10.1111/j.1432-2277.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 24.Starzl TE, Demetris AJ, Trucco M, et al. Systemic chimerism in human female recipients of male livers. Lancet. 1992;340:876–7. doi: 10.1016/0140-6736(92)93286-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander SI, Smith N, Hu M, et al. Chimerism and tolerance in a recipient of a deceased-donor liver transplant. N Engl J Med. 2008;358:369–74. doi: 10.1056/NEJMoa0707255. [DOI] [PubMed] [Google Scholar]
- 26.Tian C, Bagley J, Iacomini J. Persistence of antigen is required to maintain transplantation tolerance induced by genetic modification of bone marrow stem cells. Am J Transplant. 2006;6:2202–7. doi: 10.1111/j.1600-6143.2006.01455.x. [DOI] [PubMed] [Google Scholar]
- 27.Ko S, Deiwick A, Jager MD, et al. The functional relevance of passenger leukocytes and microchimerism for heart allograft acceptance in the rat. Nat Med. 1999;5:1292–7. doi: 10.1038/15248. [DOI] [PubMed] [Google Scholar]
- 28.Anderson CC, Matzinger P. Immunity or tolerance: opposite outcomes of microchimerism from skin grafts. Nat Med. 2001;7:80–7. doi: 10.1038/83393. [DOI] [PubMed] [Google Scholar]
- 29.Bonilla WV, Geuking MB, Aichele P, Ludewig B, Hengartner H, Zinkernagel RM. Microchimerism maintains deletion of the donor cell-specific CD8+ T cell repertoire. J Clin Invest. 2006;116:156–62. doi: 10.1172/JCI26565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993;90:8392–6. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pree I, Wekerle T. Inducing mixed chimerism and transplantation tolerance through allogeneic bone marrow transplantation with costimulation blockade. Methods Mol Biol. 2007;380:391–403. doi: 10.1007/978-1-59745-395-0_25. [DOI] [PubMed] [Google Scholar]
- 32.Bodine DM, McDonagh KT, Seidel NE, Nienhuis AW. Survival and retrovirus infection of murine hematopoietic stem cells in vitro: effects of 5-FU and method of infection. Exp Hematol. 1991;19:206–12. [PubMed] [Google Scholar]
- 33.Linhart B, Bigenzahn S, Hartl A, et al. Costimulation blockade inhibits allergic sensitization but does not affect established allergy in a murine model of grass pollen allergy. J Immunol. 2007;178:3924–31. doi: 10.4049/jimmunol.178.6.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vrtala S, Sperr WR, Reimitzer I, et al. cDNA cloning of a major allergen from timothy grass (Phleum pratense) pollen; characterization of the recombinant Phl pV allergen. J Immunol. 1993;151:4773–81. [PubMed] [Google Scholar]
- 35.Riviere I, Brose K, Mulligan RC. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci U S A. 1995;92:6733–7. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dearman RJ, Kimber I. Animal models of protein allergenicity: potential benefits, pitfalls and challenges. Clin Exp Allergy. 2009;39:458–68. doi: 10.1111/j.1365-2222.2008.03194.x. [DOI] [PubMed] [Google Scholar]
- 37.Bagley J, Tian C, Sachs DH, Iacomini J. T cells mediate resistance to genetically modified bone marrow in lethally irradiated recipients. Transplantation. 2002;74:1454–60. doi: 10.1097/00007890-200211270-00019. [DOI] [PubMed] [Google Scholar]
- 38.Kaul S, Lüttkopf D, Kastner B, et al. Mediator release assays based on human or murine immunoglobulin E in allergen standardization. Clin Exp Allergy. 2007;37:141–50. doi: 10.1111/j.1365-2222.2006.02618.x. [DOI] [PubMed] [Google Scholar]
- 39.Wekerle T, Kurtz J, Ito H, et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med. 2000;6:464–9. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 40.Pilat N, Baranyi U, Klaus C, et al. Tregtherapy allows mixed chimerism and transplantation tolerance without cytoreductive conditioning. Am J Transplant. 2010;10:751–62. doi: 10.1111/j.1600-6143.2010.03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andreola G, Chittenden M, Shaffer J, et al. Mechanisms of donor-specific tolerance in recipients of haploidentical combined bone marrow/kidney transplantation. Am J Transplant. 2011;11:1236–47. doi: 10.1111/j.1600-6143.2011.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bigenzahn S, Blaha P, Koporc Z, et al. The role of non-deletional tolerance mechanisms in a murine model of mixed chimerism with costimulation blockade. Am J Transplant. 2005;5:1237–47. doi: 10.1111/j.1600-6143.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- 43.Ginn SL, Alexander IE. Gene therapy: progress in childhood disease. J Paediatr Child Health. 2012;48:466–71. doi: 10.1111/j.1440-1754.2011.02204.x. [DOI] [PubMed] [Google Scholar]
- 44.Smarr CB, Hsu CL, Byrne AJ, Miller SD, Bryce PJ. Antigen-fixed leukocytes tolerize Th2 responses in mouse models of allergy. J Immunol. 2011;187:5090–8. doi: 10.4049/jimmunol.1100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination. Nat Rev Immunol. 2003;3:721–32. doi: 10.1038/nri1181. [DOI] [PubMed] [Google Scholar]
- 46.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 47.Finkelman FD, Katona IM, Urban JF, Jr, et al. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988;141:2335–41. [PubMed] [Google Scholar]
- 48.Snapper CM, Peschel C, Paul WE. IFN-gamma stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J Immunol. 1988;140:2121–7. [PubMed] [Google Scholar]
- 49.Linhart B, Hartl A, Jahn-Schmid B, et al. A hybrid molecule resembling the epitope spectrum of grass pollen for allergy vaccination. J Allergy Clin Immunol. 2005;115:1010–6. doi: 10.1016/j.jaci.2004.12.1142. [DOI] [PubMed] [Google Scholar]
- 50.Linhart B, Jahn-Schmid B, Verdino P, et al. Combination vaccines for the treatment of grass pollen allergy consisting of genetically engineered hybrid molecules with increased immunogenicity. FASEB J. 2002;16:1301–3. doi: 10.1096/fj.01-1012fje. [DOI] [PubMed] [Google Scholar]
- 51.Linhart B, Mothes-Luksch N, Vrtala S, Kneidinger M, Valent P, Valenta R. A hypoallergenic hybrid molecule with increased immunogenicity consisting of derivatives of the major grass pollen allergens, Phl p 2 and Phl p 6. Biol chem. 2008;389:925–33. doi: 10.1515/BC.2008.105. [DOI] [PubMed] [Google Scholar]