Abstract
A major task of the central nervous system (CNS) is to control behavioral actions, which necessitates a precise regulation of muscle activity. The final components of the circuitry controlling muscles are the motorneurons, which settle into pools in the ventral horn of the spinal cord in positions that mirror the musculature organization within the body. This ‘musculotopic’ motor-map then becomes the internal CNS reference for the neuronal circuits that control motor commands. This review describes recent progress in defining the neuroanatomical organization of the higher-order motor circuits in the cortex and spinal cord, and our current understanding of the integrative features that contribute to complex motor behaviors. We highlight emerging evidence that cortical and spinal motor command centers are loosely organized with respect to the musculotopic spatial-map, but these centers also incorporate organizational features that associate with the function of different muscle groups during commonly enacted behaviors.
Introduction
An animal’s behavioral repertoire is evolved to suit its particular survival needs and originates from the nervous system circuitries that control movements. One major strategy for an animal to organize the neuronal networks for diverse behaviors is to possess ensembles of neurons that are distributed to form maps of external space or body space. These ‘topographic’ arrangements are a common theme in the vertebrate nervous system (Box 1). Within the motor networks that are the focus of this review, topography is a major organizing principle of many critical components, including the spinal motorneurons, the motor cortex, the spinal sensory system, and spinal inter-neurons.
Box 1. Definitions and concepts.
Topography
The description of the locations of neurons within the central nervous system. It is often specifically used to describe an arrangement of neurons that has an orderly relationship to the space of an external input or output. Examples include the retinotopic maps of the visual cortex, the tonotopic map of the auditory cortex, the multiple somatosensory maps of the sensory cortex, and the musculotopic organization of spinal motorneurons.
Frame of reference
The organization of a central nervous system network relative to a given external or body-based scheme. For instance, visual processing incorporates a retinotopic frame of reference with an internal reference of eye position to form a ‘head-centered’ frame of reference [1]. In the spinal cord, both the proprioceptive system and motorneurons are organized with respect to muscles, but the nociceptive sensory fibers have a skin based frame of reference.
Dimensionality
The number of parameters encoded in a neural circuit. For instance, the parameters, or dimensions, of a visual image are form, color, and motion, and these can be encoded and processed separately. In cortical coding of a motor command, information may include multiple dimensions such as which muscles to contract, and the timing, force, and velocity of muscle contractions, and the final desired position of the limb.
There is a pleasing simplicity to the orderliness of topographic maps; however, we are just beginning to understand how the spatial organization of neuronal networks relates to their function. Many questions remain. For instance, how can multiple maps of the body with different frames of reference interact to produce coherent motor plans? How are non-spatial parameters of movement encoded together with spatial information in motor commands? How pervasive is topographic mapping as a scheme for organizing motor circuitry? How can topographic information be transmitted through intervening elements such as interneurons, which are not traditionally understood to have significant spatial or topographic organization? What is the purpose of topographic organization? This review discusses recent work related to these organizational principles and their integration within the spinal cord, as they pertain to the control of movement.
Motorneurons: the musculotopic map
As all other motor-related circuitries with their particular organizational schemes must signal to the motorneurons that control muscles, it is helpful to examine the arrangement of motorneurons first. Romanes and colleagues have used retrograde neuronal fills from the muscles or nerves in the periphery and identified discrete clusters of motor-neurons, called pools, devoted to each muscle [3–7]. These studies also identified the organizational principles of inter-pool relationships. For instance, motor pools for hip flexor muscles are located more ventrally and rostrally, while muscles of the ankle and foot are generally located more dorsally and caudally (Figure 1A). Thus, an orderly spatial arrangement exists between motor pools within the spinal cord and the muscles they innervate in the periphery – referred to as a musculotopic map.
Figure 1. Spatial and functional relationships in the spinal cord and cortex.
(A) Spinal motorneurons display a linear organizational scheme in which they form a direct connection with their respective muscle target. This type of organization represents a low dimensional topographic scheme in which motor pools are organized with respect to a musculotopic frame of reference. Each color corresponds to a muscle-specific motor pool and its corresponding target muscle.
(B) Within small regions of the motor cortex, many parameters are compressed into a two dimensional physical space. Examples include movement characteristics (green), muscle selection (pink), and types of evoked behavior (blue) – a high-dimensional organization. Thus, microstimulation of specific regions within the cortex is capable of evoking complex behaviors.
(C) Sensory afferents transform body sensations into neural cues. Proprioceptive fibers selectively form connections with motorneurons that innervate shared muscle targets. However, proprioceptive fibers also form some connections with motorneurons controlling synergistic muscles, representing divergent input onto multiple targets. Primary afferents of sensory neurons can also converge onto shared targets. For instance, individual proprioceptive neurons share common motorneuron targets. Additionally, afferents from multiple modalities can share cellular targets (multimodality convergence) to integrate information from the periphery. Thus, sensory fibers that project into the spinal cord display an intermediate dimensional organization. Cell bodies for sensory neurons are intermingled within the dorsal root ganglion, though central branches of sensory fibers have a well-established topographic organization: Nociceptive fibers important for pain sensation innervate superficial layers I and II of the spinal cord dorsal horn. Mechanoreceptive fibers that mediate touch project to dorsal lamina III–IV. Proprioceptive fibers important for detecting body position project to and branch within the deep dorsal horn. Some fibers terminate in the deep dorsal horn, while some continue ventrally toward motor pools.
(D) Examples of spinal interneurons with topographic distributions: UPPER PANEL – LEFT: CONTRALATERALLY projecting descending commissural (DC) interneurons that correlate with flexor (green) and extensor (blue) motorneuron activation are segregated within the ventral spinal cord. UPPER PANEL – RIGHT: REFLEX encoder interneurons located in the deep dorsal horn that transmit information from the nociceptive withdrawal reflex are organized with a musculotopic frame of reference, such that there is a medial to lateral arrangement of distal extensor (blue), distal flexor (green), and proximal flexor (green). LOWER PANEL, LEFT SIDE: RABIES injections into flexor and extensor muscles labels pre-motor interneurons in the deep dorsal horn. Pre-flexor (blue) and pre-extensor (red) ipsilaterally projecting interneurons overlap but display a lateral and medial bias, respectively. LOWER PANEL, RIGHT SIDE: DISTAL (red) and proximal (blue) pre-motor interneurons are positioned in overlapping regions within the ipsilateral spinal cord. Pre-motor interneurons contacting motor pools that control proximal muscles have a more significant ventral distribution when compared to those controlling distal muscles.
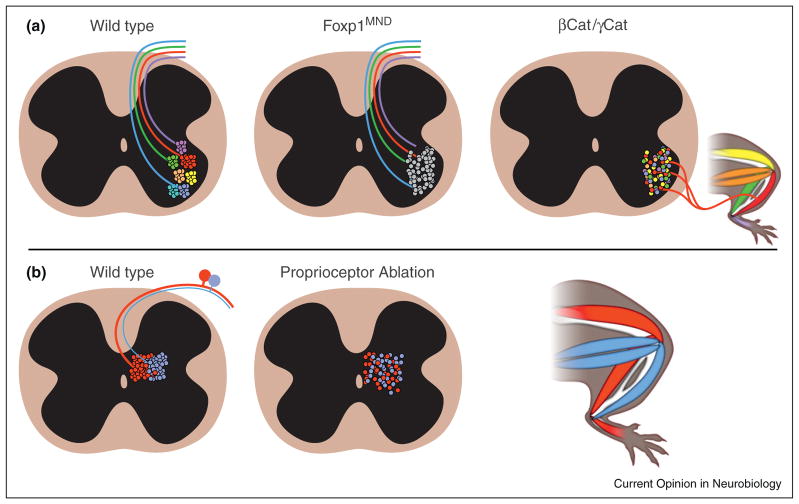
A combinatorial transcription code that defines motor-neuron pools for each muscle has been described, and it is thought that this code directs proper motor pool and muscle connectivity [8]. Interestingly, Demireva et al. have demonstrated that the positional identity of motor-neurons can be uncoupled from their transcriptional identity [9••]. By removing β-catenin and γ-catenin from motorneurons, they eliminated the stereotypical, pooled organization of motorneurons. Instead, motorneurons for a given muscle were found scattered through the motor-neuron region of the spinal cord, but still found their appropriate muscular targets in the periphery. This reveals that motorneuron cell body position is dependent on β-catenin and γ-catenin function, thereby establishing a critical role for these genes in creating the musculotopic motor-map (Figure 2A). In addition, it suggests that soma position is not a critical determinant for axon targeting of muscles.
Figure 2. Using genetics to probe the function and source of topography.
(A) The role of motor pool segregation in establishing connectivity. Left: Trajectory of proprioceptive afferents. Proprioceptive afferents form homonymous connections with spinal motorneurons to mediate the monosynaptic stretch reflex. Middle: Sensory-motor matching is impaired in mice with motor-neuron specific deletion of the Hox transcriptional cofactor, Foxp1. In, Foxp1MND animals, proprioceptive fibers project to their ‘wild type’ termination zone, but lose the ability to distinguish ‘self’ and ‘non-self’ motorneurons. Motor pool segregation is disrupted in βCat/γCat mutant animals, though axons project to their appropriate target muscle. Thus, while motorneuron topography is not required for motor axon trajectory, it is necessary for the proper wiring of sensory-motor connections.
(B) The highest density of extensor pre-motor interneurons (red) is medial to that of flexor pre-motor interneurons (blue). Using a genetically encoded toxin, proprioceptive sensory neurons were ablated in mice. In the absence of proprioceptive input, the deep dorsal pre-motor interneuron populations fail to segregate properly, indicating that sensory input is capable of shaping the spatial organization of a developing circuit.
Overall, the relationship between motorneurons and the muscles of the body is a ‘low dimensional’ musculotopic scheme (Box 1), defined by a single dominant parameter, namely the control of a single target muscle by a single motor pool (Figure 1A). This musculotopic system provides a simple substrate for relaying neural commands for movement, but redirects the question to understanding how activity among motor pools is coordinated in order to achieve complex behaviors.
Motor cortex: space and function
The first observations of topographic maps within the CNS were made using electrical stimulation of the cortex [2,10]. These classic experiments revealed that the motor cortex of mammals is organized as a ‘homonculus’ – a map of the body represented in the brain such that contiguous parts of the body are controlled by neighboring cortical regions. In primates, the primary motor area (M1) is roughly arranged with a lateral to medial progression of head, then hand, then arm, then leg, then foot (Figure 3B) [2]. In the rodent, there are two major motor subdivisions – a rostral forelimb area, and a caudal region that contains both forelimb and hindlimb areas (Figure 3A) [11,12]. Despite the appealing simplicity of this organization, from the outset Sherrington, Woolsey and colleagues recognized that this somatotopic map exists only on a gross scale, whereas in reality the fine representations of body regions are overlapping, non-continuous, and flexible [13–16]. Further refinements on the idea of a topographic map of the motor cortex are based on observations that, within a cortical domain relating to one body region, many parameters are represented including the target muscles, but also force, angle, velocity, the coordination with other commonly associated muscles or parameters, and types of movement (Figure 1B) [17–20].
Figure 3. Motor cortex anatomy.
(A) Rodent motor cortex is roughly segregated into rostral and caudal subdivisions. Rostral motor cortex and the lateral portion of caudal motor cortex contain cells that project to forelimb levels of the spinal cord (light blue). Caudal motor cortex also contains cells that project to hindlimb levels of the spinal cord (dark blue).
(B) Spatial organization of primate motor cortical cells that control movement of muscle groups are located in a medial to lateral progression of foot, leg, arm, hand and head (blue). Corticomotoneuronal (CM) cells are organized in a medial to lateral progression of proximal (red) to distal (yellow) muscle targets in a region corresponding to ‘new M1,’ a subdivision of the ‘old M1’ region (blue).
In contrast to the low-dimensional topographic arrangement of motorneurons, in which the spatial grouping of neurons correlates very well with functional output, the fine scale organization of motor cortex neurons does not have a simple relationship with either body space or behaviors. Instead, the motor cortex is a ‘high-dimensional’ system (Box 1) embedded within a coarse topographic spatial organization. Graziano and Aflalo have suggested that the spatial layout of the motor cortex reflects the reduction of these many different dimensions or parameters onto the two-dimensional physical space of the cortical sheet (Figure 1B) [21].
Recent evidence suggests that one of the main organizing principles of the motor cortex is the spatial separation of neuronal clusters that are active during complex behaviors rather than a strict mapping relationship with the motor pools and muscles. Nevertheless, behaviorally relevant movements naturally involve muscles within the same part of the body leading to a significant local clustering of neurons that control neighboring body regions. For instance, topographic representations of the forelimb can be represented spatially in multiple, potentially overlapping, regions that each relate to a type of movement, including: (1) stereotyped repetitive behaviors such as grooming or running, (2) complex voluntary movements such as bringing the hand to the mouth in feeding, or (3) fine motor manipulation skills such as independent finger movements (Box 2). We summarize evidence from new studies that demonstrate cortical spatial organization pertaining to these three different categories of movement.
Box 2. Movement Categories.
The movements of animals can be categorized along a spectrum from highly automatic to highly volitional, after Hughlings Jackson [2].
Automatic
This category includes movements related to reflexes, autonomic control, breathing, and simple postural control. These behaviors can be highly stereotyped and repetitive, and do not require voluntary control, although they can be modulated voluntarily. The neuronal circuitries for these behaviors are generally hardwired, evolutionarily ancient, and typically reside in the brainstem and spinal cord.
Stereotypical
Somewhat automatic behaviors often involve the entire body, or coordination between body regions, and they are often repetitive. They include walking and grooming. These behaviors are initiated and can be modulated by voluntary cortical and brainstem control, but the core circuitry elements that mediate these behaviors often function autonomously within the spinal cord. A classic example of these features is the central pattern generator that controls locomotion.
Voluntary
This category includes isolated, voluntary movements that involve multiple muscles, joints, and even body regions. These are generally common and behaviorally relevant, or ethological movements. For example, reaching out the forelimb, grasping an object, and retracting the forelimb as used for eating. The programs for these actions can be represented in spatially localized cortical and subcortical regions that are capable of activating the entire behavioral-routine when stimulated.
Fine
These motor acts are isolated and voluntary, like those of the category above, but they involve a high degree of precision and individuated movements. This category is a relatively recent evolutionary addition to the behavioral repertoire of animals. The most refined example is the relatively independent finger movements that are a hallmark of human hand use. This reflects primate-specific neural circuitries that mediate direct cortical control over motor outputs and these command centers appear to originate from an evolutionarily ‘new’ region of the motor cortex.
Direct observation of neuronal activity in awake-behaving mice provides a novel and powerful approach to identify where active neurons are located as different movements are performed. To study ‘stereotypical’ movements (Box 2), Tank and colleagues analyzed activity with a fluorescent calcium indicator in neurons of the caudal forelimb area of the motor cortex during running and grooming, that both rely on many of the same muscles [22•]. They observed networks of layer 2/3 cortical neurons that became active during these movements, but noted that the location of the sites of correlated-firing was different for each stereotypical behavior. Therefore, layer 2/3 cortical neuron position appears to be linked to particular behaviors that rely on complex patterns of muscle activation, rather than an organizational scheme that creates a one-to-one map with the motor pools.
A similar functional organization seems to characterize the motor cortex networks that direct isolated ‘voluntary’ movements (Box 2). Moore and colleagues first showed that long duration electrical microstimulation of primate motor cortex resulted in behaviorally relevant, also known as ethological, movements [23•]. Interestingly, specific motor cortical regions reliably produced particular behaviors. For example, stimulation of an area that represents the arm and face can result in the animal bringing its hand toward its mouth in a grip posture and opening its mouth. Stimulation of a more dorsal area produced hand movements toward the trunk or hindlimb. This work was extended to the rodent by Tuszynski and colleagues, who demonstrated distinct regions of rostral and caudal forelimb areas in the rat motor cortex that directed reach, grasp, and retraction movements [24]. Recent work using light-based activation of motor cortex microdomains identified regions within the caudal motor cortex that direct abduction or adduction of the forelimb, and confirmed this type of functional organization within the motor cortex [25].
Despite the evidence for a cortical map that segregates into neuronal clusters that elicit complex behaviors, it still remains unclear to what extent the spatial organization of movement types is based on the topographic arrangements of cortical neurons. One possibility is that local clusters of topographically distributed neurons for a given body area are grouped into bigger networks that represent compound movements of that body area. A potential substrate for this type of network has been described by Capaday and colleagues, who suggest that interconnectivity within the cortex promotes spreading waves of activation [26–28]. They found that focal activation of sites within the motor cortex produced expanding waves that cover broad regions of neurons that were experimentally determined to control multiple muscles and joints. However, during a complex behavior, many neurons in the motor cortex are recruited, and distinct behaviors can involve intermingled and distributed networks of neurons. The resulting movements cannot be predicted simply from a map of muscles activated by experimental stimulation of the relevant active cortical regions. This highlights the complexity of coding multiple movement parameters overlaying a topographic spatial system, and of communicating these diverse parameters to the motor output cells of the spinal cord.
Our understanding of the relationship between function and topography has been facilitated by the study of a primate-specific population of motor cortex cells that directly target motorneurons. In most mammals, motor-neurons are controlled through indirect pathways from the cortex. However, in primates, corticomotoneuronal (CM) cells are uniquely suited to control highly precise, individuated movements [29,30]. These cells subserve ‘fine’ motor skills (Box 2), the most voluntary movements that represent another class of behavior that has a spatially distinct control region, at least in primates.
Using retrograde trans-synaptic circuit tracing with wild-type rabies virus, Rathelot and Strick were able to map the location of CM cells within the primate motor cortex that contact motor pools for the shoulder, elbow, and finger muscles [31••]. They observed that CM cells are mostly segregated within a caudal region of the M1 motor cortex and possessed a medial–lateral progression of shoulder to elbow to finger CM cell locations. However, a significant degree of overlap was also noted between these CM populations. Their findings suggest there is an ‘old M1’ and a ‘new M1’ that contain parallel topographic maps that exist within the primate motor cortex (Figure 3B).
It is important to note that cortical organization must be framed with reference to subcortical target organization. For instance, despite the high-dimensionality of the motor cortex (Box 1), this information must eventually be reduced to the specific patterns of spatiotemporal activation of muscle contractions. It is not known how this process occurs, but recent evidence suggests that spinal cord interneurons actively translate transient cortical commands into ongoing motorneuron activation programs during the movement. Specifically, electrical recordings from motor cortex during a movement reveal that many neurons are active and correlate with specific movement parameters, but then fail to perdure during the entire movement. Similar recordings within the interneuron regions of the spinal cord identify many cells that respond to these cortical commands but consistently code movement parameters for the duration of the movement [32].
Spinal sensory system: mapping body space into motor commands
The sensory system functions in motor behaviors to detect the environment so that proper movements can be selected to mediate reflexes, to inform the central nervous system about the starting position of the body in preparing movements, and to provide ongoing feedback about body position during movement. This system has varied somatotopic organization that translates body space into the spinal cord. There are three major sensory modalities, each mediated by a subpopulation of sensory neurons in the dorsal root ganglion, where their cell bodies are spatially intermingled; they are nociception (pain), mechanoreception (touch), and proprioception (position). Importantly, within a modality, there is divergence of sensory signals to multiple targets and convergence of sensory signals onto shared targets, thus blending spatially segregated inputs (Figure 1C). In addition, some sensory target cells within the spinal cord receive inputs from sensory neurons that mediate different modalities, thus compressing multiple types of sensory information. Accordingly, the spinal sensory system can be understood as an ‘intermediate dimensional’ topographic organization of inputs (Box 1).
Specifically, primary afferent fibers of the nociceptive and mechanoreceptive systems are mostly separated by modality and organized in parallel, as they enter the spinal cord and target dorsal horn cells with a well-established topographic order based on the skin body map (Figure 1C) [33–36,70]. Proprioceptive afferents innervate muscles and joints and have two major target regions in the spinal cord. These fibers enter the spinal cord from the dorsal edge and first target interneurons in the deep dorsal horn. Some of these fibers then continue to the ventral horn to synapse with motorneurons directly and with other classes of interneurons, such as 1a inhibitory interneurons (Figure 1C) [37–39]. Proprioceptive fibers have a tight relationship with the motorneurons of the muscles they sense, preferentially innervating ‘self’ motor neurons, but also forming some connections with motor neurons that supply functionally synergistic muscle groups. This specificity is accomplished through the sensory-motor matching of neurotrophin-induced gene expression signatures, including the Ets transcription factors and cadherin adhesion molecules [40–42].
The simplicity of the mono-synaptic connection between proprioceptive sensory axons and motorneurons has facilitated the study of the relationship between these two topographic schemes. The mechanisms that direct assembly of muscle-specific sensory-motor connections have recently been investigated by work from Jessell and colleagues. Surmeli et al. examined the role of motor neuron settling position in the formation of sensory-motor connections [43••]. Motor neuron-specific deletion of the FoxP1 transcriptional cofactor for Hox proteins (referred to as Foxp1MND) strips motor pools of their specific molecular identites. In Foxp1MND animals, motor neurons effectively innervate muscle targets in the limb, and the initial trajectory of proprioceptive fibers into the ventral horn occurs normally. However, careful analysis of sensory to motorneuron contacts using fluorescent labels revealed that when motorneuron cell body positioning is scrambled along the dorsal–ventral and mediolateral axes in the Foxp1MND animals, proprioceptive fibers lose the ability to discriminate between self and non-self motor neurons. Instead, most proprioceptive fibers innervate motor neurons that are located in their ‘wild-type’ termination zone, without regard for muscle-specific motor neuron identity (Figure 2A). Therefore, the topographically organized terminations of the proprioceptive fibers within the ventral horn of the spinal cord is independent from the deletion of FoxP1 causing motorneurons to change their position and cell fate. These observations support the facilitation of proper connectivity as a potential function of topographic organization.
Spinal interneurons: integrators of motor plans
Spinal interneurons link most sensory and descending inputs to motorneuron outputs. However, they do not passively transmit this information. Rather, they translate sensory and cortical information onto the musculotopic topography of the motorneurons. A remaining question in the field is to understand how spinal interneurons are organized to fulfill the role of integrating various frames of reference of the skin, musculature, and mechanical function into coordinated motor outputs.
One of the best studies of spatial organization of inter-neurons is in an important series of reports by Schouenborg and colleagues on the nociceptive withdrawal reflex [33,44,45••,46]. The response to a noxious stimulus on the skin is to activate the muscles that withdraw that body region. For instance, a painful stimulus on the heel promotes ankle extension and knee and hip flexion. This reflex is mediated by nociceptive sensory afferents that target interneurons in the superficial dorsal horn, that relay to interneurons in the deep dorsal horn. Each of these sensory and interneuronal components has a stereotypic topographic organization (Figure 1D). Specifically, the sensory fibers and interneurons are arranged in a medial to lateral progression to control distal extensors, then distal flexors, and then more proximal flexors. Interestingly, deprivation of the sensory cues during development perturbs this arrangement [33], suggesting neuronal activity-based mechanisms are required to establish these topographically organized connections.
A few examples of defined locations for subclasses of ventral interneurons have been described. However, the relevance of these spatial distributions to topography or function has yet to be determined. A rich literature has used markers and genetics to characterize a set of four cardinal lineage-related classes of ventral interneurons identifiable using well-established molecular markers: V0, V1, V2, and V3 [47–50]. V1-derived Renshaw cells that mediate direct feedback inhibition to motor actions surround motorneurons, and V1-derived 1a inhibitory interneurons that are involved in proprioceptive reflexes are dorsal to motorneurons [51]. V0-derived V0c inter-neurons that modulate motorneuron excitability are located surrounding the central canal [52,53]. And Hb9 interneurons are only found at rostral lumbar levels, in the segments with the strongest central pattern generator drive [53,54].
Although the topographic frame of reference for molecularly defined classes of spinal interneurons is largely unknown, there are electrophysiological experiments that suggest a function-based spatial order might exist for descending commissural-projecting interneurons (DC neurons). Kiehn and colleagues found that activation of flexor motorneurons was correlated with ventrally located DC neurons, whereas extension correlated with activity in a more dorsally located group of DC cells (Figure 1D) [55].
The characterization of interneuron topography has also been greatly facilitated by retrograde trans-neuronal labeling from the motorneurons of defined muscles. Techniques such as trans-neuronal WGA-HRP labeling and trans-synaptic wild-type rabies transmission revealed likely pre-motor interneuron distributions [56,57,58,59]. Generally, spinal pre-motor interneurons are found in the contralateral medial ventral horn, and in the ipsilateral spinal cord in the deep dorsal horn, intermediate region, and ventral horn. Recently, Callaway and Young and colleagues developed a genetically modified rabies virus that allows restricted retrograde mono-trans-synaptic spread of the virus [60•]. With this technique, analysis of defined pre-motor interneurons has confirmed previously observed general distribution patterns. The comparisons of pre-motor interneuron distributions from proximal and distal muscles and from flexor and extensor muscles have revealed biases in the trends of interneuron locations (Figure 1D). Proximal muscles have pre-motor interneuron distributions that are more likely to have a significant ipsilateral ventral horn contribution, relative to more distal muscles that have a more intermediate region and dorsal horn bias. Within the deep dorsal horn inter-neuron groups, pre-extensor interneurons are more likely to be medial than pre-flexor interneurons. However, in contrast to individual motor pools that are well segregated, the pre-motor populations for multiple muscles are highly intermingled.
Very recently, a significant study by Arber and colleagues examined whether different motor pools have spatially distinct pre-motor components, using mono-synaptically restricted rabies virus [61••]. They analyzed the pre-motor interneuron populations for several muscles and identified the medial extensor/lateral flexor bias in the dorsal horn interneurons (Figure 2B). They also demonstrated that pre-motor interneurons were in turn contacted by sensory afferents. This is important because the ability to map multiple circuit elements simultaneously has yielded a much richer understanding of network integration of multiple topographic systems. Moreover, when deprived of proprioceptive signals, the bias in pre-extensor and pre-flexor interneuron distributions is eroded (Figure 2B) [61••]. This suggests that proprioceptive information can be a cue for spatial organization of interneurons, similar to the role for nociceptive/ mechnoreceptive cues in refining the nociceptive withdrawal reflex.
Perspectives
Topography of neural circuits is clearly a major organizational strategy of the CNS, and we have reviewed examples of topographic spatial organization within the spinal motorneurons, motor cortex, spinal sensory system, and spinal interneurons. These networks function together with the thalamus, basal ganglia, brainstem, cerebellum, and other brain regions to initiate and coordinate movements. Although the spatial organization of most of these other regions is not well characterized and not described in this review, there is evidence that motor circuits in the brainstem and cerebellum display some topographic order that relates to specific muscles or groups of muscles [58,62].
We are just beginning to understand how multiple topographic networks interact to produce coherent movements. On the one hand, it is a complicated problem to merge the high-dimensional cortical system, the intermediate-dimensional sensory system, and the diverse and poorly understood organizations of spinal interneurons. Each of these systems has its own frame of reference and parameters that it can encode. How can these be reduced to the low-dimensionality of motorneuron and muscle organization? On the other hand, the filtering and integration of multiple inputs to produce coherent outputs is a hallmark of neuronal function, and the principles that apply at the cellular scale may also be relevant to the way circuits process and transform information.
It is also important to ask what is the purpose of this complex spatial organization. Given the vast array of neurons, fibers, and connections in the CNS, perhaps topographic principles facilitate the embryonic and post-natal development of proper connectivity. Another possible purpose for topographic organization is to preserve ‘wiring efficiency’. Neurons that are commonly recruited together may be physically close to minimize the time and energy that must be used for their communication [63–65]. In addition, in both the cortex and spinal cord, local neuronal ensembles may be co-recruited by traveling waves of activity. Local clustering of functionally related and topographically related neurons provides a substrate for waves of activation to bind their related targets together into a compound output [27,66–69].
Recent work in this field has continued to provide rich descriptions of the extent of topographic organization in the spinal cord, and to provide a circuit-based framework for integrating the organization of the cortical, sensory, and interneuronal systems with the musculotopic motor-neuronal system. Once we better understand the spatial and connectivity principles that govern movement at the level of the spinal cord, it will become feasible and critical to examine the role of this organization in producing coordinated behaviors. For instance, can topographic maps be re-written and if so, what are the consequences for movement? Is the alteration of topographic mapping the cellular substrate used by evolution to modify behaviors across species? These types of questions will address the importance of these schemes for function and may provide the basis for understanding motor plan integration when topographic map organization is altered in training, injury, and disease.
Acknowledgments
We would like to thank Dario Bonanomi and Chris Hinckley for helpful comments on the manuscript. A.J.L. was supported by George E. Hewitt Foundation for Medical Research. K.A.L. is a National Science Foundation Graduate Research Fellow. S.L.P. is an HHMI investigator. Research in the laboratory is supported by NINDS.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Eric R, Kandel RHW. Constructing the visual image. In: Eric R, Kandel JHS, Moa Jessell Thomas, editors. Principles of Neural Science. New York: McGraw-Hill; 2000. p. 498. [Google Scholar]
- 2.Phillips CG, Porter R. Corticospinal neurones. Their role in movement. Monogr Physiol Soc. 1977;34:v–xii. 1–450. [PubMed] [Google Scholar]
- 3.Vanderhorst VG, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol. 1997;382:46–76. [PubMed] [Google Scholar]
- 4.McHanwell S, Biscoe TJ. The localization of motoneurons supplying the hindlimb muscles of the mouse. Philos Trans R Soc Lond B Biol Sci. 1981;293:477–508. doi: 10.1098/rstb.1981.0082. [DOI] [PubMed] [Google Scholar]
- 5.Nicolopoulos-Stournaras S, Iles JF. Motor neuron columns in the lumbar spinal cord of the rat. J Comp Neurol. 1983;217:75–85. doi: 10.1002/cne.902170107. [DOI] [PubMed] [Google Scholar]
- 6.Romanes GJ. The motor cell columns of the lumbo-sacral spinal cord of the cat. J Comp Neurol. 1951;94:313–363. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- 7.Romanes GJ. The motor pools of the spinal cord. Prog Brain Res. 1964;11:93–119. doi: 10.1016/s0079-6123(08)64045-5. [DOI] [PubMed] [Google Scholar]
- 8.Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- 9••.Demireva EY, Shapiro LS, Jessell TM, Zampieri N. Motor neuron position and topographic order imposed by beta- and gamma-catenin activities. Cell. 2011;147:641–652. doi: 10.1016/j.cell.2011.09.037. Here, the authors genetically altered the catenin and cadherin profile of motorneurons and observed that they no longer formed pools or possessed a topographic order within the spinal cord, but were instead scattered throughout the motorneuron region. Despite this, the motor-neurons correctly found their muscle targets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrier D. Experimental researches in cerebral physiology and pathology. J Anat Physiol. 1873;8(Pt 1):152–155. [PMC free article] [PubMed] [Google Scholar]
- 11.Akintunde A, Buxton DF. Differential sites of origin and collateralization of corticospinal neurons in the rat: a multiple fluorescent retrograde tracer study. Brain Res. 1992;575:86–92. doi: 10.1016/0006-8993(92)90427-b. [DOI] [PubMed] [Google Scholar]
- 12.Tennant KA, Adkins DL, Donlan NA, Asay AL, Thomas N, Kleim JA, Jones TA. The organization of the forelimb representation of the c57bl/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cereb Cortex. 2011;21:865–876. doi: 10.1093/cercor/bhq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips CG, Porter R. Corticospinal Neurones: Their Role in Movement. London: Academic Press; 1977. [PubMed] [Google Scholar]
- 14.Leyton SSF, Sherrington CS. Observations on the excitable cortex of the chipanzee, orang-utan and gorilla. Exp Physiol. 1917;11:135–222. [Google Scholar]
- 15.Schieber MH, Hibbard LS. How somatotopic is the motor cortex hand area? Science. 1993;261:489–492. doi: 10.1126/science.8332915. [DOI] [PubMed] [Google Scholar]
- 16.Monfils MH, Plautz EJ, Kleim JA. In search of the motor engram: motor map plasticity as a mechanism for encoding motor experience. Neuroscientist. 2005;11:471–483. doi: 10.1177/1073858405278015. [DOI] [PubMed] [Google Scholar]
- 17.Aflalo TN, Graziano MS. Relationship between unconstrained arm movements and single-neuron firing in the macaque motor cortex. J Neurosci. 2007;27:2760–2780. doi: 10.1523/JNEUROSCI.3147-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgopoulos AP, Kettner RE, Schwartz AB. Primate motor cortex and free arm movements to visual targets in three-dimensional space. II. Coding of the direction of movement by a neuronal population. J Neurosci. 1988;8:2928–2937. doi: 10.1523/JNEUROSCI.08-08-02928.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci. 1982;2:1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol. 1968;31:14–27. doi: 10.1152/jn.1968.31.1.14. [DOI] [PubMed] [Google Scholar]
- 21.Graziano MS, Aflalo TN. Mapping behavioral repertoire onto the cortex. Neuron. 2007;56:239–251. doi: 10.1016/j.neuron.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 22•.Dombeck DA, Graziano MS, Tank DW. Functional clustering of neurons in motor cortex determined by cellular resolution imaging in awake behaving mice. J Neurosci. 2009;29:13751–13760. doi: 10.1523/JNEUROSCI.2985-09.2009. In this study, the authors used a fluorescent calcium indicator to monitor the activity of neurons in the motor cortex during running and grooming behaviors. They found that neurons that were co-active were likely to be close spatially, and were somewhat segregated into a network for each behavior analyzed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Graziano MS, Taylor CS, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. This paper used long duration intracortical microstimulation of motor cortex to elicit complex, ethologically relevant, behaviors and mapped these behaviors to a coherent spatial distribution. [DOI] [PubMed] [Google Scholar]
- 24.Ramanathan D, Conner JM, Tuszynski MH. A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proc Natl Acad Sci USA. 2006;103:11370–11375. doi: 10.1073/pnas.0601065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison TC, Ayling OG, Murphy TH. Distinct cortical circuit mechanisms for complex forelimb movement and motor map topography. Neuron. 2012;74:397–409. doi: 10.1016/j.neuron.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 26.Capaday C, Ethier C, Brizzi L, Sik A, van Vreeswijk C, Gingras D. On the nature of the intrinsic connectivity of the cat motor cortex: evidence for a recurrent neural network topology. J Neurophysiol. 2009;102:2131–2141. doi: 10.1152/jn.91319.2008. [DOI] [PubMed] [Google Scholar]
- 27.Capaday C, van Vreeswijk C, Ethier C, Ferkinghoff-Borg J, Weber D. Neural mechanism of activity spread in the cat motor cortex and its relation to the intrinsic connectivity. J Physiol. 2011;589(Pt 10):2515–2528. doi: 10.1113/jphysiol.2011.206938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ethier C, Brizzi L, Darling WG, Capaday C. Linear summation of cat motor cortex outputs. J Neurosci. 2006;26:5574–5581. doi: 10.1523/JNEUROSCI.5332-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 30.Bortoff GA, Strick PL. Corticospinal terminations in two new-world primates: further evidence that corticomotoneuronal connections provide part of the neural substrate for manual dexterity. J Neurosci. 1993;13:5105–5118. doi: 10.1523/JNEUROSCI.13-12-05105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci USA. 2009;106:918–923. doi: 10.1073/pnas.0808362106. This study used wild-type rabies virus as a retrograde circuit tracer to identify the locations of cortico-motoneuronal cells in the motor cortex, with reference to specific motorneuron pools. They observed that cortico-motorneuronal cells clustered in a region of the primary cortex that they call ‘new M1’. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shalit U, Zinger N, Joshua M, Prut Y. Descending systems translate transient cortical commands into a sustained muscle activation signal. Cereb Cortex. 2012;22:1904–1914. doi: 10.1093/cercor/bhr267. [DOI] [PubMed] [Google Scholar]
- 33.Granmo M, Petersson P, Schouenborg J. Action-based body maps in the spinal cord emerge from a transitory floating organization. J Neurosci. 2008;28:5494–5503. doi: 10.1523/JNEUROSCI.0651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swett JE, Woolf CJ. The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. J Comp Neurol. 1985;231:66–77. doi: 10.1002/cne.902310106. [DOI] [PubMed] [Google Scholar]
- 35.Nyberg G, Blomqvist A. The somatotopic organization of forelimb cutaneous nerves in the brachial dorsal horn: an anatomical study in the cat. J Comp Neurol. 1985;242:28–39. doi: 10.1002/cne.902420103. [DOI] [PubMed] [Google Scholar]
- 36.Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. New York: Kluwer Academic, New York; 2004. [Google Scholar]
- 37.Eccles JC, Fatt P, Landgren S. Central pathway for direct inhibitory action of impulses in largest afferent nerve fibres to muscle. J Neurophysiol. 1956;19:75–98. doi: 10.1152/jn.1956.19.1.75. [DOI] [PubMed] [Google Scholar]
- 38.Jankowska E, Lindstrom S. Morphology of interneurones mediating ia reciprocal inhibition of motoneurones in the spinal cord of the cat. J Physiol. 1972;226:805–823. doi: 10.1113/jphysiol.1972.sp010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jankowska E, Roberts WJ. Synaptic actions of single interneurones mediating reciprocal ia inhibition of motoneurones. J Physiol. 1972;222:623–642. doi: 10.1113/jphysiol.1972.sp009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koo SJ, Pfaff SL. Fine-tuning motor neuron properties: signaling from the periphery. Neuron. 2002;35:823–826. doi: 10.1016/s0896-6273(02)00870-x. [DOI] [PubMed] [Google Scholar]
- 41.Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci. 2007;8:114–127. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- 42.Chen HH, Hippenmeyer S, Arber S, Frank E. Development of the monosynaptic stretch reflex circuit. Curr Opin Neurobiol. 2003;13:96–102. doi: 10.1016/s0959-4388(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 43••.Surmeli G, Akay T, Ippolito GC, Tucker PW, Jessell TM. Patterns of spinal sensory-motor connectivity prescribed by a dorsoventral positional template. Cell. 2011;147:653–665. doi: 10.1016/j.cell.2011.10.012. Here, the role of topography in sensory-motorneuron matching in the proprioceptive monosynaptic stretch reflex was examined. They used a genetic manipulation to alter the position and identity of motor neurons, and examined the distribution of proprioceptive afferents. They found that proprioceptive fibers projected to their normal territory despite these changes to motor neurons, suggesting that cell body location may facilitate connectivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levinsson A, Holmberg H, Broman J, Zhang M, Schouenborg J. Spinal sensorimotor transformation: relation between cutaneous somatotopy and a reflex network. J Neurosci. 2002;22:8170–8182. doi: 10.1523/JNEUROSCI.22-18-08170.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Schouenborg J, Weng HR, Kalliomaki J, Holmberg H. A survey of spinal dorsal horn neurones encoding the spatial organization of withdrawal reflexes in the rat. Exp Brain Res. 1995;106:19–27. doi: 10.1007/BF00241353. Here, the authors used electrophysiology to study the nociceptive withdrawal reflex interneurons, and they identified ‘reflex encoder’ interneurons in the deep dorsal horn. These interneurons possess a cutaneous receptive field that corresponds to the region withdrawn upon contraction of a single muscle and they are topographically organized according to the muscles that they modulate. [DOI] [PubMed] [Google Scholar]
- 46.Schouenborg J. Action-based sensory encoding in spinal sensorimotor circuits. Brain Res Rev. 2008;57:111–117. doi: 10.1016/j.brainresrev.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci. 2009;10:507–518. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol. 2009;19:572–586. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stepien AE, Arber S. Probing the locomotor conundrum: descending the ‘v’ interneuron ladder. Neuron. 2008;60:1–4. doi: 10.1016/j.neuron.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 50.Goulding M, Pfaff SL. Development of circuits that generate simple rhythmic behaviors in vertebrates. Curr Opin Neurobiol. 2005;15:14–20. doi: 10.1016/j.conb.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 51.Alvarez FJ, Jonas PC, Sapir T, Hartley R, Berrocal MC, Geiman EJ, Todd AJ, Goulding M. Postnatal phenotype and localization of spinal cord v1 derived interneurons. J Comp Neurol. 2005;493:177–192. doi: 10.1002/cne.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron. 2009;64:645–662. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson JM, Hartley R, Maxwell DJ, Todd AJ, Lieberam I, Kaltschmidt JA, Yoshida Y, Jessell TM, Brownstone RM. Conditional rhythmicity of ventral spinal interneurons defined by expression of the hb9 homeodomain protein. J Neurosci. 2005;25:5710–5719. doi: 10.1523/JNEUROSCI.0274-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hinckley CA, Hartley R, Wu L, Todd A, Ziskind-Conhaim L. Locomotor-like rhythms in a genetically distinct cluster of interneurons in the mammalian spinal cord. J Neurophysiol. 2005;93:1439–1449. doi: 10.1152/jn.00647.2004. [DOI] [PubMed] [Google Scholar]
- 55.Butt SJ, Lebret JM, Kiehn O. Organization of left-right coordination in the mammalian locomotor network. Brain Res Brain Res Rev. 2002;40:107–117. doi: 10.1016/s0165-0173(02)00194-7. [DOI] [PubMed] [Google Scholar]
- 56.Harrison PJ, Jankowska E, Zytnicki D. Lamina viii interneurones interposed in crossed reflex pathways in the cat. J Physiol. 1986;371:147–166. doi: 10.1113/jphysiol.1986.sp015965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jankowska E, Skoog B. Labelling of midlumbar neurones projecting to cat hindlimb motoneurones by transneuronal transport of a horseradish peroxidase conjugate. Neurosci Lett. 1986;71:163–168. doi: 10.1016/0304-3940(86)90552-5. [DOI] [PubMed] [Google Scholar]
- 58.Ruigrok TJ, Pijpers A, Goedknegt-Sabel E, Coulon P. Multiple cerebellar zones are involved in the control of individual muscles: a retrograde transneuronal tracing study with rabies virus in the rat. Eur J Neurosci. 2008;28:181–200. doi: 10.1111/j.1460-9568.2008.06294.x. [DOI] [PubMed] [Google Scholar]
- 59.Jankowska E. Further indications for enhancement of retrograde transneuronal transport of wga-hrp by synaptic activity. Brain Res. 1985;341:403–408. doi: 10.1016/0006-8993(85)91084-4. [DOI] [PubMed] [Google Scholar]
- 60•.Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. The authors created a genetically modified rabies virus that can spread in a retrograde fashion from one neuron to its pre-synaptic partners, and that labels these cells with bright fluorescence. This novel technique will greatly facilitate probing the neural circuitry controlling movement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61••.Tripodi M, Stepien AE, Arber S. Motor antagonism exposed by spatial segregation and timing of neurogenesis. Nature. 2011;479:61–66. doi: 10.1038/nature10538. The authors used retrograde monosynaptic circuit tracing with rabies virus to identify the distributions of pre-motor interneurons of hindlimb muscles. They found that dorsal pre-flexor interneurons are born earlier and are more lateral than their pre-extensor counterparts. [DOI] [PubMed] [Google Scholar]
- 62.Coffman KA, Dum RP, Strick PL. Cerebellar vermis is a target of projections from the motor areas in the cerebral cortex. Proc Natl Acad Sci USA. 2011;108:16068–16073. doi: 10.1073/pnas.1107904108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chklovskii DB, Schikorski T, Stevens CF. Wiring optimization in cortical circuits. Neuron. 2002;34:341–347. doi: 10.1016/s0896-6273(02)00679-7. [DOI] [PubMed] [Google Scholar]
- 64.Mitchison G. Axonal trees and cortical architecture. Trends Neurosci. 1992;15:122–126. doi: 10.1016/0166-2236(92)90352-9. [DOI] [PubMed] [Google Scholar]
- 65.Cherniak C. Neural component placement. Trends Neurosci. 1995;18:522–527. doi: 10.1016/0166-2236(95)98373-7. [DOI] [PubMed] [Google Scholar]
- 66.Ivanenko YP, Poppele RE, Lacquaniti F. Spinal cord maps of spatiotemporal alpha-motoneuron activation in humans walking at different speeds. J Neurophysiol. 2006;95:602–618. doi: 10.1152/jn.00767.2005. [DOI] [PubMed] [Google Scholar]
- 67.Yakovenko S, Mushahwar V, VanderHorst V, Holstege G, Prochazka A. Spatiotemporal activation of lumbosacral motoneurons in the locomotor step cycle. J Neurophysiol. 2002;87:1542–1553. doi: 10.1152/jn.00479.2001. [DOI] [PubMed] [Google Scholar]
- 68.Combes D, Merrywest SD, Simmers J, Sillar KT. Develo_1egation of spinal networks driving axial- and hindlimb-based locomotion in metamorphosing xenopus laevis. J Physiol. 2004;559(Pt 1):17–24. doi: 10.1113/jphysiol.2004.069542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wallen P, Williams TL. Fictive locomotion in the lamprey spinal cord in vitro compared with swimming in the intact and spinal animal. J Physiol. 1984;347:225–239. doi: 10.1113/jphysiol.1984.sp015063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, Woodbury CJ, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]