Epithelial to mesenchymal transition (EMT) is a dynamic cellular process that is essential for the development of metastatic disease. During EMT, a tumor cell with epithelial characteristics transitions to a tumor cell with mesenchymal characteristics through modulation of cell polarity and adhesion. Two hallmark EMT proteins, E-Cadherin and Vimentin, are tightly controlled during EMT through multiple signal transduction pathways. Epidermal growth factor (EGF) and transforming growth factorβ (TGFβ) promote EMT by regulating a distinct set of transcription factors, including Snail and Twist. Snail, Twist, and Slug are integral to the induction of EMT through direct regulation of genes involved in cellular adhesion, migration, and invasion. This review highlights the current literature on EMT in HNSCC. Understanding the role of EMT will provide insight to the pathogenesis of disease progression and may lead to the development of novel anti-cancer therapeutics for metastatic HNSCC.
Introduction
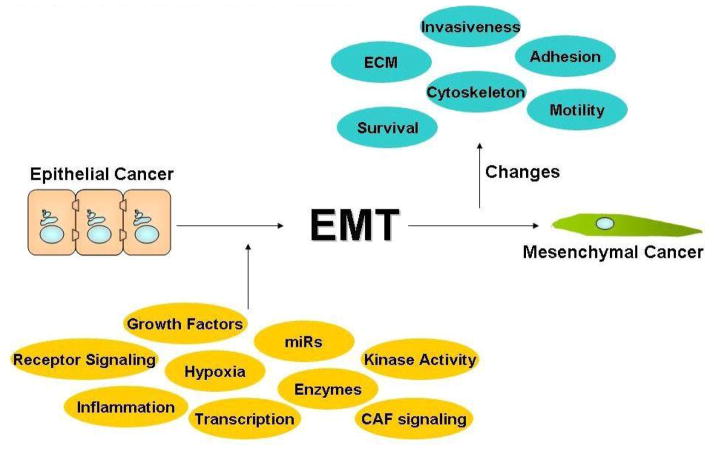
In 2011, it is estimated there will be over 11,460 deaths from head and neck squamous cell carcinoma cancers (HNSCC) in the United States and over 300,000 deaths worldwide.1,2 Most HNSCC patients present with stage III/IV disease and have a 5-year survival rate below 40%.3 HNSCC patients with metastatic disease have extremely poor prognosis and a survival rate of less than 10%.3 Gene expression profiling studies have identified gene signatures associated with NF-κB activation, epithelial to mesenchymal transition (EMT), and cell adhesion deregulation as prominent genetic alterations in HNSCC development and/or progression.4 EMT is a complex and reversible biological process, where an epithelial tumor cell alters its polar, adhesive phenotype to a mesenchymal phenotype characterized by an increase in cell migration and invasion potential, cytoskeleton remodeling, and resistance to apoptosis (Figure 1).5,6 In 2009, Kalluri and Weinberg designated EMT in cancer as type 3.6 This classification recognizes the fluidity and idiosyncrasies of EMT in cancer in comparison to the more characterized fibroblast formation (type 2 EMT) and developmental process transitions (type 1 EMT).6 A recent study reported that primary HNSCC tumors expressing a hallmark EMT signature, low E-Cadherin and high Vimentin, has a two-fold increase in the satellite’s average distance compared to primary HNSCC tumors without an EMT signature.7 Therefore, a clear understanding of the EMT process is essential to identify novel druggable targets for the development of therapeutic approaches to prevent disease progression and metastasis in HNSCC.
Figure 1. Epithelial-to-Mesenchymal Transition.
Epithelial cancer cells with a polar, adhesive phenotype are induced into cells with a mesenchymal phenotype characterized by increased invasive, motility, and survival.
Cytoskeletal, extracellular matrix, and adhesion molecules in EMT
Rearrangement of stress fibers, modulation of adhesion molecules and extracellular proteins are key events in EMT for tumor cells (Figure 2).8 Epithelial cells are characterized by stable cell-cell contacts and the formation of adherens junctions.5 These junctions and contacts are predominantly E-Cadherin-dependent and connect to actin filaments through α- or β-catenin.5 Loss of E-Cadherin and relocalization of β-catenin from the membrane to the nucleus is frequently observed in tumor cells undergoing EMT.5,6,8 Another classical marker of EMT, the intermediate filament Vimentin, is present in mesenchymal cells to control cell motility.9 In primary HNSCC tumors, low E-Cadherin and high Vimentin is associated with an increase in distant metastasis (Table 1).10 Primary HNSCC tumors with the low E-Cadherin and high Vimentin signature have a 100% metastasis rate compared to a 44% metastasis rate for primary HNSCC tumors with an incomplete or null EMT signature.10 This pivotal study supports the potential utility of E-Cadherin and Vimentin as a dual predictive biomarker for metastasis in HNSCC.10
Figure 2. Cytoskeletal, extracellular matrix, and adhesion molecules in EMT.
A key indicator of EMT is the rearrangement of stress fibers, a change in adhesion molecules, and extracellular proteins. In a cell undergoing EMT, β-catenin is transported from the cell membrane to the nucleus. E-Cadherin expression can be repressed by hypermethylation, Actin is reorganized, and Vimentin/N-Cadherin expression is increased.
Table 1.
Select EMT-Associated Biomarkers in HNSCC
| Reference | Biomarker | # Patients | OS/PFS | N+/M |
|---|---|---|---|---|
| Nijkamp, 2012 | E-Cadherin | 26 | p=0.001 (PFS) | p=0.004 (M) |
| Jouppila-Matto, 2011 | Twist + Snail | 109 | p=0.043 (OS) | NS |
| Song, 2009 | Bmil | 75 | p=0.019(OS) | NS |
| Yang, 2008 | HIF-1α | 147 | p<0.001 (PFS) | ND |
| Twist | p<0.001 (PFS) | |||
| Snail | p<0.001 (PFS) | |||
| Yang, 2010 | Twist + Bmil | 132 | p<0.001 (OS) | ND |
The Cadherin superfamily consists of transmembrane proteins involved in cell adhesion.11 E- and P-Cadherins are tumor suppressive and maintain epithelial cell adheren junctions with catenins.11 Conversely, N-Cadherin is pro-oncogenic and enhance tumor cell invasion and migration.11,12 Overexpression of Aurora-A, a mitotic serine/threonine kinase involved in bipolar spindle formation, increases active MAPK resulting in a decrease in E-Cadherin and β-catenin.13 A HNSCC cell line established from a lymph node metastasis had a spindle morphology and a decrease in E-Cadherin and β-catenin. 14 Further examination of the invasive HNSCC cell line indicated that promoter methylation may be the mechanism for E-Cadherin silencing.14 Interestingly, heterogeneous promoter methylation of E-Cadherin was observed in the invasive fronts of tumors from metastatic HNSCC patients.14 Regulation of E-Cadherin by promoter methylation is known to occur in other epithelial carcinomas, including gastric, hepatic, bladder, and lung cancer.5,15,16
There is emerging evidence that P-Cadherin suppresses EMT in HNSCC. During migration, post translational cleavage causes P-Cadherin to be shortened from 100kDa to 50kDa, resulting in decreased cell attachment.17,18 Full length P-Cadherin expression was decreased or lost in tumor cells at the invasive front.17 Ectopic expression of full-length P-Cadherin in HNSCC cells is sufficient to inactivate Snail and activate glycogen synthase kinase-3β (GSK-3β) resulting in a reversion to the epithelial phenotype.17 Work in breast carcinoma has also implicated a role for P-Cadherin in EMT and metastasis, however, the molecular mechanism remains to be fully elucidated.19
A decrease in E-Cadherin is often associated with an increase in N-Cadherin in a process called cadherin switching. Cadherin switching is associated with positive lymph nodes in HNSCC.8,20,21 Moreover, cadherin switching was reported to correlate with metastasis in spindle cell carcinoma of the head and neck (HNSpCC).22,23 HNSpCC is a rare biphasic tumor composed of a malignant spindle cell component and squamous cell carcinoma cells.21–24 HNSpCC is of particular interest in the study of EMT due to the evidence of pathogenesis from a stem cell undergoing EMT.24 Further illustration of the EMT-like phenotype in HNSpCC is the loss of desmogleins and desmocollins, components of desmosome which connects intermediate filaments in cell-cell contacts.21 Moreover, catenin alterations, in particular lost of γ-catenin (plankoglobin), were observed in a majority of HNSpCC tumor specimens. 23
Actin remodeling is a crucial event for the progression of EMT.25,26 Guaninenucleotide-binding protein α12 (Gα12) subunit modulates G coupled protein receptor (GPCR) signaling to facilitate cell migration through IQGAP1.26 Transcriptome analysis in Gα12-deficient cells revealed Actin reorganization as a critical step in cell motility in EMT.26 Independent knockdowns of both Gα12 and IQGAP1 caused a decrease in F-actin, migration, and invasion, along with loss of cell-cell adhesion and polarity.26 Knockdown of the Actin bundling protein Fascin, commonly associated with cell protrusions, resulted in a decrease in migration, adhesion, and invasion.27 HNSCC, which usually overexpress Fascin, showed a decrease Twist expression when treated with Fascin siRNA.27 These observations indicate that Fascin may be an essential regulator of EMT in HNSCC.27
Laminins are basement membrane components that are trimers of polypeptide chains.28 Laminins are secreted by the cell to facilitate the formation of a basement membrane, which is α chain dependent.29 Laminin 511, consisting of the α5 chain, is expressed in epithelial basement membranes. In contrast, Laminin 411, containing the α4 chain, is expressed in mesenchymal-originated cells.29 In Snail-induced EMT cells, laminin α4 was detected whereas laminin α5 was not present.29 Snail binds to the promoters of laminin α5 and laminin α4 to conversely regulate these two laminins during EMT.29 Interestingly, EMT tumor cells express integrin α6β1, the receptor for laminin 411.29 Taken together, these studies suggest that EMT is facilitated through selective regulation of basement membrane components, namely the switch from laminin 511 to laminin 411.29
Transcriptional factors in EMT
The transcription factors Slug, Snail, and Twist are known to bind to the E-box regulatory regions of E-Cadherin.30, 31,32 All three are the most recognized transcription factors associated with EMT, mainly due to their repression of E-Cadherin.6,8 In salivary adenoid cystic carcinoma, Slug expression correlated with advanced stage, invasion, recurrence, and distant metastasis.33 A intriguing study showed that Slug controls group migration and not individual tumor cell migration in HNSCC cells.34 In comparison to stage- and site-matched HNSCC tumors, HNSpCC tumors were observed to have elevated expression of Slug and Snail.23, 24 Furthermore, an inverse correlation between Snail and γ-catenin was observed in HNSpCC suggesting that γ-catenin may be a downstream target of Snail.23 In pharyngeal squamous cell carcinoma, Twist was expressed in 35% of primary tumors and interestingly, in 40% of accompanying stroma.34 An association between stromal expression of Twist and increased tumor stage was observed, while tumor expression correlated to lymph node metastasis.34 Moreover, Twist and Snail expression only correlated in stromal cells and not in tumor cells suggesting that EMT may reprogram tumor cells into cancer associated fibroblasts.34
Lysyl-Oxidase-Like 2 (LOXl2), a matrix remodeling enzyme, is upregulated in HNSCC and reported to stabilize Snail, through blocking GSK-3β-mediated phosphorylation, leading to E-Cadherin repression.35,36 Interestingly, 60% of the genes affected by LOXl2 are related to epidermal differentiation.36 Suppression of LOX12 resulted in an increase in E-Cadherin and a decrease in cell invasion.36 In addition to LOXl2, STAT3 has been shown to promote EMT and enhance Snail and Twist levels.37,38 A recent study showed that Snail and Twist induced CD44high/CD24low stem-cell like cells in breast carcinoma.39 In HNSCC, inhibition of STAT3 with Cucurbitacin 1 resulted in a decrease in Snail and Twist and attenuated the ability of CD44+ALDH1+ HNSCC cancer stem cells (CSCs) to metastasize in vivo.40 These results provide initial evidence linking STAT3 to EMT transcription factors, Snail and Twist, in the regulation of CSCs in HNSCC (Figure 3A).
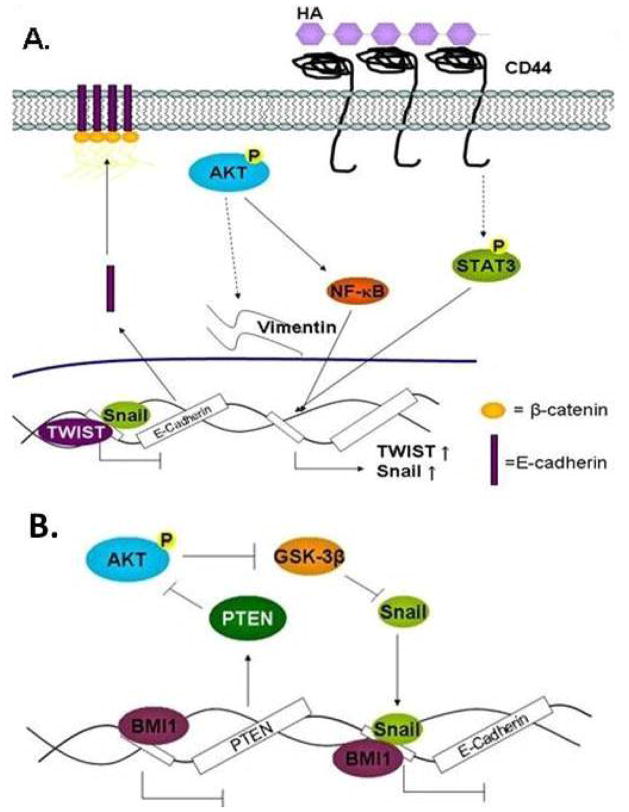
Figure 3. Snail and Twist in EMT.
(A) Phosphorylated AKT increases Snail, Twist, NF-κB, and Vimentin. HA-bound CD44 activates STAT3 which leads to the upregulation of Twist and Snail. Twist and Snail occupies E-Cadherin promoter to repress E-Cadherin transcription. (B) Bmi1 repressess PTEN allowing AKT to inhibit Snail degradation by GSK-3β. Bmi1 cooperates with Snail to inhibit E-Cadherin.
AKT is known to induce morphological changes associated with EMT, loss of cell-cell adhesion, and increased motility and invasion.41 Inhibition of AKT by blocking the PH domain with phosphatidlylinositol ether lipid analogues (PIA) resulted in a decrease in Snail and Twist expression and reversion of tumor cells back to an epithelial phenotype with high E-Cadherin and low Vimentin.42 Transcription factor B lymphoma Mo-MLV insertion region 1 homolog (Bmi1) is known to suppress INK4A and cooperate with c-Myc to promote HNSCC.43 Recently, Bmi1 was found to bind to the promoter of AKT inhibitor Phosphatase and tensin homolog (PTEN).43 Promotion of AKT activity by Bmi1 promotes EMT by blocking GSK-3β-mediated degradation of Snail.43 Interestingly, Bmi1 binds to the E-Cadherin promoter but is dependent on Snail for E-Cadherin repression (Figure 3B). These observations support Bmi1 as a player in EMT through activation of AKT, stabilization of Snail, and repression of E-Cadherin in HNSCC.
Growth Factor and Receptor Signaling in EMT
Transforming growth factorβ1 (TGFβ1) is increased in HNSCC and exposure to TGFβ is sufficient to induce a mesenchymal morphology in HNSCC cell lines (Figure 4A).44,45 There is evidence to propose a novel EMT mechanism in which TGFβ1 upregulates matrix metalloprotease 9 (MMP9) through Snail/Ets-1-dependent transcriptional regulation.44 MMPs are gelatinases that are capable of degrading the extracellular matrix components, as well as, regulating pathways and growth factors from the extracellular matrix (ECM).46 Accumulating research has shown the importance of MMPs in EMT, in particular breast, cervical, prostate, lung, and bronchial carcinomas.46,47 Also, TGFβ1 has been shown to regulate Slug through the ERK1/2 pathway and independent of Ets-1. 48 Similar to Snail, TGFβ1 was shown to promote a Slug-mediated increase in MMP9 expression.48 Alternatively, TGFβ1 can promote EMT through modulation of the SMAD signaling pathway. TGFβ1 signals through SMAD2/3 and MLCK to regulate MMP9 post-transcriptionally in HNSCC.49 SMAD6 competes with SMAD4 to inactivate SMAD signaling complexes, while SMAD7 blocks TGFβR phosphorylation.50 SMAD6 and 7 are known collectively as the anti-SMADs, acting antagonistically on TGFβ signaling.50 In HNSCC patient tumors, high expression of SMAD6 and low expression of SMAD2 correlated with better survival, whereas, low levels of SMAD7 correlated with decreased survival.51 Taken together, these studies demonstrate that TGFβ1 promotes EMT through multiple mechanisms in HNSCC.48
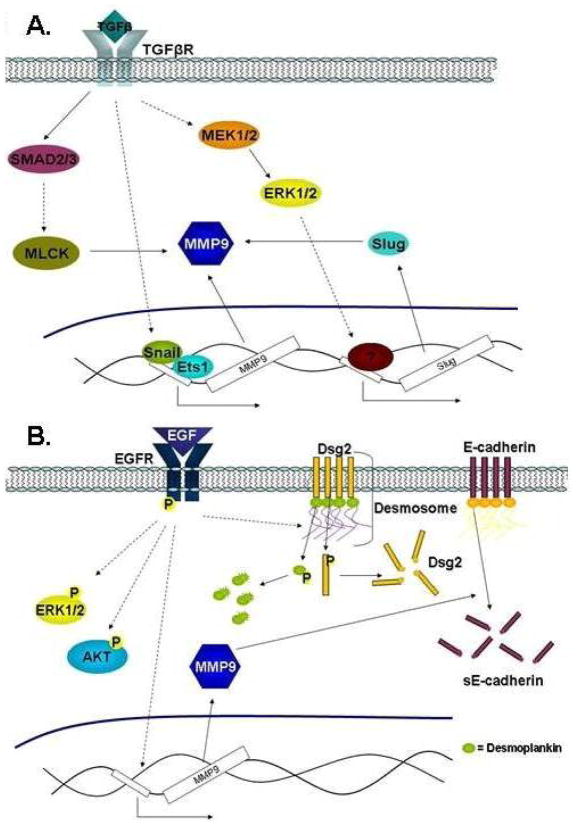
Figure 4. TGFβ and EGF in EMT.
(A) TGFβ post-transcriptionally regulates MMP9 through SMAD2/3 and MLCK to promote EMT. Transcriptional regulation of MMP9 by TGFβ is mediated through the Snail/Ets1 and MEK1/2-ERK1/2-Slug pathways. (B) EGF activates Erk1/2 and AKT pathways to promote EMT. EGF activates the EGFR to increase MMP9 resulting in degradation of E-Cadherin into sE-Cadherin. Also, the EGF/EGFR signaling pathway regulates the degradation of desmosomes.
Epidermal growth factor receptor (EGFR), a tyrosine kinase receptor, is of particular interest in HNSCC considering EGFR is almost universally overexpressed in this patient population.52,53 In several solid malignancies, including HNSCC, activation of EGFR has been shown to decrease cell adhesion leading to an increase in tumor cell invasion and migration (Figure 4B).54,55 In HNSCC cells with high endogenous EGFR, treatment with epidermal growth factor (EGF) enhanced cell motility, invasion velocity, and number of invasive cells.56 Additionally, an increase in the phosphorylation levels of EGFR, AKT, and ERK1/2 is observed following EGF exposure.56 Moreover, degradation of E-Cadherin into sE-Cadherin via MMP9 was shown following EGFR activation.56 In contrast, another group reported that total E-Cadherin was unchanged, however, E-Cadherin was re-localized from the membrane to the perinuclear area following EGF-induced EMT in HNSCC.57 It was reported that EGFR modulates cell adhesion through regulation of desmosomes, cellular structure that joins adjacent cells together.58 Inhibition of EGFR enhanced the levels of Desmoglein-2 (Dsg2) and Desmoplakin (Dsp), two desmosomal proteins, and E-Cadherin at cell borders.58 Conversely, activation of EGFR increased tyrosine phosphorylation and degradation of Dsg2 and Dsp resulting in reduced cellular adhesion.58 It was suggested that degradation of Dsg2 may be a result of EGFR-mediated MMP9 activation since Dsg2 is a known substrate of MMPs.58
A recent report demonstrated that EGF and TGFβ1 can cooperatively promote EMT in HNSCC. Stimulation of HNSCC cells with EGF and TGFβ1 induced a mesenchymal phenotype and modulated the expression of a cadre of EMT-associated proteins.59 Specifically, Vimentin and Snail were increased whereas E-Cadherin was decreased.59 Other proteins identified, such as CD44v6, have been shown to be involved in EMT in other carcinomas but have yet to be linked to HNSCC.59 An fascinating observation is that the expression of EMT-associated genes was regulated with a distinct temporal resolution suggesting that an early and late EMT program may be a possibility.59 Additional work is needed to explore this intriguing finding in order to enhance our understanding of the dynamic EMT process in HNSCC.
Vascular endothelial growth factor receptor (VEGFR) and Wnt regulates EMT in various solid malignancies.5 However, there is scant literature linking VEGFR- and Wnt-mediated EMT in HNSCC. VEGFR and its ligand vascular endothelial growth factor (VEGF) are well-recognized as essential players in angiogenesis. Treatment with VEGF in pancreatic, breast, and colorectal carcinoma cell lines lead to induction of EMT with an increase of Snail, Twist, and Slug expression.53 In HNSCC patient samples, VEGF is correlated with invasion depth and increased risk of positive lymph nodes.60 In addition to VEGF, the Wnt signaling pathway has been linked to disease progression.61 Wnt-5a has been shown to increase Slug and Snail in breast carcinoma and reported to increase tumor cell invasion in numerous carcinomas.61,62 In HNSCC, Wnt-5b enhanced tumor cell invasion and migration, but not proliferation, suggesting that Wnt-5b may regulate EMT.61 Overexpression of Wnt-5b in HNSCC cells was reported to enhance MMP10 expression.62 Interestingly, MMP10 is correlated with increased invasion, tumor stage, and lymph node metastasis in HNSCC patients.62 There is evidence to support the notion that Wnt-5b signals through MEF2A to regulate MMP10 in HNSCC, a mechanism that is similar to the TGFβ1-mediated MMP10 activation pathway in breast carcinoma.62,63
There is data to implicate neurothrophinreceptor B (TrkB), a tyrosine kinase receptor, as a regulator of EMT in HNSCC. TrkB has been shown to promote disease progression in various carcinomas.64 Examination of HNSCC patient samples revealed increased expression of TrkB and its ligand BDNF.64 Treatment with BDNF in TrkB expressing HNSCC cell lines resulted in enhanced tumor cell motility and invasion.64 Conversely, suppression of TrkB decreased Twist and N-Cadherin levels and promoted mesenchymal to epithelial transition (MET).64 Inhibition of AKT blocks BDNF/TrkB-mediated tumor cell invasion and migration indicating that AKT is downstream of the BDNF/TrkB pathway.64 Interestingly, co-culture of SCC25 HNSCC cells with fibroblasts resulted in an increase in Vimentin and TrkB levels in the SCC25 cells and an increase in the TrkB ligand, BNDF, in the fibroblasts.65 These observations suggest that the stromal cell-tumor cell interaction may efficiently activate the BDNF-TrkB signaling cascade through a paracrine manner in HNSCC. Together, these results provide emerging evidence that the TrkB modulates EMT in HNSCC.
Hypoxia and inflammation in EMT
Hypoxia, or oxygen deprivation, occurs in tumors due to inadequate vasculature to allow sufficient oxygen diffusion.66 HIF-1α, a key hypoxia-regulated gene, is an important contributor to metastasis and has been shown to induce EMT.67 HNSCC cells with high HIF-1α was demonstrated to exhibit the hallmark EMT phenotype and modulate the expression of EMT-associated genes.67 HIF-1α was shown to bind to the HRE proximal promoter element of Twist to enhance Twist expression.67 Co-expression of HIF-1α, Snail, and Twist in primary HNSCC tumors was associated with poor progression-free and overall survival.67 In a separate study, elevated HIF-1α was demonstrated to correlate with worst disease-free and overall survival confirming the importance of HIF-1α as a key regulator of disease progression in HNSCC.68 Recent work revealed that Twist regulates Bmi1 to promote EMT under hypoxic conditions.69 Twist was reported to directly enhance Bmi1 expression through Bmi1 promoter occupancy.69 In addition, Twist and Bmi1 act cooperatively to repress E-Cadherin and p16INK4a to promote EMT in HNSCC.69 Also, HIF-1α controls MMP17 promoter activity through activation of Slug in HNSCC cells under hypoxic conditions.70 Genetic knockdown of MMP17 blocked cell invasion in vitro and metastasis in vivo.70 Moreover, elevated MMP17 is associated with poor prognosis in HNSCC patients.70 These results indicate that HIF-1α regulates hypoxia-induced EMT through several independent mechanisms in HNSCC.
Inflammatory cytokines, such as GM-CSF, IL-1α, IL-6, and IL-8, are known to correlate with increased metastasis in HNSCC.71 A regulatory loop involving IL-6-induced EMT and Snail has been reported in breast carcinoma.72 Recently, IL-6 was reported to enhance Snail levels via the STAT3 pathway to promote EMT in HNSCC cells.73 Another mechanism for IL-6-mediated EMT is through modulation of Twist stability. Casein kinase 2 (CK2), an IL-6 target, stabilizes Twist through a post-translational phosphorylation event.40 Similarly to IL-6, IL-1β regulates EMT through several pathways. Treatment of HNSCC cells with IL-1β decreased E-Cadherin and increased Zeb1 expression.74 IL-1β increased binding of Zeb1 to the Ebox element on the promoter of E-Cadherin to silence E-cadherin expression.74 IL-1β has also been shown to regulate E-Cadherin through COX2-dependent upregulation of Snail.75
Conclusion
HNSCC patients with distant metastasis have a mortality rate of approximately 90%.3 EMT is a cellular process that is intimately linked to metastasis and understanding EMT biology will be essential to improve patient outcome. Downregulation of E-cadherin, upregulation of Vimentin, relocalization of β-catenin, and rearrangement of the cytoskeleton are some of the most critical cellular events during EMT. Extensive research has focused on elucidating the signal transduction pathways co-opted by tumor cells to facilitate the dynamic EMT program. Transcription factors Snail and Twist are well characterized as the most influential factors on E-cadherin repression and EMT induction. Moreover, growth factor receptor signaling, including EGFR and TGFβR, controls numerous aspects of the EMT process, in part through modulation of Snail and Twist. Despite the extensive research reported on signaling networks responsible for EMT, much remains to be understood regarding this complex and dynamic cellular process.
Mesenchymal to epithelial transition (MET) is the reverse process of EMT to transition a cell that has acquired mesenchymal characteristics back to a cell with epithelial characteristics. In contrast to the extensive literature on EMT in tumorigenesis, there has been limited work to delineate the role and requirement of MET in disease progression and metastasis. It is postulated that a tumor cell with EMT features must transition back to an epithelial tumor phenotype to proliferate and colonize at distant organ sites; however, there is scant experimental evidence to confirm this hypothesis. Thus, further research is warranted to better understand the MET process in order to provide additional insights into the metastatic tumor cell program in HNSCC.
There are several limitations in the EMT literature that needs to be addressed to better define the EMT process in HNSCC. First, HNSCC covers numerous subsites, including nasopharyngeal, oropharynx, oral, and larynx. Each subsite may have unique characteristics and thus may regulate EMT through overlapping and distinct mechanisms. Second, most of the studies did not determine or report on human papillomavirus (HPV) status. HPV is known to promote tumorigenesis through a unique mechanism, however, the influence of HPV on EMT remains to be elucidated.77 Along with the many etiological differences of HNSCC, the EMT program may have nuances that have been largely ignored. Emerging evidence reveal that early and late stage EMT have different molecular profiles suggesting that the utilization of hallmark EMT markers E-Cadherin and Vimentin may be insufficient to capture the dynamic EMT process.54,59 In addition, sub-cellular localization of markers has been shown to be a better indicator of EMT.10,14,59,78 A potential possibility is that sub-cellular localization may be an indicator of early EMT whereas total levels may be an indicator of late EMT. Additional work to clarify these potential issues will better delineate the mechanisms that regulate the dynamic EMT program in HNSCC. Enhance understanding of the complex signaling networks essential for EMT may reveal novel druggable targets for the development of anti-cancer therapeutics for managing metastatic HNSCC.
Acknowledgments
This work was supported in part by National Institutes of Health (R01CA135096) and American Cancer Society (RSG0821901).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts & Figures 2011. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 2.Boyle P, Levin B. World cancer report. Lyon: IARC Press; 2008. International agency for research on cancer; p. 330. [Google Scholar]
- 3.Glisson B, Cango M, Feigenberg S. Head and neck tumors. In: Pazdur R, Wagman L, Camphausen K, Hoskins W, editors. Cancer management: A multidisciplinary approach. 13. Manhassett: CMP Health Media; 2011. [Google Scholar]
- 4.Chung CH, Parker JS, Ely K, Carter J, Yi Y, Murphy BA, et al. Gene expression profiles identify epithelial-to-mesenchymal transition and activation of Nuclear Factor- B signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer Research. 2006;66(16):8210–8218. doi: 10.1158/0008-5472.CAN-06-1213. [DOI] [PubMed] [Google Scholar]
- 5.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nature Reviews Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 6.Kalluri R, Weinberg R. The basics of epithelial-mesenchymal transition. The Journal of Clinical Investigation. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang T, Wu C, Ko J, Wang C, Lou P, Chang Y. Significance of tumor satellite variables in reflecting the epithelial-mesenchymal transition of tongue cancer. Oral Oncology. 2011;47(8):720–724. doi: 10.1016/j.oraloncology.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. Journal of Clinical Investigation. 2009;119(6):1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari H, et al. Introducing intermediate filaments: from discovery to disease. Journal of Clinical Investigation. 2009;119(7):1763–1771. doi: 10.1172/JCI38339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nijkamp MM, Span PN, Hoogsteen IJ, Van der Kogel AJ, Kaanders JH, Bussink J. Expression of E-cadherin and vimentin correlates with metastasis formation in head and neck squamous cell carcinoma patients. Radiotherapy and Oncologyn. 2011;99(3):344–348. doi: 10.1016/j.radonc.2011.05.066. [DOI] [PubMed] [Google Scholar]
- 11.Islam S, Carey TE, Wolf GT, Wheelock MJ, Johnson KR. Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. The Journal of Cell Biology. 1996;135(6):1643–1654. doi: 10.1083/jcb.135.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous Expression of N-Cadherin in Breast Cancer Cells Induces Cell Migration, Invasion, and Metastasis. The Journal of Cell Biology. 2000;148(4):779–790. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan X, Long Z, Yan M, Xu J, Xia L, Liu L, et al. Inhibition of Aurora-A suppresses epithelial-mesenchymal transition and invasion by downregulating MAPK in nasopharyngeal carcinoma cells. Carcinogenesis. 2008;29(10):1930–1937. doi: 10.1093/carcin/bgn176. [DOI] [PubMed] [Google Scholar]
- 14.Kudo Y, Kitajima S, Ogawa I, Hiraoka M, Sargolzaei S, Keikhaee MR, et al. Invasion and Metastasis of Oral Cancer Cells Require Methylation of E-Cadherin and/or Degradation of Membranous β-Catenin. Clinical Cancer Research. 2004;10(16):5455–5463. doi: 10.1158/1078-0432.CCR-04-0372. [DOI] [PubMed] [Google Scholar]
- 15.Tamura G, Yin J, Wang S, Fleisher A, Zou T, Abraham JM, et al. E-Cadherin gene promoter hypermethylation in primary human gastric carcinomas. JNCI Journal of the National Cancer Institute. 2000;92(7):569–573. doi: 10.1093/jnci/92.7.569. [DOI] [PubMed] [Google Scholar]
- 16.Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S. Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proceedings of the National Academy of Sciences. 1995;92(16):7416–7419. doi: 10.1073/pnas.92.16.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer K, Dowejko A, Bosserhoff A, Reichert T, Bauer RJ. P-cadherin induces an epithelial-like phenotype in oral squamous cell carcinoma by GSK-3beta-mediated Snail phosphorylation. Carcinogenesis. 2009;30(10):1781–1788. doi: 10.1093/carcin/bgp175. [DOI] [PubMed] [Google Scholar]
- 18.Bauer R, Dowejko A, Driemel O, Boßerhoff A, Reichert TE. Truncated P-cadherin is produced in oral squamous cell carcinoma. FEBS Journal. 2008;275(16):4198–4210. doi: 10.1111/j.1742-4658.2008.06567.x. [DOI] [PubMed] [Google Scholar]
- 19.Kovacs A, Dhillon J, Walker R. Expression of P-cadherin, but not E-cadherin or N-cadherin, relates to pathological and functional differentiation of breast carcinomas. Molecular Pathology. 2003;56(6):318–322. doi: 10.1136/mp.56.6.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyan P, Kudo Y, Yoshida M, Kamata N, Ogawa I, Takata T. N-cadherin expression is involved in malignant behavior of head and neck cancer in relation to epithelial-mesenchymal transition. Histology and Histopathology. 2011;26(2):147–156. doi: 10.14670/HH-26.147. [DOI] [PubMed] [Google Scholar]
- 21.Zidar N, Boštjančič E, Gale N, Kojc N, Poljak M, Glavač D, et al. Down-regulation of microRNAs of the miR-200 family and miR-205, and an altered expression of classic and desmosomal cadherins in spindle cell carcinoma of the head and neck—hallmark of epithelial-mesenchymal transition. Human Pathology. 2011;42(4):482–488. doi: 10.1016/j.humpath.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen PT, Kudo Y, Yoshida M, Iizuka S, Ogawa I, Takata T. N-cadherin expression is correlated with metastasis of spindle cell carcinoma of head and neck region. Journal of Oral Pathology & Medicine. 2010;40(1):77–82. doi: 10.1111/j.1600-0714.2010.00966.x. [DOI] [PubMed] [Google Scholar]
- 23.Zidar N, Gale N, Kojc N, Volavsek M, Cardesa A, Alos L, et al. Cadherin-catenin complex and transcription factor Snail-1 in spindle cell carcinoma of the head and neck. Virchows Archiv. 2008;453(3):267–274. doi: 10.1007/s00428-008-0649-y. [DOI] [PubMed] [Google Scholar]
- 24.Kojc N, Zidar N, Gale N, Poljak M, Komlos K, Cardesa A, et al. Transcription factors Snail, Slug, Twist, and SIP1 in spindle cell carcinoma of the head and neck. Virchows Archiv. 2009;454(5):549–555. doi: 10.1007/s00428-009-0771-5. [DOI] [PubMed] [Google Scholar]
- 25.Haynes J, Srivastava J, Madson N, Wittman T, Barber DL. Dynamic Actin Remodeling During Epithelial-Mesenchymal Transition Depends on Increased Moesin Expression. Molecular Biology of the Cell. 2011;22(24):4750–4764. doi: 10.1091/mbc.E11-02-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S, Jen Y, Jiang SS, Chang J, Hsiung CA, Wang C, et al. Galpha12-Mediated Pathway Promotes Invasiveness of Nasopharyngeal Carcinoma by Modulating Actin Cytoskeleton Reorganization. Cancer Research. 2009;69(15):6122–6130. doi: 10.1158/0008-5472.CAN-08-3435. [DOI] [PubMed] [Google Scholar]
- 27.Chen S, Lin C, Chang Y, Li J, Fu E, Chang F, et al. Effects of small interfering RNAs targeting Fascin on gene expression in oral cancer cells. Journal of Oral Pathology & Medicine. 2009;38(9):722–730. doi: 10.1111/j.1600-0714.2009.00769.x. [DOI] [PubMed] [Google Scholar]
- 28.Schittny J, Yurchenco P. Basement membranes: molecular organization and function in development and disease. Current Opinion in Cell Biology. 1989;1(5):983–988. doi: 10.1016/0955-0674(89)90069-0. [DOI] [PubMed] [Google Scholar]
- 29.Takkunen M, Ainola M, Vainionpää N, Grenman R, Patarroyo M, García de Herreros A, et al. Epithelial-mesenchymal transition downregulates laminin α5 chain and upregulates laminin α4 chain in oral squamous carcinoma cells. Histochemistry and Cell Biology. 2008;130(3):509–525. doi: 10.1007/s00418-008-0443-6. [DOI] [PubMed] [Google Scholar]
- 30.Dai Y-S, Cserjesi P. The Basic Helix-Loop-Helix Factor, HAND2, Functions as a Transcriptional Activator by Binding to E-boxes as a Heterodimer. Journal of Biological Chemistry. 2002;277(15):12604–12612. doi: 10.1074/jbc.M200283200. [DOI] [PubMed] [Google Scholar]
- 31.Hajra KM, Chen DY-S, Fearon ER. The SLUG Zinc-Finger Protein Represses E-Cadherin in Breast Cancer. Cancer Research. 2002;62(6):1613–1618. [PubMed] [Google Scholar]
- 32.Vesuna F, van Diest P, Chen JH, Raman V. Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochemical and Biophysical Research Communications. 2008;367(2):235–241. doi: 10.1016/j.bbrc.2007.11.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Y, Liang X, Zheng M, Zhu Z, Zhu G, Yang J, et al. Expression of c-kit and Slug correlates with invasion and metastasis of salivary adenoid cystic carcinoma. Oral Oncology. 2010;46(4):311–316. doi: 10.1016/j.oraloncology.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Jouppila-Mättö A, Närkiö-Mäkelä M, Soini Y, Pukkila M, Sironen R, Tuhkanen H, et al. Twist and snai1 expression in pharyngeal squamous cell carcinoma stroma is related to cancer progression. BMC Cancer. 2011;11(1):350. doi: 10.1186/1471-2407-11-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barker HE, Erler JT. The potential for LOXL2 as a target for future cancer treatment. Future Oncology. 2011;7(6):707–710. doi: 10.2217/fon.11.46. [DOI] [PubMed] [Google Scholar]
- 36.Peinado H, Moreno-Bueno G, Hardisson D, Perez-Gomez E, Santos V, Mendiola M, et al. Lysyl Oxidase-Like 2 as a New Poor Prognosis Marker of Squamous Cell Carcinomas. Cancer Research. 2008;68(12):4541–4550. doi: 10.1158/0008-5472.CAN-07-6345. [DOI] [PubMed] [Google Scholar]
- 37.Masuda M, Wakasaki T, Suzui M, Toh S, Joe AK, Weinstein IB. Stat3 orchestrates tumor development and progression: The Achilles’ Heel of head and neck cancers? Current Cancer Drug Targets. 2010;10(1):117–126. doi: 10.2174/156800910790980197. [DOI] [PubMed] [Google Scholar]
- 38.Su Y, Xie T, Sano D, Myers JN. IL-6 stabilizes Twist and enhances tumor cell motility in head and neck cancer cells through activation of Casein Kinase 2. Plos One. 2011;6(4):e19412. doi: 10.1371/journal.pone.0019412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mani SA, Guo W, Liao M, Eaton EN, Ayyanan A, Zhou AY, et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Chen K, Huang P, Chen Y, Chiou G, Lo W, et al. Curcubitacin 1 suppressed stem-like property and enhanced radiation-induced apoptosis in head and neck squamous carcinoma-derived CD44+ALDH1+ cells. Molecular Cancer Therapeutics. 2010;9(11):2879–2892. doi: 10.1158/1535-7163.MCT-10-0504. [DOI] [PubMed] [Google Scholar]
- 41.Larue L, Bellacosa A. Epithelial–mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24(50):7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 42.Hong K, Kim J, Hong J, Yoon H, Lee J, Hong S, et al. Inhibition of Akt activity induces the mesenchymal-to-epithelial reverting transition with restoring E-cadherin expression in KB and KOSCC-25B oral squamous cell carcinoma cells. Journal of Experimental & Clinical Cancer Research. 2009;28(1):28. doi: 10.1186/1756-9966-28-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song L, Li J, Liao W, Feng Y, Yu C, Hu L, et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. Journal of Clinical Investigation. 2009;119(12):3626–3636. doi: 10.1172/JCI39374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun L, Diamond ME, Ottaviano AJ, Joseph MJ, Ananthanarayan V, Munshi HG. Transforming Growth Factor- 1 promotes Matrix Metalloproteinase-9 mediated oral cancer invasion through Snail expression. Molecular Cancer Research. 2008;6(1):10–20. doi: 10.1158/1541-7786.MCR-07-0208. [DOI] [PubMed] [Google Scholar]
- 45.Qiao B, Johnson NW, Gao J. Epithelial-mesenchymal transition in oral squamous cell carcinoma triggered by transforming growth factor-β1 is Snail family-dependent and correlates with matrix metalloproteinase-2 and -9 expressions. International Journal of Oncology. 2010;37(3) doi: 10.3892/ijo_00000715. [DOI] [PubMed] [Google Scholar]
- 46.Gilles C, Newgreen DF, Sato H, Thompson EW. Madame Curie Bioscience Database [Internet] Austin (TX): Landes Bioscience; 2000. Matrix Metalloproteases and Epithelial-to-Mesenchymal Transition: Implications for Carcinoma Metastasis. [Google Scholar]
- 47.Orlichenko LS, Radisky DC. Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clinical & Experimental Metastasis. 2008;25(6):593–600. doi: 10.1007/s10585-008-9143-9. [DOI] [PubMed] [Google Scholar]
- 48.Joseph MJ, Dangi-Garimella S, Shields MA, Diamond ME, Sun L, Koblinski JE, et al. Slug is a downstream mediator of transforming growth factor-β1-induced matrix metalloproteinase-9 expression and invasion of oral cancer cells. Journal of Cellular Biochemistry. 2009;108(3):726–736. doi: 10.1002/jcb.22309. [DOI] [PubMed] [Google Scholar]
- 49.Sinpitaksakul SN, Pimkhaokham A, Sanchavanakit N, Pavasant P. TGF-β1 induced MMP-9 expression in HNSCC cell lines via Smad/MLCK pathway. Biochemical and Biophysical Research Communications. 2008;371(4):713–718. doi: 10.1016/j.bbrc.2008.04.128. [DOI] [PubMed] [Google Scholar]
- 50.Singh P, Srinivasan R, Wig J. The Smad family and its role in pancreatic cancer. Indian Journal of Cancer. 2011;48(3):351. doi: 10.4103/0019-509X.84939. [DOI] [PubMed] [Google Scholar]
- 51.Mangone FR, Walder F, Maistro S, Pasini FS, Lehn CN, Carvalho MB, et al. Smad2 and Smad6 as predictors of overall survival in oral squamous cell carcinoma patients. Molecular Cancer. 2010;9(1):106. doi: 10.1186/1476-4598-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabbah M, Emami S, Redeuilh G, Julien S, Prevost G, Zimber A, et al. Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resistance Updates. 2008;11(4–5):123–151. doi: 10.1016/j.drup.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Thariat J, Etienne-Grimaldi M, Grail D, Bensadoun R, Cayre A, Penault-Liorca F, et al. Epidermal Growth Factor Receptor protein detection in head and neck cancer patients: A many-faceted picture. Clinical Cancer Research. 2012;18(5):1313–1322. doi: 10.1158/1078-0432.CCR-11-2339. [DOI] [PubMed] [Google Scholar]
- 54.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nature Reviews Molecular Cell Biology. 2005;6(8):622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 55.Barr S, Thomson S, Buck E, Russo S, Petti F, Sujka-Kwok I, et al. Bypassing cellular EGF receptor dependence through epithelial-to-mesenchymal-like transitions. Clinical & Experimental Metastasis. 2008;25(6):685–693. doi: 10.1007/s10585-007-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuo J, Zhu W, Li M, Li X, Yi H, Zeng G, et al. Activation of EGFR promotes squamous carcinoma SCC10A cell migration and invasion via inducing EMT-like phenotype change and MMP-9-mediated degradation of E-cadherin. Journal of Cellular Biochemistry. 2011;112(9):2508–2517. doi: 10.1002/jcb.23175. [DOI] [PubMed] [Google Scholar]
- 57.Holz C, Niehr F, Boyko M, Hristozova T, Distel L, Budach V, et al. Epithelial-mesenchymal-transition induced by EGFR activation interferes with cell migration and response to irradiation and cetuximab in head and neck cancer cells. Radiotherapy and Oncology. 2011;101(1):158–164. doi: 10.1016/j.radonc.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 58.Lorch JH, Klessner J, Park J, Getsios S, Wu Y, Stack M, et al. Epidermal Growth Factor Receptor inhibition promotes desmosome assembly and strengthens intercellular adhesion in squamous cell carcinoma cells. Journal of Biological Chemistry. 2004;279(35):37191–37200. doi: 10.1074/jbc.M405123200. [DOI] [PubMed] [Google Scholar]
- 59.Richter P, Umbreit C, Franz M, Berndt A, Grimm S, Vecker A, et al. EGF/TGFβ1 co-stimulation of oral squamous cell carcinoma cells causes an epithelial-mesenchymal transition cell phenotype expressing laminin 332. Journal of Oral Pathology & Medicine. 2011;40(1):46–54. doi: 10.1111/j.1600-0714.2010.00936.x. [DOI] [PubMed] [Google Scholar]
- 60.Kim S, Cho NH, Kim K, Lee JS, Koo BS, Kim JH, et al. Correlations of oral tongue cancer invasion with matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF) expression. Journal of Surgical Oncology. 2006;93(4):330–337. doi: 10.1002/jso.20461. [DOI] [PubMed] [Google Scholar]
- 61.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. Journal of Mammary Gland Biology and Neoplasia. 2010;15(2):117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deraz EM, Kudo Y, Yoshida M, Obayashi M, Tsunematsu T, Tani H, et al. MMP-10/Stromelysin-2 promotes invasion of head and neck cancer. PLoS ONE. 2011;6(10):e25438. doi: 10.1371/journal.pone.0025438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ishikawa F, Miyoshi H, Nose K, Shibanuma M. Transcriptional induction of MMP-10 by TGF-β, mediated by activation of MEF2A and downregulation of class IIa HDACs. Oncogene. 2009;29(6):909–919. doi: 10.1038/onc.2009.387. [DOI] [PubMed] [Google Scholar]
- 64.Kupferman ME, Jiffar T, El-Naggar A, Yilmaz T, Zhou G, Xie T, et al. TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma. Oncogene. 2010;29(14):2047–2059. doi: 10.1038/onc.2009.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dudás J, Bitsche M, Schartinger V, Falkeis C, Sprinzl GM, Riechelmann H. Fibroblasts produce brain-derived neurotrophic factor and induce mesenchymal transition of oral tumor cells. Oral Oncology. 2011;47(2):98–103. doi: 10.1016/j.oraloncology.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson W, Hay M. Targeting hypoxia in cancer therapy. Nature Reviews Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 67.Yang M, Wu M, Chiou S, Chen P, Chang S, Liu C, et al. Direct regulation of TWIST by HIF-1α promotes metastasis. Nature Cell Biology. 2008;10(3):295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 68.Liang X, Zheng M, Jiang J, Zhu G, Yang J, Tang Y. Hypoxia-inducible factor-1 alpha, in association with TWIST2 and SNIP1, is a critical prognostic factor in patients with tongue squamous cell carcinoma. Oral Oncology. 2011;47(2):92–97. doi: 10.1016/j.oraloncology.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 69.Yang M, Hsu DS, Wang H, Wang H, Lan H, Yang W, et al. Bmi1 is essential in Twist1-induced epithelial–mesenchymal transition. Nature Cell Biology. 2010;12(10):982–992. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 70.Chi-Hung H, Wen-Hao Y, Shyue-Yih C, Shyh-Kuan T, Cheng-Hwei T, Jung-Yie K, et al. Regulation of membrane-type 4 matrix metalloproteinase by SLUG contributes to hypoxia-mediated metastasis. Neoplasia. 2009;11(12):1371–1382. doi: 10.1593/neo.91326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Z, Malhotra PS, Thomas GR, Ondrey FG, Duffey DC, Smith CW, et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999;5(6):1369–79. [PubMed] [Google Scholar]
- 72.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-Mesenchymal Transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 73.Yadav A, Kumar B, Datta J, Teknos T, Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Molecular Cancer Research. 2011;9(12):1658–1667. doi: 10.1158/1541-7786.MCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dohadwala M, Wang G, Heinrich E, Luo J, Lau O, Shih H, et al. The role of ZEB1 in the inflammation-induced promotion of EMT in HNSCC. Otolaryngology - Head and Neck Surgery. 2010;142(5):753–759. doi: 10.1016/j.otohns.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 75.St John MA, Dohadwala M, Luo J, Wang G, Lee G, Shih H, et al. Proinflammatory mediators upregulate Snail in head and neck squamous cell carcinoma. Clinical Cancer Research. 2009;15(19):6018–6027. doi: 10.1158/1078-0432.CCR-09-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hong KO, Ji-Hong K, Ji-Soo H, Hye-Jung Y, Jae-Il L, Sam-Pyo H, et al. Inhibition of Akt activity induces the mesenchymal-to-epithelial reverting transition with restoring E-cadherin expression in KB and KOSCC-25B oral squamous cell carcinoma cells. Journal of Experimental & Clinical Cancer Research. 2009;28(28) doi: 10.1186/1756-9966-28-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Seminars in Oncology. 2004;31(6):744–754. doi: 10.1053/j.seminoncol.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 78.Schneider FT, Schanzer A, Czupalla CJ, Thom S, Engels K, Schmidt MH, et al. Sonic Hedgehog Acts as a Negative Regulator of β-Catenin Signaling in the Adult Tongue Epithelium. American Journal Of Pathology. 2010;177(1):404–414. doi: 10.2353/ajpath.2010.091079. [DOI] [PMC free article] [PubMed] [Google Scholar]