Abstract
Tomato yellow leaf curl virus (TYLCV) was first detected in China in 2006, following the introduction of Bemisia tabaci Q into China in 2003. Since then, the incidence of TYLCV in tomato fields in China has greatly increased as has the abundance and distribution of Q whiteflies containing the bacterial symbiont Hamiltonella with high frequency. This suggested that the symbiont Hamiltonella might associate with the transmission efficiency of TYLCV by the whitefly vector. Here we report the first evidence that the Hamiltonella is closely associated with the acquisition, retention, and transmission efficiency of TYLCV by the whitefly vector. Our findings combined with the outbreaks of TYLCV following the introduction of Q, provided an explanation for why Hamiltonella is being maintained at a relatively high level in Chinese B. tabaci Q and also have implications for disease and vector management.
As a vector of viruses, the whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is one of the most economically important pests of vegetables, cotton, and flowers. One of the viruses transmitted by B. tabaci is Tomato yellow leaf curl virus (TYLCV), which originated in the Middle East–Mediterranean region1, but has since been introduced into many other regions worldwide and is now among the most devastating viral diseases of tomato2. Infected tomato plants have small, curled leaflets and are stunted and bushy. Severe infections can result in complete crop loss. Like other Begomoviruses, TYLCV is transmitted in a circulative and persistent manner by B. tabaci3,4,5,6. Outbreaks caused by TYLCV and other whitefly-transmitted viruses have often coincided with invasions by particular biotypes of B. tabaci4,7,8.
Recent studies indicate that B. tabaci is species complex that contains at least 24 reproductively isolated but morphologically indistinguishable cryptic species9,10,11. Because discrimination at the species level within the B. tabaci complex has yet to be fully resolved, we have retained the commonly used term ‘biotype' to refer to the cryptic species. These biotypes differ in host range, life history traits, insecticide resistance, transmission competency for begomoviruses, and the symbionts that they harbor12,13,14,15,16. The two most invasive and destructive biotypes are B. tabaci biotype B (or simply B) and biotype Q (or simply Q). B belongs to the Middle East-Minor Asia 1 (MEAM1) genetic group, and Q belongs to the Mediterranean (MED) genetic group9.
Although B. tabaci was first recorded in the late 1940s in China17, crop losses caused by this insect did not become serious until the introduction of B in the 1990s18. Q was first found in Yunnan Province in 2003 and was considered a new, invasive whitefly in China19. Q has now displaced well-established populations of B in many areas and has become the dominant form of B. tabaci in field agricultural systems in most parts of China16,20,21.
Bemisia tabaci is host to bacterial symbionts. The maternally inherited, endosymbiotic bacteria of insects are prevalent and are broadly divided into two groups: primary symbionts (P-symbionts) and secondary symbionts (S-symbionts)22. P-symbionts benefit host insects by aiding in digestion of food or by providing nutrients that are limited or lacking in the diet23. S-symbionts, in contrast, may not be required for host survival but may play important roles in host biology and evolution24,25. To date, one P-symbiont and six S-symbionts have been reported from B. tabaci. The P-symbiont is Portiera22, and the S-symbionts are species of Hamiltonella26, Arsenophonus27, Cardinium28, Wolbachia26, Rickettsia29, and Fritschea30.
Recent research has speculated that the S-symbiont Hamiltonella was probably associated with the transmission efficiency of TYLCV by the whitefly vector31. Israeli Q lacks Hamiltonella and cannot effectively transmit TYLCV31, while Chinese Q frequently contains Hamiltonella14,15 and can efficiently transmit TYLCV to tomato plants32. Hence, we hypothesize that the ability of B. tabaci Q to acquire, retain, and transmit TYLCV is affected by Hamiltonella. To test this hypothesis, we: 1) established Hamiltonella-infected (H+) and uninfected (H−) B. tabaci Q strains with the same genetic background via antibiotic treatment and introgression; and 2) compared the ability of H+ and H− strains to acquire, retain, and transmit TYLCV.
Results
Screening for the presence of symbionts
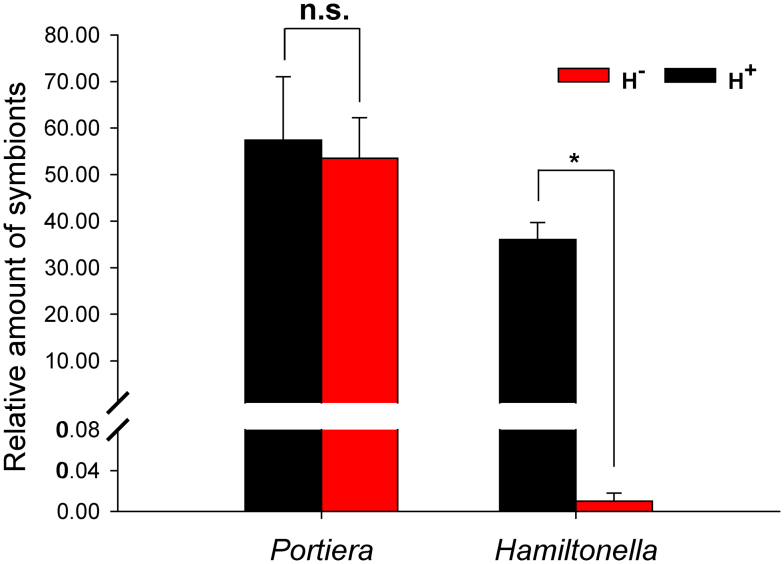
At the outset of this study, the Q population only harbored the P-symbiont Portiera and the S-symbiont Hamiltonella, the other S-symbionts Arsenophonus, Cardinium, Wolbachia, Rickettsia, and Fritschea were not detected. Continuous rearing of newly-emerged adult insects on cottons for 6 generations of introgression, the relative concentration of Portiera did not differ in the introgressed H+ vs. H− strains (F1, 4 = 0.121, P = 0.746) (Fig. 1). However, Hamiltonella almost disappeared from the H− strain, and the relative concentration of Hamiltonella was 3600-fold higher in the introgressed H+ strain than in H– strain (F1, 4 = 108.36, P < 0.0001) (Fig. 1).
Figure 1. Relative amount of Portiera and Hamiltonella (normalized to the host nuclear β-actin gene) in H+ (black bars) and H− Q (red bars) whiteflies (B. tabaci) as determined by q-PCR.
Vertical lines indicate standard deviations of the mean. The asterisk indicates that symbiont gene expression differed (P < 0.001) between H+ and H− Q, and n.s. indicates not significant (P > 0.05).
Acquisition of TYLCV DNA by H+ vs. H− Q whiteflies
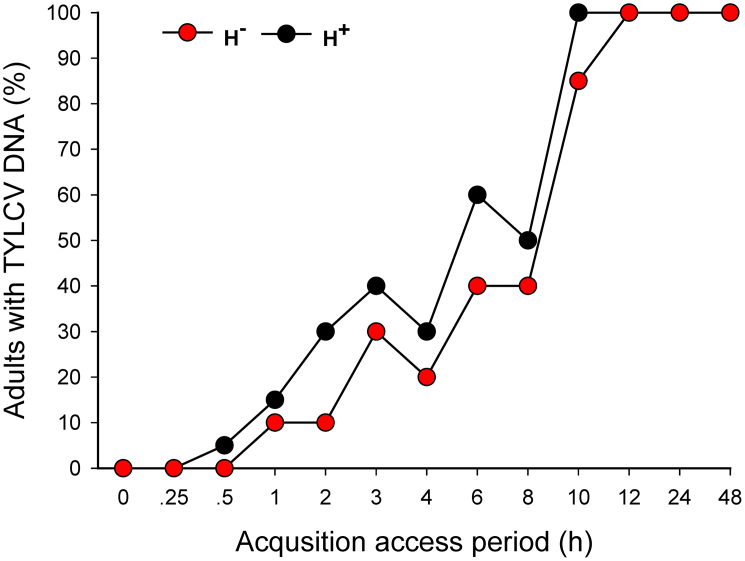
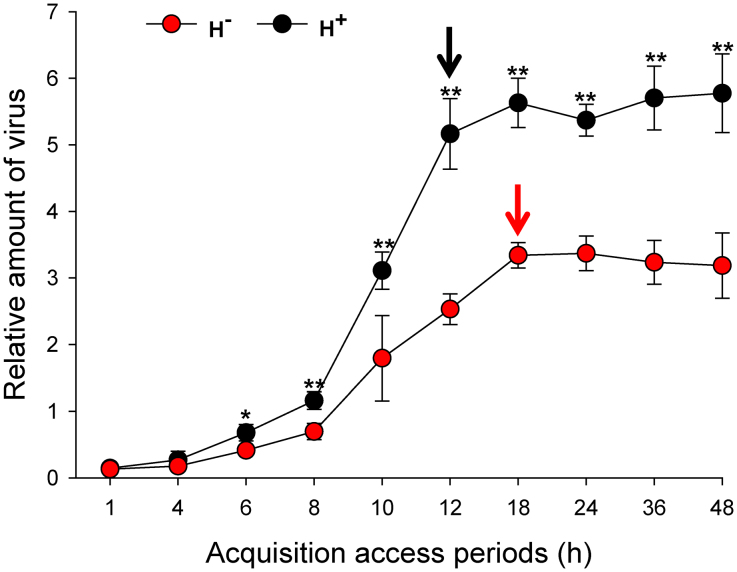
As indicated by conventional PCR, the shortest AAP for acquisition of TYLCV from infected plants was 0.5 h for H+ Q and 1.0 h for H− Q (Fig. 2). The percentage of adults with detectable TYLCV DNA increased with the length of AAP and reached 100% after a 10-h AAP for H+ Q and after a 12-h AAP for H− Q (Fig. 2). The percentage of whiteflies that acquired TYLCV was significantly affected by AAPs (Wald's χ2 = 10.525, P = 0.001), whereas not by the infection status of Hamiltonella (Wald's χ2 = 2.023, P = 0.155) and the interaction between these two factors (Wald's χ2 = 0.545, P = 0.46). Also, the relative amount of TYLCV (as indicated by q-PCR) was significantly higher in H+ Q than in H− Q after each of the eight AAPs (Fig. 3). TYLCV DNA peaked after a 12-h AAP in H+ Q and after an 18-h AAP in H− Q (Fig. 3).
Figure 2. Percentage of H+ vs. H− Q whiteflies (B. tabaci) that acquired TYLCV after 13 acquisition access periods (AAPs) as determined by conventional PCR.
For each whitefly type and AAP, 20 adults were assessed. The percentage of whiteflies that acquired TYLCV between H+ and H− Q was compared by binary logistic regression.
Figure 3. Relative amount of TYLCV (normalized to the host nuclear β-actin gene) in H+ (black dots) and H− (red dots) Q whiteflies (B. tabaci) after 10 acquisition access periods (AAPs) as determined by q-PCR.
Values are means ± standard deviation. Asterisks indicate that gene expression was significantly greater in H+ than in H− Q (* P < 0.05, ** P < 0.01). The black and red arrows indicate the time when viral titer reached a steady-state in H+ and H− Q, respectively.
Retention of TYLCV DNA by whiteflies
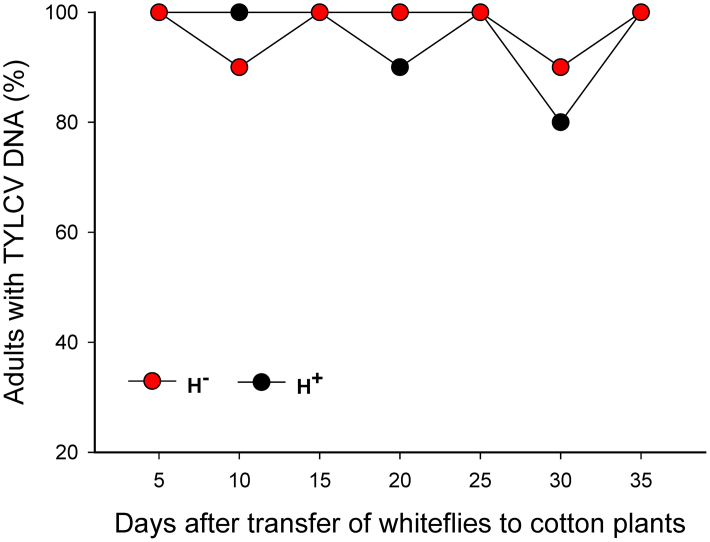
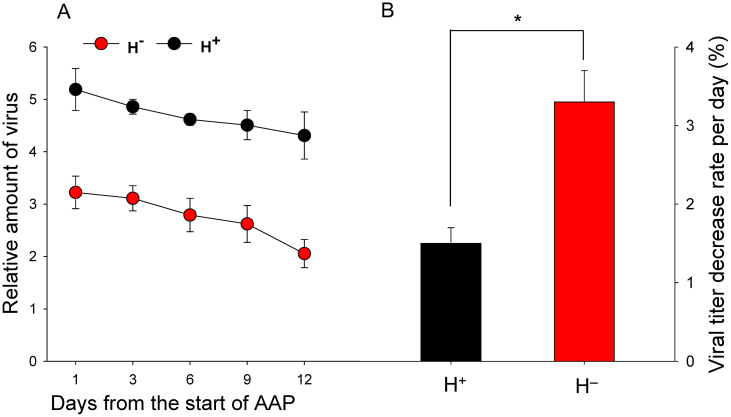
As indicated by conventional PCR, a high percentage of viruliferous adults of H+ and H− Q retained TYLCV DNA after feeding for 5 to 35 days on cotton, which is not a host for TYLCV (Fig. 4). According to q-PCR, however, the relative amounts of TYLCV per whitefly were greater in of H+ than in H− Q (Fig. 5A). The relative amount of virus decreased in both H+ and H− Q on cotton but the rate of decrease was lower for H+ Q than for H− Q (1.42% vs. 3.03% per day; F 1, 4 = 26.199, P = 0.007) (Fig. 5B).
Figure 4. Retention of TYLCV in H+ and H− Q whiteflies (B. tabaci) as indicated by conventional PCR.
After a 24-AAP on TYLCV-infected tomato, whiteflies were placed on cotton for 5 to 35 days. For each whitefly type and duration of feeding on cotton, 20 adults were assessed. The percentage of whiteflies with TYLCV did not differ between H+ and H− Q for any feeding time on cotton (P > 0.05).
Figure 5. Relative amount of TYLCV (normalized to the host nuclear β-actin gene) in H+ (black dots) and H− (red dots) Q whiteflies (B. tabaci) after a 24-h AAP on a TYLCV-infected tomato plant and 3 to 12 days of incubation on cotton.
Vertical lines on each bar represent standard deviations of the mean. Asterisks indicate significant difference with the rate of viral titer decline per day (B) between H+ and H− Q (P < 0.01).
Transmission of TYLCV by whiteflies and viral accumulation in plants
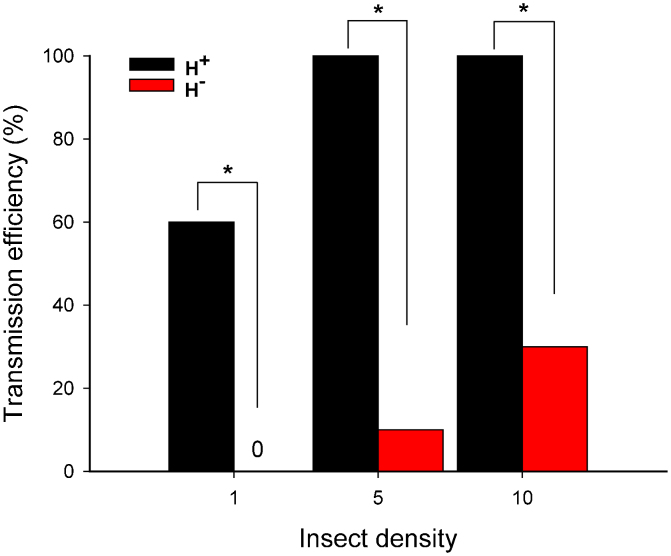
Based on the presence or absence of TYLCV symptoms on tomato plants, transmission frequency was much higher with H+ Q females than with H− Q females, and these differences were statistically significant at each of the three whitefly densities (Fig. 6). For H+ Q females, transmission frequency was high (60%) with only one female per plant and increased to 100% with 5 and 10 females per plant. For H− Q females, transmission frequency was only 0, 10, and 30% with 1, 5, and 10 females per plant, respectively (Fig. 6).
Figure 6. Transmission of TYLCV as affected by type (H+ vs. H− Q) and number of B. tabaci females.
Transmission was assessed based on the presence or absence of TYLCV symptoms 30 days after tomato plants had been exposed to whiteflies for 24 h. Each combination of whitefly type and density was represented by 20 replicate plants. Asterisks indicate significant differences between H+ and H− Q at each of three insect densities (P < 0.001).
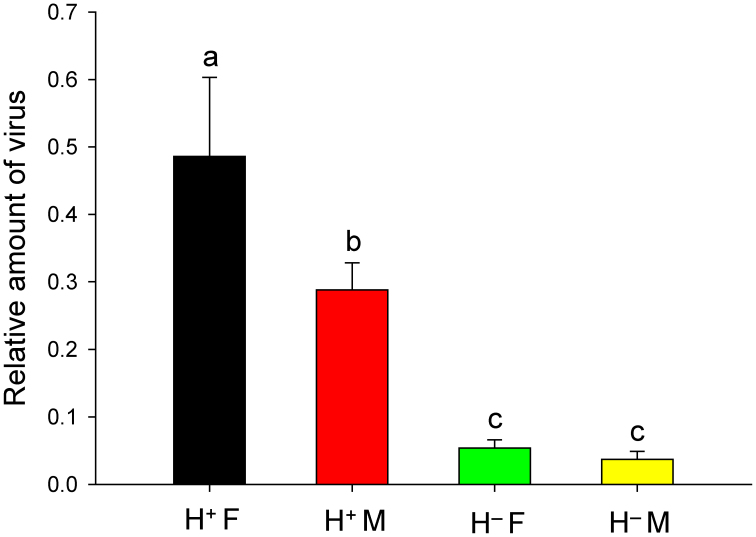
Viral accumulation in tomato plants was significantly affected by the infection status of Hamiltonella (F3, 16 = 1132.039, P < 0.0001), the sex of B. tabaci (F3, 16 = 112.333, P < 0.0001), and by the interaction between these two factors (F3, 16 = 79.434, P < 0.0001) (Fig. 7). Specifically, relative viral accumulation was 1.69-fold higher in plants exposed to one H+ Q female than to one H+ Q male (F1, 8 = 118.771, P < 0.0001). Relative viral accumulation was 9.0-fold higher in plants exposed to one H+ Q female than to one H− Q female (F1, 8 = 1003.696, P < 0.0001) and was 7.6-fold higher in plants exposed to one H+ Q male than to one H− Q male (F1, 8 = 278.252, P < 0.0001). Relative viral accumulation did not differ between plants exposed to one H− Q female and one H− Q male (F1, 8 = 3.537, P = 0.097).
Figure 7. Relative amount of TYLCV in tomato leaves as affected by type (H+ vs. H− Q) and sex of B. tabaci. H+ F: H+ females; H− F: H− females; H+ M: H+ males; H− M: H− males.
The abundance of the TYLCV gene (normalized to the abundance of the Tomato 25S ribosomal RNA gene) was assessed by q-PCR 10 days after tomato plants had been exposed to a single whitefly for 24 h. Each combination of whitefly type and sex was represented by five replicate plants. Vertical lines on each bar represent standard deviations of the mean. Different letters indicate significant differences between the four treatments (P < 0.05).
Discussion
Our studies indicate that the presence of Hamiltonella is involved in acquisition, retention, and transmission of TYLCV by B. tabaci Q and in significant differences for TYLCV accumulation in plants exposed to the whiteflies. On the other hand, the result also indicated that virus transmission efficiency seems to be more related with differences in symbiont composing than with whitefly biotypes, regardless several literature stressing the importance of the biotypes in viral transmission. This result coupled with the outbreaks of TYLCV following the introduction of Q16, provided a possible explanation for why Hamiltonella is being maintained at a relatively high level in Chinese B. tabaci Q populations15.
Although the P-symbiont Portiera was maintained in H+ and H− Q strains of the whitefly B. tabaci, the S-symbiont Hamiltonella can't detected from the H− Q strain by diagnostic PCR. After six generations of introgression, the level of Portiera did not differ between the introgressed H+ and H− Q strains, however, relative amounts of Hamiltonella were 3600-fold higher in the introgressed H+ Q than in H− Q. This indicates that the relative amount of Portiera is not affected by changes with Hamiltonella abundance in a short-term period, even though these symbionts are confined together in the bacteriocytes in the same host. The current results also confirmed that Portiera had an obligatory relationship with B. tabaci and could not be removed by antibiotic treatment. Hamiltonella, in contrast, almost disappeared from B. tabaci by antibiotic treatment, as determined by q-PCR.
The percentage of whiteflies that acquired TYLCV was significantly affected by AAPs for H+ and H− Q, and the relative amount of TYLCV acquired was greater in H+ Q than in H− Q when the access acquisition period (AAP) was ≥ 6 h. Prior study has shown that the ability of acquisition and transmission of TYLCV by whiteflies were associated with its feeding behavior33, our preliminary feeding behavior study showed that only two of twenty five parameters associated with feeding behavior were a little different between H+ Q vs. H− Q strains (BML, unpublished data). Consequently, this symbiont had little impact on the performance benefits to their feeding behavior of B. tabaci Q.
Following a 1–2 day AAP, whitefly vectors were previously found to contain TYLCV DNA for several weeks and sometimes for their entire life32,34,35,36. Following a 24-h AAP on TYLCV-infected plants in our experiments, all of viruliferous adult H+ and H− Q retained TYLCV DNA for their entire adult lifetime. Whether Begomovirus can replicate in its vector whitefly remains controversial, and only one paper has reported that TYLCV can replicate in B. tabaci37. In the current study, the rate at which viral titer decreased in B. tabaci after the insects were transferred from virus-infected tomato to virus-free cotton was 1.42% per day in H+ and 3.03% per day in H− Q. Our results are consistent with previous results obtained by membrane-hybridization of DNA from single B. tabaci B, which showed that titers of TYLCV38 and that Tomato yellow leaf curl Sardinia virus (TYLCSV)39 gradually decreased in vectors that fed on plants that were not hosts to the viruses. However, Sinisterra et al. (2005)40 reported that TYLCV titer in B. tabaci B remained constant even after 7 days of feeding on cotton plants. These discrepancies might be due to differences in the symbionts harbored by the whiteflies, environmental conditions, virus isolate, and/or the haplotype of B. tabaci used in the experiment.
A GroEL homologue produced by endosymbiotic bacteria seems to protect begomoviruses in insect haemolymph and thereby affects the ability of B. tabaci to transmit virus31,41,42. In the current study, transmission efficiency was substantially higher by H+ Q than by H− Q. This result is consistent with Gottlieb et al. (2010)31, who speculated that Hamiltonella increased the ability of B. tabaci to transmit TYLCV. Prior studies have shown that the efficiency of TYLCV transmission was affected by B. tabaci gender and age36,43. We also found that TYLCV accumulation was greater in tomato plants exposed to H+ Q females than to H+ Q males. More importantly, we found that TYLCV accumulation was much greater in tomato plants exposed to H+ Q than to H− Q regardless of sex. Lapidot et al. (2001)44 study suggested a positive correlation between TYLCV level in the plant and whitefly transmission rate. In that paper, there were two major factors affect the transmission efficiency or virus accumulation in plants: (i) host plant cultivars and (ii) starting virus level in the whitefly. With respect to our results, the factor (i) was the same, however, initial TYLCV inocula (factor (ii)) from H+ strain were more than H− strain (Fig. 3), and indirectly demonstrated H− whiteflies are less efficient vector. Therefore, these results indicate that Hamiltonella plays an important role in virus transmission by B. tabaci Q.
Taken together, our study provides sufficient evidence that the ability of B. tabaci Q to acquire, retain, and transmit TYLCV is affected by its S-symbiont Hamiltonella. Our findings also have implications for disease and vector management.
Methods
Insect source, plant cultures, and TYLCV agroinoculation
The laboratory Q population was collected from the poinsettia, Euphorbia pulcherrima Wild. ex Klotz., in Beijing, China in 200916. Since then, it was maintained in isolated whitefly-proof screen cages in a greenhouse under natural lighting and controlled temperature (26 ± 2°C). The purity of the Q population was monitored by sampling 15 adults per generation using a molecular diagnostic technique, CAPS (cleavage amplified polymorphic sequence), and a molecular marker, mitochondrial cytochrome oxidase I genes (mtCOI)20. Tomato (Solanum lycopersicum Mill. cv. zhongza 9) and cotton (Gossypium herbaceum L. cv. DP99BB) were used. Tomato is a host of TYLCV but cotton is not. Healthy tomato and cotton plants were grown in a potting mix in 1.5-L pots (one plant/pot) under natural light and with controlled temperature (26 ± 2°C) in a glasshouse. When plants grew to the 6–7 true-leaf stage, they were used in the experiments. TYLCV-infected tomato plants were obtained by agro-inoculation using a cloned TYLCV genome (GenBank accession number: AM282874) that was originally isolated from tomato plants in Shanghai, China45. Tomato plants were inoculated when they had three true leaves and were assumed to be infected with virus when they developed characteristic leaf-curl symptoms.
Detection of symbionts and TYLCV
Nucleic acids from individual whiteflies and plants were extracted using the methods of White et al. (2009)46 and Xie et al. (2002)47. The PCR procedure described by Pan et al. (2012)15 was used to detect the P-symbiont and S-symbionts in whiteflies. As indicated in the Results, the only symbionts detected in the Q population were the P-symbiont Portiera and the S-symbiont Hamiltonella. A TYLCV DNA fragment (~410 bp) was amplified using the primer pairs C473 (5′-AGTCACGGGCCCTTACA-3′) and V61 (5′-ATACTTGGACACCTAATGGC-3′)48. The resultant PCR products were electrophoresed on a 2.0% agarose gel in a 0.5×TBE buffer and visualized by Gelview staining.
Quantitative real-time polymerase chain reaction (q-PCR)
Amplifications for symbionts and TYLCV were performed with 2.5× Real Master Mix (SYBR Green) (TIANGEN Biotech (Beijing), Co., Ltd) and 5 pmol of each primer. The cycling conditions for symbionts were: 5 min activation at 94°C followed by 40 cycles of 20 s at 94°C, 30 s at 60°C, and 30 s at 72°C. The cycling conditions for TYLCV were: 5 min activation at 94°C followed by 40 cycles of 30 s at 94°C, 30 s at 60°C, and 60 s at 72°C. For each amplification run, standards were loaded in the same plate to build the appropriate standard curve. PCR reactions were carried out in 96-well optical plates in the Applied Biosystems 7500 real-time PCR instrument and the accompanying software were used for qPCR data normalization and quantification. For each whitefly sample, three replicates were amplified in each of three biologically independent experiments. For each plant sample, three replicates were amplified in each of five biologically independent experiments. The relative expression levels of the TYLCV or symbiont gene in B. tabaci (normalized to β-actin from B. tabaci) and the TYLCV gene in plants (normalized to Tomato 25S rRNA) were calculated based on the 2-ΔCt method49. The specific q-PCR primers used for quantification of Portiera, Hamiltonella, and TYLCV are listed in Table 1.
Table 1. List of primers developed and used in this study.
| Gene | Primer set | Expected size (bp) | Primer sequence (5′to 3′) | Reference |
|---|---|---|---|---|
| Portiera 16S rDNA | Port-F | 229 | TAGTCCACGCTGTAAACG | 56 |
| Port-R | AGGCACCCTTCCATCT | |||
| Hamiltonella 16S rDNA | Ham-F | 243 | GCATCGAGTGAGCACAGTT | 57 |
| Ham-R | TATCCTCTCAGACCCGCTAA | |||
| TYLCV | C473 | 410 | AGTCACGGGCCCTTACA | 48 |
| V61 | ATACTTGGACACCTAATGGC | |||
| TYLCV (q-PCR) | TY-F | 144 | GTCTACACGCTTACGCC | 16 |
| TY-R | GCAATCTTCGTCACCC | |||
| β-actin | β-actin F | 130 | TCTTCCAGCCATCCTTCTTG | 40 |
| β-actin R | CGGTGATTTCCTTCTGCATT | |||
| 25S Tomato | 25S rRNA F | 113 | ATAACCGCATCAGGTCTCCA | 39 |
| 25S rRNA R | CCGAAGTTACGGATCCATTT |
Antibiotic treatment
Antibiotic treatments were administered using Parafilm-membrane sachets for direct feeding by adults in a feeding chamber50,51. The control diet solution was 5 mM phosphate buffer (pH 7.0) with 25% sucrose, while the antibiotic diet was the same solution with the addition of 50 μg/ml rifampicin (Amersco, no. 0146). Approximately 50 newly emerged Q adults (females and males mixed) were introduced into each feeding chambers at room temperature; a 0.4-ml drop of diet was placed on the outer surface of the stretched Parafilm and covered with another layer of stretched Parafilm to enclose the solution without air bubbles between the Parafilm layers. After they had fed for 48 h, 20 of the adults were collected and subjected to PCR detection for symbionts, and the others were placed in cages containing cotton plants. Whiteflies were cured of their S-symbiont Hamiltonella after they had received rifampicin-infused sucrose (50 μg/ml) for 48 h. 30 adults from cured strain per generation were subjected to specific PCR detection to confirm elimination of the symbiont. Thereby, they were continuously reared on cottons under the conditions described above.
Introgression
In order to minimize genetic differences among individuals, we carried out an introgression series to homogenize the nuclear background of both H+ and H−strains52. We introgressed the H− Q nuclear background into the H+ Q cytotype for 6 generations to yield infected and uninfected lines that shared > 98% of their nuclear alleles24. Newly emerged virgin whitefly adults from each strain were obtained according to the method of Luan et al. (2008)53. Both introgressed H+ and H− whitefly strains have been maintained in separate net cages containing cotton plants under natural light and ambient temperature (26 ± 2°C) in a glasshouse. The introgressed H+ line and H− line were used for all experiments.
Acquisition of TYLCV by whiteflies
We randomly collected twenty newly emerged adults and transferred to a clip-cage attached to a leaf (the second to fifth leaf from the top) of TYLCV-infected tomato plants; four clip-cages, all with H+ or H− Q, were attached to each of six plants for each whitefly strain. Thereafter, two clip-cages of H+ and H− Q were randomly collected at the end of 13 acquisition access periods (AAPs: 0, 0.25, 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 24, and 48 h). The collected adults were stored at −20°C and later assayed individually to detect TYLCV DNA by conventional PCR; the results were expressed as the percentage of individuals with TYLCV DNA. For quantification of TYLCV in whiteflies, 20 adults (females and males in approximately equal numbers) of either H+ or H− Q were randomly collected at the end of each of 10 AAPs (1, 4, 6, 8, 10, 12, 18, 24, 36, and 48 h) on the TYLCV-infected tomato plants. These were stored at −20°C, and after their total DNA was extracted, they were assayed for TYLCV by q-PCR.
Retention of TYLCV DNA by whiteflies
Newly emerged non-viruliferous adult whiteflies of either H+ or H− Q were collected and caged with TYLCV-infected tomato plants to obtain viruliferous adults. After a 24-h AAP, the adults were removed from the TYLCV-infected tomato plants and placed on cotton plants; cotton is not a host for TYLCV. After 5, 10, 15, 20, 25, 30, and 35 days on cotton, 20 live adults were collected following their natural death between 30 and 40 days54. During the collection process, the adults remaining on the plant were transferred onto a new cotton plant once two weeks to avoid emergence of new adults among the progeny on the same plants. The whiteflies collected were stored at −20°C and later assayed individually for TYLCV DNA by conventional PCR, and the results were expressed as the percentage of individuals with TYLCV DNA. For quantification of TYLCV in whiteflies that first fed on TYLCV-infected tomato (24-h AAP) and then on cotton, 20 adults (females and males in approximately equal numbers) of either H+ or H− Q were randomly collected after 3, 6, 9, and 12 days on cotton. The collected insects were stored at −20°C and were later assayed for TYLCV by q-PCR as described earlier.
Transmission of TYLCV to tomato plants and viral accumulation in plants
Newly emerged females of either H+ or H− Q were collected and transferred to a TYLCV-infected tomato plant where they were caged for a 24-h AAP. The whiteflies were then removed from the infected plant and confined in a clip-cage (1, 5, or 10 females per cage) on a virus-free tomato seedling (with three true leaves) for a 24-h period of inoculation feeding. The females were removed, and the plants were kept for symptom development in insect-proof cages. Twenty plants were used for each of the six treatments (two whitefly types × three whitefly densities). After 30 days, plants were assessed for virus infection based on the presence or absence of TYLCV symptoms.
For quantification of viral accumulation in plants, a single viruliferous female or male of H+ Q or H− Q (all adults were of similar age) was placed in a clip-cage on the second leaf from the top of a same healthy tomato plant (one clip-cage per plant) with three true leaves. Since the results of virus acquisition have demonstrated that viral DNA was present in 100% of the whiteflies for both H+ and H− Q strains after a 12-h AAP on virus-infected tomato plants (Fig. 2), we only allowed the whiteflies to feed on tomato plants for 12 h to acquire the viruses, ensure that each individual should acquire the virus. After that, the adults were removed, and the plants reared in insect-proof cages. In order to compare virus load of different plants, it is advisable to use the first two youngest leaves of an infected plant39, and TYLCV reached its peak viral load at 10 or 20 days post-inoculation55. So, we collected from the first two youngest leaf of each plant at 10 days post-inoculation and pooled them to monitor viral accumulation using q-PCR developed in this study. Five plants were used for each of the four treatments (two whitefly types × two whitefly sexes).
Data analysis
The percentage of whiteflies that acquired TYLCV between H+ and H− Q was compared by binary logistic regression, with infection status and AAPs as model variables. Chi-square tests were used to compare H+ and H− Q for: the percentage of whiteflies that retained TYLCV after each of seven periods of feeding on cotton, and the percentage of tomato plants with TYLCV symptoms after exposure to viruliferous whiteflies at each of three densities. The relative quantities of symbiont and virus in H+ and H− Q after each of 10 AAPs were compared by one-way analysis of variance (ANOVA). TYLCV accumulation in plants was compared by two-factor ANOVAs, with infection status and sex of B. tabaci as fixed factors. Means for significant ANOVAs were compared by the honestly significant difference (HSD) test at P < 0.05. All statistical analyses were performed with SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Author Contributions
Y.J.Z., Q.S. and H.P.P. conceived and designed the experiments. Q.S., H.P.P., B.M.L., W.X., S.L.W., Q.J.W. and B.Y.X. performed the experiments. Q.S., H.P.P. and Y.J.Z. analyzed the data. Q.S., H.P.P., D.C. and Y.J.Z. wrote the paper.
Acknowledgments
This research was supported by the National Science Fund for Distinguished Young Scholars (31025020), the 973 Program (2013CB127602), the National Natural Science Foundation of China (31171857), and Beijing Key Laboratory for Pest Control and Sustainable Cultivation of Vegetables. The granting agencies have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Cohen S. & Harpaz I. Periodic, rather than continual acquisition of a new tomato virus by its vector, the tobacco whitefly (Bemisia tabaci Gennadius). Entomol. Exp. Appl. 7, 155–166 (1964). [Google Scholar]
- Lefeuvre P. et al. The spread of tomato yellow leaf curl virus from the Middle East to the world. PLoS Pathog. 6, e1001164 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. K. Molecular markers for the identification and global tracking of whitefly vector-begomovirus complexes. Virus Res. 71, 233–260 (2000). [DOI] [PubMed] [Google Scholar]
- Brown J. K. in Bionomics and Management of a Global Pest, (eds Stansly, P. A. & Naranjo, S. E.) 31–67 (Springer Ltd., 2010). [Google Scholar]
- Brown J. K. & Czosnek H. Whitefly transmission of plant viruses. Adv. Bot. Res. 36, 65–76 (2002). [Google Scholar]
- Gill R. & Brown J. K. in Bionomics and Management of a Global Pest, (eds Stansly, P. A. & Naranjo, S. E.) 5–29 (Springer Ltd., 2010). [Google Scholar]
- Varma A. & Malathi V. G. Emerging geminivirus problems: a serious threat to crop production. Ann. Appl. Biol. 142, 145–164 (2003). [Google Scholar]
- Seal S. E., VandenBosch F. & Jeger M. J. Factors influencing begomovirus evolution and their increasing global significance: implications for sustainable control. Crit. Rev. Plant Sci. 25, 23–46 (2006). [Google Scholar]
- Dinsdale A., Cook L., Riginos C., Buckley Y. M. & De Barro P. J. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase I to identify species level genetic boundaries. Ann. Entomol. Soc. Am. 103, 196–208 (2010). [Google Scholar]
- Xu J., De Barro P. J. & Liu S. S. Reproductive incompatibility among genetic groups of Bemisia tabaci supports the proposition that the whitefly is a cryptic species complex. Bull. Entomol. Res. 100, 359–366 (2010). [DOI] [PubMed] [Google Scholar]
- De Barro P. J., Liu S. S., Boykin L. M. & Dinsdale A. B. Bemisia tabaci: a statement of species status. Annu. Rev. Entomol. 56, 1–19 (2011). [DOI] [PubMed] [Google Scholar]
- Brown J. K., Frohlich D. R. & Rosell R. C. The sweetpotato or silverleaf whiteflies: biotypes of Bemisia tabaci or a species complex? Annu. Rev. Entomol. 40, 511–534 (1995). [Google Scholar]
- Jones D. R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 109, 195–219 (2003). [Google Scholar]
- Chu D. et al. Further insights into the strange role of bacterial endosymbionts in whitefly, Bemisia tabaci: Comparison of secondary symbionts from biotypes B and Q in China. Bull. Entomol. Res. 101, 477–486 (2011). [DOI] [PubMed] [Google Scholar]
- Pan H. P. et al. Factors affecting population dynamics of maternally transmitted endosymbionts in Bemisia tabaci. PLoS One 7, e30760 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H. P. et al. Rapid spread of Tomato yellow leaf curl virus in China is aided differentially by two invasive whiteflies. PLoS One 7, e34817 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. The list of whiteflies in China. China Entomol. 3, 1–18 (1949). [Google Scholar]
- Luo C. et al. The use of mitochondrial cytochrome oxidase mtCOI gene sequences for the identification of biotypes of Bemisia tabaci (Gennadius) in China. Acta. Entomol. Sin. 45, 759–763 (2002). [Google Scholar]
- Chu D. et al. The introduction of the exotic Q biotype of Bemisia tabaci from the Mediterranean region into China on ornamental crops. Fla. Entomol. 89, 168–174 (2006). [Google Scholar]
- Chu D., Wan F. H., Zhang Y. J. & Brown J. K. Change in the biotype composition of Bemisia tabaci in Shandong Province of China from 2005 to 2008. Environ. Entomol. 39, 1028–1036 (2010). [DOI] [PubMed] [Google Scholar]
- Pan H. P. et al. Further spread of and domination by Bemisia tabaci (Hemiptera: Aleyrodidae) biotype Q on field crops in China. J. Econ. Entomol. 104, 978–985 (2011). [DOI] [PubMed] [Google Scholar]
- Baumann P. Biology bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Entomol. 59, 155–189 (2005). [DOI] [PubMed] [Google Scholar]
- Feldhaar H. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol. Entomol. 36, 533–543 (2011). [Google Scholar]
- Himler A. G. et al. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 332, 254–256 (2011). [DOI] [PubMed] [Google Scholar]
- Charlat S. et al. The joint evolutionary histories of Wolbachia and mitochondria in Hypolimnas bolina. BMC Evol. Biol. 9, 64 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zchori-Fein E. & Brown J. K. Diversity of prokaryotes associated with Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Ann. Entomol. Soc. Am. 95, 711–718 (2002). [Google Scholar]
- Thao M. L. & Baumann P. Evidence for multiple acquisition of Arsenophonus by whitefly species (Sternorrhyncha: Aleyrodidae). Curr. Microbiol. 48, 140–144 (2004). [DOI] [PubMed] [Google Scholar]
- Weeks A. R., Velten R. & Stouthamer R. Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. P. Roy. Soc. B. Biol. Sci. 270, 1857–1865 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb Y. et al. Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Appl. Environ. Microbiol. 72, 3646–3652 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett K. D., Thao M., Horn M., Dyszynski G. E. & Baumann P. Novel chlamydiae in whiteflies and scale insects: endosymbionts′Candidatus Fritschea bemisiae′ strain Falk and ′Candidatus Fritschea eriococci′ strain Elm. Int. J. Syst. Evol. Micr. 55, 1581–1587 (2005). [DOI] [PubMed] [Google Scholar]
- Gottlieb Y. et al. The transmission efficiency of tomato yellow leaf curl virus by the whitefly Bemisia tabaci is correlated with the presence of a specific symbiotic bacterium species. J. Virol. 84, 9310–9317 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Hu J., Xu F. C. & Liu S. S. Transmission of Tomato Yellow Leaf Curl Virus by two invasive biotypes and a Chinese indigenous biotype of the whitefly Bemisia tabaci. Int. J. Pest Mange. 56, 275–280 (2010). [Google Scholar]
- Jiang Y. X., Blas C. D., Barrios L. & Fereres A. Correlation between whitefly (Homoptera: Aleyrodidae) feeding behavior and transmission of tomato yellow leaf curl virus. Ann. Entomol. Soc. Am. 93, 573–579 (2000). [Google Scholar]
- Rubinstein G. & Czosnek H. Long-term association of Tomato yellow leaf curl virus with its whitefly vector Bemisia tabaci: effect on the insect transmission capacity, longevity and fecundity. J. Gen. Virol. 78, 2683–2689 (1997). [DOI] [PubMed] [Google Scholar]
- Rosell R. C., Torres-Jerez I. & Brown J. K. Tracing the geminivirus-whitefly transmission pathway by polymerase chain reaction in whitefly extracts saliva, hemolymph, and honeydew. Phytopathology 89, 239–246 (1999). [DOI] [PubMed] [Google Scholar]
- Czosnek H., Ghanim M. & Ghanim M. The circulative pathway of begomoviruses in the whitefly vector Bemisia tabaci-insights from studies with Tomato yellow leaf curl virus. Ann. Appl. Biol. 140, 215–231 (2002). [Google Scholar]
- Mehta P., Wyman J. A., Nakhla M. K. & Maxwell D. P. Transmission of tomato yellow leaf curl geminivirus by Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 87, 1291–1297 (1994). [DOI] [PubMed] [Google Scholar]
- Caciagli P. & Bosco D. Quantitation over time of tomato yellow leaf curl geminivirus DNA in its whitefly vector. Phytopathology 87, 610–613 (1997). [DOI] [PubMed] [Google Scholar]
- Mason G., Caciagli P., Accotto G. P. & Noris E. Real-time PCR for the quantitation of Tomato yellow leaf curl Sardinia virus in tomato plants and in Bemisia tabaci. J. Virol. Methods 147, 282–289 (2008). [DOI] [PubMed] [Google Scholar]
- Sinisterra X. H., McKenzie C. L., Hunter W. B., Powell C. A. & Shatters R. G. Jr. Differential transcriptional activity of plant pathogenic begomoviruses in their whitefly vector (Bemisia tabaci, Gennadius: Hemiptera Aleyrodidae). J. Gen. Virol. 86, 1525–1532 (2005). [DOI] [PubMed] [Google Scholar]
- Morin S. et al. A GroEL homologue from endosymbiotic bacteria of the whitefly Bemisia tabaci is implicated in the circulative transmission of tomato yellow leaf curl virus. Virology 256, 75–84 (1999). [DOI] [PubMed] [Google Scholar]
- Morin S., Ghanim M., Sobol I. & Czosnek H. The GroEL protein of the whitefly Bemisia tabaci interacts with the coat protein of transmissible and nontransmissible begomoviruses in the yeast two-hybrid system. Virology 276, 404–416 (2000). [DOI] [PubMed] [Google Scholar]
- Muniyappa V. et al. Tomato leaf curl virus from Bangalore (ToLCV-Ban4): sequence comparison with Indian ToLCV isolates, detection in plants and insects, and vector relationships.Arch. Virol. 145, 1583–1598 (2000). [DOI] [PubMed] [Google Scholar]
- Lapidot M., Friedmann M., Pilowsky M., Ben-Joseph R. & Cohen S. Effect of host plant resistance to Tomato yellow leaf curl virus (TYLCV) on virus acquisition and transmission by its whitefly vector. Virology 91, 1209–1213 (2001). [DOI] [PubMed] [Google Scholar]
- Wu J. B., Dai F. M. & Zhou X. P. First report of Tomato yellow leaf curl virus in China. Ann. Appl. Biol. 155, 439–448 (2006). [DOI] [PubMed] [Google Scholar]
- White J. A., Kelly S. E., Perlman S. J. & Hunter M. S. Cytoplasmic incompatibility in the parasitic wasp Encarsia inaron: disentangling the roles of Cardinium and Wolbachia symbionts. Heredity 102, 483–489 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Zhou X. P., Zhang Z. K. & Qi Y. J. Tobacco curly shoot virus isolated in Yunnan is a distinct species of Begomovirus. Chinese Sci. Bull. 47, 197–200 (2002). [Google Scholar]
- Ghanim M., Sobol I., Ghanim M. & Czosnek H. Horizontal transmission of begomoviruses between Bemisia tabaci biotypes. Arthropod–Plant Inte. 1, 195–204 (2007). [Google Scholar]
- Livak K. G. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- Ruan Y. M., Xu J. & Liu S. S. Effects of antibiotics on fitness of the B biotype and a non-B biotype of the whitefly Bemisia tabaci. Entomol. Exp. Appl. 121, 159–166 (2006). [Google Scholar]
- Ahmed M. Z. et al. Prevalence of endosymbionts in Bemisia tabaci populations and their in vivo sensitivity to antibiotics. Curr. Microbiol. 61, 322–328 (2010). [DOI] [PubMed] [Google Scholar]
- Brelsfoard C. L., Sechan Y. & Dobson S. L. Interspecific hybridization yields strategy for South Pacific filariasis vector elimination. PLoS Neglect. Trop. D. 2, e129 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan J. B., Ruan Y. M., Zhang L. & Liu S. S. Pre-copulation intervals, copulation frequencies, and initial progeny sex ratios in two biotypes of whitefly, Bemisia tabaci. Entomol. Exp. Appl. 129, 316–324 (2008). [Google Scholar]
- Jiao X. G. et al. Differences in host selection and performance between B and Q putative species of Bemisia tabaci on three host plants. Entomol. Exp. Appl. 10.1111/eea.12040 (2013). [Google Scholar]
- Péréfarres F. et al. A novel synthetic quantification standard including virus and internal report targets: application for the detection and quantification of emerging begomoviruses on tomato. Virol. J. 8, 389 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H. P. et al. Relative amount of symbionts in insect hosts changes with host-plant adaptation and insecticide resistance. Envoron. Entomol. 42, 74–78 (2013). [DOI] [PubMed] [Google Scholar]
- Brumin M., Kontsedalov S. & Ghanim M. Rickettsia influences thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Sci. 18, 57–66 (2010). [Google Scholar]