Abstract
Objective
Emerging evidence suggests that some individuals may be simultaneously more responsive to the effects from environmental adversity and enrichment (i.e., differential susceptibility). Given that parenting behavior and a variable number tandem repeat polymorphism in the 3′untranslated region of the dopamine transporter (DAT1) gene are each independently associated with ADHD, our goal was to evaluate the potential interactive effects of child DAT1 genotype with positive and negative parenting behaviors on childhood ADHD.
Method
We recruited an ethnically-diverse sample of 150 six to nine year-old boys and girls with and without ADHD. Children were genotyped for a common polymorphism of the DAT1 gene, and objective counts of observed parenting behavior (i.e., negativity and praise) were obtained from a valid parent-child interaction task. Structural equation modeling was used to examine the interactive effects of DAT1 and observed parenting with a latent ADHD factor.
Results
We detected a significant interaction between observed praise and child DAT1 (coded additively) which suggested that praise was associated with increased ADHD, but only among youth with the 9/10 genotype. In addition, a marginally significant interaction between DAT1 (coded additively and recessively) and observed negativity emerged for ADHD, such that negativity was positively associated with ADHD but only for youth with the 9/9 genotype.
Conclusions
Although differential susceptibility theory was not fully supported, These preliminary results suggest that interactive exchanges between parenting behavior and child genotype potentially contribute to the development of ADHD. Clinical implications for interactions between parenting behavior and child genotype are discussed.
Keywords: Parenting, ADHD, gene-environment interaction, DAT1, differential susceptibility
Attention-deficit/hyperactivity disorder (ADHD) affects 8% of children in the U.S. and 8–12% of school-aged children worldwide (Froehlich et al., 2007; Faraone et al., 2003). ADHD disrupts social, academic, behavioral, and emotional functioning across development, including elevated rates of criminality, substance problems, school failure, and occupational instability (Langley et al., 2010; Molina et al., 2009). It is also associated with substantial economic consequences due to mental health and educational services (Pelham, Foster & Robb, 2007).
Gene × Environment Interplay and ADHD
Although ADHD is highly heritable (Thapar et al., 2000), genetic studies are complicated by environmental influences on ADHD, and that genetic influences on psychopathology are likely to depend on the experience of varying environmental conditions. That is, among children exposed to environmental adversity (e.g., abuse), some youth develop psychopathology whereas others do not, suggesting a potential gene x environment interaction (G×E) (Waldman, 2007). Relatively little research has assessed how biological and environmental factors interact in the development of ADHD (Moffitt, Caspi, & Rutter, 2006) as the nature of these influences may vary across development. Variants regulating dopamine moderated the association of prenatal exposure to teratogens (Kahn et al., 2003; Knopik et al., 2006), pregnancy complications (Milberger et al., 1997), and early maltreatment (Laucht et al., 2007) with ADHD, suggesting genetic influences on increased sensitivity to early adversity for ADHD. However, given that ADHD is developmentally-sensitive, prospective longitudinal studies are needed to determine whether G×E affects the trajectory of ADHD (Sonuga-Barke & Halperin, 2010; Kuntsi, Rijsdijk, Ronald, Asherson, & Plomin, 2006). Developmental theory suggests that environmental influences interact with biological risk factors throughout the course of development, such that incremental changes to brain structures and functions eventuate in psychiatric disorder (Sonuga-Barke & Halperin, 2010). Developmental theories and perspectives of G×E are necessary to disentangle what are likely to be multiple etiological pathways underlying ADHD.
Genetic studies of ADHD are not only complicated by unmeasured interactions with environmental factors, but also from emerging evidence that genetic influences may confer sensitivity (rather than risk) to both negative and positive environmental conditions (Belsky & Pluess, 2009). For example, an individual with genetic vulnerability may be at heightened risk for psychopathology when he/she experiences adversity, but the same individual may respond particularly well if reared in an enriched environment (i.e., “differential susceptibility;” Belsky, Bakermans-Kranenburg, & Van IJzendoorn, 2009). Adults with the SS genotype of the serotonin transporter gene exhibited the most depression if they experienced severe emotional abuse, physical abuse and/or poor quality parenting (i.e., frequent parental fighting, lack of warmth) during childhood relative to all other genotypes (Taylor et al., 2006). However, SS genotype also had the lowest level of depression if they were raised in a supportive family environment during childhood (Taylor et al., 2006). Similarly, increased parental sensitivity (through a parent training intervention) was more effective in reducing externalizing problems in preschool-aged children carrying the 7-repeat variant of D4 receptor gene (DRD4) than in children without this variant (Bakermans-Kranenberg et al., 2008), suggesting that genetically sensitive individuals also show the strongest response to therapeutic intervention. Crucially, gene expression and brain plasticity may be more sensitive to environmental effects during particular stages of development (Belsky & de Haan, 2010) such that early childhood adversity may have more enduring effects on brain development and future outcome than adult experiences (Fox, Levitt, & Nelson, 2010; Branchi, 2009). Even “less-memorable,” albeit chronic negative life experiences that accumulate over time (e.g., poor parent-child interactions) are associated with poor brain development (Kishiyama et al., 2009). Thus, G×E effects may be especially salient during childhood.
Parenting and ADHD
Parenting quality such as involvement, warmth, and supportiveness, may interact with genetic factors in ways that are consistent with differential susceptibility in the development of ADHD. Compared to parents of typically-developing children, parents of children with ADHD made more harsh and critical statements, and were less physically involved (Wells et al., 2000). Negative parenting may also exacerbate the course of ADHD (Sonuga-Barke & Halperin, 2010) given that coercive parenting and maternal rejection predicted more ADHD and the emergence of comorbid conduct disorder (CD) (Morrell & Murray, 2003). However, negative parenting behavior may also be in response to ADHD and other disruptive behaviors (Burke, Pardini, & Loeber, 2008; Seipp & Johnston, 2005), thus necessitating diverse methods (e.g., longitudinal, experimental) to disentangle child effects. In addition, dimensions of positive parenting (i.e., high involvement, warmth, and praise) show unique patterns with ADHD relative to negative parenting, which is consistent with the empirical independence of positive and negative parenting behavior (Pettit, Bates, & Dodge, 1997). Relatively few studies of ADHD and behavior problems more generally have made this important distinction. In a prospective study of preschool children with ADHD, observed positive parenting (but not negative parenting) was inversely associated with CD symptoms eight years later (Chronis et al., 2007). Negative and positive parenting may be empirically distinct (rather than reflecting opposite ends of an underlying continuum) and may interact with genetic factors in ways that support differential susceptibility. However, this proposition requires significant empirical scrutiny.
Parenting quality and nurturing behavior more generally are associated with offspring neural changes (Belsky & de Haan, 2010), a key consideration for selecting plausible environmental risk factors for G×E and psychopathology (Rutter, Moffitt, & Caspi, 2006). Poor parent-infant attachment disrupts hypothalamic-pituitary-adrenocortical axis (HPA) functioning (Schore, 2001), which regulates stress reactivity and mental health outcomes (Twardosz & Lutzker, 2010). Parenting quality may be most salient during a sensitive period in development (i.e., infancy to childhood). Institutionalized youth who were adopted between 10 and 92 months showed significantly reduced left and right superior-lateral lobes of the cerebellum and poorer visual memory and planning abilities at age nine compared to non-adopted controls (Bauer, Hanson, Pierson, Davidson, & Pollak, 2009). Increased myelination in the rat brain was limited to socially-housed infant rats, but not in adults rats reared in complex housing environments (Markham et al., 2009). Similarly, rats reared by genetically unrelated mothers who were frequently licked/groomed (LG) showed increased hippocampal expression, decreased hypothalamic corticotrophin releasing factor expression, and reduced HPA stress reactivity compared to rats that were raised by infrequent LG foster mothers (Liu et al., 1997). Overall, early parental behavior may negatively affect brain and cognitive development in offspring, suggesting the possibility that negative and nurturing parenting behavior may affect neurobiological systems in ways that may be influenced by biological or genetic variation.
DAT1 and ADHD
Prevailing theories on the biological etiology of ADHD converge around the centrality of dopamine (DA) regulation in the frontosubcortical system, which is the primary mechanism of action in pharmacological treatments for ADHD (Cook et al., 1995; Faraone & Biederman, 1998). The 40 base-pair (bp) variable number tandem repeat (VNTR) polymorphism in the 3′ untranslated region (UTR) in the DA transporter gene (DAT1; SLC6A3) is a compelling candidate for ADHD because stimulant medication reduces ADHD by inhibiting the DA transporter and increasing extracellular DA (Spencer et al., 2007). DAT1 is also a potentially important biomarker for ADHD given the centrality of DA on attentional and motor abilities (Faraone & Biederman, 1998). In particular, DAT1 10-repeat homozygosity has been associated with increased mRNA expression (Mill et al., 2002) as well as decreased DA transporter binding compared to non-10-repeat homozygotes (Heinz et al., 2000). More importantly, striatal DA levels persisted in the extracellular space 100 times longer among mice with DAT inactivated (i.e., homozygotes) compared to mice with partial activation of DAT (i.e., heterozygotes and wild-type mice), suggesting that homozygosity leads to significant changes in neurochemistry and resultant animal behavior (Giros et al., 1996). Methylphenidate was less efficacious in the treatment of ADHD for youth with the 10/10 versus other DAT1 genotypes, suggesting that the 10/10 DAT1 genotype increases susceptibility for ADHD (Roman et al., 2002). In contrast, a longitudinal study found that individuals with the 9/10 DAT1 genotype had more behavioral and attention problems than 10/10 homozygotes from childhood through adulthood (Barkley, Smith, Fischer, & Navia, 2006), although Froehlich et al. (2011) and Joober et al. (2007) that found that individuals with the 9/10 and 10/10 DAT1 genotypes displayed significantly more positive effects from methylphenidate treatment than individuals with the 9/9 genotype. Null findings between DAT1 and ADHD have also been reported (e.g., Franke et al., 2009; Bruggemann et al., 2007). Inconsistent findings may reflect differences in phenotypic measurement (e.g., dimensional versus categorical), research design (e.g., cross-sectional versus prospective), and phenomenology (e.g., age and sex differences).
Despite its biological plausibility, studies have yet to examine the interaction of parenting and child DAT1 for ADHD. Beyond parenting, other environmental risk factors have interacted with DAT1 to predict ADHD, including prenatal exposure to teratogens (Kahn et al., 2003) and maltreatment (Laucht et al., 2007). The association between prenatal alcohol exposure and offspring ADHD was moderated by the 30-bp VNTR polymorphism in intron 8 of DAT1 such that youth with the 10/3-repeat haplotype had increased odds for ADHD in the presence of maternal alcohol use during pregnancy compared to youth without the haplotype (Brookes et al., 2006). Severe institutional deprivation was prospectively associated with increased ADHD symptoms among Romanian youth with the 10/6-repeat haplotype for the 40-bp VNTR in the 3′UTR and the 30-bp VNTR in the intron 8 polymorphism in DAT1, relative to youth without this haplotype (Stevens et al., 2009). Thus, exposure to environmental risk may interact with DAT1 genotypes to predict psychopathology. However, few studies have examined negative and nurturing environmental conditions and their interaction with genotype. Furthermore, despite the need to incorporate more rigorous methods of environmental assessment for G×E studies, most studies continue to rely on potentially biased methods (i.e., self-reported or retrospective measures) (Dunn et al., 2011). For example, a structured observational assessment of parenting behavior was psychometrically superior to maternal self-report and naturalistic observations of child behavior and functioning (Zaslow et al., 2006). Yet, G×E studies with structured observational approaches to measure parenting-influenced environmental factors are rare (Fortuna et al., 2011; Deater-Deckard, 2000).
The Present Study
We tested the association of observed parenting behavior from a structured parent-child interaction task, child DAT1, and their respective interactions with multidimensional (e.g., diagnostic status, symptom counts) and multi-informant (parents and teachers) ratings of child ADHD using structural equation modeling (SEM). Given that positive and negative parenting are partially distinct (Pettit, Bates & Dodge, 1997), we hypothesized that DAT1 genotype would interact with positive and negative parenting in opposite directions: children with the more transcriptionally-active 10/10 DAT1 genotype would exhibit more ADHD as a function of parental negativity than children with the 9/9 and 9/10 DAT1 genotypes whereas children with the 10/10 genotype would be less likely to have ADHD and have fewer ADHD symptoms as a function of parental praise than 9/9 and 9/10 genotype youth.
Method
Participants
We recruited 150 6 to 9 year-old children (mean age = 7.4, SD = 1.1) with (n = 84) and without ADHD (n = 66) (56% Caucasian, 9% African America, 8% Hispanic or Latino, and 25% Mixed or Other). We mailed flyers to local schools, placed advertisements in public locations, made presentations to self-help groups, and received referrals from mental health service providers. Study eligibility required all participants to have a Full Scale IQ above 70, to live with one biological parent at least half the time, and to be fluent in English. Exclusion criteria included diagnoses of mental retardation, seizure, autism, or pervasive developmental disorders. ADHD diagnostic status was determined from the Diagnostic Interview Schedule for Children, 4th edition, parent version (DISC-IV; Shaffer et al., 2000), a fully structured diagnostic interview keyed to Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (American Psychiatric Association [DSM-IV], 1994) criteria (e.g., age of onset). ADHD probands consisted of youth who met full diagnostic criteria for ADHD on the DISC-IV. Interviews were conducted by advanced graduate students in clinical psychology and trained research assistants. To avoid recruiting a non-ADHD group that was unrealistically high functioning, youth were placed in the non-ADHD group if they met diagnostic criteria for any disorder other than ADHD (see Table 1). This strategy has been used in other studies of childhood ADHD (Lahey et al., 1998). Although teacher data were obtained, they were not used to make diagnostic designations, partly because teacher data were obtained from only two-thirds of the sample. That is, we did not want to confound the availability of teacher data with the increased probability of meeting diagnostic criteria for ADHD (i.e., adopting strategies such as the “or” rule would increase positive ADHD diagnoses in the presence of parent + teacher versus parent only data).
Table 1.
Bivariate correlations, means and standard deviations for all observed variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. DISC Inatt | -- | ||||||||||||
| 2. DISC Hyp | .53** | -- | |||||||||||
| 3. CBCL AttProb (T) | .73** | .46** | -- | ||||||||||
| 4. T-DBDRS Inatt | .37** | .42** | .43** | -- | |||||||||
| 5. T-DBDRS Hyp | .31** | .57** | .30** | .60** | -- | ||||||||
| 6. TRF AttProb (T) | .37** | .43** | .41** | .79** | .76** | -- | |||||||
| 7. DPICS Negativity | .15* | .15* | .10 | .18* | .18* | .23* | -- | ||||||
| 8. DPICS Praise | .09 | .08 | .01 | .12 | .14 | .08 | −.01 | -- | |||||
| 9. Child Age | −.07 | −.26** | −.08 | −.08 | −.22** | −.21* | −.13 | −.14 | -- | ||||
| 10. Child Sex | .07 | −.19* | .31** | −.12 | −.09 | .06 | −.09 | .08 | .01 | -- | |||
| 11. Parent BDI | .08 | .10 | .09 | −.06 | .06 | .07 | .14* | .02 | .04 | .08 | -- | ||
| 12. DISC ODD | .47** | .66** | .25** | .14* | .40** | .29** | .02 | .05 | −.06 | −.03 | .22 | -- | |
| 13. DPICS Noncomp | .21** | .18* | .13* | .21* | .03 | .12* | .50** | −.07 | −.19* | −.14* | .11 | .12 | -- |
| Mean | 4.55 | 3.41 | 62.77 | 3.72 | 3.13 | 60.71 | 0.38 | 0.52 | 7.42 | -- | 6.79 | 2.16 | 0.19 |
| SD | 3.14 | 3.06 | 10.77 | 3.24 | 3.10 | 10.00 | 0.37 | 0.46 | 1.15 | -- | 6.01 | 2.39 | 0.27 |
p < .05,
p < .01;
DISC = Diagnostic Interview Schedule for Children, Version IV; CBCL = Child Behavior Checklist; T-DBDRS = Disruptive Behavior Disorder Rating Scale, Teacher Version; TRF = Teacher Report Form; DPICS = Dyadic Parent-Child Interaction Coding System; BDI = Beck Depression Inventory.
Procedures
Eligibility was determined after parents completed a telephone screening. Eligible families who were interested in participating were mailed rating scales and families were then invited to our laboratory for in-person assessments. Following parent consent and child assent, parents completed the DISC-IV and other measures related to parenting, child behavior, family functioning, and their own psychopathology. Approximately 85% of children (mostly treated with stimulants) were assessed in the lab without medication. Similarly, parents and teachers were each asked to complete rating scales based on the child’s unmedicated behavior. All interviews were initially blind to the child’s diagnostic status. The IRB approved all study procedures.
The parent and child completed a structured parent-child interaction task (Eyberg, Nelson, Duke, & Boggs, 2005) with demonstrated predictive validity and sensitivity to intervention effects (Thomas & Zimmer-Gembeck, 2007). Toys were placed strategically around an observation room, consisting of Legos, Lincoln Logs, Mr. Potato Head, Etch-A-Sketch, and a jungle-themed puzzle. Parents and their offspring were instructed to engage in three play-oriented conditions using the toys provided: (1) 10 minutes of child-led play, (2) 10 minutes parent-led play, and (3) 5 minutes of parent-led cleanup time. The assessor entered the room at 5-minute intervals to ensure that the dyad was fully engaged in the task.
Genotyping
DNA from the biological parents and offspring was extracted from saliva using DNA Genotek Oragene™ Self-Collection Kits (DNA Genotek, Inc., Ottawa, Canada). Genotyping was conducted on six genetic markers: 5-HTTLPR, DRD4, DRD2, MAO-A, COMT, and DAT1. However, we only examined DAT1 in this study and the results reported herein are not the result of widespread statistical tests (across different variants) and selective reporting based on statistical significance. DAT1 (SLC6A3) contains a 40-bp VNTR polymorphism in the 3′UTR. The 9-repeat (440 bp) and 10-repeat (480 bp) polymorphisms are the two most common alleles in the population. The primer sequences were: forward, 5′TGTGGTGTAGGGAACGGCCTGAG-3′ (fluorescently labeled), and reverse: 5′CTTCCTGGAGGTCACGGCTCAAGG-3′. Given that the literature is unclear about whether DAT1 follows a dominant, additive, or recessive genetic model, we tested and reported the results for all three genetic models (Uher & McGuffin, 2010). DAT1 genotype frequencies in our sample were distributed as follows: 9/9 (n = 11, 7.3%), 9/10 (n = 54, 36%), and 10/10 (n = 85, 56.7%). These frequencies were in Hardy-Weinberg equilibrium (X2 = 0.25, df = 1, p = .62).
Measures
Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996)
The BDI is a 21 item adult self-report inventory of current depression symptoms criteria from DSM-IV. Parents (88% of whom were mothers) were asked to rate the severity of their depression symptoms over the past two weeks from a four-point likert scale. We used total scores from BDI-II to control for parental depression in each of our models. The reliability of the BDI-II was adequate in our sample (α = .85).
Diagnostic Interview Schedule for Children, Version IV, Parent Version (DISC; Shaffer et al., 2000)
The DISC is a computer-assisted, fully structured diagnostic interview with the parent used to assess child psychopathology based on DSM-IV criteria. The ADHD module of the DISC has good psychometric properties, including high test-retest reliability (r = .79 after one year) and internal consistency (α = .84 for symptoms and α = .77 for criterion) for parent ratings in a large community sample (Shaffer et al., 2000). We separately analyzed the number of DSM-IV inattention and hyperactivity symptoms. ODD symptoms from the DISC were also used as a covariate in each of our models.
Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001)
The CBCL is a 113-item, parent rating scale that yields eight narrowband syndrome scales. Parents rated each behavior based on the preceding 6 months as “Not True” (0), “Somewhat or Sometimes True” (1), or “Very True or Often True” (2). Normative data are available for 6–18 youth and scales have shown to discriminate well between referred and non-referred populations (Chen, Faraone, Biederman, & Tsuang, 1994). We used T-scores on the Attention Problems narrowband scale.
Teacher Report Form (TRF; Achenbach & Rescorla, 2001)
The TRF is a 120 item, teacher rating scale based on the same eight narrowband syndrome scales of the CBCL. Teachers were asked to rate the frequency of problem behaviors in the classroom over the past two months. Similar to the CBCL, the TRF exhibits good reliability and validity (Achenbach & Rescorla, 2001) and the syndrome scales discriminate well between clinic and nonclinic samples. We used the T-scores on the Attention Problems narrowband scale.
Disruptive Behavior Disorder Rating Scale, Teacher Version (T-DBDRS, respectively; Pelham, Gnagy, Greenslade, & Milich, 1992)
The T-DBDRS is rating scale adapted from DSM-III-R symptoms for disruptive behavior disorders. Teachers rated on the frequency on DBD symptoms by selecting from four-response options: “Not at All” (0), “Just a Little” (1), “Pretty Much” (2), or “Very Much” (3). The DBDRS had excellent internal consistency in our sample (α = .93). We separately analyzed the number of inattention and hyperactivity symptoms. Teacher data were available for 88 of the original 150 participants. No significant differences were observed between youth with vs. without teacher data on demographic (i.e., age, sex, race-ethnicity, income, education), child (i.e., ODD, anxiety, depression) and parenting variables.
Dyadic Parent Child Interaction Coding System (DPICS; Eyberg et al., 2005)
We used the DPICS, a well-validated system of rating parent-child interaction in children with disruptive behavior disorders (Chronis-Tuscano et al., 2008). 88% of parents who participated in the study were mothers. Discrete parent and child behaviors were coded continuously. We created composite categories of parenting quality that appear in the literature (Chronis-Tuscano et al., 2008; Eyberg et al., 2001), including parental negativity, praise, and child noncompliance. Parental negativity was coded when parents utilized a harsh or brash tone of voice, made critical or hostile comments (e.g., “that’s a terrible drawing,” “you better try harder or else you’ll be punished”), issued negative commands (e.g., “stop doing that right now!”), or made sarcastic and condescending remarks (e.g., “you think you’re real good at building that don’t you?”). Examples of praise included positive appraisals for a child’s behavior or attribute, or a product that the child created (e.g., “you’re a good builder,” “that’s a really pretty picture of a dog you drew”). Child noncompliance was coded when the child refused parental commands and/or ignored questions. We tallied the total counts of parent and child behaviors across each condition (e.g., number of times the parent praised the child) and divided this by the total minutes that were coded. Interactions were recorded using a digital recorder.
Research assistants were intensively trained in the DPICS coding procedures until at least 70% agreement was attained. Coders participated in a full day of training followed by two months of practice where each coding category was discussed with reviews/quizzes. We held weekly coding meetings to ensure reliability and to resolve disagreements. 20% of the videos was randomly selected and coded by two separate raters to estimate reliability. DPICS composite categories have shown moderate to substantial inter-rater and test-retest reliability (Chronis-Tuscano et al., 2008). The intra-class correlations (ICC) for our categories were as follows: negativity (ICC = .75), praise (ICC = .88), and child noncompliance (ICC = .78).
Statistical Analysis
Data were analyzed using structural equation modeling (SEM) in Stata 12.1 (StataCorp, 2011) using maximum likelihood estimation of the full covariance matrix. To characterize ADHD with maximal precision, we evaluated two competing models and used the better model in to test G×E. In the first model, we formed separate latent ADHD variables based on parent and teacher data to represent ADHD at home and school, respectively (DeYoung, Peterson, Seguin, & Tremblay, 2008). Latent factors representing parent- and teacher-ADHD were allowed to correlate in the model so that any unique variance could be attributed to context-specific ADHD. In the second model, we specified another latent variable representing the shared variance between these two factors to represent a general ADHD latent factor. In other words, we hypothesized that ADHD could be represented by a general factor, regardless of the context. Normand, Flora, Toplak, and Tannock (2012) recently found that a general ADHD factor and two specific factors best accounted for parent and teacher reports of ADHD symptoms in a large sample of 6–9 year old boys and girls. We evaluated model fit with the likelihood ratio chi-square test, the comparative fit index (CFI; Bentler, 1990), the root-mean-square error of approximation (RMSEA; Browne & Cudeck, 1993), and standardized root-mean-square residual (SRMR), which represents the average correlation among residuals.
After satisfactory model fit was established, we conducted hierarchical regression analyses with two steps. In the first step, we examined the effect of parental negativity, praise, and child DAT1 on the latent ADHD factor, controlling for child age, sex, ODD symptoms, observed noncompliance from the DPICS, and parent BDI (i.e., main effects model). In the second step, we added the cross-products between DAT1 and negativity and praise (separately) to this model and estimated its effects on the latent factor (i.e., fully saturated model).
Results
Population Stratification and Gene-Environment Correlation
Population stratification can produce spurious effects and confound the interpretation of potential G×E. DAT1 was unrelated to race-ethnicity (χ2(7) = 11.37, p = .12) and ADHD status (χ2(1) = .08, p = .78). Passive and evocative gene-environment correlations (rGE) may also confound tests of G×E (Jaffee & Price, 2007). To address these concerns, we first tested the association of parent DAT1 genotype with parenting behaviors, controlling for demographic factors (i.e., parental education, income level), parental psychopathology (i.e., depression) and child disruptive behavior (i.e., child ODD and noncompliance). Parent DAT1 genotype was unrelated to parental negativity (B = .13, SE = .11, p = .22) and praise (B = −.01, SE = .13, p = .99) (n = 150). Thus, our conceptualization of parenting as the “environment” in the context of G×E was not complicated by the influence of parental DAT1 genotype. To address evocative rGE, where the child’s genotype predicted exposure to the environment, negativity and praise did not differ significantly by offspring DAT1 genotype [F(1, 149) = .04, p = .59 and F(1,149) = .11, p = .48, respectively]. Thus, our findings are robust to rGE at this DAT1 locus.
Structural Equation Models
Correlations, means and standard deviations among all measured variables are presented in Table 1. We found positive correlations between parent- and teacher rated dimensions of ADHD (p’s < .01), including for the inattention and hyperactivity clusters, suggesting that their covariation may be related to a general latent construct of ADHD. Parental negativity was also positively correlated (p < .05) with parent- and teacher-rated dimensions of ADHD, but not for CBCL attention problems. Interestingly, praise was unrelated to all measures of ADHD and to parental negativity. Finally, the number of child ODD symptoms and DPICS noncompliance were positively associated with all measures of ADHD (p’s < .05).
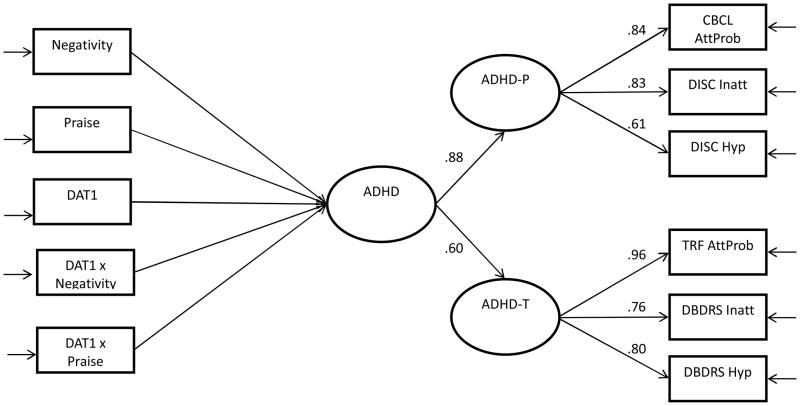
Next, we compared the model fit between the context-specific vs. general-ADHD models. When parent and teacher-ADHD were treated as separate latent variables (without a general factor), the model fit was excellent (χ2(10) = 8.19, p = .61; CFI = .99; RMSEA = .01; SRMR = .03). When the general factor of ADHD was included, the model also yielded an excellent fit to the data (χ2(10) = 7.11, p = .47; CFI = .99; RMSEA = .01; SRMR = .02). In both models, the factor loadings of the individual observed variables on the latent factors were excellent (all > .6) (Figure 1). Thus, we conducted regressions using a general latent ADHD factor rather than the context-specific model to prioritize model parsimony. Furthermore, when G×E analyses were conducted using context-specific latent variables as outcomes, the results were consistent for both outcomes (results available upon request).
Figure 1.
Unspecified SEM model with ADHD as a general latent construct
Note. ADHD-P = parent-rated ADHD based on CBCL and DISC; ADHD-T = teacher-rated ADHD based on TRF and T-DBDRS; the left side of the model is unspecified given that separate regression analyses were conducted based on additive, dominant vs. recessive DAT1-coded genotypes. Table 2 shows the parameter estimates for each of these analyses.
Regressions
Additive model
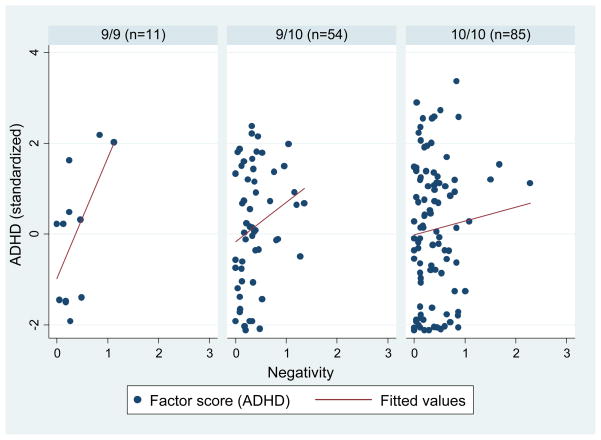
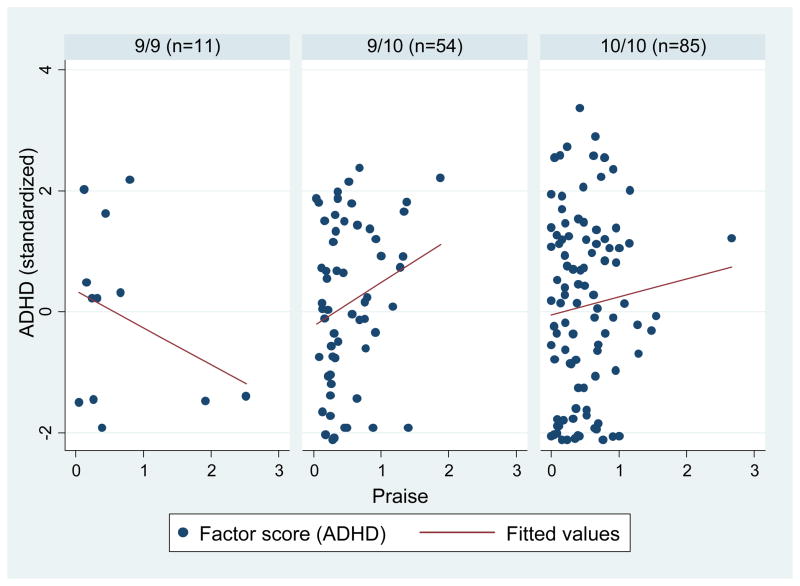
DAT1 was coded as a dummy variable with three groups: 9/9, 9/10, and 10/10 (9/9 was the reference class). We regressed parental negativity, praise, and DAT1 on the latent ADHD factor, controlling for age, sex, child ODD symptoms, observed noncompliance, and parental BDI. There were no significant main effects for negativity, praise or DAT1. However, in the fully saturated model, a significant interaction between DAT1 (9/9 vs. 9/10) and praise and a marginally significant interaction between DAT1 (9/9 vs. 10/10) and negativity emerged for ADHD (Table 2). Specifically, praise was positively associated with ADHD, but only for children with the 9/10 genotype (B = .83, SE = .40, p < .05) and not for children with the 9/9 or 10/10 genotypes (B = .84, SE = .76, p = .33 and B = .06, SE = .33, p = .85, respectively). Negativity was also positively associated with ADHD, but only for children with the 9/9 genotype (B = 2.58, SE = 1.00, p < .05) and not for children with the 9/9 or 10/10 genotypes (B = .75, SE = .61, p = .22 and B = .27, SE = .42, p = .53, respectively).
Table 2.
Additive, dominant and recessive DAT1 regression models
| B | SE | p | B | SE | p | B | SE | p | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Additive | 9-Dominant | 9-Recessive | |||||||||
| Age | −.05 | .09 | .59 | Age | −.04 | .10 | .66 | Age | −.04 | .10 | .72 |
| Sex | .20 | .21 | .35 | Sex | .26 | .22 | .25 | Sex | .22 | .22 | .33 |
| Parental BDI | .02 | .02 | .31 | Parental BDI | .02 | .02 | .41 | Parental BDI | .02 | .02 | .41 |
| Noncompliance | .81 | .38 | < .05 | Noncompliance | .27 | .53 | .61 | Noncompliance | .33 | .52 | .53 |
| ODD symptoms | .26 | .04 | < .01 | ODD symptoms | .26 | .04 | < .01 | ODD symptoms | .26 | .04 | < .01 |
| Negativity | .51 | .33 | .13 | Negativity | .95 | .54 | .08 | Negativity | 2.44 | 1.17 | < .05 |
| Praise | .18 | .23 | .43 | Praise | .17 | .34 | .63 | Praise | −.67 | .50 | .19 |
| DAT1 | -- | -- | -- | DAT1 | .39 | .40 | .32 | DAT1 | .41 | .69 | .56 |
| 9/10 | .32 | .43 | .46 | -- | -- | -- | -- | -- | -- | -- | -- |
| 10/10 | .38 | .42 | .36 | -- | -- | -- | -- | -- | -- | -- | -- |
| DAT1 9/10 x Neg. | −1.94 | 1.29 | .13 | DAT1 x Neg. | −.70 | .64 | .28 | DAT1 x Neg. | −2.06 | 1.21 | .09 |
| DAT1 10/10 x Neg. | −2.18 | 1.23 | .08 | -- | -- | -- | -- | -- | -- | -- | -- |
| DAT1 9/10 x Praise | 1.62 | .68 | < .01 | DAT1 x Praise | −.03 | .47 | .95 | DAT1 x Praise | 1.07 | .56 | .06 |
| DAT1 10/10 x Praise | .79 | .60 | .18 | -- | -- | -- | -- | -- | -- | -- | -- |
Note. Results represent the fully-saturated regression models (i.e., step 2). Additive = 9/9 vs. 9/10 vs. 10/10; Dominant = 9/9 + 9/10 vs. 10/10; Recessive = 9/9 vs. 9/10 vs. 10/10
Dominant model
Comparing 9/9 and 9/10 genotypes with 10/10 genotypes, negativity, praise and DAT1 were unrelated to ADHD in the main effects only model, controlling for all the covariates previously mentioned (negativity: B = .50, SE = .33, p = .47; praise: B = .17, SE = .23, p = .48; DAT1: B = .12, SE = .21, p = .59). In the fully saturated model, DAT1 x negativity and DAT1 x praise interactions were unrelated to ADHD (Table 2).
Recessive model
Comparing the 9/9 genotype to the 9/10 and 10/10 genotypes, there were no main effects for negativity, praise or DAT1 for ADHD, controlling for the covariates (negativity: B = .52, SE = .40, p = .37; praise: B = .18, SE = .23, p = .43; DAT1: B = .36, SE = .40, p = .38). In the fully saturated model, marginally significant interactions emerged for DAT1 x negativity and DAT1 x praise for ADHD (Table 2). Negativity was positively associated with ADHD among children with the 9/9 genotype (B = 2.57, SE = .71, p < .05), but not for children with the 9/10 or 10/10 genotypes (B = .37, SE = .34, p = .28). Praise was unrelated to ADHD in both genotypes (9/9: B = .89, SE = .38, p = .12; 9/10 + 10/10: B = .40, SE = .26, p = .13).
Discussion
Although parenting quality is reliably correlated with ADHD (Wells et al., 2000; Chronis-Tuscano et al., 2008), few studies examined its interaction with DAT1 using differentiated measures of negative and positive parenting, despite their divergent roles in gene expression (Zhu et al., 2010; Belsky & Pluess, 2009). We used SEM with a well-characterized sample of 150 children with and without ADHD and detected a significant interaction between observed praise and child DAT1 (coded additively) where praise was modestly associated with increased ADHD, but only among youth with the 9/10 genotype. In addition, a marginally significant interaction emerged between DAT1 (coded additively and recessively) and observed negativity for ADHD, such that negativity was positively associated with ADHD but only for youth with the 9/9 genotype.
Our findings provide additional support to the emerging literature that child genotype interacts with parenting quality for ADHD (Li & Lee, 2012). However, the association of the 9-repeat allele and ADHD differs from two previous G×E studies of ADHD which implicated 10-repeat homozygosity, although these previous studies were based on prenatal exposure to teratogens and psychosocial adversity rather than parenting behavior (Kahn et al., 2003; Laucht et al., 2007). Importantly, the precise biological functionality of the DAT1 genotype is unknown (Brookes et al., 2006). Single photon emission computed tomography demonstrated that DAT1 was associated with DA transporter availability and binding potential, but the direction of these effects was unclear (Heinz et al., 2000). Although the 10-repeat homozygosity was positively correlated with DAT1 mRNA expression in human post-mortem midbrain tissue, comparisons with the 9-repeat allele were prohibitive due to limited sample size (Brookes et al., 2007). Human post-mortem studies based on larger sample sizes (e.g., Fuke et al., 2001; VanNess, Owens & Kilts, 2005) did not find functional differences, perhaps due to the fact that most human cell lines do not naturally express DA (Brookes et al., 2007). Stronger evidence for functional differences in the DAT1 genotype are suggested by fMRI studies where greater activation in the striatum and basal ganglia (i.e., regions that are rich in DA neurotransmission and are involved in motor and reward pathways) during reward-based, go/no-go inhibition tasks among individuals with at least one 9-repeat allele (Congdon et al., 2009). However, conflicting or null results have also been reported (Caldu et al., 2007). Thus, DA expression in the brain is likely to be influenced by additional factors beyond DAT1. They may also reflect the influence of other unmeasured variants that are in linkage disequilibrium with the DAT1 VNTR. Finally, statistical geneticists have proposed that these so called “allele flips” may reflect genuine differences in which both “sets” of findings are valid (Clarke & Cardon, 2010).
We speculate that DA may play an especially crucial role in response to parenting, given that the association between parental negativity and praise with ADHD only applied to youth with at least a single 9-repeat DAT1 allele. The rate of DA binding may depend on the salience of the environmental stimulus (Tripp & Wickens, 2008). DA neurons exhibit the fastest firing rate prior to a salient reward, but the firing rate falls below baseline in anticipation of a harsh punishment (Mirenowicz & Schultz, 1996). Similarly, alterations in the environment can profoundly influence DA binding and expression: individually housed monkeys in impoverished conditions exhibited increased DA and concurrent down-regulation of D2 receptors levels in the prefrontal cortex (Morgan et al., 2002). Monkeys that were later exposed to social housing (i.e., enriched environment) increased their D2 receptor binding and exhibited “normal” levels synaptic DA, however (Morgan et al., 2002). These findings may also help explain why praise was positively associated with ADHD among DAT1 9/10 youth as this construct may not have been sufficiently salient for DA activation. In our study, observed parental praise occurred far more frequently than negativity, suggesting that the use of praise may have less impact than negativity on child behavior. There is also evidence on the “detrimental” effects of praise and other forms of positive reinforcement on child behavior or performance, especially if they are not delivered genuinely (Henderlong & Lepper, 2002). Future G×E studies should prioritize salient markers for positive and negative environmental conditions and to test whether the direction of these environmental effects is consistent with differential susceptibility versus diathesis stress.
The significant interaction between offspring DAT1 and observed parenting is strengthened by its derivation from a latent variable model for ADHD featuring parent and teacher data given that G×E effects on ADHD were more likely to reflect stable factors in the etiology of ADHD rather than context- or informant-specific effects (Thapar et al., 2006). Given that the assessment of ADHD is complicated by problems with method variance, future studies should consider using latent variable approaches over separate categorical or dimensional definitions, which can inflate type I error with multiple tests. However, we also acknowledge that traditional approaches may reflect qualitatively different phenotypes (Thapar et al., 2006). In addition to the methodological strengths of this study, the G×E findings have potential clinical implications. Children with the 10/10 genotype were protected against environmental risk, in that exposure to parental negativity did not increase their risk for ADHD compared to children with the 9/9 or 9/10 genotypes (these children also did not have decreased ADHD as a function of praise, either). Thus, environmental risks may interact with genetic factors to predict not only vulnerability, but also resilience (Kim-Cohen & Gold, 2009). Incorporating genetic markers into psychosocial research may help identify the biological factors that promote resilience and prevent psychopathology among individuals who experience severe and/or chronic psychosocial adversity (Kim-Cohen & Gold, 2009). Our findings did not support the differential susceptibility hypothesis, as praise and negativity were both positively associated (albeit modestly) with ADHD for children with at least one 9-repeat allele. However, a visual inspection of the interaction figures showed that children in the 9/9 genotype group, specifically, had increased sensitivity to both negativity and praise conditions (i.e., more ADHD with high observed negativity, less ADHD with high observed praise). The limited sample size (n = 11) within this group likely complicated any potential association with praise reaching statistical significance. Nonetheless, with larger sample sizes, these investigations may have important implications for intervention, including targeted populations and interventions that focus on environmental enrichment (Belsky, 2007).
There were important limitations to our study. First, observations from the parent-child interaction task may not have reflected parenting behavior outside of the lab. This includes the unexpected finding that praise was positively associated with ADHD, for which praise may not have reflected positive parenting behaviors per se. However, the use of observational coding of parenting behavior may have provided more objective data given that self-report data may reflect psychopathology or negative attributions about the child (Gardner, 2000). Second, despite the use of SEM, our analysis may have been underpowered due to the limited sample size. Thus, we emphasize that our findings are preliminary and we await replication in larger samples Third, despite the fact that parents and teachers were asked to rate the child’s unmedicated behaviors, there may have been medication-effects nonetheless because children with ADHD often take medication at school, but not at home (Molina et al., 1998). Thus, teachers may be more sensitive to the effects of medication than parents when rating the child’s behavior. Interestingly, Antrop et al. (2002) found that parent and teacher ratings of ADHD did not differ as a function of medication status. Next, our findings were robust to stringent control of potentially confounding variables such as parental depression, and child ODD and noncompliance during the parent-child interaction, thereby suggesting that the G×E was specific to ADHD. However, we acknowledge that covarying ODD and noncompliance may not have provided sufficient safeguards the effects of these behaviors on parenting. Finally, teacher data were only available for 60% of the sample. To address this concern, we specified maximum likelihood estimation for missing data in our SEM analyses. However, we await replication for our multi-informant findings of G×E, especially given the relatively smaller sample size of teacher data. Future G×E studies should also attempt to improve the characterization of the phenotype and the environmental criterion (Li & Lee, 2010).
The current study suggests that offspring DAT1 genotype may interact with parental negativity and praise to increase ADHD. Given that DA transmission is influenced by positive and negative aspects of the environment, future G×E studies must avoid narrow definitions of environmental adversity. More importantly, the present findings underscore the unique contributions that are afforded by genetically-sensitive designs that simultaneously incorporate careful measurement of biologically-plausible environmental conditions. Further research is needed on the mechanisms of G×E, which may indicate potential targets for environmental intervention. We conclude by emphasizing that parent-child interaction constitutes and dynamically represents important forms of interplay (i.e., correlation, interaction) between genetic and environmental influences (Jaffee & Price, 2007).
Figure 2.
DAT1 and observed parental negativity on ADHD
Figure 3.
DAT1 and observed parental praise on ADHD
Acknowledgments
This work was supported by the Consortium of Neuropsychiatric Phenomics (CNP) (NIH Roadmap for Medical Research grant UL1-DE019580, RL1DA024853).
Footnotes
POTENTIAL SUBSCRIBERS TO JOURNAL
Rena Repetti, Ph.D., Theodore Robles, Ph.D., Lara A. Ray, Ph.D., UCLA Department of Psychology, 1285 Franz Hall, Box 951563, Los Angeles, CA 90095-1563
Adam Leventhal, Ph.D., University of Southern California, Soto Street Building, SSB, 2001 N. Soto St., 3rd Floor, MC9239, Los Angeles, CA 90089-9045
Contributor Information
James J. Li, Email: jamesjli26@gmail.com.
Steve S. Lee, Email: stevelee@psych.ucla.edu.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age: Forms & Profiles. ASEBA; 2001. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. Washington, DC: Author; 1994. [Google Scholar]
- Antrop I, Roeyers H, Oosterlaan J, Van Oost P. Agreement between parent and teacher ratings of disruptive behavior disorders in children with clinically diagnosed ADHD. Journal of Psychopathology and Behavioral Assessment. 2002;24:67–73. doi: 10.1023/A:1014057325752. [DOI] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IMH, Pijlman FT, Mesman J, Juffer F. Experimental evidence for differential susceptibility: Dopamine D4 receptor polymorphism (DRD4 VNTR) moderates intervention effects on toddlers’ externalizing behavior in a randomized controlled trial. Developmental Psychology. 2008;44:293–300. doi: 10.1037/0012-1649.44.1.293. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Smith KM, Fischer M, Navia B. An examination of the behavioral and neuropsychological correlates of three ADHD candidate gene polymorphisms (DRD4 7+, DBH TaqI A2, and DAT1 40 bp VNTR) in hyperactive and normal children followed to adulthood. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2006;141:487–498. doi: 10.1002/ajmg.b.30326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PM, Hanson JL, Pierson RK, Davidson RJ, Pollak SD. Cerebellar volume and cognitive functioning in children who experienced early deprivation. Biological Psychiatry. 2009;66:1100–1106. doi: 10.1016/j.biopsych.2009.06.014. http://dx.doi.org/10.1016/j.biopsych.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II (BDI-II) San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Belsky J, Bakermans Kranenburg MJ, Van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. Retrieved from http://cdp.sagepub.com/ [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, de Haan M. Annual Research Review: Parenting and children’s brain development: the end of the beginning. Journal of Child Psychology and Psychiatry. 2011;52(4):409–428. doi: 10.1111/j.1469-7610.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107(2):238–346. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Branchi I. The mouse communal nest: investigating the epigenetic influences of the early social environment on brain and behavior development. Neuroscience & Biobehavioral Reviews. 2009;33(4):551–559. doi: 10.1016/j.neubiorev.2008.03.011. Retrieved from http://dx.doi.org/10.1016/j.neubiorev.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Brookes KJ, Mill J, Guindalini C, Curran S, Xu X, Knight J, et al. A common haplotype of the dopamine transporter gene associated with attention-deficit/hyperactivity disorder and interacting with maternal use of alcohol during pregnancy. Archives of General Psychiatry. 2006;63:74–81. doi: 10.1001/archpsyc.63.1.74. Retrieved from http://archpsyc.ama-assn.org/ [DOI] [PubMed] [Google Scholar]
- Brookes KJ, Neale BM, Sugden K, Khan N, Asherson P, D’Souza UM. Relationship between VNTR polymorphisms of the human dopamine transporter gene and expression in post mortem midbrain tissue. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144:1070–1078. doi: 10.1002/ajmg.b.30572. [DOI] [PubMed] [Google Scholar]
- Bruggemann D, Sobanski E, Alm B, Schubert T, Schmalzried H, Philipsen A, et al. No association between a common haplotype of the 6 and 10-repeat alleles in intron 8 and the 3′UTR of the DAT1 gene and adult attention deficit hyperactivity disorder. Psychiatric Genetics. 2007;17:121. doi: 10.1097/YPG.0b013e32801231d4. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Burke JD, Pardini DA, Loeber R. Reciprocal relationships between parenting behavior and disruptive psychopathology from childhood through adolescence. Journal of Abnormal Child Psychology. 2008;36(5):679–692. doi: 10.1007/s10802-008-9219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldú X, Vendrell P, Bartrés-Faz D, Clemente I, Bargalló N, Jurado MÁ, et al. Impact of the COMT Val108/158 Met and DAT genotypes on prefrontal function in healthy subjects. Neuroimage. 2007;37:1437–1444. doi: 10.1016/j.neuroimage.2007.06.021. http://dx.doi.org/10.1016/j.neuroimage.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Raggi V, Clarke T, Rooney M, Diaz Y, Pian J. Associations between maternal attention-deficit/hyperactivity disorder symptoms and parenting. Journal of Abnormal Child Psychology. 2008;36:1237–1250. doi: 10.1007/s10802-008-9246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis AM, Lahey BB, Pelham WE, Jr, Williams SH, Baumann BL, Kipp H, et al. Maternal depression and early positive parenting predict future conduct problems in young children with attention-deficit/hyperactivity disorder. Developmental Psychology. 2007;43:70–82. doi: 10.1037/0012-1649.43.1.70. [DOI] [PubMed] [Google Scholar]
- Clarke GM, Cardon LR. Aspects of observing and claiming allele flips in association studies. Genetic Epidemiology. 2010;34:266–274. doi: 10.1002/gepi.20458. [DOI] [PubMed] [Google Scholar]
- Congdon E, Constable RT, Lesch KP, Canli T. Influence of SLC6A3 and COMT variation on neural activation during response inhibition. Biological Psychology. 2009;81:144–152. doi: 10.1016/j.biopsycho.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EHJ, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, Leventhal BL. Association of attention-deficit disorder and the dopamine transporter gene. American Journal of Human Genetics. 1995;56:993–998. Retrived from www.cell.com/AJHG. [PMC free article] [PubMed] [Google Scholar]
- Deater-Deckard K. Parenting and child behavioral adjustment in early childhood: A quanititative genetic approach to studying family processes. Child Development. 2000;71:468–484. doi: 10.1111/1467-8624.00158. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Peterson JB, Séguin JR, Tremblay RE. Externalizing behavior and the higher order factors of the Big Five. Journal of Abnormal Psychology. 2008;117:947. doi: 10.1037/a0013742. [DOI] [PubMed] [Google Scholar]
- Dunn EC, Uddin M, Subramanian SV, Smoller JW, Galea S, Koenen KC. Research review: Gene-environment interaction research in youth depression – a systematic review with recommendations for future research. Journal of Child Psychology and Psychiatry. 2011;52:1223–1238. doi: 10.1111/j.1469-7610.2011.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyberg S, Nelson M, Duke M, Boggs S. Manual for the dyadic parent-child interaction coding system. Gainesville: University of Florida; 2005. Retrieved from http://pcit.phhp.ufl.edu/measures. [Google Scholar]
- Eyberg SM, Funderburk BW, Hembree-Kigin TL, McNeil CB, Querido JG, Hood KK. Parent-child interaction therapy with behavior problem children: One and two year maintenance of treatment effects in the family. Child and Family Behavior Therapy. 2001;23:1–20. doi: 10.1300/J019v23n04_01. [DOI] [Google Scholar]
- Faraone SV, Biederman J. Neurobiology of attention-deficit hyperactivity disorder. Biological Psychiatry. 1998;44:951–958. doi: 10.1016/S0006-3223(98)00240-6. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2:104–113. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1525089/ [PMC free article] [PubMed] [Google Scholar]
- Fortuna K, van IJzendoorn MH, Mankuta D, Kaitz M, Avinun R, Ebstein RP, et al. Differential genetic susceptibility to child risk at birth in predicting observed maternal behavior. PLoS ONE. :e19765. doi: 10.1371/journal.pone.0019765. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SE, Levitt P, Nelson CA., III How the timing and quality of early experiences influence the development of brain architecture. Child Development. 2010;81:28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke B, Vasquez AA, Johansson S, Hoogman M, Romanos J, Boreatti-Hummer A, et al. Multicenter analysis of the SLC6A3/DAT1 VNTR haplotype in persistent ADHD suggests differential involvement of the gene in childhood and persistent ADHD. Neuropsychopharmacology. 2010;35:656–664. doi: 10.1038/npp.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, Recognition, and Treatment of Attention-Deficit/Hyperactivity Disorder in a National Sample of US Children. Archives of Pediatrics and Adolescent Medicine. 2007;161:857–864. doi: 10.1001/archpedi.161.9.857. [DOI] [PubMed] [Google Scholar]
- Froehlich TE, Epstein JN, Nick TG, Castro MSM, Stein MA, Brinkman WB, Kahn RS. Pharmacogenetic predictors of methylphenidate dose-response in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50:1129–1139. doi: 10.1016/j.jaac.2011.08.002. Retrieved from http://dx.doi.org/10.1016/j.jaac.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics. 2001;1:152–156. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- Gardner F. Methodological issues in the direct observation of parent–child interaction: do observational findings reflect the natural behavior of participants? Clinical Child and Family Psychology Review. 2000;3:185–198. doi: 10.1023/A:1009503409699. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Henderlong J, Lepper MR. The effects of praise on children’s intrinsic motivation: A review and synthesis. Psychological Bulletin. 2002;128:774–795. doi: 10.1037/0033-2909.128.5.774. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Jaffee S, Price T. Gene–environment correlations: a review of the evidence and implications for prevention of mental illness. Molecular Psychiatry. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joober R, Grizenko N, Sengupta S, Amor LB, Schmitz N, Schwartz G, et al. Dopamine transport 3′-UTR VNTR genotype and ADHD: A pharmaco-behavioural genetic study with methylphenidate. Neuropsychopharmacology. 2007;32:1370–1376. doi: 10.1038/sj.npp.1301240. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Khoury J, Nichols WC, Lanphear BP. Role of dopamine transporter genotype and maternal prenatal smoking in childhood hyperactive-impulsive, inattentive, and oppositional behaviors. Journal of Pediatrics. 2003;143:104–110. doi: 10.1016/S0022-3476(03)00208-7. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Gold AL. Measured gene-environment interactions and mechanisms promoting reslient development. Current Directions in Psychological Science. 2009;18:138–142. Retrieved from http://cdp.sagepub.com/ [Google Scholar]
- Kishiyama MM, Boyce WT, Jimenez AM, Perry LM, Knight RT. Socioeconomic disparities affect prefrontal function in children. Journal of Cognitive Neuroscience. 2009;21:1106–1115. doi: 10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Jacob T, Slutske WS, Bucholz KK, Madden PAF, et al. Maternal alcohol use disorder and offspring ADHD: disentangling genetic and environmental effects using a children-of-twins design. Psychological Medicine. 2006;36:1461–1471. doi: 10.1017/S0033291706007884. http://dx.doi.org/10.1017/S0033291706007884. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Rogers H, Swinard G, Borger N, van der Meere J, Rijsdijk F, Asherson P. Reaction time, inhibition, working memory and ‘delay aversion’ performance: genetic influences and their interpretation. Psychological Medicine. 2006;36:1613–1624. doi: 10.1017/S0033291706008580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Stein MA, Loney J, Trapani C, Nugent K, et al. Validity of DSM-IV attention-deficit/hyperactivity disorder for younger children. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:695–702. doi: 10.1097/00004583-199807000-00008. [DOI] [PubMed] [Google Scholar]
- Langley K, Fowler T, Ford T, Thapar AK, van den Bree M, Harold G, et al. Adolescent clinical outcomes for young people with attention-deficit hyperactivity disorder. The British Journal of Psychiatry. 2010;196:235–240. doi: 10.1192/bjp.bp.109.066274. [DOI] [PubMed] [Google Scholar]
- Laucht M, Skowronek MH, Becker K, Schmidt MH, Esser G, Schulze TG, et al. Interacting effects of the dopamine transporter gene and psychosocial adversity on attention-deficit/hyperactivity disorder symptoms among 15-year-olds from a high-risk community sample. Archives of General Psychiatry. 2007;64:585–590. doi: 10.1001/archpsyc.64.5.585. Retrieved from http://archpsyc.ama-assn.org/ [DOI] [PubMed] [Google Scholar]
- Li JJ, Lee SS. Latent class analysis of antisocial behavior: Interaction of serotonin transporter genotype and maltreatment. Journal of Abnormal Child Psycholology. 2010;38:789–801. doi: 10.1007/s10802-010-9409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Lee SS. Association of positive and negative parenting behavior with childhood ADHD: Interactions with offspring monoamine oxidase-A (MAO-A) genotype. Journal of Abnormal Child Psychology. 2012;40:165–175. doi: 10.1007/s10802-011-9553-z. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Markham JA, Herting MM, Luszpak AE, Juraska JM, Greenough WT. Myelination of the corpus callosum in male and female rats following complex environment housing during adulthood. Brain Research. 2009;1288:9–17. doi: 10.1016/j.brainres.2009.06.087. http://dx.doi.org/10.1016/j.brainres.2009.06.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Guite J, Tsuang MT. Pregnancy, delivery and infancy complications and attention deficit hyperactivity disorder: Issues of gene-environment interaction. Biological Psychiatry. 1997;41:65–75. doi: 10.1016/0006-3223(95)00653-2. http://dx.doi.org/10.1016/0006-3223(95)00653-2. [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3 UTR VNTR: Evidence from brain and lymphocytes using quantitative RT PCR. American Journal of Medical Genetics. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Measured gene-environment interactions in psychopathology. Perspectives on Psychological Science. 2006;1:5–27. doi: 10.1111/j.1745-6916.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE, Blumenthal J, Galiszewski E. Agreement among teachers’ behavior ratings of adolescents with a childhood history of attention deficit hyperactivity disorder. Journal of Clinical Child Psychology. 1998;27:330–339. doi: 10.1207/s15374424jccp2703_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al. The MTA at 8 years: Prospective follow-up of children treated for combined-type ADHD in a multisite study. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: Dopamine D2 receptors and cocaine self-administration. Nature Neuroscience. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Morrell J, Murray L. Parenting and the development of conduct disorder and hyperactive symptoms in childhood: A prospective longitudinal study from 2 months to 8 years. Journal of Child Psychology and Psychiatry. 2003;44:489–508. doi: 10.1111/1469-7610.t01-1-00139. [DOI] [PubMed] [Google Scholar]
- Normand S, Flora DB, Toplak ME, Tannock R. Evidence for a general ADHD factor from a longitudinal general school population study. Journal of Abnormal Child Psychology. 2011:1–13. doi: 10.1007/s10802-011-9584-5. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Foster EM, Robb JA. The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. Ambulatory Pediatrics:The Official Journal of the Ambulatory Pediatric Association. 2007;7:121–131. doi: 10.1016/j.ambp.2006.08.002. http://dx.doi.org/10.1016/j.ambp.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Pettit GS, Bates JE, Dodge KA. Supportive parenting, ecological context, and children’s adjustment: A seven-year longitudinal study. Child Development. 1997;68:908–923. doi: 10.1111/j.1467-8624.1997.tb01970.x. [DOI] [PubMed] [Google Scholar]
- Roman T, Szobot C, Martins S, Biederman J, Rohde LA, Hutz MH. Dopamine transporter gene and response to methylphenidate in attention-deficit/hyperactivity disorder. Pharmacogenetics and Genomics. 2002;12:497–499. doi: 10.1097/00008571-200208000-00011. Retrieved from http://journals.lww.com/jpharmacogenetics/ [DOI] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: Multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Schore AN. Effects of a secure attachment relationship on right brain development, affect regulation, and infant mental health. Infant Mental Health Journal. 2001;22:7–66. Retrieved from http://onlinelibrary.wiley.com/journal/10.1002/%28ISSN%291097-0355. [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Seipp CM, Johnston C. Mother–son interactions in families of boys with attention-deficit/hyperactivity disorder with and without oppositional behavior. Journal of Abnormal Child Psychology. 2005;33:87–98. doi: 10.1007/s10802-005-0936-x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Halperin JM. Developmental phenotypes and causal pathways in attention deficit/hyperactivity disorder: potential targets for early intervention? Journal of Child Psychology and Psychiatry. 2010;51(4):368–389. doi: 10.1111/j.1469-7610.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Adler LA, McGough JJ, Muniz R, Jiang H, Pestreich L. Efficacy and safety of dexmethylphenidate extended-release capsules in adults with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:1380–1387. doi: 10.1016/j.biopsych.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Stevens SE, Kumsta R, Kreppner JM, Brookes KJ, Rutter M, Sonuga Barke EJS. Dopamine transporter gene polymorphism moderates the effects of severe deprivation on ADHD symptoms: Developmental continuities in gene–environment interplay. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009;150:753–761. doi: 10.1002/ajmg.b.31010. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biological Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. doi: http://dx.doi.org/10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Thapar A, Harrington R, Ross K, McGuffin P. Does the definition of ADHD affect heritability? Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:1528–1536. doi: 10.1097/00004583-200012000-00015. doi: http://dx.doi.org/10.1097/00004583-200012000-00015. [DOI] [PubMed] [Google Scholar]
- Thapar A, Langley K, O’donovan M, Owen M. Refining the attention deficit hyperactivity disorder phenotype for molecular genetic studies. Molecular Psychiatry. 2006;11:714–720. doi: 10.1038/sj.mp.4001831. [DOI] [PubMed] [Google Scholar]
- Tripp G, Wickens JR. Research review: Dopamine transfer deficit: A neurobiological theory of altered reinforcement mechanisms in ADHD. Journal of Child Psychology and Psychiatry. 2008;49:691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- Thomas R, Zimmer-Gembeck MJ. Behavioral outcomes of parent-child interaction therapy and triple P – positive parenting program: A review and meta-analysis. Journal of Abnormal Child Psychology. 2007;35:476–495. doi: 10.1007/s10802-007-9104-9. [DOI] [PubMed] [Google Scholar]
- Twardosz S, Lutzker JR. Child maltreatment and the developing brain: A review of neuroscience perspectives. Aggression and Violent Behavior. 2010;15:59–68. doi: 10.1016/j.avb.2009.08.003. [DOI] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Molecular Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- VanNess S, Owens M, Kilts C. The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC genetics. 2005;6:55–66. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman ID. Gene-environment interactions reexamined: does mother’s marital stability interact with the dopamine receptor D2 gene in the etiology of childhood attention-deficit/hyperactivity disorder? Development and Psychopathology. 2007;19:1117–1128. doi: 10.1017/S0954579407000570. [DOI] [PubMed] [Google Scholar]
- Wells KC, Epstein JN, Hinshaw SP, Conners CK, Klaric J, Abikoff HB, et al. Parenting and family stress treatment outcomes in attention deficit hyperactivity disorder (ADHD): An empirical analysis in the MTA study. Journal of Abnormal Child Psychology. 2000;28:543–553. doi: 10.1023/A:1005131131159. [DOI] [PubMed] [Google Scholar]
- Zaslow MJ, Weinfield NS, Gallagher M, Hair EC, Ogawa J, Egeland B, et al. Longitudinal prediction of child outcomes from differing measures of parenting in a low-income sample. Developmental Psychology. 2006;42:27–37. doi: 10.1037/0012-1649.42.1.27. [DOI] [PubMed] [Google Scholar]
- Zhu X, Li T, Peng S, Ma X, Chen X, Zhang X. Maternal deprivation-caused behavioral abnormalities in adult rats relate to a non-methylation-regulated D2 receptor levels in the nucleus accumbens. Behavioural Brain Research. 2010;209:281–288. doi: 10.1016/j.bbr.2010.02.005. [DOI] [PubMed] [Google Scholar]