Abstract
Caveolin-1 (Cav1) is a scaffolding protein that serves to regulate the activity of several signaling molecules. Its loss has been implicated in the pathogenesis of several types of cancer, but its role in the development and progression of cutaneous squamous cell carcinoma (cSCC) remains largely unexplored. Herein, we use the keratinocyte cell line PAM212, a murine model of cSCC, to determine the function of Cav1 in skin tumor biology. We first show that Cav1 overexpression decreases cell and tumor growth, whereas Cav1 knockdown increases these attributes in PAM212 cells. In addition, Cav1 knockdown increases the invasive ability and incidence of spontaneous lymph node metastasis. Finally, we demonstrate that Cav1 knockdown increases extracellular signaling–related kinase 1/2 mitogen-activated protein kinase/activator protein-1 pathway activation. We attribute the growth and invasive advantage conferred by Cav1 knockdown to increased expression of activator protein-1 transcriptional targets, including cyclin D1 and keratin 18, which show inverse expression in PAM212 based on the expression level of Cav1. In summary, we demonstrate that loss of Cav1 affects several characteristics associated with aggressive human skin tumors and that this protein may be an important modulator of tumor growth and invasion in cSCC.
Nonmelanoma skin cancer, comprising both basal and squamous cell carcinomas, is the most prevalently diagnosed malignancy among white populations, and its incidence is increasing worldwide.1–3 Unlike basal cell carcinomas, cutaneous squamous cell carcinomas (cSCCs) can be aggressive cancers that carry a significant risk of metastasis. Of the 700,000 cSCCs diagnosed yearly in the United States,3 approximately 5% will metastasize to lymph nodes and distant organs.4,5 Various markers are used to assess the risk for metastatic progression in these lesions: tumor size and depth, degree of differentiation, and involvement of vascular or lymphatic vessels, among others.6,7 Thus, given the prevalence of SCCs and their potential for developing into life-threatening malignancies, the identification of novel mechanisms contributing to tumor development and progression into invasive lesions could provide better prognostic markers to predict disease outcome and improve therapeutic treatments.
Caveolae are a specialized form of membrane lipid raft characterized as flask-shaped cavities in the cell membrane.8,9 One of their main biological functions is signal transduction, accomplished through the proteins that preferentially localize to these organelles.10 The essential protein components of these cellular structures are the caveolins, encoded by three different genes (CAV1, CAV2, and CAV3) that vary in their tissue specificity.9,11,12 Caveolin-1 (Cav1) contains a scaffolding domain that compartmentalizes a multitude of signaling molecules within caveolae, modulating their activity and preferentially binding them in their inactive state.9 Cav1, therefore, functions as a negative regulator of numerous signaling molecules, and its misregulation, deletion, or mutation has been implicated in the pathological characteristics of a variety of human diseases, including cancer.
Despite the substantial amount of research on the function of Cav1 in various cancer types, its role in the pathogenesis of nonmelanoma skin cancer remains largely unexplored. Previous research has shown that Cav1 ablation in murine skin increases basal layer proliferation13 and benign papilloma incidence, multiplicity, and size after carcinogenic treatment.14 In addition, in human skin, Cav1 is lost during the progression of psoriasis15,16 and significantly decreased in cutaneous squamous cell carcinoma.17 To further examine the role of Cav1 in squamous cell carcinoma development, we used a murine keratinocyte cell line, PAM212, that is able to form squamous cell carcinomas in vivo.18 By using this system, we were able to investigate how altered Cav1 expression affects aspects of cancer development and progression.
In the current study, we show that overexpression of Cav1 in PAM212 cells results in decreased cell proliferation in vitro, which corresponds to a dramatic decrease in tumor incidence and size in vivo. In contrast, the knockdown of Cav1 in these cells increases tumor growth and enhances their invasive ability both in vitro and in vivo. Mechanistically, Cav1 knockdown is associated with hyperactivation of the extracellular signaling–related kinase (Erk) 1/2 mitogen-activated protein kinase (MAPK) signaling pathway and increased activator protein (AP)-1 transcription factor activation in response to two different growth stimuli. We implicate several AP-1–responsive genes as mediators of the proproliferative and invasive phenotype in Cav1 knockdown PAM212 cells, as determined by quantitative RT-PCR (RT-qPCR). Finally, we show that Cav1 overexpression in the human cSCC cell line SCC13 decreases in vitro proliferation, migration, and invasion, indicating that our results are able to translate to human skin cancer. In summary, these results demonstrate that loss of Cav1 negatively affects several markers for metastatic potential in human skin tumors, including tumor size and invasiveness, and this is mechanistically associated with MAPK/AP-1 hyperactivation.
Materials and Methods
Materials
Antibodies and their sources were as follows: Cav1 (N-20), cyclin D1, cyclin A, and matrix metalloproteinase 2 were from Santa Cruz Biotechnology (Santa Cruz, CA). Cav2 and epidermal growth factor receptor (EGFR) were from BD Biosciences (Franklin Lakes, NJ). Keratin 14 (K14) and keratin 10 (K10) were from Covance (Princeton, NJ). Keratin 18 (K18) and CD31 were from Abcam (Cambridge, MA). Keratin 8 (K8) was from Epitomics (Burlingame, CA). p-Histone H3 (S10) was from Upstate (Billerica, MA). p-Erk (T202/Y204), Erk, p-EGFR (Y1173), c-Fos, p-c-Jun (S73), c-Jun, and p-c-Jun N-terminal kinase (JNK; T183/Y185) were from Cell Signaling Technology (Beverly, MA). Glyceraldehyde-3-phosphate dehydrogenase was from Fitzgerald (Acton, MA), and β-actin and β-tubulin were from Sigma-Aldrich (St. Louis, MO). U0126 and SP600125 were from Cell Signaling Technology and LC Laboratories (Woburn, MA), respectively.
Cell Culture
PAM212 cells were a generous gift from Dr. Ulrich Rodeck (Thomas Jefferson University, Philadelphia, PA). They were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). HaCaT human immortalized keratinocytes were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS. SCC13 cells were obtained from The Harvard Skin Disease Research Center (Boston, MA).19,20 They were maintained in a 1:1 mix of Keratinocyte Serum Free Medium (Life Technologies, Grand Island, NY) and DF-K medium (a 1:1 mix of calcium-free Dulbecco’s modified Eagle’s medium and Ham’s F-12 Nutrient Mixture; Life Technologies) supplemented with 25 μg/mL bovine pituitary extract, 0.2 ng/mL EGF, and 0.3 mmol/L calcium chloride. All cells were kept in an incubator at 37°C with 5% CO2.
Stable Cell Lines
PAM212 and SCC13 cells were stably transduced to express either pBabe or pBabe-Cav1.21 To stably knock down Cav1, shRNA scramble control and predesigned shRNAs targeting nucleotides 185 to 205 and 482 to 502 of Cav1 mRNA (NM_007616.4) were obtained from Invitrogen (Carlsbad, CA) and subcloned into the pQCXIP-GFP retroviral vector (Clontech, Mountain View, CA). PAM212 cells were infected, and a stable cell population was selected, as previously described.22 Successful overexpression and knockdown of Cav1 were verified by using Western blot analysis. For Cav1 re-expression in Cav1 knockdown cells, short hairpin RNAs (shRNAs) specific for Cav1 (sh-Cav1) PAM212 cells were stably transduced to express pBabe-Cav1 as above. The Cav1 expressed by this vector is resistant to the sh-Cav1 constructs already being expressed in these cells because of nucleotide mismatch.
Western Blot Analyses
Cells were lysed in a modified radioimmunoprecipitation assay buffer and analyzed as previously described.23 Briefly, protein was separated via an SDS-PAGE gel and transferred to a nitrocellulose membrane. Membranes were blocked in 5% bovine serum albumin (BSA; Sigma-Aldrich) in Tris-buffered saline with 0.1% Tween. Primary antibody diluted in blocking buffer was added for either 1 hour at room temperature or overnight at 4°C. Membranes were washed three times in wash buffer, and horseradish peroxidase–conjugated secondary antibody was added for 1 hour at room temperature. After washing, signal was developed using Pierce Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL).
Confocal Microscopy
Cells were double immunostained for Cav1 and Cav2, as previously described.21 Pro-Long Gold Antifade reagent (Molecular Probes, Eugene, OR) was used to mount the coverslips, which were subsequently imaged by confocal microscopy (LSM 510.META.Confocal; Carl Zeiss Inc., Jena, Germany).
Growth Curves and Proliferation Assay
Proliferation was measured using a 5-bromodeoxyuridine (BrdU) incorporation enzyme-linked immunosorbent assay (Roche Diagnostics, Indianapolis, IN). Cells were labeled with BrdU in RPMI 1640 medium with 10% FBS for 4 hours. Growth curves of PAM212 and SCC13 cells were generated by plating 10.5 cells/cm2 and counting cell number daily for 4 days. Alternatively, growth curves were generated by MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assays (Promega, Madison, WI) and performed according to the manufacturer’s instructions.
Tumor/Metastasis Studies
PAM212 cells (5 × 106) were intradermally injected into the back skin of 5- to 6-week-old BALB/c nude mice, as previously described.24 Overexpression and knockdown cells were pair injected with their respective controls. Tumor volume was calculated weekly using the following equation: (width2 × length)/2. For spontaneous metastasis studies, one cell type per mouse was injected as previously described. Tumors were surgically excised after 5 weeks, and mice were sacrificed 4 weeks after excision and examined for visible (macroscopic) lymph node metastasis.25 Mice were maintained in an animal barrier facility with a 12-hour light/dark cycle with ad libitum access to chow. All mouse work was conducted in accordance with Institutional Animal Care and Use Committee approval.
RT-qPCR Data
RT-qPCR was performed using ready-to-use primers (Real Time Primers, LLC, Elkins Park, PA) and SYBR master mix (Applied Biosystems by Life Technologies). Quantitative expression data were acquired using the ABI-Prism 7900HT Sequence Detection System (Applied Biosystems), and results were analyzed by the ΔΔCT method.26
IHC Analysis
Tumor sections were stained for K18 expression using standard immunohistochemical (IHC) techniques.14 Briefly, paraffin-embedded sections were rehydrated and antigen retrieval was performed using citrate buffer. Endogenous peroxidase activity was blocked with 3% H2O2, and sections were blocked in 10% goat serum and incubated with primary antibody overnight. After washing, sections were incubated with biotinylated secondary antibody for 30 minutes, washed again, and incubated with streptavidin for 30 minutes. After signal development with 3,3′-diaminobenzidene, slides were counterstained with hematoxylin, dehydrated, and mounted.
Growth Factor Stimulation
PAM212 cells were plated in normal medium, serum starved for 18 hours in serum-free medium (SFM) with 0.1% BSA, and treated with 50 ng/mL EGF (Peprotech, Rocky Hill, NJ) in SFM with 0.1% BSA. After treatment, cells were lysed and used in Western blot analysis, as previously described.
Inhibitor Treatments
PAM212 cells were pre-incubated with dimethyl sulfoxide or 40 μmol/L inhibitor in SFM for 2 hours, and then stimulated with 10% FBS with dimethyl sulfoxide or inhibitor for 1 hour. After treatment, cells were lysed and used in Western blot analysis, as previously described.
Migration and Invasion Assays
For PAM212 transwell assays, 105 cells (migration) or 1.5 × 105 cells (invasion) were suspended in 0.5 mL SFM with 0.1% BSA and added to 8-μm transwell chambers (BD Biosciences) without (migration) or with (invasion) Matrigel. Cells were allowed to migrate for 6 hours or invade for 18 hours, after which cells that failed to migrate or invade were removed with a cotton swab. Cells were fixed and stained with 0.5% crystal violet in methanol. For assays using EGF, 50 ng/mL EGF in SFM with 0.1% BSA was used as the chemoattractant in the bottom well, and cells were allowed to migrate for 24 hours or invade for 48 hours. For inhibitor assays, dimethyl sulfoxide or inhibitor was added to both the top chamber and the bottom well, and cells were allowed to migrate for 24 hours or invade for 48 hours. For SCC13 assays, 7.5 × 104 cells (migration) or 105 cells (invasion) were plated using 10% FBS as the chemoattractant and allowed to migrate or invade for 24 hours.
Statistical Analysis
All results are represented as means ± SEM. Statistical analyses were performed using Prism software version 4.0 (Graph Pad Software, Inc., San Diego, CA).
Results
Cav1 Overexpression Decreases in Vitro Proliferation and in Vivo Tumor Incidence and Growth in PAM212 Keratinocytes
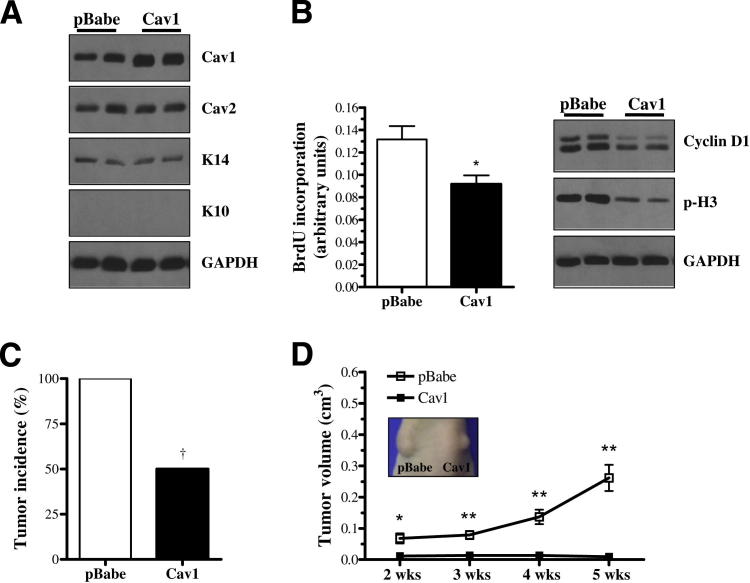
PAM212 murine keratinocytes were stably transduced to express either pBabe empty vector or pBabe-Cav1 overexpression vector. PAM212-Cav1 cells showed an approximately twofold increase in Cav1 expression, whereas Cav2, which colocalized with Cav1, remained unaffected. In addition, the expression of K14, a marker of proliferating keratinocytes, and K10, a marker of differentiating keratinocytes, were unchanged by Cav1 overexpression (Figure 1A). Furthermore, Cav1 overexpression diminished the proliferative ability of PAM212 cells, as evidenced by decreased BrdU incorporation and expression of cyclin D1 and p-histone H3 (Figure 1B).
Figure 1.
Cav1 overexpression decreases in vitro proliferation and in vivo tumor incidence and growth in PAM212 keratinocytes. A: Western blot analysis of PAM212 cells retrovirally transduced with either empty pBabe plasmid (pBabe) or pBabe-Cav1 overexpression plasmid (Cav1) shows increased Cav1 expression, but no change in the expression of Cav2; K14, a marker of proliferating keratinocytes; or K10, a marker of differentiating keratinocytes. B: Cav1 PAM212 cells display reduced proliferation, as determined by a BrdU incorporation assay (4 hours; n = 16 per group) and decreased expression of cyclin D1 and p-histone H3 proteins. Cav1 overexpression dramatically reduces tumor incidence (C) and growth (D) in PAM212 cells intradermally injected in the back skin of mice (n = 10 per group). †P < 0.05 (by Fisher’s exact test). Inset: Tumor growth 4 weeks after injection. Results are reported as means ± SEM. *P < 0.05, **P < 0.01 by the unpaired t-test. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
To examine the in vivo impact of Cav1 overexpression, pBabe and Cav1 cells were intradermally pair injected into the back skin of Nude mice. Beginning at 2 weeks after injection, analysis of tumor growth revealed that Cav1 overexpression significantly decreased the tumorigenicity of PAM212 cells (tumor incidence) from 100% to 50% (Figure 1C). In addition, overexpression of Cav1 reduced tumor volume by approximately 27-fold (Figure 1D) and tumor weight by 30-fold (data not shown) by 5 weeks after injection. These data indicated that Cav1 functions as a negative regulator of cell and tumor growth in PAM212 cells.
Cav1 Knockdown Increases in Vitro Proliferation and in Vivo Tumor Growth in PAM212 Keratinocytes
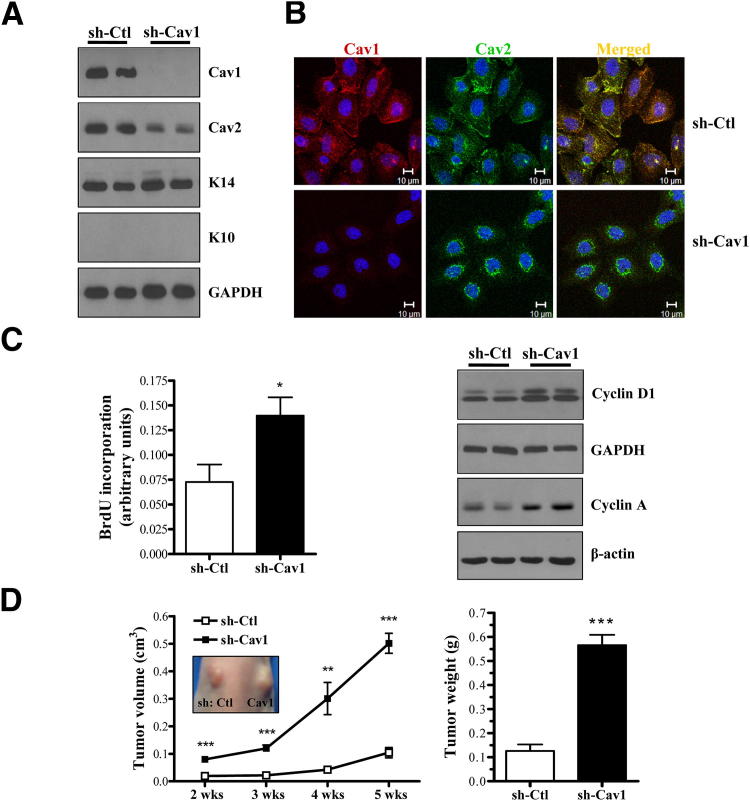
PAM212 cells that were stably transduced to express sh-Cav1 displayed complete absence of Cav1 protein with a concurrent decrease in Cav2 expression levels. Immunoblot analysis showed that K14 and K10 levels were unaffected by Cav1 knockdown (Figure 2A). As previously described in cells and the Cav1 knockout mouse, Cav1 and Cav2 colocalize,27 and Cav1 is required to stabilize and transport Cav2 to the plasma cell membrane.12 Accordingly, confocal microscopy analysis revealed that Cav1 and Cav2 colocalized as normal in short hairpin control (shCtl) cells, whereas in sh-Cav1 cells, Cav2 was mainly localized around the nucleus (Figure 2B). These results indicated that Cav1 knockdown in these cells closely mimicked the phenotype observed in Cav1 knockout mice in that Cav2 was reduced and failed to localize to the membrane.
Figure 2.
Cav1 knockdown increases in vitro proliferation and in vivo tumor growth in PAM212 cells. A: Western blot analysis of PAM212 cells stably transduced via retroviral infection with either scramble shCtl or shCav1. Cav1 knockdown results in a decrease in Cav2 expression, whereas K14 and K10 expression is unaffected. B: Confocal microscopy of shCtl and shCav1 PAM212 cells immunostained with antibodies against Cav1 and Cav2. Hoechst 33342 is shown as a nuclear marker. In the absence of Cav1, Cav2 remains sequestered around the nucleus. C: Cav1 knockdown increases the proliferative ability of PAM212 cells, as determined by a BrdU incorporation assay (4 hours; n = 4 per group) and increased expression of cyclins A and D1. D: In vivo tumor growth was assessed as in Figure 1D. Cav1 knockdown results in a dramatic increase in tumor volume and weight. Inset: Tumor growth 4 weeks after injection. Results are reported as means ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 by the unpaired t-test. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
In addition, Cav1 knockdown significantly enhanced the proliferation of PAM212 cells in vitro, as demonstrated by increased BrdU incorporation and expression of cyclins D1 and A (Figure 2C). To examine in vivo growth, Cav1 knockdown and control cells were pair injected into the back skin of Nude mice. Analysis of tumor volume revealed that Cav1 knockdown PAM212 cells formed tumors that were significantly larger compared with shCtl cells throughout the entire study (Figure 2D). By 5 weeks after injection, shCav1 tumors were approximately fivefold larger in volume and fourfold larger in mass (Figure 2D). In addition, the effect of Cav1 knockdown on in vitro proliferation and in vivo tumor growth was validated using a second shRNA construct (Supplemental Figure S1). Cav1 ablation in these cells increased BrdU incorporation by 1.9-fold, tumor volume by 3.6-fold, and tumor weight by 2.3-fold. Finally, we showed that re-expressing an shRNA-resistant Cav1 in both shCav1 cell lines rescued the increase in cell growth caused by Cav1 knockdown in these cells (Supplemental Figure S2). Collectively, these data illustrated the growth-inhibitory function of Cav1 in PAM212 cells.
Cav1 Knockdown Increases the Invasive and Metastatic Ability of PAM212 Cells
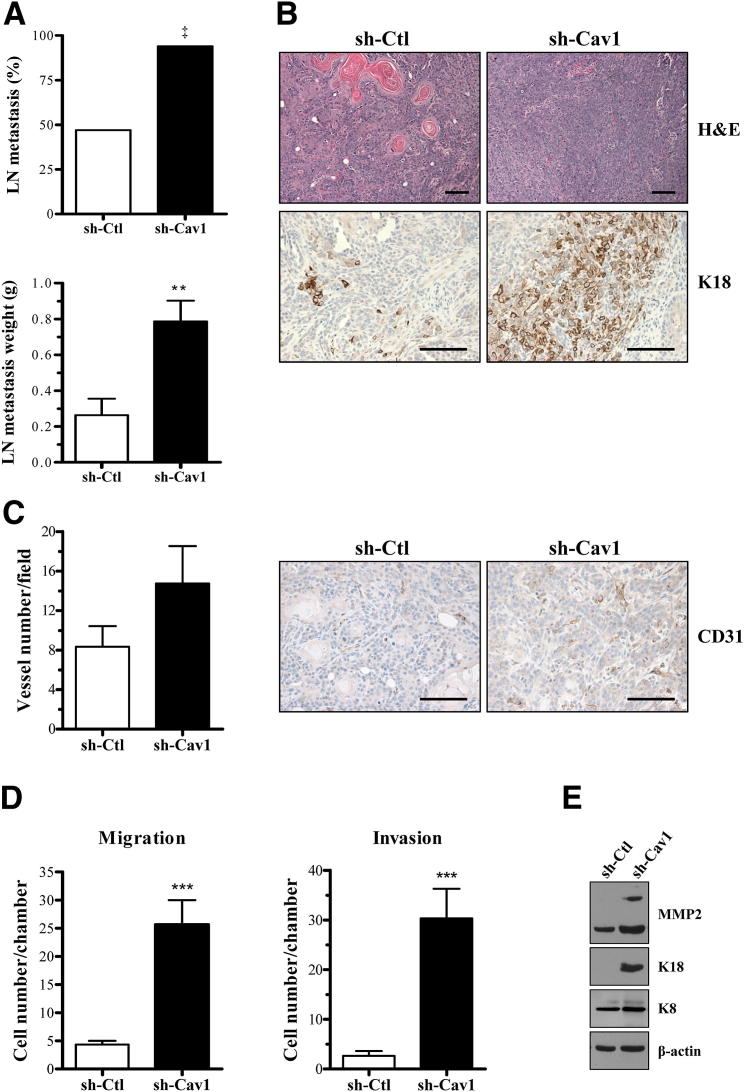
To determine whether Cav1 knockdown affected the metastatic ability of these cells, we undertook a spontaneous metastasis study. Tumors were allowed to grow for 5 weeks and were excised, and animals were examined for evidence of visible lymph node metastasis 4 weeks after excision. Interestingly, down-regulation of Cav1 significantly increased the incidence of visible lymph node metastasis from approximately 50% to approximately 100%. In addition, lymph nodes dissected from animals with metastasis were significantly larger (approximately threefold) in mice injected with shCav1 cells versus shCtl-injected animals (Figure 3A).
Figure 3.
Cav1 knockdown increases the invasive and metastatic ability of PAM212. A: Spontaneous metastasis formation was examined 9 weeks after intradermal injection of shCtl and shCav1 cells. Cav1 knockdown results in an increase in the incidence of visible lymph node metastasis (approximately twofold) and weight (n ≥ 15 per group). ‡P < 0.01 by Fisher’s exact test. B: Representative H&E staining of paired shCtl and shCav1 primary tumors demonstrate that both cell types form squamous cell carcinomas. There is increased expression of K18 in shCav1 tumors, indicative of a less-differentiated tumor. C: CD31 IHC staining reveals a trend toward increased vessel formation in Cav1 knockdown cells (n ≥ 4 per group). D: By using 10% serum as a chemoattractant, transwell migration and invasion assays show that Cav1 knockdown dramatically increases the ability of PAM212 to migrate through a transwell chamber and invade through Matrigel (n = 6 per group). E: Western blot analysis of invasive markers shows that Cav1 knockdown increases the expression of matrix metalloproteinase (MMP) 2, K18, and its binding partner, K8. Results are reported as means ± SEM. **P < 0.01, ***P < 0.001 by unpaired t-test. Scale bar = 10 μm.
To determine why shCav1 tumors colonized a secondary site with greater frequency, we examined the histological characteristics of the primary tumors. Accordingly, histological analysis revealed abundant keratin pearls (pink deposits) in shCtl tumors, an indication of a well-differentiated lesion.28 shCav1 primary tumors also showed a dramatic increase in the expression of K18, a marker for less-differentiated and more invasive SCCs29–33 (Figure 3B). In addition, CD31 staining suggested that shCav1 tumors showed a trend toward greater vessel density (Figure 3C), a requirement for tumor growth and metastasis.34,35
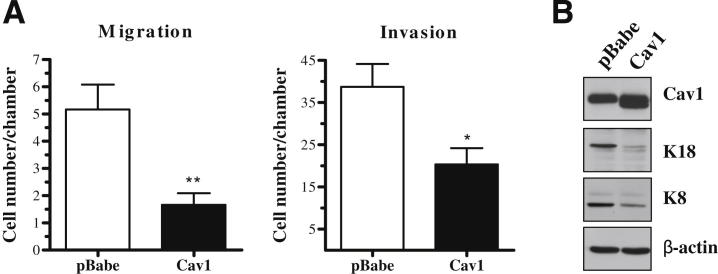
Finally, we examined the in vitro migratory and invasive ability of these cells using transwell assays. Cav1 knockdown in PAM212 conferred an increased ability to migrate through a transwell chamber by approximately fourfold and to invade through Matrigel by 11-fold (Figure 3D). This enhanced invasion was associated with increased expression of matrix metalloproteinase 2, a protein involved in extracellular matrix breakdown during tumor cell invasion and metastasis36,37; K18; and its binding partner K8, which is also associated with invasion29–33 (Figure 3E and Supplemental Figure S1). In addition, overexpression of Cav1 had the opposite effect on migration, invasion, and K8 and K18 expression (Figure 4, A and B). Cav1 overexpression decreased K8 and K18 expression and migratory ability by threefold and invasive ability by 1.9-fold. Collectively, these data suggested that Cav1 knockdown positively affected both the in vitro and in vivo invasive capacity of these cells.
Figure 4.
Cav1 overexpression decreases migration, invasion, and expression of K8 and K18 in PAM212 cells. A: By using 10% serum as a chemoattractant, transwell assays show that Cav1 overexpression decreases the ability of PAM212 to migrate and invade (n ≥ 3 per group). B: Western blot analysis shows that Cav1 overexpression decreases the expression of both K8 and K18. Results are reported as means ± SEM. *P < 0.05, **P < 0.01 by the unpaired t-test.
Cav1 Knockdown Increases MAPK Pathway Activation in Response to Serum or Stimulation with EGF
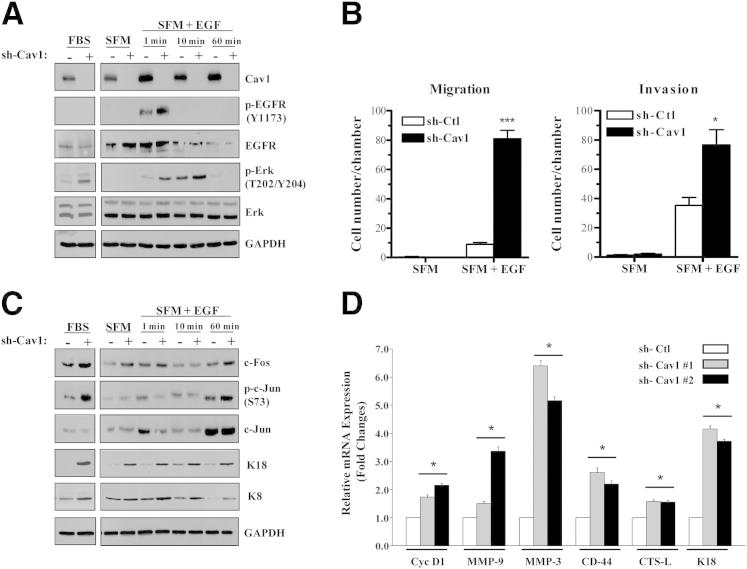
To determine a mechanism by which Cav1 knockdown enhanced tumor growth and invasion in PAM212 cells, we examined signaling pathway activation in response to various growth stimuli. When grown in medium containing FBS, shCav1 PAM212 cells showed increased Erk1/2 activation, whereas p-EGFR expression levels were unchanged. Serum-starved shCav1 PAM212 cells treated with EGF displayed enhanced p-EGFR at 1 minute after treatment, and p-Erk1/2 expression levels increased after 1 and 10 minutes (Figure 5A). Notably, Cav1 knockdown increased the ability of PAM212 cells to migrate through a transwell chamber (approximately ninefold) and invade through Matrigel (approximately twofold) when EGF was used as a chemoattractant (Figure 5B).
Figure 5.
Cav1 knockdown increases MAPK pathway activation in response to serum or stimulation with EGF. A: Western blot analysis of shCtl and shCav1 PAM212 cells grown in complete media with serum (left panel) or serum starved for 18 hours and treated with 50 ng/mL EGF (right panel). Cav1 knockdown cells treated with EGF show an increase in EGFR activation. When grown in either complete or EGF-supplemented media, Cav1 knockdown cells show an increase in activated Erk1/2. B: Cav1 knockdown increases the ability of PAM212 to migrate and invade through transwell chambers when EGF is used as the chemoattractant in serum-free conditions (n ≥ 3 per group). Results are reported as means ± SEM. *P < 0.05, ***P < 0.001 by the unpaired t-test. C: Examination of signaling molecules downstream of MAPK activation reveals that shCav1 cells grown in serum or EGF show an increase in total c-Fos protein and activated c-Jun, an indication that these cells have increased activity of the AP-1 transcription factor. In addition, Cav1 knockdown increases expression of K18, an AP-1–responsive gene, and its binding partner, K8. D: RT-qPCR analysis shows increased expression of AP-1 target genes in Cav1 knockdown PAM212 cells (n = 3 per group). Results are reported as means ± SEM. *P < 0.05 by Dunnet’s multiple comparisons test.
An examination of signaling molecules downstream of Erk1/2 activation revealed that shCav1 PAM212 cells grown in medium containing FBS showed increased total c-Fos and p-c-Jun levels. Similarly, serum-starved cells treated with EGF showed increased total c-Fos and p-c-Jun levels by 60 minutes after treatment, indicating that loss of Cav1 enhanced MAPK and AP-1 transcription factor activation (Figure 5C). Finally, shCav1 cells stimulated by either serum or EGF showed an increase in the expression of K18, an AP-1–responsive gene,32,38–41 and its binding partner, K8. This phenotype was reversed by Cav1 overexpression (Figure 4B). These findings were consistent with our in vivo results (Figure 3B) and with previous studies that have shown that K8 and K18 expression positively correlated with invasive potential in several cell types,42–45 including PAM212,33 and in mouse and human skin tumors.29,33 In addition, RT-qPCR analysis of AP-1 target genes in Cav1 knockdown cells revealed increased expression of genes associated with tumor growth and invasion (Figure 5D).
Collectively, these data indicated that the MAPK pathway was hyperactivated in Cav1 knockdown cells in response to two different growth stimuli, which, in turn, increased AP-1 activation and increased transcription of AP-1 target genes.
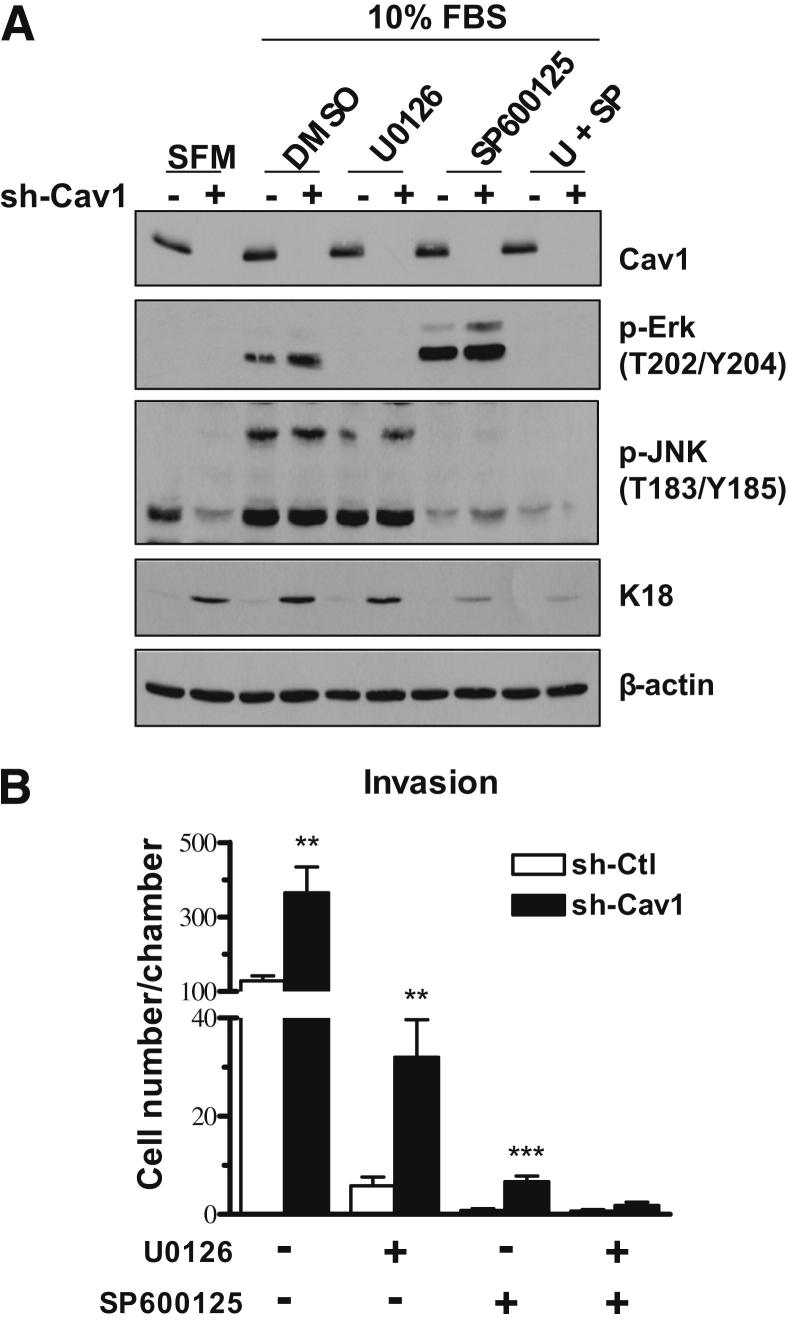
Dual Inhibition of Erk1/2 and JNK Decreases Expression of K18 and Eliminated the Invasive Advantage Conferred by Cav1 Knockdown in PAM212 Cells
To assess the functional significance of MAPK activation in shCav1 PAM212 cells, we treated cells with inhibitors for both the Erk1/2 and JNK pathways. JNK is another activator of AP-1 signaling through its ability to phosphorylate c-Jun. U0126 is an MAPK inhibitor, a molecule that activates Erk1/2, and SP600125 is a JNK inhibitor. We confirmed that 40 μmol/L of each inhibitor was sufficient to decrease its respective signaling pathways (Figure 6A). In addition, we verified that inhibiting these pathways decreased the expression of K18. Maximum inhibition of K18 expression occurred when both pathways were inhibited, indicating both pathways were contributing to the expression of this protein. Finally, an invasion assay performed with these inhibitors showed that both Erk1/2 and JNK inhibition dramatically decreased the invasive ability of both shCtl and shCav1 PAM212 cells; however, dual inhibition was necessary to abolish the significant increase in invasion observed in shCav1 cells (Figure 6B). These results indicated that the invasive advantage conferred by Cav1 knockdown was mediated through two different MAPK pathways: Erk1/2 and JNK. In addition, K18 could be a downstream mediator of the invasive phenotype, because this was one protein that was affected by inhibition of these two pathways.
Figure 6.
Dual inhibition of Erk1/2 and JNK signaling decreases expression of K18 and abolishes the invasive difference conferred by Cav1 knockdown in PAM212. A: Western blot analysis of PAM212 cells treated with 40 μmol/L of U0126, an MAPK inhibitor, or SP600125, a JNK inhibitor, either singly or in combination. Dual inhibition results in the greatest decrease in K18. B: A Matrigel invasion assay shows that inhibition of either Erk1/2 or JNK decreases the overall invasive ability of both shCtl and shCav1 PAM212 cells, but does not eliminate the difference between the two groups. When both inhibitors are used in combination, the significant difference in invasive ability conferred by Cav1 knockdown is abolished (n = 3 per group). Results are reported as means ± SEM. **P < 0.01, ***P < 0.001 by the unpaired t-test. DMSO, dimethyl sulfoxide.
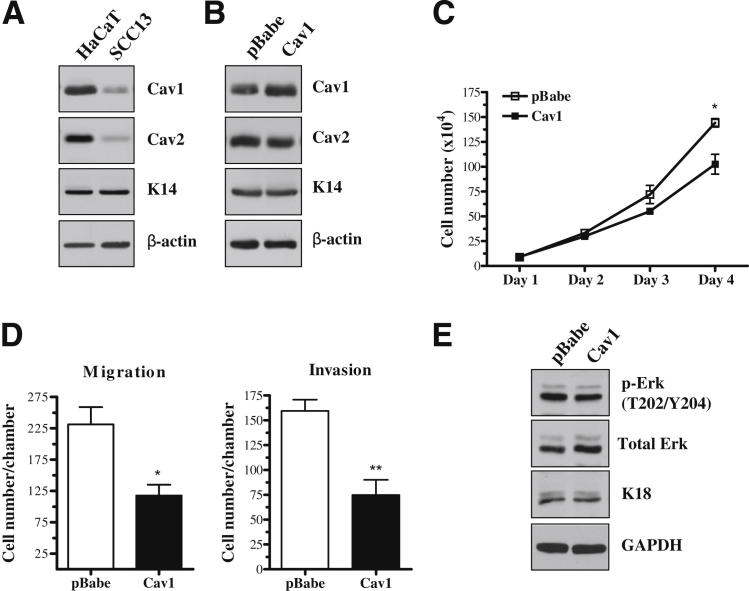
Cav1 Expression Is Decreased in the Human cSCC Cell Line, SCC13, and Cav1 Overexpression Decreases in Vitro Proliferation, Migration, and Invasion
To assess whether our results in PAM212 translated to human cells, we used the human cSCC cell line, SCC13. We first compared Cav1 expression in these cells with that of the human immortalized (ie, non-transformed) keratinocyte cell line, HaCaT (Figure 7A). SCC13 carcinoma cells showed a dramatic decrease in Cav1 and Cav2 expression compared with noncancerous cells. Next, we successfully expressed either pBabe empty vector or Cav1 overexpression vector in these cells without altering Cav2 expression (Figure 7B). Cav1 overexpression resulted in a significant decrease in cell growth, as evidenced by a growth curve (Figure 7C), and in cell migration and invasion (Figure 7D), as evidenced by transwell chamber assays. Surprisingly, despite the similar phenotype conferred by Cav1 overexpression in PAM212 and SCC13, Erk activation and K18 expression levels were largely unchanged by Cav1 overexpression in SCC13 cells (Figure 7E). These results indicated that the inhibitory role of Cav1 in growth and invasion acted through a different mechanism than that observed in PAM212. However, these data indicated that the phenotype conferred by Cav1 in murine PAM212 cells could be recapitulated in the human cell line, SCC13.
Figure 7.
Cav1 expression is decreased in the human cSCC cell line, SCC13, and Cav1 overexpression decreases in vitro proliferation, migration, and invasion. A: Western blot analysis shows that Cav1 expression is decreased in SCC13 compared with the immortalized human keratinocyte cell line, HaCaT. Cav2 expression is also decreased, whereas K14 expression is unaffected. B: Western blot analysis shows successful overexpression of Cav1 in SCC13, whereas Cav2 and K14 expression levels are unaffected. C: Cav1 overexpression decreases cell growth in SCC13, as indicated by a growth curve (n = 3 per group). D: Transwell assays show that overexpression of Cav1 decreases both the migratory and invasive ability of these cells (n = 4 per group). E: Western blot analysis shows that Cav1 overexpression does not affect Erk activation or the expression of K18. Results are reported as means ± SEM. *P < 0.05, **P < 0.01 by the unpaired t-test. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Discussion
In the present study, we have examined the function of Cav1 in the development and progression of skin cancer. By using a murine model of cutaneous squamous cell carcinoma, the PAM212 cell line, we provide evidence that loss of Cav1 functions in primary tumor growth and also in the progression of primary tumors into less-differentiated and metastatic lesions. PAM212 cells are spontaneously transformed BALB/c keratinocytes.18 They are commonly used in skin research to study in vivo tumor growth,18 gene and protein expression changes during tumor progression,46–51 and response to cytokines and chemicals.50–53 PAM212 cells form tumors with 100% incidence but display a lower incidence of metastatic dissemination,48,54 making them an ideal model to study changes in both tumor growth and progression. By altering Cav1 expression in these cells, we assessed the effect of this protein on both primary and secondary tumor formation.
We first show that Cav1 overexpression decreases cell and tumor growth, whereas Cav1 knockdown increases these attributes in PAM212 cells. Interestingly, knockdown of Cav1 also affects the invasive characteristics of these cells, as evidenced by an increase in the following: i) in vitro migratory and invasive ability, ii) vessel density and expression of K18 in primary tumors, and iii) incidence of spontaneous lymph node metastasis. Finally, we show that, in PAM212 cells stimulated with either serum or EGF, Cav1 knockdown increases Erk1/2 MAPK pathway and AP-1 transcription factor activation. We attribute the increase in invasive ability observed in Cav1 knockdown cells to downstream mediators of the MAPK and JNK pathways, because dual inhibition of these pathways abolishes the increase in invasion conferred by Cav1 knockdown. We suggest K18 as one of these mediators. To our knowledge, we provide the first evidence that Cav1 functions in the development and progression of cutaneous squamous cell carcinoma.
Significantly, we corroborated some of our results in a human cSCC cell line, SCC13. SCC13 has substantially lowered levels of both Cav1 and Cav2 compared with a non-transformed human keratinocyte cell line. Overexpression of Cav1 decreased in vitro proliferation, migration, and invasion, but it did not affect Erk1/2 activation or K18 expression, as observed in PAM212 cells. One possible explanation is that the level of Cav1 overexpression in SCC13 is lower than that observed in PAM212 cells, potentially making the phenotype less striking. These results indicate that Cav1 functions in the modulation of the malignant phenotype through a different, as yet undetermined, mechanism that is independent of MAPK signaling in these cells.
Our work demonstrates that altered Cav1 expression affects the growth of primary PAM212 tumors and also their level of differentiation and their ability to invade surrounding tissues and metastasize. Given this, we sought to determine a mechanism for the effect of Cav1 on these aspects of tumor biological characteristics. The MAPK pathways are key regulators of cellular proliferation55,56 and are frequently hyperactivated in cancer.57–59 One of the downstream mediators of both the Ras-Erk1/2 and the JNK MAPK cascades is the AP-1 transcription factor, composed of both c-Fos and c-Jun proteins.60–62 Previous work has implicated Cav1 in the negative regulation of the Ras-Erk1/2 MAPK cascade63,64 and AP-1 transcription factor activation.65,66 Our current work establishes Cav1 as a key regulator of Erk1/2 MAPK pathway activation in PAM212. By using two different stimuli, we showed increased Erk1/2 activation in Cav1 knockdown PAM212 cells. In turn, this corresponded to an increase in the expression and activation of AP-1 members, specifically c-Fos and c-Jun. Interestingly, MAPK and AP-1 activation affects the expression of proteins that promote both proliferation and invasion.
The Ras-Erk1/2 MAPK cascade is a critical controller of cell cycle progression in many cell types. Activated Erk1/2 positively regulates cell cycle progression in several ways by contributing to ribosomal RNA and protein synthesis in preparation for translation and inducing positive regulators of cell cycle progression.56,59 In addition, Erk1/2 activation is essential to G1-to-S phase progression in many cell types. This is accomplished through increasing the expression and stabilization of AP-1 transcription factor members, which, in turn, promote expression of cyclin D1.55 Interestingly, we show that overexpression of Cav1 decreases cyclin D1 expression, whereas knockdown increases expression in PAM212. Our results indicate that Cav1 knockdown results in hyperactivation of the MAPK pathway, which, in turn, drives proliferation in these cells.
Erk1/2 and AP-1 activation have also been implicated as positive regulators of migration, invasion, and metastasis. Specifically, activated Erk1/2 phosphorylates members of focal adhesions promoting motility and, via AP-1, it can induce expression of matrix metalloproteinases that promote extracellular matrix breakdown and invasion.58,59 In addition, previous work has shown that the K18 gene has an AP-1–responsive element.32,38–41 K18 and its binding partner, K8, are intermediate filament molecules normally expressed in non-stratified epithelia.32 Interestingly, Cav1 knockdown cells show an increase in both K8 and K18, whereas cells in which Cav1 is overexpressed show a decrease in these keratins. K8 and K18 have been associated with increased invasion in a variety of cell types.42–45 Recently, Yamashiro et al33 have shown that lymph node metastatic derivatives of PAM212 (LY-1 and 2) overexpress both K8 and K18 compared with parental PAM212 cells. Furthermore, the overexpression of K8 and K18 in parental PAM212 confers a more invasive phenotype. Previous work has also shown that both keratins are aberrantly expressed in skin tumors, particularly in less-differentiated areas, and are associated with invasion in human cSCC.29,33 Given this work, we hypothesized that K8 and K18 are mediators of the phenotype observed in Cav1 knockdown PAM212 cells. Although previous work has shown that the K8 promoter contains binding sites for the Ras/Erk1/2-responsive Ets transcription factors, relatively little is known about K8 gene regulation32,67; therefore, we sought to inhibit K18 expression by exploiting its known regulation by AP-1. We show that Erk1/2 inhibition modestly decreases K18 expression; to achieve maximum inhibition of K18, it is necessary to dually inhibit both Erk1/2 and JNK, another activator of AP-1 activity. Dual inhibition rescues the increase observed in invasive ability when Cav1 is knocked down in these cells. This dual contribution of the Erk1/2 and JNK MAPK pathways to cancer development has also been reported in melanoma, in which the two pathways can operate in a feed-forward mechanism, contributing to transcription of downstream target genes, such as cyclin D1.68 These results indicate that Cav1 knockdown increases activation of Erk1/2 and AP-1, which, in turn, increase invasive ability; in addition, one of the potential downstream mediators of this phenotype is K18. Our results are interesting given previous studies showing a potential role for Cav1 in both normal skin homeostasis and skin tumor biological characteristics.
Previous work has shown that Cav1 is strongly expressed in the basal layer of both murine and human skin,14,69 and that expression of Cav1 is lost in a portion of human cSCC tumors.17 Mechanistically, loss of Cav1 in a subpopulation of human tumors could be an independent event in tumor development or could occur secondarily to other signaling events. As an autonomous event, Cav1 expression or function is lost in various cancers because of the following: i) loss of the chromosomal fragile site where the human CAV1 gene is located,70–72 ii) aberrant promoter methylation,73–76 or iii) protein mislocalization due to mutation.73,77 Alternatively, loss of Cav1 in skin tumors could be a consequence of other molecular events. For example, previous work has shown that cellular transformation with the H-RasG12V oncogene decreases Cav1 promoter activity78 and results in down-regulation of both caveolin-1 mRNA and protein.79 Moreover, p53 is a positive regulator of Cav1 gene transcription.80 Therefore, p53 loss or Ras activation, two alterations commonly observed in skin cancer,81–84 could both negatively affect Cav1 expression, providing a potential mechanism by which a decrease in Cav1 protein levels is observed in a subpopulation of cutaneous squamous cell carcinomas. However, the amount of Cav1 expressed in skin tumors with these other molecular alterations could subsequently affect cellular signaling, altering the course of tumor development and progression in this type of cancer. In relation to our current findings, loss of Cav1 after Ras activation could result in a positive feedback loop with even greater Erk1/2 MAPK activation and increased expression of downstream molecules, such as cyclin D1 and K18. Interestingly, we find that our PAM212 cells express constitutively active Ras (RasG12V) and that the expression of this protein is increased by Cav1 knockdown and decreased by Cav1 overexpression (Supplemental Figure S3). These results show that, in the context of oncogenic Ras signaling, loss of Cav1, in this case through genetic manipulation, is able to function in a positive feedback loop by augmenting expression of the oncogene. Presumably, this augmentation could exacerbate the malignant phenotype, as demonstrated herein by our work in PAM212. The expression level of Cav1, therefore, could be an important modifier of the skin tumor phenotype.
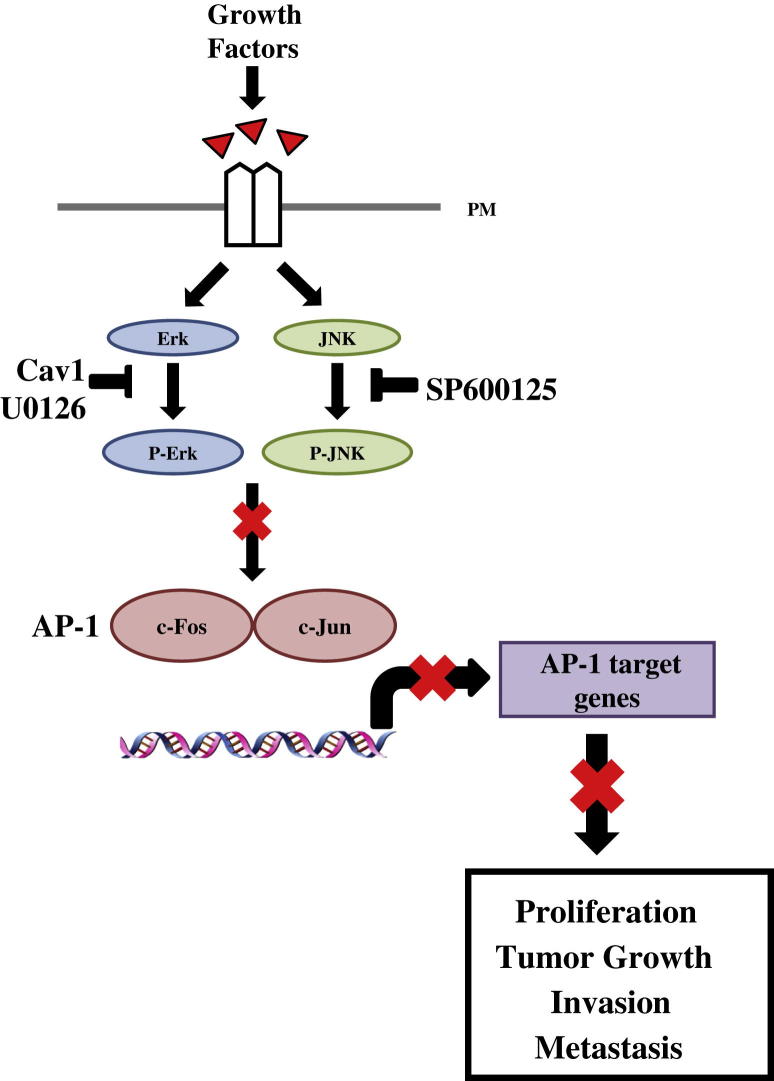
Our results show that Cav1 affects two major aspects of skin tumor biological characteristics: proliferation and growth of primary tumors and invasion and metastasis to secondary sites. We implicate Cav1 as a modulator of the Ras-Erk1/2 MAPK cascade and AP-1 transcription factor activity. These proteins affect both proliferation and invasion (Figure 8). Our data show that Cav1 knockdown increases the ability of PAM212 cells to colonize a secondary site. As in many cancers, patient mortality with cSCC is significantly increased in metastatic disease.5,85 In human tumors, characteristics such as tumor size, degree of differentiation, and involvement of vasculature are used to assess risk of metastasis.6,7 By using a murine model of cSCC, we show that Cav1 knockdown increases tumor size, vessel density, and invasiveness. To our knowledge, this is the first study to indicate that Cav1 could be a modulator of invasive and metastatic potential in cutaneous squamous cell carcinoma. In conclusion, we show that Cav1 negatively regulates both the proliferative and invasive capacity of murine cSCC cells and that this is mechanistically associated with inhibition of MAPK and AP-1 activation.
Figure 8.
Proposed model for Cav1-mediated inhibition of tumor growth and metastasis in cSCC. Cav1 functions to suppress cell signaling in response to growth factors (eg, serum or EGF) in PAM212 cells, resulting in reduced activation of Erk1/2. This, in turn, decreases expression and activation of c-Fos and c-Jun proteins that combine to form the AP-1 transcription factor, subsequently decreasing transcription of AP-1 target genes. The end result is a decrease in proliferation, tumor growth, invasion, and metastasis. PM, plasma membrane.
Footnotes
Supported by an American Heart Association grant (Beginning Grant-in-Aid to F.C.), NIH/National Cancer Institute grants (R01CA120876 and R01-CA-098779 to M.P.L.), the Susan G. Komen Breast Cancer Foundation (M.P.L.), the Margaret Q. Landenberger Research Foundation (M.P.L.), in part by the Pennsylvania Department of Health (M.P.L.), and an NIH Graduate Training Program grant (T32-CA09678 to C.T.).
A guest editor acted as editor-in-chief for this manuscript. No person at Thomas, Jefferson University was involved in the peer review process or final disposition of this article.
Current address of F.S. and M.P.L., Breakthrough Breast Cancer Research Unit, The University of Manchester, Manchester, United Kingdom.
Contributor Information
Michael P. Lisanti, Email: michael.lisanti@manchester.ac.uk.
Franco Capozza, Email: franco.capozza@jefferson.edu.
Supplemental Data
Cav1 knockdown using a second shRNA construct confirms that loss of Cav1 in PAM212 increases cell proliferation, migration, and invasion and in vivo tumor growth. A: Western blot analysis shows successful knockdown of Cav1 protein expression using a second shRNA construct. Similar to the original sh-Cav1 examined (sh-Cav1 £1), Cav1 knockdown using sh-Cav1 £2 decreases expression of Cav2 but has no effect on K14 and K10 expression. B: Knockdown of Cav1 using a second shRNA decreases cell proliferation, as shown via BrdU incorporation and a growth curve. The growth curve also shows cell growth for the original sh-Cav1 construct (sh-Cav1 £1), and both sh-Cav1 £1 and £2 display similar rates of cell growth. Results for the growth curve are reported as mean ± SEM. *P < 0.05 by Dunnett’s multiple comparisons test. C: sh-Cav1 £2 PAM212 cells show a significant increase in tumor growth beginning at 4 weeks after injection. By 5 weeks after injection, tumor weight is approximately twofold higher in Cav1 knockdown tumors. D:Cav1 knockdown using sh-Cav1 £2 decreases both the migratory and invasive ability of PAM212 cells when serum is used as a chemoattractant. In addition, decreased Cav1 expression increases the expression of K8 and K18 in these cells, similar to what we observe in the original sh-Cav1 cell line. Results are reported as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 by the unpaired t-test. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Overexpression of shRNA-resistant Cav1 in Cav1 knockdown cells rescues their proliferative advantage. A:Cav1 knockdown using two different shRNA constructs (sh-Cav1 £1 and £2) results in a significant increase in cell growth, as indicated by a cell vitality MTS assay measured over 4 days. B: Re-expression of Cav1 in these knockdown cells results in the rescue of this growth advantage, as indicated by an MTS assay measured over 4 days. Re-expression of Cav1 in shCav 1 cells actually decreases cell vitality to levels significantly lower than that of shCtl cells, whereas re-expression of Cav1 in shCav1 2 cells decreases vitality to levels similar to that of shCtl cells. Results are reported as mean ± SEM. **P < 0.01 by Dunnett’s multiple comparisons test.
The expression of constitutively active RasG12V is regulated by Cav1. Western blot analysis showing that Cav1 knockdown positively affects the expression of RasG12V whereas Cav1 overexpression decreases the expression of RasG12V. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
References
- 1.Gray D.T., Suman V.J., Su W.P., Clay R.P., Harmsen W.S., Roenigk R.K. Trends in the population-based incidence of squamous cell carcinoma of the skin first diagnosed between 1984 and 1992. Arch Dermatol. 1997;133:735–740. [PubMed] [Google Scholar]
- 2.Miller D.L., Weinstock M.A. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 3.Rogers H.W., Weinstock M.A., Harris A.R., Hinckley M.R., Feldman S.R., Fleischer A.B., Coldiron B.M. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2011;146:283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 4.Czarnecki D., Staples M., Mar A., Giles G., Meehan C. Metastases from squamous cell carcinoma of the skin in southern Australia. Dermatology. 1994;189:52–54. doi: 10.1159/000246783. [DOI] [PubMed] [Google Scholar]
- 5.Joseph M.G., Zulueta W.P., Kennedy P.J. Squamous cell carcinoma of the skin of the trunk and limbs: the incidence of metastases and their outcome. Aust N Z J Surg. 1992;62:697–701. doi: 10.1111/j.1445-2197.1992.tb07065.x. [DOI] [PubMed] [Google Scholar]
- 6.Alam M., Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 7.Cherpelis B.S., Marcusen C., Lang P.G. Prognostic factors for metastasis in squamous cell carcinoma of the skin. Dermatol Surg. 2002;28:268–273. doi: 10.1046/j.1524-4725.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 8.Razani B., Lisanti M.P. Caveolins and caveolae: molecular and functional relationships. Exp Cell Res. 2001;271:36–44. doi: 10.1006/excr.2001.5372. [DOI] [PubMed] [Google Scholar]
- 9.Cohen A.W., Hnasko R., Schubert W., Lisanti M.P. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 10.Lisanti M.P., Scherer P.E., Tang Z., Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 1994;4:231–235. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 11.Li S., Song K.S., Lisanti M.P. Expression and characterization of recombinant caveolin: purification by polyhistidine tagging and cholesterol-dependent incorporation into defined lipid membranes. J Biol Chem. 1996;271:568–573. [PubMed] [Google Scholar]
- 12.Razani B., Engelman J.A., Wang X.B., Schubert W., Zhang X.L., Marks C.B., Macaluso F., Russell R.G., Li M., Pestell R.G., Di Vizio D., Hou H., Jr., Kneitz B., Lagaud G., Christ G.J., Edelmann W., Lisanti M.P. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 13.Roelandt T., Giddelo C., Heughebaert C., Denecker G., Hupe M., Crumrine D., Kusuma A., Haftek M., Roseeuw D., Declercq W., Feingold K.R., Elias P.M., Hachem J.P. The “caveolae brake hypothesis” and the epidermal barrier. J Invest Dermatol. 2009;129:927–936. doi: 10.1038/jid.2008.328. [DOI] [PubMed] [Google Scholar]
- 14.Capozza F., Williams T.M., Schubert W., McClain S., Bouzahzah B., Sotgia F., Lisanti M.P. Absence of caveolin-1 sensitizes mouse skin to carcinogen-induced epidermal hyperplasia and tumor formation. Am J Pathol. 2003;162:2029–2039. doi: 10.1016/S0002-9440(10)64335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell L., Gumbleton M. Aberrant caveolin-1 expression in psoriasis: a signalling hypothesis. IUBMB Life. 2000;50:361–364. doi: 10.1080/713803750. [DOI] [PubMed] [Google Scholar]
- 16.Campbell L., Laidler P., Watson R.E., Kirby B., Griffiths C.E., Gumbleton M. Downregulation and altered spatial pattern of caveolin-1 in chronic plaque psoriasis. Br J Dermatol. 2002;147:701–709. doi: 10.1046/j.1365-2133.2002.05009.x. [DOI] [PubMed] [Google Scholar]
- 17.Langlois S., Cowan K.N., Shao Q., Cowan B.J., Laird D.W. The tumor-suppressive function of Connexin43 in keratinocytes is mediated in part via interaction with caveolin-1. Cancer Res. 2011;70:4222–4232. doi: 10.1158/0008-5472.CAN-09-3281. [DOI] [PubMed] [Google Scholar]
- 18.Yuspa S.H., Hawley-Nelson P., Koehler B., Stanley J.R. A survey of transformation markers in differentiating epidermal cell lines in culture. Cancer Res. 1980;40:4694–4703. [PubMed] [Google Scholar]
- 19.Rheinwald J.G., Beckett M.A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultures from human squamous cell carcinomas. Cancer Res. 1981;41:1657–1663. [PubMed] [Google Scholar]
- 20.Schon M., Rheinwald J.G. A limited role for retinoic acid and retinoic acid receptors RAR alpha and RAR beta in regulating keratin 19 expression and keratinization in oral and epidermal keratinocytes. J Invest Dermatol. 1996;107:428–438. doi: 10.1111/1523-1747.ep12363411. [DOI] [PubMed] [Google Scholar]
- 21.Capozza F., Cohen A.W., Cheung M.W., Sotgia F., Schubert W., Battista M., Lee H., Frank P.G., Lisanti M.P. Muscle-specific interaction of caveolin isoforms: differential complex formation between caveolins in fibroblastic vs. muscle cells. Am J Physiol Cell Physiol. 2005;288:C677–C691. doi: 10.1152/ajpcell.00232.2004. [DOI] [PubMed] [Google Scholar]
- 22.Trimmer C., Sotgia F., Whitaker-Menezes D., Balliet R.M., Eaton G., Martinez-Outschoorn U.E., Pavlides S., Howell A., Iozzo R.V., Pestell R.G., Scherer P.E., Capozza F., Lisanti M.P. Caveolin-1 and mitochondrial SOD2 (MnSOD) function as tumor suppressors in the stromal microenvironment: a new genetically tractable model for human cancer associated fibroblasts. Cancer Biol Ther. 2011;11:383–394. doi: 10.4161/cbt.11.4.14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capozza F., Combs T.P., Cohen A.W., Cho Y.R., Park S.Y., Schubert W., Williams T.M., Brasaemle D.L., Jelicks L.A., Scherer P.E., Kim J.K., Lisanti M.P. Caveolin-3 knockout mice show increased adiposity and whole body insulin resistance, with ligand-induced insulin receptor instability in skeletal muscle. Am J Physiol Cell Physiol. 2005;288:C1317–C1331. doi: 10.1152/ajpcell.00489.2004. [DOI] [PubMed] [Google Scholar]
- 24.Trimmer C., Whitaker-Menezes D., Bonuccelli G., Milliman J.N., Daumer K.M., Aplin A.E., Pestell R.G., Sotgia F., Lisanti M.P., Capozza F. CAV1 inhibits metastatic potential in melanomas through suppression of the integrin/Src/FAK signaling pathway. Cancer Res. 2011;70:7489–7499. doi: 10.1158/0008-5472.CAN-10-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta P.B., Kuperwasser C., Brunet J.P., Ramaswamy S., Kuo W.L., Gray J.W., Naber S.P., Weinberg R.A. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nat Genet. 2005;37:1047–1054. doi: 10.1038/ng1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaffl M.W., Horgan G.W., Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scherer P.E., Lewis R.Y., Volonte D., Engelman J.A., Galbiati F., Couet J., Kohtz D.S., van Donselaar E., Peters P., Lisanti M.P. Cell-type and tissue-specific expression of caveolin-2: caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem. 1997;272:29337–29346. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- 28.Yanofsky V.R., Mercer S.E., Phelps R.G. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2011;2011:210813. doi: 10.1155/2011/210813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markey A.C., Lane E.B., Churchill L.J., MacDonald D.M., Leigh I.M. Expression of simple epithelial keratins 8 and 18 in epidermal neoplasia. J Invest Dermatol. 1991;97:763–770. doi: 10.1111/1523-1747.ep12486607. [DOI] [PubMed] [Google Scholar]
- 30.Larcher F., Bauluz C., Diaz-Guerra M., Quintanilla M., Conti C.J., Ballestin C., Jorcano J.L. Aberrant expression of the simple epithelial type II keratin 8 by mouse skin carcinomas but not papillomas. Mol Carcinog. 1992;6:112–121. doi: 10.1002/mc.2940060206. [DOI] [PubMed] [Google Scholar]
- 31.Caulin C., Bauluz C., Gandarillas A., Cano A., Quintanilla M. Changes in keratin expression during malignant progression of transformed mouse epidermal keratinocytes. Exp Cell Res. 1993;204:11–21. doi: 10.1006/excr.1993.1003. [DOI] [PubMed] [Google Scholar]
- 32.Oshima R.G., Baribault H., Caulin C. Oncogenic regulation and function of keratins 8 and 18. Cancer Metastasis Rev. 1996;15:445–471. doi: 10.1007/BF00054012. [DOI] [PubMed] [Google Scholar]
- 33.Yamashiro Y., Takei K., Umikawa M., Asato T., Oshiro M., Uechi Y., Ishikawa T., Taira K., Uezato H., Kariya K. Ectopic coexpression of keratin 8 and 18 promotes invasion of transformed keratinocytes and is induced in patients with cutaneous squamous cell carcinoma. Biochem Biophys Res Commun. 2011;399:365–372. doi: 10.1016/j.bbrc.2010.07.077. [DOI] [PubMed] [Google Scholar]
- 34.Carmeliet P., Jain R.K. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 35.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 36.John A., Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- 37.Kleiner D.E., Stetler-Stevenson W.G. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43(Suppl):S42–S51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- 38.Pankov R., Umezawa A., Maki R., Der C.J., Hauser C.A., Oshima R.G. Oncogene activation of human keratin 18 transcription via the Ras signal transduction pathway. Proc Natl Acad Sci U S A. 1994;91:873–877. doi: 10.1073/pnas.91.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhodes K., Oshima R.G. A regulatory element of the human keratin 18 gene with AP-1-dependent promoter activity. J Biol Chem. 1998;273:26534–26542. doi: 10.1074/jbc.273.41.26534. [DOI] [PubMed] [Google Scholar]
- 40.Oshima R.G., Abrams L., Kulesh D. Activation of an intron enhancer within the keratin 18 gene by expression of c-fos and c-jun in undifferentiated F9 embryonal carcinoma cells. Genes Dev. 1990;4:835–848. doi: 10.1101/gad.4.5.835. [DOI] [PubMed] [Google Scholar]
- 41.Pankov R., Neznanov N., Umezawa A., Oshima R.G. AP-1, ETS, and transcriptional silencers regulate retinoic acid-dependent induction of keratin 18 in embryonic cells. Mol Cell Biol. 1994;14:7744–7757. doi: 10.1128/mcb.14.12.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alam H., Kundu S.T., Dalal S.N., Vaidya M.M. Loss of keratins 8 and 18 leads to alterations in alpha6beta4-integrin-mediated signalling and decreased neoplastic progression in an oral-tumour-derived cell line. J Cell Sci. 2011;124:2096–2106. doi: 10.1242/jcs.073585. [DOI] [PubMed] [Google Scholar]
- 43.Messai Y., Noman M.Z., Derouiche A., Kourda N., Akalay I., Hasmim M., Stasik I., Ben Jilani S., Chebil M., Caignard A., Azzarone B., Gati A., Ben Ammar Elgaaied A., Chouaib S. Cytokeratin 18 expression pattern correlates with renal cell carcinoma progression: relationship with Snail. Int J Oncol. 2011;36:1145–1154. doi: 10.3892/ijo_00000597. [DOI] [PubMed] [Google Scholar]
- 44.Chu Y.W., Seftor E.A., Romer L.H., Hendrix M.J. Experimental coexpression of vimentin and keratin intermediate filaments in human melanoma cells augments motility. Am J Pathol. 1996;148:63–69. [PMC free article] [PubMed] [Google Scholar]
- 45.Bordeleau F., Galarneau L., Gilbert S., Loranger A., Marceau N. Keratin 8/18 modulation of protein kinase C-mediated integrin-dependent adhesion and migration of liver epithelial cells. Mol Biol Cell. 2011;21:1698–1713. doi: 10.1091/mbc.E09-05-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong G., Loukinova E., Chen Z., Gangi L., Chanturita T.I., Liu E.T., Van Waes C. Molecular profiling of transformed and metastatic murine squamous carcinoma cells by differential display and cDNA microarray reveals altered expression of multiple genes related to growth, apoptosis, angiogenesis, and the NF-kappaB signal pathway. Cancer Res. 2001;61:4797–4808. [PubMed] [Google Scholar]
- 47.Loercher A., Lee T.L., Ricker J.L., Howard A., Geoghegen J., Chen Z., Sunwoo J.B., Sitcheran R., Chuang E.Y., Mitchell J.B., Baldwin A.S., Jr., Van Waes C. Nuclear factor-kappaB is an important modulator of the altered gene expression profile and malignant phenotype in squamous cell carcinoma. Cancer Res. 2004;64:6511–6523. doi: 10.1158/0008-5472.CAN-04-0852. [DOI] [PubMed] [Google Scholar]
- 48.Chen Z., Smith C.W., Kiel D., Van Waes C. Metastatic variants derived following in vivo tumor progression of an in vitro transformed squamous cell carcinoma line acquire a differential growth advantage requiring tumor-host interaction. Clin Exp Metastasis. 1997;15:527–537. doi: 10.1023/a:1018474910432. [DOI] [PubMed] [Google Scholar]
- 49.Smith C.W., Chen Z., Dong G., Loukinova E., Pegram M.Y., Nicholas-Figueroa L., Van Waes C. The host environment promotes the development of primary and metastatic squamous cell carcinomas that constitutively express proinflammatory cytokines IL-1alpha, IL-6, GM-CSF, and KC. Clin Exp Metastasis. 1998;16:655–664. doi: 10.1023/a:1006559811429. [DOI] [PubMed] [Google Scholar]
- 50.Dong G., Chen Z., Kato T., Van Waes C. The host environment promotes the constitutive activation of nuclear factor-kappaB and proinflammatory cytokine expression during metastatic tumor progression of murine squamous cell carcinoma. Cancer Res. 1999;59:3495–3504. [PubMed] [Google Scholar]
- 51.Loukinova E., Chen Z., Van Waes C., Dong G. Expression of proangiogenic chemokine Gro 1 in low and high metastatic variants of Pam murine squamous cell carcinoma is differentially regulated by IL-1 alpha, EGF and TGF-beta1 through NF-kappaB dependent and independent mechanisms. Int J Cancer. 2001;94:637–644. doi: 10.1002/ijc.1514. [DOI] [PubMed] [Google Scholar]
- 52.Ridd K., Dhir S., Smith A.G., Gant T.W. Defective TPA signalling compromises HaCat cells as a human in vitro skin carcinogenesis model. Toxicol In Vitro. 2010;24:910–915. doi: 10.1016/j.tiv.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 53.Miyazaki Y., Yokozeki H., Awad S., Igawa K., Minatohara K., Satoh T., Katayama I., Nishioka K. Glucocorticoids augment the chemically induced production and gene expression of interleukin-1alpha through NF-kappaB and AP-1 activation in murine epidermal cells. J Invest Dermatol. 2000;115:746–752. doi: 10.1046/j.1523-1747.2000.00101.x. [DOI] [PubMed] [Google Scholar]
- 54.Chen Z., Rosten S.I., Lord E.M., Gaspari A.A. Murine Pam 212 cutaneous squamous cell carcinoma is nonimmunogenic in normal syngeneic hosts and resistant to immune effector mechanisms. Reg Immunol. 1993;5:285–292. [PubMed] [Google Scholar]
- 55.Cargnello M., Roux P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meloche S., Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 57.Wagner E.F., Nebreda A.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 58.Kim E.K., Choi E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2011;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Boutros T., Chevet E., Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 60.Plotnikov A., Zehorai E., Procaccia S., Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta. 2011;1813:1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 61.Turjanski A.G., Vaque J.P., Gutkind J.S. MAP kinases and the control of nuclear events. Oncogene. 2007;26:3240–3253. doi: 10.1038/sj.onc.1210415. [DOI] [PubMed] [Google Scholar]
- 62.Whitmarsh A.J. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim Biophys Acta. 2007;1773:1285–1298. doi: 10.1016/j.bbamcr.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 63.Engelman J.A., Chu C., Lin A., Jo H., Ikezu T., Okamoto T., Kohtz D.S., Lisanti M.P. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo: a role for the caveolin-scaffolding domain. FEBS Lett. 1998;428:205–211. doi: 10.1016/s0014-5793(98)00470-0. [DOI] [PubMed] [Google Scholar]
- 64.Galbiati F., Volonte D., Engelman J.A., Watanabe G., Burk R., Pestell R.G., Lisanti M.P. Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J. 1998;17:6633–6648. doi: 10.1093/emboj/17.22.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams T.M., Lee H., Cheung M.W., Cohen A.W., Razani B., Iyengar P., Scherer P.E., Pestell R.G., Lisanti M.P. Combined loss of INK4a and caveolin-1 synergistically enhances cell proliferation and oncogene-induced tumorigenesis: role of INK4a/CAV-1 in mammary epithelial cell hyperplasia. J Biol Chem. 2004;279:24745–24756. doi: 10.1074/jbc.M402064200. [DOI] [PubMed] [Google Scholar]
- 66.Engelman J.A., Wykoff C.C., Yasuhara S., Song K.S., Okamoto T., Lisanti M.P. Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J Biol Chem. 1997;272:16374–16381. doi: 10.1074/jbc.272.26.16374. [DOI] [PubMed] [Google Scholar]
- 67.Blumenberg M. Transcriptional Regulation of Keratin Gene Expression. In: J Paramio., editor. Landes Bioscience; Georgetown, TX: 2006. pp. 93–109. [Google Scholar]
- 68.Lopez-Bergami P., Huang C., Goydos J.S., Yip D., Bar-Eli M., Herlyn M., Smalley K.S., Mahale A., Eroshkin A., Aaronson S., Ronai Z. Rewired ERK-JNK signaling pathways in melanoma. Cancer Cell. 2007;11:447–460. doi: 10.1016/j.ccr.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sando G.N., Zhu H., Weis J.M., Richman J.T., Wertz P.W., Madison K.C. Caveolin expression and localization in human keratinocytes suggest a role in lamellar granule biogenesis. J Invest Dermatol. 2003;120:531–541. doi: 10.1046/j.1523-1747.2003.12051.x. [DOI] [PubMed] [Google Scholar]
- 70.Engelman J.A., Zhang X.L., Galbiati F., Lisanti M.P. Chromosomal localization, genomic organization, and developmental expression of the murine caveolin gene family (Cav-1, -2, and -3): Cav-1 and Cav-2 genes map to a known tumor suppressor locus (6-A2/7q31) FEBS Lett. 1998;429:330–336. doi: 10.1016/s0014-5793(98)00619-x. [DOI] [PubMed] [Google Scholar]
- 71.Engelman J.A., Zhang X.L., Lisanti M.P. Genes encoding human caveolin-1 and -2 are co-localized to the D7S522 locus (7q31.1), a known fragile site (FRA7G) that is frequently deleted in human cancers. FEBS Lett. 1998;436:403–410. doi: 10.1016/s0014-5793(98)01134-x. [DOI] [PubMed] [Google Scholar]
- 72.Zenklusen J.C., Thompson J.C., Klein-Szanto A.J., Conti C.J. Frequent loss of heterozygosity in human primary squamous cell and colon carcinomas at 7q31.1: evidence for a broad range tumor suppressor gene. Cancer Res. 1995;55:1347–1350. [PubMed] [Google Scholar]
- 73.Engelman J.A., Zhang X.L., Lisanti M.P. Sequence and detailed organization of the human caveolin-1 and -2 genes located near the D7S522 locus (7q31.1): methylation of a CpG island in the 5′ promoter region of the caveolin-1 gene in human breast cancer cell lines. FEBS Lett. 1999;448:221–230. doi: 10.1016/s0014-5793(99)00365-8. [DOI] [PubMed] [Google Scholar]
- 74.Sunaga N., Miyajima K., Suzuki M., Sato M., White M.A., Ramirez R.D., Shay J.W., Gazdar A.F., Minna J.D. Different roles for caveolin-1 in the development of non-small cell lung cancer versus small cell lung cancer. Cancer Res. 2004;64:4277–4285. doi: 10.1158/0008-5472.CAN-03-3941. [DOI] [PubMed] [Google Scholar]
- 75.Menendez L., Walker D., Matyunina L.V., Dickerson E.B., Bowen N.J., Polavarapu N., Benigno B.B., McDonald J.F. Identification of candidate methylation-responsive genes in ovarian cancer. Mol Cancer. 2007;6:10. doi: 10.1186/1476-4598-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coolen M.W., Stirzaker C., Song J.Z., Statham A.L., Kassir Z., Moreno C.S., Young A.N., Varma V., Speed T.P., Cowley M., Lacaze P., Kaplan W., Robinson M.D., Clark S.J. Consolidation of the cancer genome into domains of repressive chromatin by long-range epigenetic silencing (LRES) reduces transcriptional plasticity. Nat Cell Biol. 2010;12:235–246. doi: 10.1038/ncb2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hayashi K., Matsuda S., Machida K., Yamamoto T., Fukuda Y., Nimura Y., Hayakawa T., Hamaguchi M. Invasion activating caveolin-1 mutation in human scirrhous breast cancers. Cancer Res. 2001;61:2361–2364. [PubMed] [Google Scholar]
- 78.Engelman J.A., Zhang X.L., Razani B., Pestell R.G., Lisanti M.P. p42/44 MAP kinase-dependent and -independent signaling pathways regulate caveolin-1 gene expression: activation of Ras-MAP kinase and protein kinase a signaling cascades transcriptionally down-regulates caveolin-1 promoter activity. J Biol Chem. 1999;274:32333–32341. doi: 10.1074/jbc.274.45.32333. [DOI] [PubMed] [Google Scholar]
- 79.Koleske A.J., Baltimore D., Lisanti M.P. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci U S A. 1995;92:1381–1385. doi: 10.1073/pnas.92.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Razani B., Altschuler Y., Zhu L., Pestell R.G., Mostov K.E., Lisanti M.P. Caveolin-1 expression is down-regulated in cells transformed by the human papilloma virus in a p53-dependent manner: replacement of caveolin-1 expression suppresses HPV-mediated cell transformation. Biochemistry. 2000;39:13916–13924. doi: 10.1021/bi001489b. [DOI] [PubMed] [Google Scholar]
- 81.Bolshakov S., Walker C.M., Strom S.S., Selvan M.S., Clayman G.L., El-Naggar A., Lippman S.M., Kripke M.L., Ananthaswamy H.N. p53 Mutations in human aggressive and nonaggressive basal and squamous cell carcinomas. Clin Cancer Res. 2003;9:228–234. [PubMed] [Google Scholar]
- 82.Brash D.E. Roles of the transcription factor p53 in keratinocyte carcinomas. Br J Dermatol. 2006;154(Suppl 1):8–10. doi: 10.1111/j.1365-2133.2006.07230.x. [DOI] [PubMed] [Google Scholar]
- 83.Pierceall W.E., Goldberg L.H., Tainsky M.A., Mukhopadhyay T., Ananthaswamy H.N. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196–202. doi: 10.1002/mc.2940040306. [DOI] [PubMed] [Google Scholar]
- 84.Khavari T.A., Rinn J. Ras/Erk MAPK signaling in epidermal homeostasis and neoplasia. Cell Cycle. 2007;6:2928–2931. doi: 10.4161/cc.6.23.4998. [DOI] [PubMed] [Google Scholar]
- 85.Clayman G.L., Lee J.J., Holsinger F.C., Zhou X., Duvic M., El-Naggar A.K., Prieto V.G., Altamirano E., Tucker S.L., Strom S.S., Kripke M.L., Lippman S.M. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759–765. doi: 10.1200/JCO.2005.02.155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cav1 knockdown using a second shRNA construct confirms that loss of Cav1 in PAM212 increases cell proliferation, migration, and invasion and in vivo tumor growth. A: Western blot analysis shows successful knockdown of Cav1 protein expression using a second shRNA construct. Similar to the original sh-Cav1 examined (sh-Cav1 £1), Cav1 knockdown using sh-Cav1 £2 decreases expression of Cav2 but has no effect on K14 and K10 expression. B: Knockdown of Cav1 using a second shRNA decreases cell proliferation, as shown via BrdU incorporation and a growth curve. The growth curve also shows cell growth for the original sh-Cav1 construct (sh-Cav1 £1), and both sh-Cav1 £1 and £2 display similar rates of cell growth. Results for the growth curve are reported as mean ± SEM. *P < 0.05 by Dunnett’s multiple comparisons test. C: sh-Cav1 £2 PAM212 cells show a significant increase in tumor growth beginning at 4 weeks after injection. By 5 weeks after injection, tumor weight is approximately twofold higher in Cav1 knockdown tumors. D:Cav1 knockdown using sh-Cav1 £2 decreases both the migratory and invasive ability of PAM212 cells when serum is used as a chemoattractant. In addition, decreased Cav1 expression increases the expression of K8 and K18 in these cells, similar to what we observe in the original sh-Cav1 cell line. Results are reported as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 by the unpaired t-test. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Overexpression of shRNA-resistant Cav1 in Cav1 knockdown cells rescues their proliferative advantage. A:Cav1 knockdown using two different shRNA constructs (sh-Cav1 £1 and £2) results in a significant increase in cell growth, as indicated by a cell vitality MTS assay measured over 4 days. B: Re-expression of Cav1 in these knockdown cells results in the rescue of this growth advantage, as indicated by an MTS assay measured over 4 days. Re-expression of Cav1 in shCav 1 cells actually decreases cell vitality to levels significantly lower than that of shCtl cells, whereas re-expression of Cav1 in shCav1 2 cells decreases vitality to levels similar to that of shCtl cells. Results are reported as mean ± SEM. **P < 0.01 by Dunnett’s multiple comparisons test.
The expression of constitutively active RasG12V is regulated by Cav1. Western blot analysis showing that Cav1 knockdown positively affects the expression of RasG12V whereas Cav1 overexpression decreases the expression of RasG12V. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.