Abstract
Previous postmortem and neuroimaging studies have repeatedly suggested alterations in serotonin 5-HT2A receptor (5-HT2AR) binding associated with the pathophysiology of schizophrenia. These studies were performed with ligands, such as ketanserin, altanserin and LSD, that may bind with high-affinity to different structural or functional conformations of the 5-HT2AR. Interpretation of results may also be confounded by chronic antipsychotic treatment and suicidal behavior in the schizophrenia group. We quantified 5-HT2AR density by radioligand binding assays in postmortem prefrontal cortex of antipsychotic-free (n=29) and antipsychotic-treated (n=16) schizophrenics, suicide victims with other psychiatric diagnoses (n=13), and individually matched controls. [3H]Ketanserin binding, and its displacement by altanserin or the LSD-like agonist DOI, was assayed. Results indicate that the number of [3H]ketanserin binding sites to the 5-HT2AR was increased in antipsychotic-free (128±11%), but not in antipsychotic-treated (92±12%), schizophrenic subjects. In suicide victims, [3H]ketanserin binding did not differ as compared to controls. Aging correlated negatively with [3H]ketanserin binding in schizophrenia, suicide victims and controls. The fraction of high-affinity sites of DOI displacing [3H]ketanserin binding to the 5-HT2AR was increased in antipsychotic-free schizophrenic subjects. Functional uncoupling of heterotrimeric G proteins led to increased fraction of high-affinity sites of altanserin displacing [3H]ketanserin binding to the 5-HT2AR in schizophrenic subjects, but not in controls. Together, these results suggest that the active conformation of the 5-HT2AR is up-regulated in prefrontal cortex of antipsychotic-free schizophrenic subjects, and may provide a pharmacological explanation for discordant findings previously obtained.
1. Introduction
5-Hydroxytryptamine (serotonin, 5-HT) receptors constitute a large family of evolutionary conserved seven transmembrane G protein-coupled receptors (GPCRs) (Millan et al., 2008). One of the serotonin receptor subtypes, the 5-HT2AR, has been involved in the symptoms of schizophrenia, and proposed to contribute to the molecular mechanisms by which atypical antipsychotic drugs, such as clozapine, olanzapine and risperidone, induce their clinical effects (Gonzalez-Maeso and Sealfon, 2009; Fribourg et al., 2011; Kurita et al., 2012). Second generation, or atypical, antipsychotic drugs all have in common a high-affinity for the 5-HT2AR, and a lower affinity for the dopamine D2 receptor (Miyamoto et al., 2005). Hallucinogenic 5-HT2AR agonists, such as mescaline, psilocybin and lysergic acid diethylamide (LSD), produce in normal subjects symptom profiles comparable to those seen in acutely ill unmedicated or first-episode schizophrenics (Young, 1974; Hermle et al., 1992; Vollenweider et al., 1998; Gouzoulis-Mayfrank et al., 2005; Quednow et al., 2011). Hallucinogenic drugs aggravate psychosis in schizophrenia patients (Hoch et al., 1952). Of particular interest is also the finding of hallucinogenic (LSD)-induced psychotic symptoms in relatives of schizophrenic patients (Anastasopoulos and Photiades, 1962), suggesting that individuals with greater predisposition to schizophrenia are more susceptible to the psychotic responses that require activation of the 5-HT2AR. In murine models, we and others have demonstrated that 5-HT2AR-regulated pathways on cortical pyramidal neurons are necessary to mediate the signaling pattern and behavioral responses to hallucinogenic drugs (see [Gonzalez-Maeso and Sealfon, 2009] for review).
Theory and experimental evidence suggest that GPCRs adopt multiple structural conformations when bound to different ligands. Drugs that increase or decrease the function of GPCRs are thought to modulate the proportion of receptors that are in the structurally active conformations relative to those in inactive, non-signaling conformations, respectively. Agonists bind with higher affinity to the active conformations of the receptor, whereas inverse agonists are ligands that preferentially bind and stabilize inactive conformational states. Interestingly, recent observations suggest that most clinically effective antipsychotic drugs are, in fact, 5-HT2AR inverse agonists rather than simply neutral antagonists—ligands that compete for the same orthosteric binding site and prevent the cellular responses induced by agonists and inverse agonists (Egan et al., 1998; Fribourg et al., 2011). Since activation and decrease in the basal activity of the receptor represent a common feature of all hallucinogenic and atypical antipsychotic drugs, respectively, these findings suggest that the 5-HT2AR may be involved in the mechanisms responsible for psychotic symptoms in schizophrenic patients.
Frontal cortex plays an important role in cognition and perception, and has been implicated more recently in schizophrenia and other psychotic disorders (Gonzalez-Maeso et al., 2008; Gonzalez-Maeso and Sealfon, 2009; Kurita et al., 2012). Many laboratories, including ours, have investigated the level of expression of 5-HT2AR protein in frontal cortex of schizophrenic subjects. Most of this work has been performed in postmortem tissue samples with the use of radioligands, including [3H]ketanserin (Reynolds et al., 1983; Mita et al., 1986; Laruelle et al., 1993; Burnet et al., 1996; Dean and Hayes, 1996; Dean et al., 1996; Dean et al., 1998; Dean et al., 1999; Marazziti et al., 2003; Matsumoto et al., 2005; Dean et al., 2008; Gonzalez-Maeso et al., 2008; Kang et al., 2009) and [3H]LSD (Bennett et al., 1979; Whitaker et al., 1981; Joyce et al., 1993; Gurevich and Joyce, 1997), as well as in untreated first-episode schizophrenic patients by positron emission tomography (PET) with [18F]altanserin (Rasmussen et al., 2010). Remarkably, there are striking differences in the results obtained: some studies suggested up-regulation of 5-HT2AR binding sites, whereas others pointed toward absence of alterations or down-regulation in the number of binding sites. Recent reviews discuss the possible role of demographic and clinical measures, such as age, treatment with antipsychotic or other psychotropic drugs, and suicide as cause of death, in these discrepant results between studies (Dean, 2003; Dean et al., 2008; Gonzalez-Maeso and Sealfon, 2009). Here, we extend our previous observations with evidence that [3H]ketanserin binding sites are up-regulated in postmortem frontal cortex of antipsychotic-free schizophrenic subjects, and demonstrate that the density of [3H]ketanserin binding sites is affected by antipsychotic drug treatment and aging. Our findings also suggest that this up-regulation may not be related to suicidal behavior, since the number of [3H]ketanserin binding sites was unchanged in suicide victims with other neuropsychiatric disorders. With the use of ligands that preferentially bind different structural and/or functional receptor conformations, our findings could also provide a pharmacological explanation that may unify previous efforts on the quantification of 5-HT2AR density in frontal cortex of schizophrenic subjects and controls.
2. Experimental procedures
2.1. Postmortem human brain samples
Human brains were obtained at autopsies performed in the Basque Institute of Legal Medicine, Bilbao, Spain, in compliance with policies of research and ethical boards for postmortem brain studies between 1992 and 2008. Deaths were subjected to retrospective searching for previous medical diagnosis and treatment using examiner’s information and records of hospitals and mental health centers. After searching of antemortem information was fulfilled, 45 subjects who had met inclusion criteria of schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R, DSM-IV or DSM-IV-R) were selected. Diagnosis of schizophrenia had been established by clinicians in charge of patients before death (Supplementary Tables 1 and 2). In the schizophrenia group, 37 subjects were suicide victims and the other 8 (4 antipsychotic-free and 4 antipsychotic-treated subjects) died of natural causes or motor vehicle accident. In order to explore the relationship between suicide completion and 5-HT2AR density, an independent group of 13 suicide victims with other antemortem psychiatric disorders was collected (Supplementary Table 3). Of note, some of the antemortem psychiatric diagnoses are not included in the Diagnostic and Statistical Manual of Mental Disorders (e.g., reactive depression and neurotic depression).
A toxicological screening for antipsychotics, antidepressants, other drugs and ethanol was performed on blood, urine, liver and gastric contents samples. Subjects who gave negative results for antipsychotic drugs in the toxicological screening were considered antipsychotic-free at death. The toxicological assays were performed at the National Institute of Toxicology, Madrid, Spain, using a variety of standard procedures including radioimmunoassay, enzymatic immunoassay, high-performance liquid chromatography and gas chromatography-mass spectrometry.
Controls for the present study were chosen among the collected brains on the basis, whenever possible, of the following cumulative inclusion criteria: (1) negative medical information on the presence of neuropsychiatric disorders or drug abuse; (2) appropriate gender, age (mean of differences 2 years, range 0–9 years), and postmortem delay defined as the time between death and tissue dissection/freezing (mean of differences 12 h, range 0–51 h), to match each subject in the schizophrenia or suicide group; (3) sudden and unexpected death (motor vehicle accidents); and (4) toxicological screening for psychotropic drugs with negative results except for ethanol.
Specimens of prefrontal cortex (Brodmann’s area 9) were dissected at autopsy (0.5–1 g tissue) on an ice-cooled surface following standard procedures (Rajkowska and Goldman-Rakic, 1995), and immediately stored at −80°C until use. Tissue pH values were as follow: control subjects, 6.5±0.08; schizophrenic subjects, 6.4±0.09; suicide victims, 6.3±0.03. Brain samples were also assayed for RNA integrity number (RIN) using the Agilent 2100 Bioanalyzer (Agilent Technologies) (control subjects: 7.8±0.1; schizophrenic subjects: 7.3±0.3; suicide victims, 7.3±0.2). The definitive pairs of antipsychotic-free schizophrenics, antipsychotic-treated schizophrenics, suicide victims with other psychiatric disorders, and individually matched controls are shown in Supplementary Tables 1, 2 and 3, respectively. Younger and older control subjects were included to investigate the effect of aging on 5-HT2AR binding (Supplementary Table 4).
2.2. Mouse brain samples
Experiments were performed on adult (8–12 weeks old) 5-HT2AR knockout (KO) and wild-type male 129S6/Sv mice (Gonzalez-Maeso et al. 2008). Animals were housed at 12 h light/dark cycle at 23°C with food and water ad libitum. The day of the experiment, mice were sacrificed by cervical dislocation, and bilateral frontal cortex was dissected and frozen at −80°C, or immediately processed for radioligand binding assays. This brain region (bregma 1.90 to 1.40 mm) includes motor cortex and somatosensory cortex. The coordinates were taken according to a published atlas of the 129S6/Sv mouse strain (Hof et al., 2000). The Institutional Animal Use and Care Committee at Mount Sinai School of Medicine approved all experimental procedures.
2.3. Materials and drugs
1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), guanosine 5′-[β,γ-imido]triphosphate trisodium salt hydrate [Gpp(NH)p], GTPγS, lysergic acid diethylamide (LSD), and ethylenediaminetetraacetic acid (EDTA) were purchased from Sigma-Aldrich. [3H]Ketanserin was obtained from PerkinElmer Life and Analytical Sciences, Inc. Altanserin and methysergide were purchased from Tocris Cookson Inc. All other chemicals were obtained from standard sources.
2.4. Radioligand binding
Tissue samples of frontal cortex were homogenized using an Ultra-Turrax T8 homogenizer in 1 ml of homogeneization buffer (5 mM Tris-HCl; 0.25 M sucrose, pH 7.4). The crude homogenate was centrifuged at 1,000μg for 5 min at 4°C, and the supernatant was re-centrifuged at 40,000μg for 15 min at 4°C. The resultant pellet (P2 fraction) was washed twice in incubation buffer (50 mM Tris-HCl; pH 7.4) and re-centrifuged in similar conditions. Aliquots were stored at −80°C until assay.
[3H]Ketanserin binding assays were performed as previously reported with minor modifications (Gonzalez-Maeso et al., 2008). Briefly, [3H]ketanserin binding (10 nM) was measured at equilibrium in 250 μl aliquots (incubation buffer) of membrane preparations (20–40 μg protein per well) which were incubated in 96-well plates at 37°C for 60 min. We selected this concentration of [3H]ketanserin because our previous findings in mouse and postmortem human frontal cortex membrane preparations demonstrate that at 10 nM the number of binding sites obtained is similar to the maximum density of receptors (Bmax) estimated by non-linear regression analysis of [3H]ketanserin binding saturation curves (Gonzalez-Maeso et al., 2008). Competition curves were carried out by incubating DOI (10−12-10−4 M; eighteen concentrations), altanserin (10−16-10−5 M; thirteen concentrations) or LSD (10−10-10−4 M; fourteen concentrations) in binding buffer containing 2.0 nM [3H]ketanserin. This concentration of [3H]ketanserin is similar to its affinity (KD) value (see [Gonzalez-Maeso et al., 2008]). In order to evaluate the affinity of competitive ligands for inactive states of the 5-HT2AR, in postmortem human brain, some of the competition binding experiments were performed in incubation buffer supplemented with Gpp(NH)p (100 μM), EDTA (1 mM), and NaCl (140 mM). Likewise, in mouse brain, some of the experiments were performed in incubation buffer supplemented with GDP (50 μM), GTPγS (0.5 nM) EGTA (1 mM), MgCl2 (3 mM) and NaCl (100 mM). Non-specific binding was determined in the presence of 10 μM methysergide. Reactions were incubated for 60 min at 37°C. Free ligand was separated from bound ligand by rapid filtration under vacuum (1450 FilterMate Harvester, PerkinElmer) through GF/C glass fiber filters. The filters were then rinsed three times with 300 μl binding buffer, dried (90°C, 10 min), and counted for radioactivity by liquid scintillation spectrometry using a MicroBeta TriLux counter (PerkinElmer).
2.5. Statistical analyses
Subject pairing was selected as the best design to control for experimental variance by the parallel processing of tissue samples from each subject pair (case and matched control). Therefore, analyses of antipsychotic-free and antipsychotic-treated schizophrenic subjects were conducted in separate along the study. [3H]Ketanserin binding data at 10 nM followed a Gaussian distribution analyzed by the D’Agostino and Pearson omnibus normality test when expressed both as binding density in fmol/mg protein and as percentage of specific binding relative to individually matched controls. Importantly, when determining the number of [3H]ketanserin binding sites in schizophrenic subjects (see Figs. 1 and 2 below), values are analyzed as percentage of specific binding relative to individually matched controls in order to avoid intra-group differences mostly due to aging (see below for discussion).
Fig. 1.
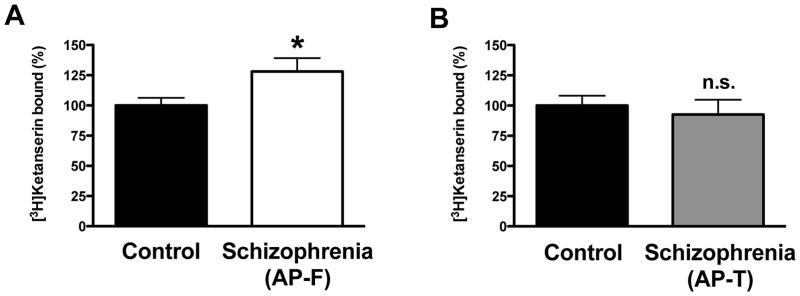
Density of 5-HT2AR shown as [3H]ketanserin specific binding (10 nM) in frontal cortex of schizophrenic subjects. (A) Antipsychotic-free (AP-F) schizophrenic subjects (n=29) and controls (n=29). (B) Antipsychotic-treated (AP-T) schizophrenic subjects (n=16) and controls (n=16). Data are shown as percentage of [3H]ketanserin specific binding relative to individually matched controls. *p<0.05; n.s., not significant; Student’s t-test. See Supplementary Tables 1 and 2 for demographic information. All values represent means±SEM.
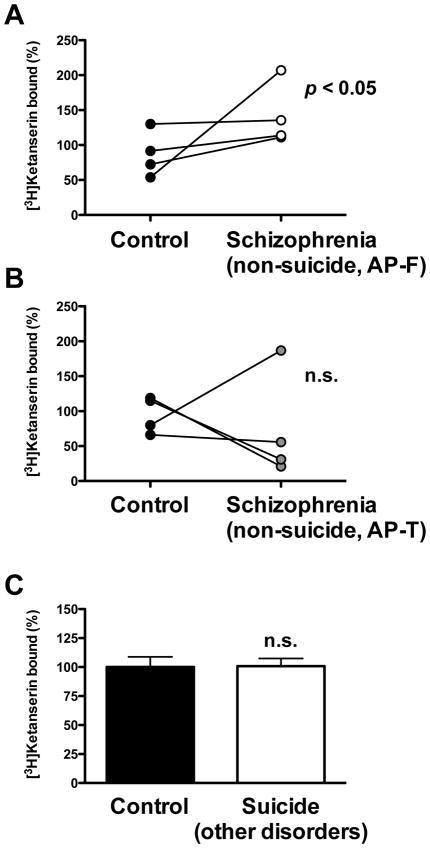
Fig. 2.
(A) Individual representation of [3H]ketanserin specific binding (10 nM) in frontal cortex of non-suicide—antipsychotic-free (AP-F) schizophrenic subjects (n=4) and controls (n=4). (B) Individual representation of [3H]ketanserin specific binding in frontal cortex of non-suicide—antipsychotic-treated (AP-T) schizophrenic subjects (n=4) and controls (n=4). (C) [3H]Ketanserin specific binding in frontal cortex of suicide victims with other psychiatric disorders (n=13) and controls (n=13). Data are shown as percentage of [3H]ketanserin specific binding relative to individually matched controls. p<0.05; n.s., not significant; Student’s t-test. See Supplementary Tables 1, 2 and 3 for demographic information. All values represent means±SEM.
Radioligand binding displacement curves were analyzed using a nonlinear curve fitting (GraphPad Prism and InVivoStat softwares). The selection between models of competition curves by DOI, LSD, or altanserin against [3H]ketanserin binding was made by the extra-sum-of-squares (F test). Following the nonlinear curve fitting, Ki values for DOI, LSD, and altanserin were calculated from the corresponding IC50 values. Differences in the binding profiles were assessed by unpaired Student’s t-test of normalized parameters. Pearson’s coefficient for simple correlation was calculated to test for possible association among variables. The influence of age as potential confounding variable on [3H]ketanserin binding was assessed with ANCOVA. A further two-way ANOVA was used to evaluate the potential interaction between suicide and schizophrenia. The level of significance was chosen at p=0.05. All data are presented as mean±SEM.
3. Results
3.1. [3H]Ketanserin Binding is Increased in Postmortem Frontal Cortex of Antipsychotic-Free Schizophrenic Subjects
[3H]Ketanserin binds with high-affinity to both 5-HT2A and 5-HT2C receptors. Using the 5-HT2 receptor ligand methysergide (10 μM) to define non-specific binding, we found that [3H]ketanserin binding sites are abolished in frontal cortex of 5-HT2A knockout (KO) mice as compared to wild type littermate controls (Supplementary Fig. 1). These findings validate the use of specific [3H]ketanserin biding as a tool to measure 5-HT2AR density.
We first investigated the number of [3H]ketanserin binding sites in postmortem frontal cortex of schizophrenic subjects and controls. We compared two different groups of subjects: those either antipsychotic-free or antipsychotic-treated at time of death. Each of these two groups included individually matched controls (see Supplementary Tables 1 and 2 for demographic and toxicological information). Similar to our previous observations (Gonzalez-Maeso et al., 2008), [3H]ketanserin binding to the 5-HT2AR was higher (128±11%) in antipsychotic-free schizophrenic subjects as compared to individually matched controls (Fig. 1A). However, no differences (92±12%) were observed between schizophrenic subjects treated with antipsychotic drugs and individually matched controls (Fig. 1B). Of note, 25 (13 antipsychotic-free and 12 antipsychotic-treated) out of these 45 schizophrenic subjects (29 antipsychotic-free and 16 antipsychotic-treated) and control pairs were included in our previous study (Gonzalez-Maeso et al., 2008). In a effort to control for confounding influence of age, we performed an ANCOVA with control, antipsychotic-free and antipsychotic-treated as the grouping effect, [3H]ketanserin binding as the dependent variable and age as covariate. Consistent with the previous finding, [3H]ketanserin binding to the 5-HT2AR was higher (F[2,86]=3.71, p<0.05) in antipsychotic-free subjects as compared to controls (p<0.05, Fisher’s post-hoc test), but did not differ between antipsychotic-treated subjects and controls (p>0.05, Fisher’s post-hoc test).
Postmortem brain studies of suicide victims have suggested a relationship between 5-HT2AR density in frontal cortex and suicidal behavior (Oquendo et al., 2006). We found that up-regulation of [3H]ketanserin binding to the 5-HT2AR remained statistically significant in non-suicide antipsychotic-free schizophrenic subjects (Fig. 2A), without changes in non-suicide antipsychotic-treated schizophrenic subjects (Fig. 2B). However, the low number of cases in these groups of non-suicide schizophrenic subjects (n=4 per group) prompted us to investigate the number of [3H]ketanserin binding sites in frontal cortex of suicide victims with other psychiatric disorders and individually matched controls (see Supplementary Table 3 for demographic information). We found no significant differences in [3H]ketanserin binding to the 5-HT2AR between suicide subjects and controls (Fig. 2C). Among antipsychotic-free subjects, two-way ANOVA demonstrated the absence of interaction between schizophrenia and suicide (F[1,83]=1.99, p>0.05) with significant effect of schizophrenia condition (F[1,83]=6.78, p<0.01) but not of suicide (F[1,83]=2.11, p>0.05). In contrast, antipsychotic-treated subjects did not show influence of schizophrenia condition (F[1,68]=1.47, p>0.05), suicide (F[1,68]=1.22, p>0.05) or interaction between both factors (F[1,68]=1.10, p>0.05).
3.2. Effect of age on [3H]ketanserin binding
The [3H]ketanserin binding to postmortem frontal cortex membrane preparations displayed a negative correlation with the age at death (R=−0.39; p<0.0001) (Fig. 3, see also Supplementary Table 4 for additional control subjects not included in Supplementary Tables 1–3). Previous findings suggest that a second order polynomial equation (parabola) provides the best fit to the data describing the effect of aging on [3H]ketanserin binding in postmortem human frontal cortex (Gross-Isseroff et al., 1990b). We found that a first order polynomial equation (straight line) provided the best fit to the data (first order polynomial versus second order polynomial: F[1,106]=0.15, p>0.05, all subjects in Supplementary Tables 1–4). Similar findings were obtained in antipsychotic-free schizophrenics (Supplementary Table 1), antipsychotic-treated schizophrenics (Supplementary Table 2), suicide victims with other psychiatric disorders (Supplementary Table 3), and controls (Supplementary Tables 1–4), (data not shown). Based on this, the first order polynomial equation was chosen as the best-fit model. According to this linear model, the average decrease per decade for [3H]ketanserin binding was 6.67% relative to the estimated value in a 10-year-old subject. Further analysis showed that the differences between the slopes of controls and antipsychotic-treated schizophrenics are significant (F[1,65]=6.02, p<0.01). No significant differences were observed between the slopes of controls and antipsychotic-free schizophrenics (F[1,76]=0.27, p>0.05). Slopes of linear regressions were as follows: −2.25±0.79 (controls in Supplementary Tables 1–4); −3.55±2.55 (antipsychotic-free schizophrenics); −8.05±2.84 (antipsychotic-treated schizophrenics). No significant correlation was observed between [3H]ketanserin binding and postmortem delay (time between death and tissue dissection/freezing; R=0.02; p>0.05), freezing storage time at −80°C (R=−0,02; p>0.05), RIN values (R=0,06; p>0.05), or tissue pH (R=−0.05; p>0.05).
Fig. 3.
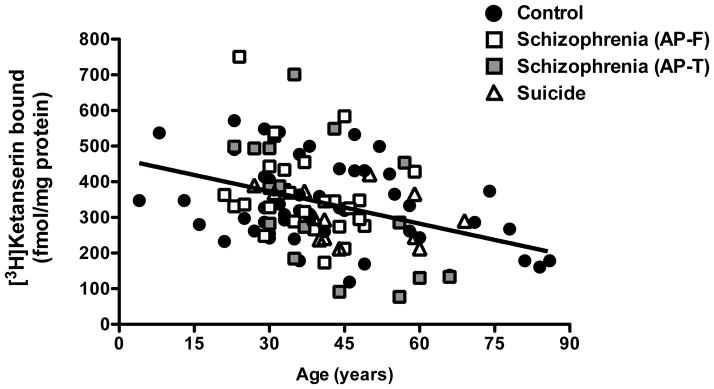
Age-related changes in [3H]ketanserin specific binding (10 nM) in frontal cortex of antipsychotic-free (AP-F) schizophrenics (n=29), antipsychotic-treated (AP-T) schizophrenics (n=16), suicide victims with other psychiatric disorders (n=13), and controls (n=55). Pearson’s correlation: R=−0.37; p<0.0001.
3.3. Fraction of high-affinity sites of DOI displacing [3H]ketanserin binding is increased in antipsychotic-free schizophrenic subjects
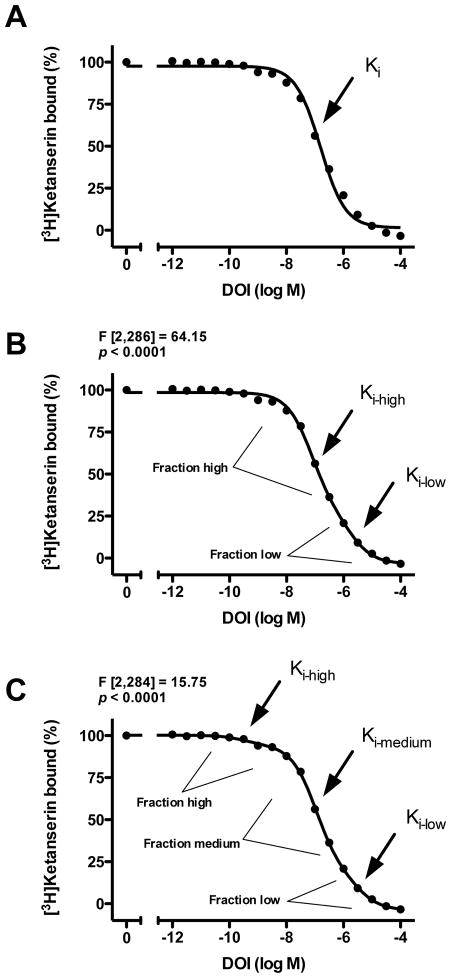
We first examined the pharmacological parameters of the 5-HT2AR agonist DOI displacing [3H]ketanserin binding in postmortem frontal cortex. As shown in Fig. 4, we found that DOI displaced [3H]ketanserin binding in a triphasic manner (monophasic versus biphasic: F[2,286]=64.15, p<0.0001; biphasic versus triphasic: F[2,284]=15.75, p<0.0001).
Fig. 4.
(A,B,C) Displacement curves of [3H]ketanserin specific binding (2 nM) by DOI in frontal cortex of control subjects (n=24). The same dataset was analyzed as monophasic (A), biphasic (B) and triphasic (C) displacement curve. Data were best fit to a biphasic (B) compared to monophasic (A) displacement curve. Data were best fit to a triphasic (C) compared to biphasic (B) displacement curve. All values represent means±SEM. Standard errors are not depicted when they are smaller than the symbols.
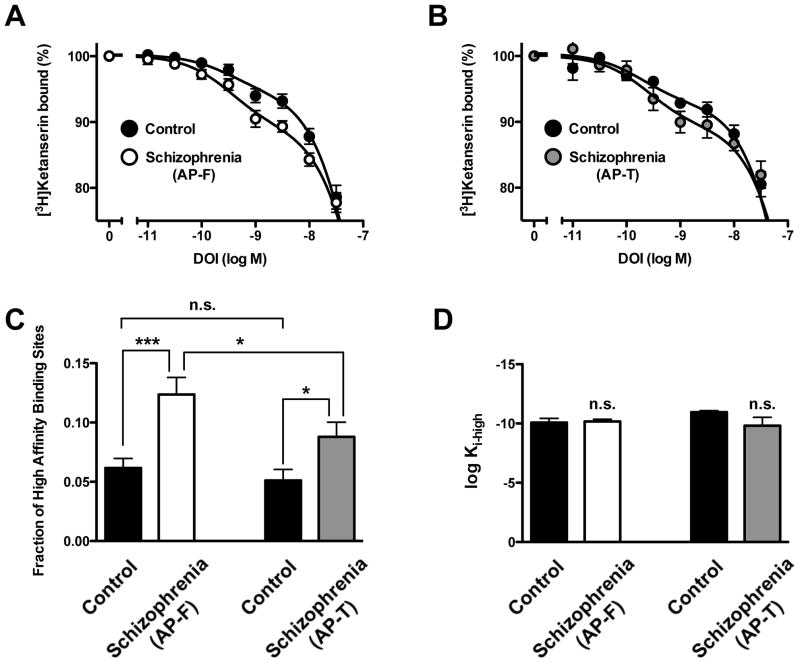
Next, we compared the bindings sites for DOI displacing [3H]ketanserin binding in schizophrenic subjects and controls. Interestingly, the fraction of high-affinity sites of DOI displacing [3H]ketanserin binding was significantly increased in antipsychotic-free as well as in antipsychotic-treated schizophrenic subjects compared to individually matched controls (Fig. 5A, Fig. 5B, Fig. 5C, and Table 1). Notably, this fraction of high-affinity binding sites was increased in antipsychotic-free as compared to antipsychotic-treated schizophrenic subjects (Fig. 5C, and Table 1). No statistically significant differences were found between Ki-high, Ki-medium, Ki-low, or fractions of medium and low affinity sites of DOI displacing [3H]ketanserin binding in untreated schizophrenics, treated schizophrenics, and individually matched controls (Figs. 5A–5D, and Table 1). No differences were found between the two groups of control subjects (Fig. 5C, and Table 1).
Fig. 5.
(A) [3H]Ketanserin specific binding (2 nM) displacement curves by DOI in postmortem frontal cortex of antipsychotic-free (AP-F) schizophrenic subjects and controls. (B) [3H]Ketanserin specific binding (2 nM) displacement curves by DOI in postmortem frontal cortex of antipsychotic-treated (AP-T) schizophrenic subjects and controls. (C) Fraction of high-affinity sites of DOI displacing [3H]ketanserin specific binding. (D) Normalized high-affinity (log Ki-high) of DOI displacing [3H]ketanserin specific binding in schizophrenic subjects and individually matched controls. *p<0.05; ***p<0.001; n.s., not significant; Student’s t-test. See Supplementary Tables 1 and 2 for demographic information. See Table 1 for statistical analysis. All values represent means±SEM.
Table 1.
[3H]Ketanserin binding displacement curves by DOI in frontal cortex membrane preparations of antipsychotic-free (AP-F) schizophrenics, antipsychotic-treated (AP-T) schizophrenics, and controls.
| Control | Schizophrenia(AP-F) | Control | Schizophrenia (AP-T) | |
|---|---|---|---|---|
|
|
||||
| log Ki-high | −10.21 ± 0.32 | −10.20 ± 0.15*** | −10.78 ± 0.33 | −10.53 ± 0.33*,+ |
| Fraction high (%) | 5.51 ± 1.06 | 10.34 ± 0.88*** | −05.75 ± 1.01 | 8.76 ± 1.51*,+ |
| log Ki-medium | −7.70 ± 0.05 | −7.55 ± 0.03*** | 0-7.69 ± 0.04 | −7.56 ± 0.07*,+ |
| Fraction medium (%) | 72.5 ± 5.31 | 73.8 ± 1.68*** | --78.0 ± 2.74 | 75.2 ± 4.20*,+ |
| log Ki-low | −6.25 ± 0.16 | −5.91 ± 0.19*** | 0-6.06 ± 0.23 | −5.94 ± 0.37*,+ |
p<0.05;
p<0.001 when compared with individually matched control subjects by Student’s t-test.
p<0.05 when compared with antipsychotic-free schizophrenic subjects by Student’s t-test.
Data were best fit to a triphasic compared to biphasic displacement curve by F-test (p<0.0001).
See Supplementary Tables 1 and 2 for demographic information. All values represent means ± SEM.
3.4. Functional uncoupling of G proteins increases the fraction of high-affinity sites of altanserin displacing [3H]ketanserin binding in schizophrenic subjects, but not in controls
The highly specific alterations of both [3H]ketanserin and DOI binding to the 5-HT2AR in postmortem frontal cortex of antipsychotic-free schizophrenic subjects (see Fig. 1 and Fig. 5 above), together with previous findings by PET scan showing decrease in [18F]altanserin binding in antipsychotic-naïve patients with first-episode schizophrenia (Rasmussen et al., 2010), led us to examine the pharmacological profile of altanserin displacing [3H]ketanserin binding in antipsychotic-free and antipsychotic-treated schizophrenic subjects, and individually matched controls. In order to provide additional information regarding the affinity of altanserin for active and inactive states of the 5-HT2AR, we also investigated the effect of functional uncoupling of heterotrimeric G proteins by the non-hydrolyzable GTP analog Gpp(NH)p on the affinity of altanserin displacing [3H]ketanserin binding.
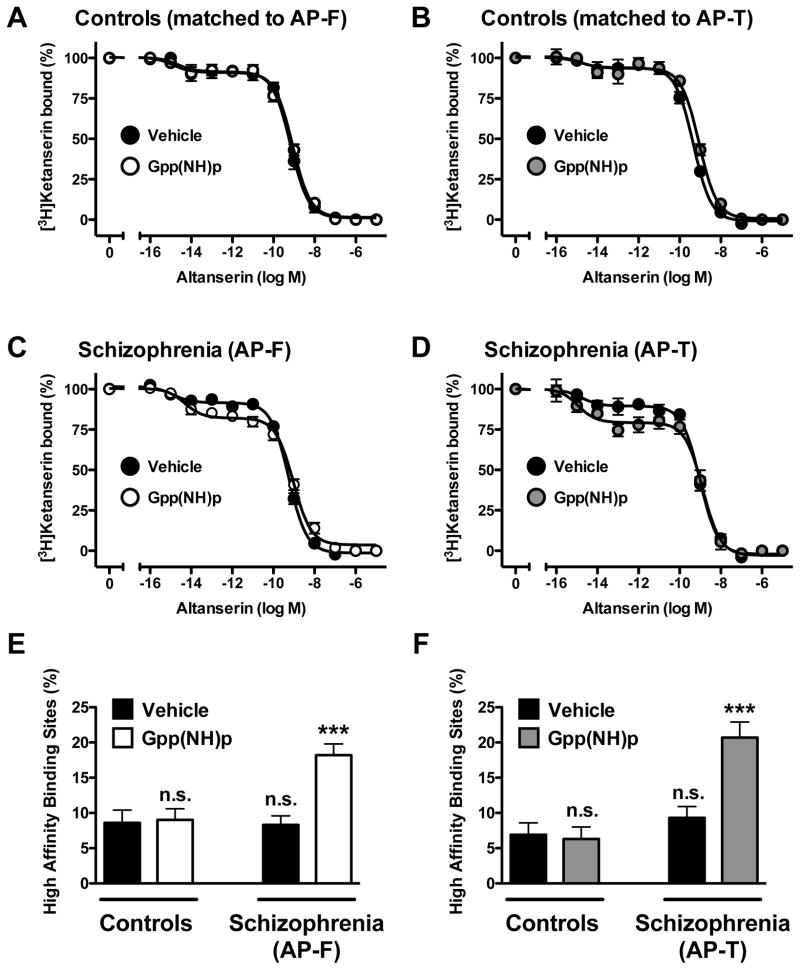
Non-hydrolyzable GTP analogs are characterized for their ability to uncouple heterotrimeric G proteins from their respective GPCRs, shifting agonist or inverse agonist competition curves to lower or higher overall affinities, respectively (see Supplementary Fig. 2). In control subjects, displacement of [3H]ketanserin binding by altanserin was best described by a two-site model (Fig. 6A, Fig. 6B, and Table 2). We also found that the affinities of altanserin displacing [3H]ketanserin binding were not affected in the presence of Gpp(NH)p. In schizophrenic subjects, altanserin also displaced [3H]ketanserin binding in a biphasic manner, with no differences between antipsychotic-free or antipsychotic-treated schizophrenics, and individually matched controls (Fig. 6C, Fig. 6D, and Table 2). Notably, the presence Gpp(NH)p significantly increased the fraction of high-affinity sites of altanserin, without changes in Ki-high and Ki-low binding (Fig. 6C, Fig. 6D, and Table 2). This effect of Gpp(NH)p on altanserin binding was observed in both antipsychotic-free and antipsychotic-treated schizophrenics (Fig. 6E and Fig. 6F). No differences were found between the two groups of control subjects (Fig. 6 and Table 2).
Fig. 6.
(A,B,C,D) [3H]Ketanserin specific binding (2 nM) displacement curves by altanserin in postmortem frontal cortex of schizophrenic subjects (C,D) and controls (A,B) in the presence and in the absence of the non-hydrolyzable GTP analog Gpp(NH)p. The presence of Gpp(NH)p increases the fraction of high-affinity binding of altanserin displacing [3H]ketanserin binding in antipsychotic-free (AP-F) (C,E) and antipsychotic-treated (AP-T) (D,F) schizophrenics, but not in individually matched controls (A,B,E,F). ***p<0.001; n.s., not significant; Student’s t-test. See Supplementary Tables 1 and 2 for demographic information. See Table 2 for statistical analysis. All values represent means±SEM.
Table 2.
[3H]Ketanserin binding displacement curves by altanserin in frontal cortex membrane preparations of antipsychotic-free (AP-F) schizophrenics, antipsychotic-treated (AP-T) schizophrenics, and controls. Experiments were performed in the presence or in the absence of Gpp(NH)p.
| Control | Schizophrenia (AP-F) | Control | Schizophrenia (AP-T) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Vehicle | Gpp(NH)p | Vehicle | Gpp(NH)p | Vehicle | Gpp(NH)p | Vehicle | Gpp(NH)p | |
|
|
||||||||
| log Ki-high | −15.2 ± 0.60 | −15.2 ± 0.05 | −15.3 ± 0.40 | −14.9 ± 0.01*** | −15.4 ± 0.80 | −15.0 ± 0.80 | −15.4 ± 0.50 | −15.3 ± 0.32** |
| Fraction high (%) | 8.6 ± 1.80 | 9.0 ± 1.60 | 8.3 ± 1.30 | 18.2 ± 1.61*** | 6.3 ± 1.70 | 6.3 ± 1.70 | 9.3 ± 1.60 | 20.7 ± 2.21** |
| log Ki-low | −9.8 ± 0.05 | −9.4 ± 0.02 | −9.9 ± 0.04 | −9.3 ± 0.05*** | −10.1 ± 0.05 | −9.4 ± 0.06 | −9.7 ± 0.05 | −9.2 ± 0.08** |
p<0.01;
p<0.001 when compared with individually matched control subjects (vehicle) by Student’s t-test.
Data were best fit to a biphasic compared to monophasic displacement curve by F-test (p<0.0001). See Supplementary Tables 1 and 2 for demographic information. All values represent means ± SEM.
Additional control experiments were performed with the 5-HT2AR agonists DOI and LSD displacing [3H]ketanserin binding in the presence or in the absence of a non-hydrolyzable GTP analog (Supplementary Fig. 3A and Fig. 3B). As expected, we found that uncoupling of heterotrimeric G proteins leads to lower affinities of DOI and LSD for the 5-HT2AR. Together, these findings suggest the existence in schizophrenic subjects, but not in controls, of a fraction of 5-HT2ARs that is sensitive to G protein uncoupling by non-hydrolyzable GTP analogs.
4. Discussion
In the present study we characterized the number of [3H]ketanserin binding sites in postmortem frontal cortex of antipsychotic-free schizophrenic subjects, schizophrenic subjects treated with antipsychotic drugs, and control subjects individually matched by gender, age, and postmortem delay. We found that [3H]ketanserin binding was increased in frontal cortex of antipsychotic-free schizophrenic subjects, with absence of changes in antipsychotic-treated schizophrenics. Since specific binding was absent in frontal cortex of 5-HT2A-KO mice, these data suggest that changes in [3H]ketanserin binding represent alterations at the 5-HT2AR in frontal cortex of antipsychotic-free schizophrenics. Most of the schizophrenic subjects included in this study had committed suicide. Our findings, suggest that these findings are not affected by suicidal behavior, as [3H]ketanserin binding was unchanged in suicide victims with other psychiatric disorders. We also found that the fraction of high-affinity sites of the hallucinogenic 5-HT2AR agonist DOI displacing [3H]ketanserin binding was significantly increased in antipsychotic-free schizophrenic as compared to individually matched controls, and that this increase was partially reversed in antipsychotic-treated schizophrenic subjects. Additionally, functional uncoupling of receptors and heterotrimeric G proteins significantly increased the fraction of high-affinity sites of altanserin to the 5-HT2AR in schizophrenic subjects, yet this effect was not observed in controls. These data support the hypothesis of a selective up-regulation of [3H]ketanserin binding in frontal cortex of antipsychotic-free schizophrenic subjects, with an specific increase in the fraction of active states of the 5-HT2AR. Other hypotheses, including down-regulation of radioligand binding, have been proposed to account for the potential role of the 5-HT2AR in schizophrenia frontal cortex. In the course of this study, we have tested several of these alternative explanations for our results.
4.1. Effect of antipsychotic drugs on 5-HT2AR binding
In this study, our findings suggest that [3H]ketanserin binding to the 5-HT2AR is increased in frontal cortex of antipsychotic-free schizophrenic subjects, and that treatment with antipsychotic drugs decreases this number of binding sites to control levels. Similar findings were previously reported in postmortem frontal cortex of antipsychotic-free and antipsychotic-treated schizophrenic subjects (Gonzalez-Maeso et al., 2008). This also correlates with our previous findings that chronic treatments with the atypical antipsychotics clozapine and risperidone down-regulate [3H]ketanserin binding in mouse cortical neurons, an effect that is not observed after chronic treatment with the typical antipsychotic haloperidol (Gonzalez-Maeso et al., 2008; Kurita et al., 2012). Since only two of the schizophrenic subjects included in this study were treated with haloperidol (see Supplementary Table 2), these and our previous findings in postmortem human brain and mouse models support the hypothesis that chronic treatment with atypical antipsychotic drugs decreases the number of binding sites for [3H]ketanserin in frontal cortex.
Some researchers have suggested that [3H]ketanserin binding is decreased or unchanged in schizophrenia. Most (Reynolds et al., 1983; Laruelle et al., 1993; Burnet et al., 1996; Dean and Hayes, 1996; Dean et al., 1998; Dean et al., 1999; Matsumoto et al., 2005; Dean et al., 2008; Kang et al., 2009), but not all (Mita et al., 1986; Laruelle et al., 1993), of these studies were performed in frontal cortex of treated schizophrenic subjects. Our findings in rodent models (Gonzalez-Maeso et al., 2008) and postmortem human brain (Fig. 1) suggest that absence of changes and/or decreased [3H]ketanserin binding in postmortem frontal cortex of treated schizophrenic subjects may be a consequence of the effect of chronic treatment with atypical antipsychotic drugs. These effects of chronic treatment with atypical antipsychotic drugs are consistent with the fact that the slopes of linear regression between [3H]ketanserin binding and age were significantly steeper (more negative) in antipsychotic-treated, but not in antipsychotic-free, schizophrenic subjects as compared to controls (see below for further discussion regarding the effect of aging on [3H]ketanserin binding). Overall, our results suggest up-regulation of [3H]ketanserin binding in frontal cortex of antipsychotic-free schizophrenics. Although further investigation is needed, it is tempting to speculate that this up-regulation of 5-HT2AR in frontal cortex may be associated to psychotic symptoms in schizophrenia patients, hypothesis that is also supported by findings in postmortem temporal cortex of parkinsonian subjects with visual hallucinations treated with medications that were not antipsychotics (Huot et al., 2010). Previous findings suggest absence of effect of ethanol present in postmortem toxicological analyses (Gross-Isseroff et al., 1990b), and absence of differences in [3H]ketanserin binding between alcoholics and controls (Underwood et al., 2008). However, further investigation is also needed to determine the effect, if any, of ethanol and psychoactive drugs such as amphetamine and tetrahydrocannabinol on 5-HT2AR binding in postmortem human frontal cortex.
Together with the effects of treatment with antipsychotic drugs, potential explanations for these discrepancies in [3H]ketanserin binding findings are also parameters intrinsic to the studies carried out in postmortem human brain tissue samples such as age, inclusion of control subjects that are not individually matched by demographic variables, and the reliability of postmortem psychiatric diagnosis mostly based on postmortem family interviews. In schizophrenia patients, hallucinations and delusions typically attenuate with aging (Davidson et al., 1995). This clinical measure is further supported by the negative correlation of [3H]ketanserin binding and age at time of death that we and others have found in postmortem frontal cortex tissue samples (Fig. 3 and [Gross-Isseroff et al., 1990b; Gonzalez-Maeso et al., 2008]), and by the decline in [18F]altanserin binding relative to age in healthy volunteers (Moses-Kolko et al., 2011). The significant effect of aging on [3H]ketanserin binding highlights the importance of employing study designs that include schizophrenic subjects individually, and not collectively, matched to control subjects by demographic factors such as gender, age and postmortem delay (see Supplementary Tables 1–3). This profound effect of aging also provides the rationale for the statistical analysis of data presented as percentage of specific [3H]ketanserin binding in schizophrenic subjects relative to individually matched controls, and not as binding density in fmol/mg protein, in order to avoid intra-group differences (i.e., age-related differences in [3H]ketanserin binding within the control group). The two most commonly used approaches for assessing psychiatric diagnoses in postmortem human brain research are clinical record reviews and postmortem interviews with family and peers. Recent publications suggest that psychiatric diagnoses are difficult to ascertain based on postmortem family interviews (Deep-Soboslay et al., 2005). Our findings were obtained in postmortem tissue samples of subjects with antemortem diagnosis of schizophrenia or other neuropsychiatric disorders based on the clinical information obtained at mental health centers (see Experimental procedures, above).
Previous findings demonstrate an age-dependent decrease in [3H]ketanserin binding that reaches a minimum in the 5th decade followed by an age-dependent increase in older subjects (6th and 7th decade) (Gross-Isseroff et al., 1990b). Our findings suggest that linear regression provides the best fit to the data describing the effect of aging on [3H]ketanserin binding in postmortem human frontal cortex (ages ranged from 4 to 86 years, see Supplementary Tables 1–4). These divergent results might be explicable by differences in experimental approaches (radioligand binding in plasma membrane preparations or tissue sections) as well as the effect of age at different anatomical regions in frontal cortex.
4.2. Different findings with ketanserin, altanserin and LSD
Agonists bind with greatest affinity to the active (G protein-coupled) state of the receptor. Our findings with the hallucinogenic 5-HT2AR agonist DOI show a selective increase in the fraction of high-affinity sites displacing [3H]ketanserin binding as an alteration in postmortem frontal cortex of antipsychotic-free as well as antipsychotic-treated schizophrenics when compared to control subjects. Taking into consideration that the density of [3H]ketanserin binding sites was unchanged in antipsychotic-treated schizophrenics (Fig. 1), further investigation is needed to better understand the persistence of these changes in the fraction of high-affinity binding sites of DOI displacing [3H]ketanserin binding after antipsychotic treatment. However, and importantly, these changes with the hallucinogen DOI were significantly more pronounced in antipsychotic-free as compared with antipsychotic-treated schizophrenics, thereby suggesting that treatment with atypical antipsychotic drugs partially reverses alterations in the functionally active conformations of the 5-HT2AR in schizophrenia frontal cortex. Similar findings have been previously observed by using other hallucinogenic 5-HT2AR radioligands, with up-regulation of [3H]LSD binding in postmortem frontal cortex of untreated, but not treated, schizophrenic subjects (Whitaker et al., 1981; Joyce et al., 1993; Gurevich and Joyce, 1997). In markedly contrast, however, a series of experiments focused on PET imaging convincingly demonstrate that [18F]altanserin binding is decreased in frontal cortex of drug-naïve first-episode schizophrenic patients (Rasmussen et al., 2010). A potential explanation for these apparently discrepant findings is the different functional outcomes of LSD-like drugs and altanserin, as well as their affinity for all the structural conformations of the 5-HT2AR. Biochemical findings in postmortem frontal cortex suggest that, opposite to DOI and LSD, the ligand altanserin presents a higher affinity for the inactive G protein-uncoupled conformation of the 5-HT2AR in schizophrenic subjects. Thus, heterotrimeric G protein uncoupling increases the fraction of high-affinity sites of altanserin binding to the 5-HT2AR in schizophrenic subjects—indicating that altanserin behaves as a 5-HT2AR inverse agonist in schizophrenic subjects, but not in controls (see also Supplementary Fig. 2). The alterations in both altanserin (Fig. 6) and DOI (Fig. 5) displacing [3H]ketanserin binding that we found in postmortem frontal cortex of schizophrenic subjects point toward up-regulation in the fraction of 5-HT2ARs that are susceptible of being uncoupled from heterotrimeric G proteins. Together, these data suggest that up-regulation in the fraction of active G protein-coupled 5-HT2AR will lead to both increased binding of DOI (Fig. 5), and, possibly, decreased binding of altanserin (Rasmussen et al., 2010) in schizophrenic subjects. Further work studying 5-HT2AR-G protein coupling is definitely needed to validate this hypothesis, as well as the molecular mechanisms underlying the differences in the effect of Gpp(NH)p in schizophrenic subjects and controls.
4.3. The 5-HT2AR and suicide
Numerous abnormalities have been reported in the serotonergic system in suicide victims. In this study, there were no differences in [3H]ketanserin binding to the 5-HT2AR in a group of suicide victims with psychiatric disorders such as dysthymic disorder, alcoholism, and anorexia nervosa, among others. Similarly, previous work has shown that [3H]ketanserin binding is unaffected in postmortem frontal cortex of suicide victims with major depression (Owen et al., 1983; Crow et al., 1984; Cheetham et al., 1988; Arranz et al., 1994; Lowther et al., 1994; Stockmeier et al., 1997; Rosel et al., 2000). Few studies using [3H]ketanserin reported that the 5-HT2AR is altered in suicide victims without psychiatric diagnosis (Gross-Isseroff et al., 1990a; Turecki et al., 1999). [3H]Ketanserin binding has also been shown to correlate with lifetime aggression in suicide (Oquendo et al., 2006). Our preliminary findings in non-suicide antipsychotic-free schizophrenic subjects suggest that up-regulation of [3H]ketanserin binding sites is related to schizophrenia and not to suicidal behavior (Fig. 2A and 2B). However, a possible explanation for the absence of changes in [3H]ketanserin binding that we found in suicides (Fig. 3C) may be related to the psychiatric diagnoses of the subjects included in the study. We still cannot exclude the possibility of dysregulations in [3H]ketanserin binding that differ (up-regulation, down-regulation and/or absence of change) in the variety of diagnoses of the suicide victims, which limits the validity of our findings in non-schizophrenic suicide completers (Supplementary Table 3). Adding more complexity, overrepresentation of suicide among the schizophrenia groups could potentially reflect special characteristics of the schizophrenic subjects examined that not fully represent the spectrum of the disorder. Nonetheless, it appears that suicide does not statistically interact with 5HT2A receptor changes neither in antipsychotic-free nor in antipsychotic-treated subjects. The apparent consistency of this finding suggests that increase in [3H]ketanserin binding is more likely related to schizophrenia status than to suicidal behavior in the disease process. However, further work is clearly needed to determine the impact, if any, of suicide on 5-HT2AR binding.
Concluding Remarks
In summary, we find that [3H]ketanserin binding is increased in postmortem frontal cortex of antipsychotic-free schizophrenic subjects. We demonstrate up-regulation of high-affinity binding sites for the hallucinogenic 5-HT2AR agonist DOI in schizophrenia frontal cortex. Our findings also suggest that altanserin behaves as a 5-HT2AR inverse agonist in schizophrenic subjects, but not in controls. These data may help explain the differences previously reported with use of ligands that bind with different affinities to distinct 5-HT2AR conformations. The 5-HT2AR has been shown to form heterocomplexes with GPCRs such as the metabotropic glutamate 2 receptor (Gonzalez-Maeso et al., 2008; Fribourg et al., 2011). At present, it is unknown if the relationships between the 5-HT2AR and other neurotransmitter receptors as GPCR heterocomplexes are involved in schizophrenia and other neuropsychiatric disorders: a subject under active investigation in our laboratory. Our findings may be potentially useful for a better understanding of the biochemical alterations responsible for schizophrenia and other psychotic disorders.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anastasopoulos G, Photiades H. Effects of LSD-25 on relatives of schizophrenic patients. J Ment Sci. 1962;108:95–98. doi: 10.1192/bjp.108.452.95. [DOI] [PubMed] [Google Scholar]
- Arranz B, Eriksson A, Mellerup E, Plenge P, Marcusson J. Brain 5-HT1A, 5-HT1D, and 5-HT2 receptors in suicide victims. Biol Psychiatry. 1994;35:457–463. doi: 10.1016/0006-3223(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Bennett JP, Jr, Enna SJ, Bylund DB, Gillin JC, Wyatt RJ, Snyder SH. Neurotransmitter receptors in frontal cortex of schizophrenics. Arch Gen Psychiatry. 1979;36:927–934. doi: 10.1001/archpsyc.1979.01780090013001. [DOI] [PubMed] [Google Scholar]
- Burnet PW, Eastwood SL, Harrison PJ. 5-HT1A and 5-HT2A receptor mRNAs and binding site densities are differentially altered in schizophrenia. Neuropsychopharmacology. 1996;15:442–455. doi: 10.1016/S0893-133X(96)00053-X. [DOI] [PubMed] [Google Scholar]
- Cheetham SC, Crompton MR, Katona CL, Horton RW. Brain 5-HT2 receptor binding sites in depressed suicide victims. Brain Res. 1988;443:272–280. doi: 10.1016/0006-8993(88)91621-6. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Cross AJ, Cooper SJ, Deakin JF, Ferrier IN, Johnson JA, Joseph MH, Owen F, Poulter M, Lofthouse R, et al. Neurotransmitter receptors and monoamine metabolites in the brains of patients with Alzheimer-type dementia and depression, and suicides. Neuropharmacology. 1984;23:1561–1569. doi: 10.1016/0028-3908(84)90100-x. [DOI] [PubMed] [Google Scholar]
- Davidson M, Harvey PD, Powchik P, Parrella M, White L, Knobler HY, Losonczy MF, Keefe RS, Katz S, Frecska E. Severity of symptoms in chronically institutionalized geriatric schizophrenic patients. Am J Psychiatry. 1995;152:197–207. doi: 10.1176/ajp.152.2.197. [DOI] [PubMed] [Google Scholar]
- Dean B. The cortical serotonin2A receptor and the pathology of schizophrenia: a likely accomplice. J Neurochem. 2003;85:1–13. doi: 10.1046/j.1471-4159.2003.01693.x. [DOI] [PubMed] [Google Scholar]
- Dean B, Crossland N, Boer S, Scarr E. Evidence for altered post-receptor modulation of the serotonin 2a receptor in schizophrenia. Schizophr Res. 2008;104:185–197. doi: 10.1016/j.schres.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Dean B, Hayes W. Decreased frontal cortical serotonin2A receptors in schizophrenia. Schizophr Res. 1996;21:133–139. doi: 10.1016/0920-9964(96)00034-5. [DOI] [PubMed] [Google Scholar]
- Dean B, Hayes W, Hill C, Copolov D. Decreased serotonin2A receptors in Brodmann’s area 9 from schizophrenic subjects. A pathological or pharmacological phenomenon? Mol Chem Neuropathol. 1998;34:133–145. doi: 10.1007/BF02815075. [DOI] [PubMed] [Google Scholar]
- Dean B, Hayes W, Opeskin K, Naylor L, Pavey G, Hill C, Keks N, Copolov DL. Serotonin2 receptors and the serotonin transporter in the schizophrenic brain. Behav Brain Res. 1996;73:169–175. doi: 10.1016/0166-4328(96)00091-5. [DOI] [PubMed] [Google Scholar]
- Dean B, Hussain T, Hayes W, Scarr E, Kitsoulis S, Hill C, Opeskin K, Copolov DL. Changes in serotonin2A and GABA(A) receptors in schizophrenia: studies on the human dorsolateral prefrontal cortex. J Neurochem. 1999;72:1593–1599. doi: 10.1046/j.1471-4159.1999.721593.x. [DOI] [PubMed] [Google Scholar]
- Deep-Soboslay A, Akil M, Martin CE, Bigelow LB, Herman MM, Hyde TM, Kleinman JE. Reliability of psychiatric diagnosis in postmortem research. Biol Psychiatry. 2005;57:96–101. doi: 10.1016/j.biopsych.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Egan CT, Herrick-Davis K, Teitler M. Creation of a constitutively activated state of the 5-hydroxytryptamine2A receptor by site-directed mutagenesis: inverse agonist activity of antipsychotic drugs. J Pharmacol Exp Ther. 1998;286:85–90. [PubMed] [Google Scholar]
- Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R, Park G, Adney SK, Hatcher C, Eltit JM, Ruta JD, Albizu L, Li Z, Umali A, Shim J, Fabiato A, Mackerell AD, Jr, Brezina V, Sealfon SC, Filizola M, Gonzalez-Maeso J, Logothetis DE. Decoding the Signaling of a GPCR Heteromeric Complex Reveals a Unifying Mechanism of Action of Antipsychotic Drugs. Cell. 2011;147:1011–1023. doi: 10.1016/j.cell.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Sealfon SC. Psychedelics and schizophrenia. Trends Neurosci. 2009;32:225–232. doi: 10.1016/j.tins.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, Stoll M, Stock C, Obradovic M, Kovar KA. Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): a double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry. 2005;38:301–311. doi: 10.1055/s-2005-916185. [DOI] [PubMed] [Google Scholar]
- Gross-Isseroff R, Salama D, Israeli M, Biegon A. Autoradiographic analysis of [3H]ketanserin binding in the human brain postmortem: effect of suicide. Brain Res. 1990a;507:208–215. doi: 10.1016/0006-8993(90)90274-f. [DOI] [PubMed] [Google Scholar]
- Gross-Isseroff R, Salama D, Israeli M, Biegon A. Autoradiographic analysis of age-dependent changes in serotonin 5-HT2 receptors of the human brain postmortem. Brain Res. 1990b;519:223–227. doi: 10.1016/0006-8993(90)90081-l. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Joyce JN. Alterations in the cortical serotonergic system in schizophrenia: a postmortem study. Biol Psychiatry. 1997;42:529–545. doi: 10.1016/S0006-3223(97)00321-1. [DOI] [PubMed] [Google Scholar]
- Hermle L, Funfgeld M, Oepen G, Botsch H, Borchardt D, Gouzoulis E, Fehrenbach RA, Spitzer M. Mescaline-induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: experimental psychosis as a tool for psychiatric research. Biol Psychiatry. 1992;32:976–991. doi: 10.1016/0006-3223(92)90059-9. [DOI] [PubMed] [Google Scholar]
- Hoch PH, Cattell JP, Pennes HH. Effects of mescaline and lysergic acid (d-LSD-25) Am J Psychiatry. 1952;108:579–584. doi: 10.1176/ajp.108.8.579. [DOI] [PubMed] [Google Scholar]
- Hof PR, Young WG, Bloom FE, Belichenko PV, Celio MR. Comparative cytoarchitectonic atlas of the C57BL/6 and 129/Sv mouse brains. Elsevier; 2000. [Google Scholar]
- Huot P, Johnston TH, Darr T, Hazrati LN, Visanji NP, Pires D, Brotchie JM, Fox SH. Increased 5-HT2A receptors in the temporal cortex of parkinsonian patients with visual hallucinations. Mov Disord. 2010;25:1399–1408. doi: 10.1002/mds.23083. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Shane A, Lexow N, Winokur A, Casanova MF, Kleinman JE. Serotonin uptake sites and serotonin receptors are altered in the limbic system of schizophrenics. Neuropsychopharmacology. 1993;8:315–336. doi: 10.1038/npp.1993.32. [DOI] [PubMed] [Google Scholar]
- Kang K, Huang XF, Wang Q, Deng C. Decreased density of serotonin 2A receptors in the superior temporal gyrus in schizophrenia--a postmortem study. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:867–871. doi: 10.1016/j.pnpbp.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Kurita M, Holloway T, Garcia-Bea A, Kozlenkov A, Friedman AK, Moreno JL, Heshmati M, Golden SA, Kennedy PJ, Takahashi N, Dietz DM, Mocci G, Gabilondo AM, Hanks J, Umali A, Callado LF, Gallitano AL, Neve RL, Shen L, Buxbaum JD, Han MH, Nestler EJ, Meana JJ, Russo SJ, Gonzalez-Maeso J. HDAC2 regulates atypical antipsychotic responses through the modulation of mGlu2 promoter activity. Nat Neurosci. 2012;15:1245–1254. doi: 10.1038/nn.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Casanova MF, Toti R, Weinberger DR, Kleinman JE. Selective abnormalities of prefrontal serotonergic receptors in schizophrenia. A postmortem study. Arch Gen Psychiatry. 1993;50:810–818. doi: 10.1001/archpsyc.1993.01820220066007. [DOI] [PubMed] [Google Scholar]
- Lowther S, De Paermentier F, Crompton MR, Katona CL, Horton RW. Brain 5-HT2 receptors in suicide victims: violence of death, depression and effects of antidepressant treatment. Brain Res. 1994;642:281–289. doi: 10.1016/0006-8993(94)90932-6. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Giannaccini G, Giromella A, Betti L, Pesce D, Nardi I, Rossi A, Lucacchini A, Cassano GB. [3H]-ketanserin binding sites in different psychiatric disorders. Neurochem Int. 2003;42:511–516. doi: 10.1016/s0197-0186(02)00093-1. [DOI] [PubMed] [Google Scholar]
- Matsumoto I, Inoue Y, Iwazaki T, Pavey G, Dean B. 5-HT2A and muscarinic receptors in schizophrenia: a postmortem study. Neurosci Lett. 2005;379:164–168. doi: 10.1016/j.neulet.2004.12.059. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Marin P, Bockaert J, Mannoury la Cour C. Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol Sci. 2008;29:454–464. doi: 10.1016/j.tips.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Mita T, Hanada S, Nishino N, Kuno T, Nakai H, Yamadori T, Mizoi Y, Tanaka C. Decreased serotonin S2 and increased dopamine D2 receptors in chronic schizophrenics. Biol Psychiatry. 1986;21:1407–1414. doi: 10.1016/0006-3223(86)90332-x. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Price JC, Shah N, Berga S, Sereika SM, Fisher PM, Coleman R, Becker C, Mason NS, Loucks T, Meltzer CC. Age, Sex, and Reproductive Hormone Effects on Brain Serotonin-1A and Serotonin-2A Receptor Binding in a Healthy Population. Neuropsychopharmacology. 2011;36:2729–2740. doi: 10.1038/npp.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Russo SA, Underwood MD, Kassir SA, Ellis SP, Mann JJ, Arango V. Higher postmortem prefrontal 5-HT2A receptor binding correlates with lifetime aggression in suicide. Biol Psychiatry. 2006;59:235–243. doi: 10.1016/j.biopsych.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Owen F, Cross AJ, Crow TJ, Deakin JF, Ferrier IN, Lofthouse R, Poulter M. Brain 5-HT-2 receptors and suicide. Lancet. 1983;2:1256. doi: 10.1016/s0140-6736(83)91310-7. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Kometer M, Geyer MA, Vollenweider FX. Neuropsychopharmacology. 2011. Psilocybin-Induced Deficits in Automatic and Controlled Inhibition are Attenuated by Ketanserin in Healthy Human Volunteers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex. 1995;5:323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Rasmussen H, Erritzoe D, Andersen R, Ebdrup BH, Aggernaes B, Oranje B, Kalbitzer J, Madsen J, Pinborg LH, Baare W, Svarer C, Lublin H, Knudsen GM, Glenthoj B. Decreased frontal serotonin2A receptor binding in antipsychotic-naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 2010;67:9–16. doi: 10.1001/archgenpsychiatry.2009.176. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Rossor MN, Iversen LL. Preliminary studies of human cortical 5-HT2 receptors and their involvement in schizophrenia and neuroleptic drug action. J Neural Transm Suppl. 1983;18:273–277. [PubMed] [Google Scholar]
- Rosel P, Arranz B, San L, Vallejo J, Crespo JM, Urretavizcaya M, Navarro MA. Altered 5-HT(2A) binding sites and second messenger inositol trisphosphate (IP(3)) levels in hippocampus but not in frontal cortex from depressed suicide victims. Psychiatry Res. 2000;99:173–181. doi: 10.1016/s0925-4927(00)00076-7. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Dilley GE, Shapiro LA, Overholser JC, Thompson PA, Meltzer HY. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology. 1997;16:162–173. doi: 10.1016/S0893-133X(96)00170-4. [DOI] [PubMed] [Google Scholar]
- Turecki G, Briere R, Dewar K, Antonetti T, Lesage AD, Seguin M, Chawky N, Vanier C, Alda M, Joober R, Benkelfat C, Rouleau GA. Prediction of level of serotonin 2A receptor binding by serotonin receptor 2A genetic variation in postmortem brain samples from subjects who did or did not commit suicide. Am J Psychiatry. 1999;156:1456–1458. doi: 10.1176/ajp.156.9.1456. [DOI] [PubMed] [Google Scholar]
- Underwood MD, Mann JJ, Huang YY, Arango V. Family history of alcoholism is associated with lower 5-HT2A receptor binding in the prefrontal cortex. Alcohol Clin Exp Res. 2008;32:593–599. doi: 10.1111/j.1530-0277.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Whitaker PM, Crow TJ, Ferrier IN. Tritiated LSD binding in frontal cortex in schizophrenia. Arch Gen Psychiatry. 1981;38:278–280. doi: 10.1001/archpsyc.1981.01780280046004. [DOI] [PubMed] [Google Scholar]
- Young BG. A phenomenological comparison of LSD and schizophrenic states. Br J Psychiatry. 1974;124:64–74. doi: 10.1192/bjp.124.1.64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.