Abstract
Background/Methods
The objective of this study was to determine whether relinquishing cognitive, physical, and social activities is associated with an increased risk of cognitive decline in patients with age-related macular degeneration (AMD). We conducted a 3-year longitudinal study of 206 nondemented patients with AMD.
Results
Twenty-three subjects (14.4%) declined cognitively. Age, sex, education, decline in visual acuity, and number of dropped activities were associated with cognitive decline; each additional dropped activity increased the risk by 58%. Subjects who relinquished three activities were 3.87 times (95% confidence interval, 1.95–7.76) more likely to become demented than subjects who relinquished no activities; those who relinquished five activities were 9.54 times (95% confidence interval, 3.05–30.43) more likely. A multivariate model demonstrated that number of dropped activities was a powerful predictor of cognitive decline after controlling for relevant risk factors, particularly for subjects younger than 80 years of age.
Conclusions
Relinquishing valued activities is associated with an increased risk of cognitive decline in older patients with vision loss caused by AMD. These data suggest the importance of promoting optimal cognitive and physical health in patients with AMD and perhaps other chronic diseases.
Keywords: Activity loss, Cognitive decline, Vision loss
1. Introduction
Recent studies suggest that complex mental activity, physical exercise, and social engagement might prevent cognitive decline in older persons [1–9]. It is uncertain, however, whether medical conditions that decrease participation in these activities are associated with cognitive decline. We investigated that possibility in older persons with age-related macular degeneration (AMD). AMD is a highly disabling degenerative disease of the macular region of the retina that leads to geographic atrophy (one type of dry AMD) or choroidal neovascularization (wet AMD) [10]. It is the leading cause of legal blindness in older persons in the United States, affects more than 10 million people, and prevents many from reading, driving, socializing, and pursuing hobbies [11–14].
Some researchers have noted intriguing relationships between AMD and Alzheimer’s disease (AD) [15]. In both conditions, misfolded amyloid beta peptides accumulate (in the retina and in the brain, respectively) and might play a central role in their onset [16]. If this is the case, cognitive and vision impairment in AMD might reflect a shared pathogenesis. Alternatively, vision loss might lead to cognitive decline via deafferentation of the visual system from the sensory cortex [17]. Vision loss might also result in behavioral changes (eg, relinquishing valued activities like reading and socializing) that indirectly and adversely affect neural function and can lead to or unmask incipient cognitive decline [6].
In this study, we examined whether relinquishing cognitive, physical, and/or social activities is associated with an increased risk of cognitive decline by using data from the Preventing Depression in AMD Trial [18]. This was a 12-month randomized controlled clinical trial comparing the efficacy of problem-solving treatment (PST) versus usual care to prevent depressive disorders in older patients with AMD. At the baseline visit of the clinical trial, we assessed the extent to which the 206 enrolled subjects had relinquished specific activities that other research suggests prevent cognitive decline. Then 3 years later, we interviewed the 160 available knowledgeable informants of subjects who were originally enrolled in the clinical trial to ascertain whether subjects had declined cognitively. We tested the hypothesis that relinquishing more valued activities was associated with an increased risk for cognitive decline.
2. Background
We recruited 206 subjects from December 2001 to July 2005 from the retinovitreous clinics associated with Wills Eye Institute in Philadelphia, PA, enrolling those with newly diagnosed neovascular AMD (NV-AMD) in one eye (within the preceding 6 months) and preexisting AMD in the fellow eye. The other inclusion criteria were age older than 64 years and visual acuity in the better eye of 20/70 or worse. We chose these parameters to identify older patients with recent bilateral visual impairment who were at high risk for vision-related disability. The exclusion criteria were the presence of Diagnostic and Statistical Manual of Mental Disorders, fourth revision (DSM-IV) diagnoses of depressive or other axis I disorders, current treatment for depression, cognitive impairment, and confounding eye conditions. All subjects signed an informed consent form approved by Thomas Jefferson University’s Institutional Review Board.
We reviewed fluorescein angiogram reports, confirmed clinical diagnoses with treating ophthalmologists, and sent introductory letters to 602 potentially eligible patients. Of these, 230 (38%) were eligible (on the basis of telephone screening) and were enrolled in the clinical trial. The major reasons for nonparticipation were refusal (242, 40%), inability to contact (66, 11%), cognitive impairment (18, 3%), depression on screening (8, 1%), and other (38, 6%). Subjects who did not participate did not differ from participating subjects on demographic characteristics, visual acuity, or a screening measure of depression (data not shown).
We screened for cognitive impairment at baseline for the clinical trial by using an abbreviated version of the Mini-Mental Status Examination (MMSE) that omits vision-dependent items [19]. This version included orientation to time (ie, day, month, and year), spelling “world” backwards, and delayed recall of three words. Possible scores ranged from 0 to 11, with higher scores indicating better cognitive function. To be eligible, subjects needed to answer the three orientation questions correctly, score at least 3 of 5 on “world” backwards, and recall two of three words on delayed recall. We repeated this version of the MMSE 1 year later at the end of the clinical trial.
At baseline, we asked subjects to rate their participation in (including whether they had relinquished any) complex cognitive activities, physical exercise, and social engagement by using the corresponding items of the National Eye Institute Vision Function Questionnaire (NEI VFQ-17) [20]. We targeted the following six activities: reading, exercise/sports, hobbies, spectator activities, social activities, and cards/games. Each was scored categorically as to whether the subject continued to engage in (scored 0) or had relinquished the activity (scored 1). Scores were summed (range, 0 to 6), with higher scores indicating a greater number of relinquished activities.
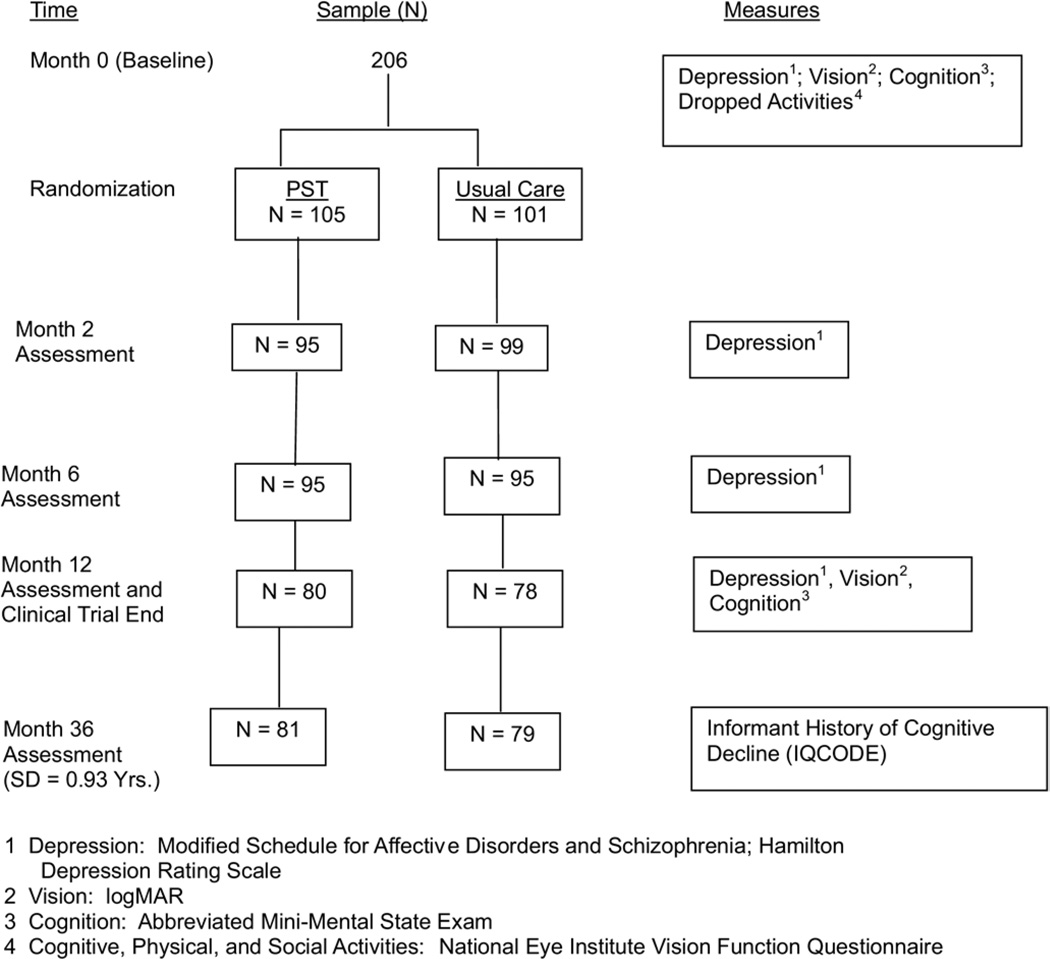
After the baseline assessment, 206 of the 230 (90%) subjects were randomized to the PST and control groups in a 1:1 allocation ratio (Fig. 1). Twenty-four subjects were not randomized because of consent withdrawal (N = 19), depression (N = 4), or cognitive impairment (N = 1) on further screening. From January 2002 to August 2005, PST-trained therapists administered six weekly 30- to 45-minute PST sessions during a period of 8 weeks to PST-assigned subjects. PST is a manual-driven psychological treatment that teaches problem-solving skills to define problems, establish realistic goals, generate and implement solutions, and evaluate outcomes [21]. Subjects in the control condition received usual care from their ophthalmologists and other health care providers.
Fig. 1.
Flow chart of sample and study measures.
3. Assessments during the clinical trial
Research nurses with extensive training in ophthalmology and psychiatry obtained informed consent and conducted clinical examinations in subjects’ homes. Demographic characteristics included age, race, sex, years of education, and marital status. We assessed best-corrected distance visual acuity in each eye separately by using the Lighthouse Ferris-Bailey ETDRS Chart at baseline and month 12 [22]. Scores were converted to the logarithm of the minimum angle of resolution (logMAR), with higher scores indicating greater impairment [23]. Medical comorbidity was assessed by using the Chronic Disease Score, which is derived from a weighted sum of medications taken for chronic illness, at baseline and month 12 [24]. The score predicts health care utilization, costs, hospitalization rates, and mortality, with higher scores indicating greater medical disease burden [25]. We created a vascular conditions variable at baseline (ie, hypertension, diabetes, and stroke) to examine as a possible risk factor for cognitive decline. Incident DSM-IV defined-depressive disorders were identified at months 2, 6, and 12 by using the Modified Schedule for Affective Disorders and Schizophrenia and the Structured Interview Guide for the Hamilton Rating Scale [26,27].
4. Follow-up informant interviews after completion of the clinical trial
From May to September 2006, we administered the Informant Questionnaire for Cognitive Decline in the Elderly (IQCODE) on one occasion by telephone to each of the 160 knowledgeable informants who were available for follow-up [28]. The 160 subjects (76.6% of the 206 subjects enrolled in the clinical trial) on whom informants provided IQCODE scores did not differ in their demographic or clinical characteristics (including number of dropped activities, abbreviated MMSE scores at baseline, and incidence rates of depressive disorder during the trial) from the 46 subjects for whom IQCODE scores were unavailable (data not shown). The average length of time (and standard deviation) from subjects’ baseline evaluation to the IQCODE administration was 3.00 (0.93) years. The standard deviation reflects the relatively longer time to enroll the sample (ie, 3.5 years) in relation to the 4-month time period during which informants were interviewed.
The IQCODE measures cognitive decline from a premorbid level by using informant reports (eg, decline in recalling recent conversations, knowing the day and month) [28]. A 16-item short form of the original 26-item version correlates 0.98 with the full-length version and has equally strong discriminatory power and internal consistency (alpha, 0.93) [29]. Each of the 16 items is scored on a 5-point scale from 1, better than before to 5, much worse (3, no change). Ratings are averaged to give an overall score from 1 to 5. Factor analyses show that the IQCODE measures a single general factor of cognitive decline [28]. A number of studies have demonstrated its reliability and validity against measures of cognitive change over time, clinical diagnoses, and neuropathology and neuroimaging results [28]. Jorm et al [28] cited a variety of cut scores in different populations that maximize sensitivity and specificity: in community populations; cut scores range from 3.3 to 3.6 and in patient samples from 3.4 to 4.0. None of the samples studied, however, closely resembles the visually impaired older adults that we studied. For this reason, we took a conservative, empirical approach to define an appropriate cut score that maximized specificity (to avoid false positives). We defined subjects as cognitively impaired if their IQCODE scores exceeded one standard deviation above the mean for this population. A cut score of 3.81 met this criterion.
5. Statistical methods
Because of the variability in follow-up time, the measure of interest in describing cognitive decline is the incidence density ratio (IDR), which is the number of new cases divided by the total person-years of follow-up. Poisson regression was used to model the incidence of cognitive decline while adjusting for different amounts of follow-up time. Generalized estimating equation (GEE) methods were used to adjust standard errors and P values [30]. Clinically relevant variables were considered independent predictors of cognitive decline if they had a P value of .10 or less at the bivariate level. A variable was considered a confounder of the association between dropped activities and cognitive decline if coefficients of terms in the model change by more than 15%. A variable was called an effect modifier if the P value for the interaction term was less than .05.
6. Results
The mean age and education ± standard deviation of the sample were 81.1 ± 5.8 and 12.5 ± 3.1 years, respectively; 70% were women; 98.1% were white; and 41.5% lived alone. The distribution of IQCODE scores was skewed to the right and had the following characteristics: range, 2.63 to 5.00; median, 3.13; and mean, 3.30 ± 0.51.
Twenty-three subjects (14.4% of the sample) met the criteria for cognitive decline, representing an incidence density of 48.6 cases per 1,000 person-years at risk. Table 1 presents descriptive statistics for the sample and the bivariate relationships between the study measures and dementia status at follow-up. The relationships are reported as incidence density ratios, which are based on Poisson regressions with number of years from the 1-year assessment to follow-up as an offset variable. Table 1 shows that age, sex, education, change in visual acuity, and number of dropped activities were associated with dementia at follow-up; each 5-year increment in age increased dementia risk by 57%; each 4-year increment of education increased the risk by 36%; and each additional dropped activity increased the risk by 57%. Thus, subjects who relinquished three activities, for example, were 3.87 times (95% confidence interval [CI], 1.95 to 7.76) more likely to become demented than subjects who relinquished no activities. The risk for subjects who relinquished five activities increased to 9.54 times (95% CI, 3.05 to 30.43).
Table 1.
Bivariate IDRs of study measures with dementia status at follow-up (n = 160)
| Descriptive statistics | IDR | 95% CI | P value | |
|---|---|---|---|---|
| Baseline variables | ||||
| Age, mean (SD)* | 81.1 (5.8) | 1.57 | 1.12–2.19 | .008 |
| Education, mean (SD)† | 12.51 (3.13) | 1.36 | 1.04–1.79 | .027 |
| Female, n (%) | 112 (70) | 1.93 | 0.92–4.07 | .083 |
| Abbreviated MMSE, mean (SD) | 10.3 (.96) | 0.96 | 0.74–1.23 | .745 |
| Number of dropped activities, mean (SD) | 1.8 (1.4) | 1.57 | 1.25–1.98 | <.001 |
| Visual acuity in better eye, mean (SD) | 0.58 (.36) | 0.79 | 0.20–3.11 | .74 |
| Chronic Disease Score, mean (SD) (n = 159) | 5.16 (3.07) | 0.96 | 0.85–1.11 | .64 |
| Vascular conditions (ie, stroke, hypertension, diabetes) (n, %) | 113 (71) | 0.77 | 0.34–1.71 | .52 |
| Assigned to PST (n, %) | 81 (51) | 0.72 | 0.33–1.56 | .40 |
| Longitudinal variables | ||||
| MMSE, ≥1 point decline from baseline to 12 months (n, %) (n = 154) | 20 (13%) | 0.53 | 0.22–1.28 | .157 |
| Visual acuity, change from baseline to 12 months, mean (SD) (n = 153) | −0.13 (0.32) | 0.48 | 0.20–1.15 | .098 |
| Depression diagnosis at months 2, 6, or 12 (n, %) | 55 (34) | 1.40 | 0.65–3.01 | .38 |
| Chronic Disease Score, change from baseline to 12 months, mean (SD) (n = 154) | −0.02 (2.04) | 1.04 | 0.85–1.27 | .722 |
Based on 5-year increments.
Based on 4-year increments.
Baseline abbreviated MMSE score, visual acuity in the better eye, medical comorbidity (ie, Chronic Disease Score), vascular risk factors, and treatment assignment (PST vs usual care) were unrelated to dementia risk. During the 1-year clinical trial, decline by more than one point on the abbreviated MMSE, incident depressive disorder, and changes in medical comorbidity were also unrelated to dementia risk. There was no significant correlation between number of dropped activities and baseline abbreviated MMSE (r = 0.05, P = .525) or change in MMSE scores from baseline to 12 months (r = 0.10, P = .213).
Table 2 shows the results of a multivariate model that included age, number of dropped activities, and their interaction. Although education, sex, and change in visual acuity were significant at the bivariate level, when added to the model they did not meet the criteria for confounding and were thus not included in the final model. The interaction term of age × dropped activities was significant (P = .013), indicating effect modification by age. For those older than age 80, each additional dropped activity increased the risk of cognitive decline by 27% (IDR, 1.27; 95% CI, 0.99 to 1.63; P = .065). For those younger than 80, the effect was stronger; each additional activity dropped increased the risk of cognitive decline by nearly 200% (IDR, 2.96; 95% CI, 1.59 to 5.52; P ≤ .001).
Table 2.
Poisson generalized estimating equations model predicting cognitive decline
| Variable | IDR* (95% CI) | P value |
|---|---|---|
| Number of dropped activities: subjects <80 years old | 2.96 (1.59–5.52) | <.001 |
| Number of dropped activities: subjects ≥80 years old | 1.27 (0.99–1.63) | .065 |
Increased risk associated with each additional dropped activity.
7. Discussion
We found that relinquishing valued activities was associated with an increased risk of cognitive decline in patients with recent bilateral vision loss caused by AMD. The effect was particularly strong in subjects younger than 80 years of age, consistent with other studies that have reported a more aggressive trajectory of decline in younger persons [31,32].
These findings are best understood, however, in the context of the study’s limitations. First, the subjects we studied were not representative of most patients with AMD or the general population of older persons; thus, the results might have limited generalizability. Second, we conducted no formal clinical or neuropsychological assessments at baseline to accurately characterize the cognitive function of the sample. At follow-up, we had no clinical validation of dementia diagnoses or precise knowledge of the temporal relationship between cognitive decline and loss of activities. The abbreviated MMSE that we used was likely insensitive to mild cognitive impairment and might have misclassified subjects at baseline or was insensitive to change over time. Other researchers, however, have used similar MMSE versions and have found equivalent power to distinguish demented from nondemented individuals. Regarding the IQCODE, although the validity of the cut score that we used is uncertain, it was more stringent than is commonly used. However, we excluded patients from the study who had a history or objective evidence of cognitive impairment and found no correlation between activity loss and cognitive measures at baseline or with cognitive change during a period of 1 year. The latter suggests that a dementing process was not evident at the time of enrollment. Moreover, the incidence rate of cognitive decline that we observed is comparable to that reported in similar age cohorts (ranging from 32.6 to 59.9 cases per 1,000 patient-years), which supports the validity of our findings [33]. Nevertheless, we cannot exclude the possibility that activity loss might well have represented an early sign of dementia in some subjects.
Alternatively, activity loss might have triggered or hastened the onset of cognitive decline, although the association that we found between activity loss and cognitive decline does not demonstrate this possibility. Epidemiologic and clinical studies have established a link between vision loss and cognitive decline, however. Crews and Campbell [34] examined the 1994 Second Supplement on Aging involving 9,447 older persons and found that those with self-reported vision loss were 2.2 times more likely to be cognitively impaired than those without vision loss. Reyes- Ortiz et al [35], by using the population-based Hispanic Established Populations for Epidemiologic Studies of the Elderly, found that objective measures of vision impairment were associated with cognitive decline. In patients with AMD, the Age-Related Eye Disease Study Research Group (2006) found that increased macular abnormalities and reduced vision were associated with lower cognitive function, and that persons with vision acuity worse than 20/40 in both eyes were more likely to be cognitively impaired than persons with better visual acuity [36].
The mechanisms linking vision loss, as a result of AMD in particular, with cognitive decline are uncertain, although existing research suggests a number of possibilities. AMD and AD, for example, share histopathologic features that suggest a common pathogenesis. Amyloid beta deposition and inflammatory proteins characterize both ocular drusen and the neuritic plaques of AD [15]. Some studies showed that amyloid beta increases vascular endothelial growth factor and might lead to retinal pigment epithelium atrophy [16]. In AD, amyloid beta–induced neurotoxicity appears to be central to neurodegeneration [37]. Moreover, previous studies have observed degeneration of the optic nerve and retina in AD [38]. AMD and AD also share vascular risk factors (eg, atherosclerosis) but have different genetic risks (ie, the APOE ε4 allele increases the risk for AD but decreases the risk for AMD) [39,40].
In AD, we know that cognitive activities such as reading or playing board games or musical instruments have the potential to reduce the risk of dementia, as do physical and social activities [1–4,7]. The mechanism by which these activities maintain cognition is uncertain but might involve enhancing the brain’s capacity to tolerate neuropathology (ie, increased neural reserve) via beneficial effects on neuroplasticity, neurogenesis, or cortisol regulation [41,42]. Although other mechanisms are possible, our finding that activity loss is associated with cognitive decline in AMD suggests the importance of maintaining activities in the face of vision loss. This finding might also apply to patients with other chronic diseases that lead to the loss of or restrict participation in valued activities. If so, promoting optimal cognitive and physical health might have wide relevance to the care of the growing population of older adults with chronic disabilities.
Acknowledgements
This work was supported by NIMH grant RO 1 MH61331 and the Farber Institute for Neurosciences of Thomas Jefferson University.
References
- 1.Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 2.Wilson RS, Mendes de Leon CF, Barnes LL, Schneider JA, Bienias JL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer’s disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 3.Wang JYJ, Zhou HD, Li J, Zhang M, Deng J, et al. Leisure activity and the risk of cognitive impairment: the Chong Qing Aging Study. Neurology. 2006;66:911–913. doi: 10.1212/01.wnl.0000192165.99963.2a. [DOI] [PubMed] [Google Scholar]
- 4.Sturman MT, Morris MC, Mendes de Leon CF, Bienias J, Wilson RS, et al. Physical activity, cognitive activity, and cognitive decline in a biracial community population. Arch Neurol. 2005;62:1750–1754. doi: 10.1001/archneur.62.11.1750. [DOI] [PubMed] [Google Scholar]
- 5.Rockwood K, Middleton L. Physical activity and the maintenance of cognitive function. Alzheimer’s and Dementia. 2007;3(Suppl 1):S38–S44. doi: 10.1016/j.jalz.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Hendrie HC, Albert MS, Butters MA, Gao S, Knopman DS, et al. The NIH Cognitive and Emotional Health Project: report of the critical evaluations study committee. Alzheimer’s and Dementia. 2006;2:12–32. doi: 10.1016/j.jalz.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in older people: a longitudinal cohort study. Lancet Neurol. 2006;5:406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 8.Bassuk S, Glass TA, Berkman LF. Social disengagement and incident cognitive decline in community-dwelling elderly persons. Ann Intern Med. 1999;131:165–173. doi: 10.7326/0003-4819-131-3-199908030-00002. [DOI] [PubMed] [Google Scholar]
- 9.Seeman TE, Lusignolo TM, Albert M, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur Studies of Successful Aging. Health Psychol. 2001;20:243–255. doi: 10.1037//0278-6133.20.4.243. [DOI] [PubMed] [Google Scholar]
- 10.The Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 11.Williams RA, Brody BL, Thomas RG, Kaplan RM, Brown SI. The psychosocial impact of macular degeneration. Arch Ophthalmol. 1998;116:514–520. doi: 10.1001/archopht.116.4.514. [DOI] [PubMed] [Google Scholar]
- 12.Stelmack J. Quality of life of low-vision patients and outcomes of low-vision rehabilitation. Optom Vis Sci. 2001;78:335–342. doi: 10.1097/00006324-200105000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Mangione CM, Gutierrez PR, Lowe G, Orav EJ, Seddon JM. Influence of age-related maculopathy on visual functioning in health-related quality of life. Am J Ophthalmol. 1999;128:45–53. doi: 10.1016/s0002-9394(99)00169-5. [DOI] [PubMed] [Google Scholar]
- 14.Chia EM, Mitchell P, Rochthina E, Foran S, Golding M, Jie JW. Association between vision and hearing impairments and their combined effects on quality of life. Arch Ophthalmol. 2006;124:1465–1470. doi: 10.1001/archopht.124.10.1465. [DOI] [PubMed] [Google Scholar]
- 15.Klaver CCW, Ott A, Hofman A, Assink JM, Bereteler MMB, et al. Is age-related maculopathy associated with Alzheimer’s disease?: the Rotterdam Study. Am J Epidemiol. 1999;150:963–968. doi: 10.1093/oxfordjournals.aje.a010105. [DOI] [PubMed] [Google Scholar]
- 16.Wyoshida T, Ohno-Matsui K, Ichinose S, Sato T, Iwata N, et al. The potential role of amyloid beta in the pathogenesis of age-related macular degeneration. J Clin Invest. 2005;115:2793–2800. doi: 10.1172/JCI24635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- 18.Rovner B, Casten R, Hegel M, Leiby B, Tasman W. Preventing depression in age-related macular degeneration. Arch Gen Psychiatry. 2007;64:886–892. doi: 10.1001/archpsyc.64.8.886. [DOI] [PubMed] [Google Scholar]
- 19.Folstein M, Folstein S, McHugh P. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Massof RW, Fletcher DC. Evaluation of the NEI visual functioning questionnaire as an interval measure of visual ability in low vision. Vision Res. 2001;41:397–413. doi: 10.1016/s0042-6989(00)00249-2. [DOI] [PubMed] [Google Scholar]
- 21.D’Zurilla TJ. Problem-solving therapy: a social competence approach to clinical intervention. New York: Springer Pub; 2000. pp. 95–150. [Google Scholar]
- 22.Rubin GS, Bandeen Roche K, Prasada-Rao P, Fried LP. Visual impairment and disability in older adults. Optom Vis Sci. 1994;12:750–760. doi: 10.1097/00006324-199412000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Holladay JT, Prager TC. Mean visual acuity. Am J Ophthalmol. 1991;111:372–374. doi: 10.1016/s0002-9394(14)72328-1. [DOI] [PubMed] [Google Scholar]
- 24.Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45:197–203. doi: 10.1016/0895-4356(92)90016-g. [DOI] [PubMed] [Google Scholar]
- 25.Clark DO, Von Korff M, Saunders K, Baluch WM, Simon GE. A chronic disease score with empirically derived weights. Med Care. 1995;33:783–795. doi: 10.1097/00005650-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Parmelee PA, Katz IR, Lawton MP. The relation of pain to depression among institutionalized aged. J Gerontol. 1991;46:P15–P21. doi: 10.1093/geronj/46.1.p15. [DOI] [PubMed] [Google Scholar]
- 27.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 28.Jorm AF. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): a review. Int Psychogeriatr. 2004;16:275–293. doi: 10.1017/s1041610204000390. [DOI] [PubMed] [Google Scholar]
- 29.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 30.Carter RE, Lipsitz SR, Tilley BC. Quasi-likelihood estimation for relative risk regression models. Biostatistics. 2005;6:39–44. doi: 10.1093/biostatistics/kxh016. [DOI] [PubMed] [Google Scholar]
- 31.Mungas D, Reed BR, Ellis WG, Jagust W. The effects of aging on rate of progression of Alzheimer’s disease and dementia with associated cerebrovascular disease. Arch Neurol. 2001;58:1243–1247. doi: 10.1001/archneur.58.8.1243. [DOI] [PubMed] [Google Scholar]
- 32.Bäckman L, Jones S, Small BJ, Agüero-Torres H, Fratiglioni L. Rate of cognitive decline in preclinical Alzheimer’s disease: the role of comorbidity. J Gerontol Psychol Social Sci. 2003;58:228–236. doi: 10.1093/geronb/58.4.p228. [DOI] [PubMed] [Google Scholar]
- 33.Breitner JCS. Dementia-epidemiological considerations, nomenclature, and a tacit consensus definition. J Geriatr Psychiatry Neurol. 2006;19:129–136. doi: 10.1177/0891988706291081. [DOI] [PubMed] [Google Scholar]
- 34.Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling older Americans: implications for health and functioning. Am J Public Health. 2004;94:823–829. doi: 10.2105/ajph.94.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reyes-Ortiz CA, Kuo WS, DiNuzzo AR, Ray LA, Raji MA, et al. Near vision impairment predicts cognitive decline: data from the Hispanic Established Populations for Epidemiologic Studies of the Elderly. J Am Geriatr Soc. 2005;53:681–686. doi: 10.1111/j.1532-5415.2005.53219.x. [DOI] [PubMed] [Google Scholar]
- 36.Age-Related Eye Disease Study Research Group. Cognitive impairment in the age-related eye disease study. AREDS report no. 16. Arch Ophthalmol. 2006;124:537–543. doi: 10.1001/archopht.124.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gandy S. The role of cerebral amyloid beta accumulation in common forms of Alzheimer’s disease. J Clin Invest. 2005;115:1–9. doi: 10.1172/JCI25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med. 1986;315:485–487. doi: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt S, Klaver CCW, Saunders AM, Postel EA, DeLaPaz MA, et al. A pooled case-controlled study of the apolipoprotein E (APOE) gene in age-related maculopathy. Ophthalmic Genet. 2002;23:209–223. doi: 10.1076/opge.23.4.209.13883. [DOI] [PubMed] [Google Scholar]
- 40.Bird TD. Genetic factors in Alzheimer’s disease. N Engl J Med. 2005;352:862–864. doi: 10.1056/NEJMp058027. [DOI] [PubMed] [Google Scholar]
- 41.Burke D, Hickie I, Breakspear M, Goatz J. Possibilities for the prevention and treatment of cognitive impairment and dementia. Br J Psychiatry. 2007;190:371–372. doi: 10.1192/bjp.bp.106.033407. [DOI] [PubMed] [Google Scholar]
- 42.Lee BK, Glass TA, McAtee MJ, Wand GS, Bandeen-Roche K, et al. Associations of salivary cortisol with cognitive function in the Baltimore Memory Study. Arch Gen Psychiatry. 2007;64:810–818. doi: 10.1001/archpsyc.64.7.810. [DOI] [PubMed] [Google Scholar]