Abstract
Individuals with schizophrenia are impaired in processing social signals such as facial expressions of emotion. Perceiving facial expressions is a complex process that depends on a distributed neural network of regions involved in affective, cognitive, and visual processing. We examined repetition priming, a non-conscious form of perceptual learning, to explore the visual-perceptual processes associated with perceiving facial expression in people with schizophrenia. Functional magnetic resonance imaging (fMRI) was also employed to probe the sensitivity of face-responsive regions in the ventral pathway to the repetition of stimuli. Subjects viewed blocks of novel and repeated faces displaying fear expressions and neutral expressions and identified each face as male or female. Gender decisions were faster for repeated encoding relative to initial encoding of faces, indicating significant priming for facial expressions. Priming was normal in schizophrenia patients, but, as expected, recognition memory for the expressions was impaired. Neuroimaging findings showed that priming-related activation for patients was reduced in the left fusiform gyrus, relative to controls, regardless of facial expression. The findings suggest that schizophrenia patients have altered neural sensitivity in regions of the ventral visual processing stream that underlie early perceptual learning of objects and faces.
Keywords: Repetition priming, Face perception, Facial expression, Fusiform gyrus, Ventral visual cortex
1. Introduction
Schizophrenia is associated with deficits in social cognition that diminish the ability to perceive, interpret, and benefit from social experiences (Penn et al., 2008). Perception of facial expression is one such deficit that persists throughout the course of the illness and has a significant impact on social functioning (Mandal et al., 1998; Edwards et al., 2002; Kohler et al., 2010). Research has established that visual-perceptual processes play a significant role in the disturbances of emotion perception in this disorder (e.g. Butler et al., 2009; Chen et al., 2009; Norton et al., 2009; Lee et al., 2010; McBain et al., 2010). However, few studies have examined the sensitivity of visual-perceptual mechanisms to repetition and learning. Here we examined repetition priming to study how the repetition of facial expressions modulates performance in a visual-perceptual task of face processing and neural activity in the ventral visual pathway. We specifically were interested in whether schizophrenia patients exhibited normal experience-dependent changes in occipito-temporal regions that are critical for processing faces.
Repetition priming refers to the facilitation in processing a stimulus as a result of a prior encounter with the same stimulus. Behaviorally, it is expressed as improved accuracy or faster reaction time to identify repeated items. Repetition priming is an implicit or non-conscious form of learning that is distinguished from explicit memory measured in tests of recall and recognition (Graf and Schacter, 1985; Tulving and Schacter, 1990; Schacter et al., 2004). The characteristic neural response associated with repetition priming is a reduction in neural activity, referred to as repetition suppression or neural priming (Buckner et al., 1995; Schacter and Buckner, 1998; Henson, 2003; Grill-Spector et al., 2006). Theoretically, a collection of processes drive the repetition effect, such as response preparation, facilitated motor response, stimulus-response learning, and enhanced processing of specific stimulus attributes (Wiggs and Martin, 1998; Schacter et al., 2004; Schnyer et al., 2007).
Studies of repetition priming have been particularly useful in identifying components of object and face processing and the neural pathways that underlie these components (Henson et al. 2003, for review). Repetition of facial stimuli is associated with reduced activity in regions of the occipito-temporal cortex that respond preferentially to faces and facial features relative to non-face objects. For example, there is reduced activity in the right inferior occipital gyrus (occipital face area) and bilateral regions of the fusiform gyrus extending anteriorly into the fusiform face area (FFA) for repeated faces with neutral expressions (Henson et al., 2002; Henson, 2003; Eger et al., 2005; Rotshstein et al., 2005; Pitcher et al., 2011). Repetition of faces displaying emotional expressions (e.g., fear, anger) is also associated with attenuated activity in the right inferior occipital gyrus and bilateral fusiform gyri (Suzuki et al., 2011; Xu and Biederman, 2010), as well as in the right superior temporal sulcus (STS) (Winston et al., 2004). The occipital face area (OFA), fusiform face area (FFA), and superior temporal sulcus (STS) form the core of a distributed neural network in the occipito-temporal cortex for face perception. The OFA is involved in early visual processing of faces and face parts (Gauthier et al., 2000; Rotshstein et al., 2005; Pitcher et al., 2007, 2011), the FFA mediates the processing of internal and external features (Andrews et al., 2010) and facial identity (Kanwisher et al., 1997; Haxby et al., 2000) and the STS responds to eye-gaze direction and facial expressions (Haxby et al., 2000). The findings of repetition effects in these face-sensitive regions in the occipito-temporal cortex suggest that repetition facilitates the processing of stimulus attributes such as facial features or identity.
The question addressed here is whether or not repetition modulates the processing of facial expressions and the neural regions associated with face perception in individuals with schizophrenia. We utilized a perceptual priming paradigm in which the identical visual stimulus was repeated following initial encoding to provide a window into the early visual stages of face processing that might support implicit memory for facial expressions. At the behavioral level, several studies in schizophrenia have shown that repetition priming in a variety of implicit memory tasks is not impaired (Clare et al., 1993, Schwartz et al., 1993; Gras-Vincendon et al., 1994; Doniger et al., 2001; Soler et al., 2011). But these studies have examined priming for words and common objects and not facial expressions. Therefore we do not know whether repetition priming in tasks with facial expressions is preserved in schizophrenia. At the neural level, data suggest that schizophrenia patients have bilateral structural and functional abnormalities in the fusiform gyrus (Onitsuka et al., 2003, 2006; Quintana et al., 2003; Johnston et al., 2005; although see Yoon et al., 2006), a key neural substrate of face perception. In particular, functional neuroimaging studies showed that schizophrenia patients, relative to controls, had reduced activity in the lateral fusiform gyrus in response to facial expressions, irrespective of whether or not subjects identified the emotional expression (Gur et al., 2002a; Quintana et al., 2003; Johnston et al., 2005). However, the neural activity associated with repetition priming of faces displaying emotion has not been studied in schizophrenia.
Deficits in social perception are widely recognized to play a significant role in the functional outcomes of adults with schizophrenia (e.g., Couture et al., 2006). Although many studies have been conducted to better understand the affective and cognitive processes responsible for the impairment in facial affect perception, no studies have examined early perceptual learning or priming of facial expressions. This approach may help isolate the visual processes and neural systems that are impaired, and those that are preserved, in the processing of facial expressions. Such findings could have implications for remediation strategies designed to target impairments of social cognition in people with schizophrenia.
The aims of this study were first, to test whether repetition facilitated performance in a gender decision task with facial expressions in patients with schizophrenia, and second, to test whether patients showed the expected reduction in activity in the occipito-temporal cortex with repetition. To closely compare the behavioral and neural effects of repetition in this patient group, we used the same paradigm to study behavioral priming and neural priming. This design, comparing initial encoding to repeated encoding of stimuli within a short timespan, was modeled after functional magnetic resonance imaging (fMRI) studies of repetition priming used to study the neural basis of implicit memory (Demb et al., 1995; Gabrieli et al., 1996). As repetition effects are known to diminish rapidly with intervening items (Henson et al., 2003), this paradigm maximizes repetition facilitation by repeating sets of stimuli immediately, albeit in a different order, following initial encoding. Thus, activation visualized with fMRI reflects repetition facilitation at its maximum. The same design was used for the behavioral and fMRI studies in order to gauge whether the magnitude of priming from the smaller sample of the fMRI study was comparable to that obtained from a larger group of subjects in a laboratory setting. In the first experiment, blocks of faces with fear expressions and neutral expressions were presented twice, initially and immediately repeated, and subjects identified the gender of the face on each presentation. Repetition priming is evidenced by faster responses to identify the gender upon repeated relative to initial encoding of faces. We also assessed recognition memory for the same facial expressions to compare performance between implicit (gender decision) and explicit (recognition) memory tasks. In the second study, we used fMRI to test whether repetition priming in schizophrenia patients was associated with reduced neural activity in object-sensitive areas of occipito-temporal cortex.
2. Materials and methods
2.1. Behavioral study
2.1.1. Subjects
Patients (21M, 1F) were recruited from outpatient mental health services at the Washington DC Veterans Affairs Medical Center. All met criteria for a diagnosis of schizophrenia (N = 15) or schizoaffective disorder (N = 7) using the Structured Clinical Interview for DSM-IV (First et al., 1997) and chart review. Structured interviews were conducted by psychologists and doctoral-level students in psychology. All patients were treated with atypical antipsychotic medications (N = 21) with the exception of one patient who received a conventional antipsychotic medication. Control subjects (14M, 4F) were recruited from advertisements posted at the Medical Center. Exclusion criteria were past or current psychiatric disorder, alcohol and substance use disorder, neurological disorder, or current serious medical illness. The groups did not differ in terms of age (Patient: M = 47.68, S.D. = 9.33; Control: M = 48.9, S.D. = 6.62), and pre-morbid IQ as measured by the revised National Adult Reading Test (NART; Blair and Spreen, 1989) (Patient: M = 104.3, S.D. = 7.53; Control: M = 105.5, S.D. = 9.54, all P values > 0.05). However, controls had completed on average one more year of education relative to the patients (Patient: M = 13.0, S.D. = 1.35; Control = M = 14.2, S.D. = 2.10, P < 0.05).
2.1.2. Materials and procedure
The stimuli consisted of 120 faces: 60 unique faces each shown with a neutral expression and a fear expression. Faces were selected from the NimStim Set of Facial Expressions (Tottenham et al., 2009) and the University of Pennsylvania database of facial expressions (Gur et al., 2002b, 2010). Although the NimStim face stimuli are a relatively new set of facial expressions, our prior work using these materials in participants with schizophrenia has shown that performance in an implicit task varied as a function of the facial expression (Schwartz et al., 2010). The stimuli were divided into two lists of 60 unique faces. Each list comprised 30 neutral expressions and 30 fear expressions. A face with a fear expression on List 1 appeared with a neutral expression on List 2, and vice versa. Half of the subjects received List 1 and the remaining half received List 2.
Stimuli were presented using E-Prime (Version 1.0 Psychology Software Tools Inc.). Subjects viewed a continuous sequence of 10 blocks of trials that alternated between fear and neutral expressions. The blocked design was used to keep the design in the behavioral study parallel to the imaging study. In each block, six unique faces with the same facial expression were presented for initial encoding and then immediately repeated in a different random order (see Fig. 1). The sequence for each trial was a fixation trial, consisting of a crosshair to alert subjects of the imminent stimulus presentation (1000 ms), face stimulus (1000 ms), and blank screen (1000 ms). Subjects were instructed to identify the face as male or female (gender decision) using the mouse key pad. The left key was labeled “M” for male and the right key was labeled “F” for female. Accuracy and response latency to novel and repeated faces were recorded.
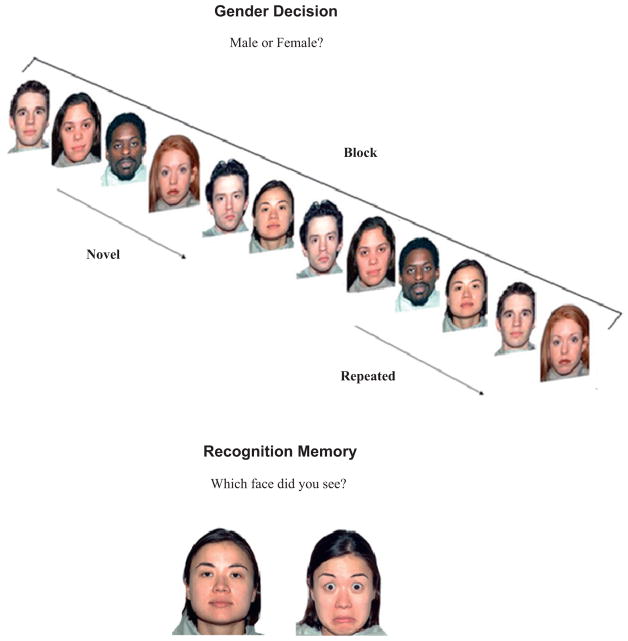
Fig. 1.
Examples of a block of neutral faces in the gender decision task and a pair of faces in the recognition memory task. In the gender decision task, subjects responded male or female for each face. After initial presentation of the six faces in the block (novel faces), the set of six faces was repeated in a different random order (repeated faces). In the recognition memory task, subjects identified which one of the two faces they saw in the previous sequence of trials. Subjects were told that they had seen the model in the photograph with only one of the facial expressions.
A test of recognition memory was administered immediately after the gender decision task to compare implicit versus explicit memory for facial expressions. Subjects viewed 60 pairs of faces of the same person: one face displayed a neutral expression and the other face displayed a fear expression (Fig. 1). Subjects were instructed to choose the face with the expression they had previously seen. Each pair was presented for 5 s. Responses were said aloud and the experimenter recorded accuracy.
2.2. Imaging study
2.2.1. Subjects
Another group of eight patients (seven male, one female) and eight controls (seven male, one female) participated in the imaging study and met the same inclusion and exclusion criteria as those described above. Participants in the patient and control groups did not differ in terms of age (Patient: M = 52.13, S.D. = 6.0; Control: M = 50.5, S.D. = 6.82), education (Patient: M = 13.75, S.D. = 2.05; Control: M = 13.38; S.D. = 0.92), or pre-morbid IQ (Patient: M = 102.8, S.D. = 12.30; Control: M = 100.98, S.D. = 13.09), all P values > 0.05.
2.2.2. Task procedure
The gender decision task described above was used during the scanning session. The task was split into two runs of five blocks. Each block was followed by a fixation trial of 9 s. The timing and structure (initial and repeated encoding of sets of stimuli comprising one block and emotional expression alternating across blocks) of the trial sequence remained the same as described above. Subjects pressed a right hand-held button when the face was male and a left hand-held button when the face was female.
2.2.3. fMRI acquisition
Images were acquired on a 3T Siemens magnet (Siemens Magnetom Trio, Erlangen, Germany). Head movement was minimized with foam padding placed in the head coil. Stimuli were generated in E-prime (Version 2.0 Psychology Software Tools Inc) and back-projected onto a screen (209 × 279 cm2) that was viewed via a coil-mounted mirror. Functional images (77/run) were acquired using a T2-sensitive gradient EPI sequence. The first two scans in each run were acquired for signal stabilization, before the task began, and thus were discarded from analysis, resulting in 75 images in each run. Fifty axial slices (3.2 × 3.2 × 3.2 mm3) were acquired covering the whole brain using the following parameters: TR = 3000 ms, TE = 30 ms, 205 × 205 mm2 FOV, and 90° flip angle. Immediately following functional imaging, a high resolution sagittal T1-weighted structural scan was obtained for the purpose of anatomical localization. The scan was acquired using a 3D MPRAGE sequence with TR = 1600 ms, TE = 4.4 ms, 256 × 256 mm2 FOV, 160-mm slab with 1-mm-thick slices, 256 × 256 × 160 matrix (effective resolution of 1.0 mm3), 1 excitation and a 15° flip angle.
2.2.4. fMRI data analysis
Data were analyzed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB (Version 7.0, Mathworks, Inc., Sherborn, MA). Images for individual subjects were realigned to the first image of each run. No subject displayed more than 3 mm of motion in the x, y, and z directions. Functional images were normalized into MNI anatomical space and aligned to the high-resolution T1structural image for the individual subject. Normalized images were smoothed with an isotropic 8 mm full width half-maximum Gaussian kernel and temporally filtered (high-pass filter: SPM default calculated based upon trial frequency). fMRI responses were modeled by canonical hemodynamic response function with a boxcar function lasting for the duration of each set of stimuli with the block. For each subject, a linear contrast identified activation during repetition priming (Initial > Repeated encoding) separately for faces with neutral and fearful expressions
For second-level analysis, we first examined whether significant priming-related activation was observed in occipito-temporal cortex, irrespective of facial expression, in each group using one-sample t-tests. Next, we conducted a Group (Patients, Controls) × Emotion (Neutral, Fear) analysis of variance (ANOVA) on the repetition priming contrast to identify: (1) regions showing a main effect of Group assessing differences in priming-related activation between patients and controls irrespective of facial expression; and (2) regions showing a Group × Emotion interaction assessing group differences in priming-related activation by facial expression. Each of these analyses was restricted to our apriori area of interest, occipito-temporal cortex, by using an anatomical mask of regions created from the AAL atlas (Tzourio-Mazoyer et al., 2002) including inferior temporal gyrus, fusiform gyrus, lingual gyrus, and visual cortex. For each analysis, maps were thresholded at P < 0.005, k = 35 which is an overall significance level of P < 0.05 corrected for multiple comparisons based on Monte Carlo simulation of random noise distribution [using 3dClustSim module of AFNI (Forman et al., 1995)].
3. Results
3.1. Behavioral study
Performance for the gender decision and recognition memory tasks is shown in Fig. 2. In the gender decision task, a trial was scored as correct if the subject correctly identified the face as male or female. The total number of errors and the mean response time (RT) to make the decision were calculated for each expression condition at each presentation for each participant. Errors were excluded from the RT analysis. A separate 2 (Group) × 2 (Expression) × 2 (Presentation) analysis of variance (ANOVA) was performed for RT and accuracy, with Group as a between-subject factor and Expression and Presentation as within-subject factors. Because novel items presented for initial encoding occurred in multiple blocks, we were able to perform trend analyses to assess practice effects by determining whether gender decisions improved across blocks. For the recognition task, the total number of errors in each expression condition was calculated and analyzed in a 2 (Group) × 2 (Expression) ANOVA.
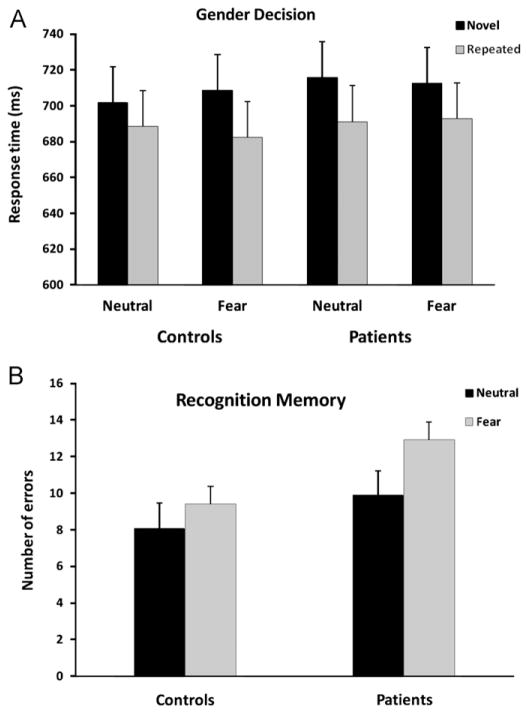
Fig. 2.
(A) Mean RT to identify the face as male or female as a function of Presentation condition (novel, repeated), Expression condition (neutral, fear), and Group (control, patient). (B) Mean number of errors in the recognition memory test as a function of Expression condition and Group. Error bars for each task are presented as the standard error of the mean.
Repetition priming was observed as evidenced by shorter RTs for faces upon repeated relative to initial encoding, F(1, 38) = 36.81, P < 0.0001. Priming did not differ between the groups, F < 1, and did not vary as a function of Expression, F < 1. There were no significant two-way or three-way interactions, all Fs < 1. Priming was not confounded by practice effects, as novel items were not identified faster over blocks (linear trend for Neutral faces: F(1,38) = 0.12, P > 0.05; linear trend for Fear faces: F(1, 38) = 1.61, P > 0.05). Analysis of the error data indicated more errors in the Fear condition (M = 1.31, S.D. = 1.47) than in the Neutral condition (M = 0.89, S.D. = 1.14), F(1, 38) = 5.03, P < 0.05. However, there was no difference in the error rate between the groups, F(1, 38) = 2.00, P > 0.05, or between initial and repeated encoding of faces, F < 1.
As expected, error rate in recognition memory was higher in the patient group, F(1, 38) = 12.70, P < 0.001, but errors did not vary as a function of Expression, F(1, 38) = 1.56, P > 0.05, and there was no interaction of Group × Expression, F < 1. To rule out the possibility that the groups differed for recognition performance due to differences in education level, we performed an analysis of covariance on recognition errors. The results did not change: errors remained higher in the patient group, F(1, 37) = 8.96, P < 0.01, and there was no effect of Expression, P > 0.05 or an interaction of Group × Expression, P > 0.05.
The findings above demonstrate repetition priming as gender decisions were faster for repeated than initial presentation of faces. We were also interested in assessing whether repeated faces were identified faster than novel images of different faces. This comparison is more similar to standard paradigms of repetition priming in which studied items are compared to items not previously encountered. The block design allowed us to compare performance for repeated faces from one block (e.g., repeated faces in block 1) with performance for faces in the next block that were subsequently presented (e.g., novel faces in block 2). Repeated faces were identified faster than novel images of different faces indicating significant priming in both the Neutral, t(39) = 3.89, P < 0.0001 and Fear conditions, t(39) = 3.28, P < 0.01. To compare these two measures of priming, we conducted a 2 × 2 × 2 ANOVA, with Expression and Type of Priming as within-subjects factors, and Group as a between-subjects factor. The results indicated that priming measured in the gender decision task did not vary as a function of Group, Expression, or Type of Priming, all P values > 0.05
To sum up, repetition priming was observed for faces with fear expressions and neutral expressions irrespective of whether repeated items were compared with novel images of the same face (i.e., initial encoding) or a different face. Priming of gender decisions for facial expressions was not impaired in schizophrenia. In contrast, explicit recognition memory for the facial expressions was impaired. However, in the absence of a significant Group × Expression interaction, we are unable to ascertain whether the deficit in recognition memory for schizophrenia patients is due to deficits in identifying and remembering emotional stimuli or simply due to general problems in explicit memory.
3.2. Imaging study
3.2.1. Behavioral results
The results for the gender decision task performed during the scanning session yielded similar results to those reported above. There was a significant reduction in mean RT for faces upon repeated than initial encoding indicating repetition priming (initial: M = 649.40 ms, S.D. = 98.71; repeated: M = 626.37; S.D. = 87 ms), F(1, 16) = 12.05, P < 0.01. There was no statistical difference in overall performance between the groups, (Patient: M = 651.46 ms, S.D. = 121.8; Control: M = 624.25 ms, S.D. = 52.7), F < 1, no difference between Expression conditions (Fear: M = 636.72 ms, S.D. = 95.69; Neutral: M = 639.1 ms, S.D. = 89.5), F < 1, and no interaction of Group × Expression, F < 1. Furthermore, there were no significant main effects or interactions for accuracy, P values > 0.05.
3.2.2. Imaging results
All reported coordinates are converted from MNI to Talairach space using the algorithm mni2tal (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach).
One-sample t-tests indicated that priming-related activation was evident in occipito-temporal cortex in each group (see Table 1, Fig. 3A). For participants with schizophrenia, priming was associated with a reduction in the posterior fusiform gyrus in the right hemisphere. For control participants, significant reductions were found bilaterally in the right fusiform gyrus, right inferior occipital gyrus, and left fusiform gyrus.
Table 1.
Summary of regions showing a significant reduction in activity with the repetition of faces for patients and controls.
| Structure | Brodmann area | Cluster size (voxel) | Z-value | Peak coordinates
|
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Controls | ||||||
| Left fusiform gyrus | 18 | 926 | 4.03 | −38 | −88 | −10 |
| Right inferior occipital gyrus | 19 | 279 | 3.65 | 42 | −82 | −10 |
| Right fusiform gyrus | 37 | 110 | 4.24 | 44 | −44 | −28 |
| Patients | ||||||
| Right fusiform gyrus | 19 | 102 | 3.72 | 34 | −72 | −14 |
Fig. 3.
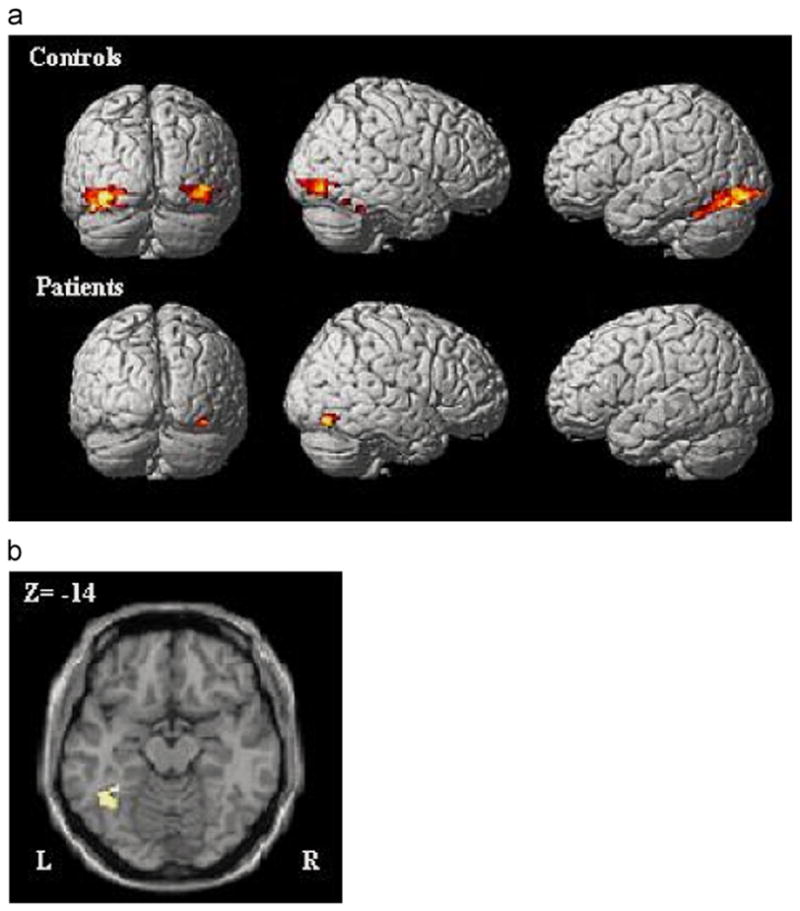
(A) Regions showing significant priming-related activation (initial encoding > repeated encoding) irrespective of Expression in a one-sample t-test for controls (top row) and patients (bottom row), p < 0.05 corrected. Activation in the fusiform gyrus was right lateralized for patients and bilateral for controls. (B) Region in the left fusiform gyrus that showed a significant difference in priming-related activation between patients and controls (main effect of Group), p < 0.05 corrected.
Results of the Group × Expression ANOVA revealed a significant main effect of Group in one large cluster in left fusiform gyrus (x = −36, y = −44, z = −14, 123 voxels, Z = 3.07) such that priming-related activation was greater in control subjects relative to patients, irrespective of facial expression (see Fig. 3B). No regions showed a Group × Expression interaction indicating that priming-related activation in occipito-temporal cortex did not vary by facial expression for either control or patient group.
It appears that the type of expression (fear vs. neutral) affected neither behavioral priming nor priming-related brain activity (cf. Suzuki et al., 2011). One possibility is that naming the gender of the face, rather than identifying the facial expression, reduced encoding of the emotional aspects of the faces. The priming paradigm was used to assess occipito-temporal regions associated with face perception (e.g., fusiform gyrus) rather than regions associated with emotional processing (e.g., amygdala). The finding that priming-related activity was observed in the ventral visual cortex suggests that visual perceptual properties of faces rather than emotional aspects were encoded. Thus priming-related differences between the groups in lateral fusiform gyrus reflect differences in neural activity that are not specific to the emotional expression but can nonetheless affect the processing of facial expressions.
4. Discussion
The aim of the study was to examine behavioral priming and priming-related neural activity for faces with emotional expressions in patients with schizophrenia. There were three main findings: (1) repetition priming was unimpaired in schizophrenia patients; (2) there was a reduction in neural activity for repeated facial expressions in occipito-temporal regions, and (3) group differences in priming-related activation were observed in the left lateral fusiform gyrus.
The behavioral findings showed repetition priming in the gender decision task for facial expressions, as decisions were faster for repeated compared to initial presentations of faces. These findings replicate prior reports of repetition priming for (unfamiliar) faces (Goshen-Gottstein and Ganel, 2000; Martin et al., 2010). In contrast, recognition memory for facial expressions was impaired in participants with schizophrenia. Consistent with previous reports, we observed that implicit memory is preserved in the context of impaired explicit memory in individuals with schizophrenia (Clare et al., 1993; Schwartz et al., 1993; Marie et al., 2001; Soler et al., 2011). Thus, the present results extend the findings of intact implicit memory for words and common objects in schizophrenia to include priming for faces with emotional expressions.
The neuroimaging data revealed the predictable reduction in neural activity associated with stimulus repetition (e.g. Schacter and Buckner, 1998; Henson, 2003; Grill-Spector et al., 2006). Specifically, there was a reduction in the neural response for repeated faces in the right inferior occipital cortex and in bilateral posterior regions of the fusiform gyrus (see also Eger et al., 2005; Pitcher et al., 2007). There was overlap between the two groups in priming-related neural activity in right ventral cortex. However, group differences were observed in the spatial extent and laterality of neural activity. Participants with schizophrenia did not show priming-related reduction in left fusiform gyrus. These findings suggest a disturbance in experience-related neural modulation in schizophrenia (see also, Jeong and Kubicki, 2010).
Research suggests that there is a hierarchical organization of priming-related neural activity that shows lateralized specialization (e.g., Koutstaal et al., 2001; Eger et al., 2005; see also Marsolek et al., 1992; Marsolek, 1995; Vaidya et al., 1998). Priming-related activation that is sensitive to changes in the perceptual features of an item tends to be right lateralized, whereas activity that is less sensitive to such changes is left lateralized. Furthermore, as one proceeds from posterior to anterior regions in the ventral visual processing stream, priming-related activation is less dependent on the repetition of the identical image of a face, suggesting that anterior regions code for more abstract information (Eger et al., 2005). The finding in the patient group that priming-related activity was observed in right occipito-temporal regions associated with early visual face and object processing suggests that repetition modified a more feature-dependent representation of faces. By comparison, bilateral activation extending into more anterior regions of the fusiform gyrus in the control group signals broader activation of abstract, possibly categorical (male/female) representations. As abstract representations support explicit memory, it is possible that deficits in explicit memory in schizophrenia were associated to the lack of sensitivity in anterior left fusiform gyrus to repetition.
One limitation of the study is that we did not include stimuli other than faces to localize regions that respond specifically to faces. This limits our ability to conclude with greater certainty that repetition of stimuli reduced regions that are selective for faces relative to other objects. Another limitation is that the larger number of males than females in the sample could have potentially biased performance in the priming task, especially since the task required subjects to identify the gender of each face. In addition, the small sample size in the neuroimaging study limited statistical power to identify priming-related neural activation in other cortical regions (Wig et al., 2009). Future studies with a larger sample of patients will also allow us to examine the relation between repetition facilitation and clinical symptoms of schizophrenia.
Repetition priming served as a valuable tool to explore visual-perceptual processes associated with face processing in people with schizophrenia. The findings suggest that the repetition paradigm can help isolate mechanisms of implicit memory in the ventral visual cortex. In this study, initial encoding was compared to repeated encoding of the identical visual stimulus to assess a form of perceptual implicit memory. Thus normal priming effects in patients with schizophrenia suggest that enhanced gender decisions for facial expressions can be made on the basis of perceptual features of the stimulus (e.g., face parts, hairstyle). It is also possible to vary stimulus attributes (e.g., unfamiliar vs. familiar faces) or the type of response (e.g., stimulus-response mapping) to test whether abstract or conceptual forms of implicit memory are intact in people with schizophrenia. These studies are important to identifying areas of preserved versus impaired processing of facial expressions in this disorder.
Although we did not observe a deficit in priming of gender decisions for facial expressions for the patients, the data suggest abnormal repetition-related activity, particularly in the left fusiform gyrus. It is likely that altered sensitivity to stimulus repetition disrupts memory and affective processes that depend on specialized information processing mediated by the ventral processing stream. Future studies will need to determine the scope and impact of this deficit and ways to enhance the neural response to socio-emotional stimuli in schizophrenia.
Acknowledgments
The work was supported by a grant from the Brain and Behavior Research Foundation (NARSAD grant) and the Essel Foundation to Barbara Schwartz and by NIMH (No. MH084961) to Chandan Vaidya. The authors would like to thank Katalina McInerney for assistance in testing subjects and Sonia Bansal for assistance with data analysis. Portions of this research were previously presented at meetings of the International Congress of Schizophrenia Research in April, 2009 and the American College of Neuropsychopharmacology in December, 2010.
References
- Andrews TJ, Davies-Thompson J, Kingstone A, Young AW. Internal and external features of the face are represented holistically in face-selective regions of visual cortex. Journal of Neuroscience. 2010;30:3544–3552. doi: 10.1523/JNEUROSCI.4863-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair JR, Spreen O. Predicting premorbid I.Q.: a revision of the National Adult Reading Test. Clinical Neuropsychologist. 1989;43:129–136. [Google Scholar]
- Buckner RL, Petersen SE, Ojemann JG, Miezin FM, Squire LR, Raichle ME. Functional anatomical studies of explicit and implicit memory retrieval tasks. Journal of Neuroscience. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Abeles IY, Weiskopf NG, Tambini A, Jalbrzikowski M, Legatt ME, Javitt DC. Sensory contributions to impaired emotion processing in schizophrenia. Schizophrenia Bulletin. 2009;35:1095–1107. doi: 10.1093/schbul/sbp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Norton D, McBain R, Öngür D, Heckers S. Visual and cognitive processing of face information in schizophrenia: detection, discrimination and working memory. Schizophrenia Research. 2009;107:92–98. doi: 10.1016/j.schres.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare L, McKenna PJ, Mortimer AM, Baddeley AD. Memory in schizophrenia: What is impaired and what is preserved? Neuropsychologia. 1993;31:1225–1241. doi: 10.1016/0028-3932(93)90070-g. [DOI] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophrenia Bulletin. 2006;31:21–42. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. Journal of Neuroscience. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniger GM, Silipo G, Rabinowicz EF, Snodgrass JG, Javitt DC. Impaired sensory processing as a basis for object-recognition deficits in schizophrenia. American Journal of Psychiatry. 2001;158:1818–1826. doi: 10.1176/appi.ajp.158.11.1818. [DOI] [PubMed] [Google Scholar]
- Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clinical Psychology Review. 2002;22:789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- Eger E, Schweinberger SR, Dolan RJ, Henson RN. Familiarity enhances invariance of face representations in human ventral visual cortex: fMRI evidence. Neuroimage. 2005;26:1128–1139. doi: 10.1016/j.neuroimage.2005.03.010. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. User’s guide for the Structured Clinical Interview for DSM-IV Axis 1 Disorders—Clinician Version (SCID–CV) American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Desmond JE, Demb JB, Wagner AD, Stone MV, Vaidya CJ, Glover CJ. Functional magnetic resonance imaging of semantic memory processes in the frontal lobes. Psychological Science. 1996;7:278–283. [Google Scholar]
- Gauthier I, Tarr MJ, Moylan J, Skudlarski P, Gore JC, Anderson AW. The fusiform “face area” is part of a network that processes faces at the individual level. Journal of Cognitive Neuroscience. 2000;12:495–504. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- Goshen-Gottstein Y, Ganel T. Repetition priming for familiar and unfamiliar faces in a sex-judgment task: evidence for a common route for the processing of sex and identity. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:1198–1214. doi: 10.1037//0278-7393.26.5.1198. [DOI] [PubMed] [Google Scholar]
- Graf P, Schacter DL. Implicit and explicit memory for new associations in normal and amnesic patients. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1985;11:501–518. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- Gras-Vincendon A, Danion JM, Grange D, Bilik M, Willard-Schroeder D, Sichel JP, Singer L. Explicit memory, repetition priming and cognitive skill learning in schizophrenia. Schizophrenia Research. 1994;13:117–126. doi: 10.1016/0920-9964(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends in Cognitive Sciences. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Gur RE, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI, Gur RC. An fMRI study of facial emotion processing in patients with schizophrenia. American Journal of Psychiatry. 2002a;159:1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bagcsy R, Posner A, Gur RE. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. Journal of Neuroscience Methods. 2002b;115:137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. Journal of Neuroscience Methods. 2010;187:254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Gorno-Tempini ML, Dolan RJ. Face repetition effects in implicit and explicit memory tests as measured by fMRI. Cerebral Cortex. 2002;12:178–186. doi: 10.1093/cercor/12.2.178. [DOI] [PubMed] [Google Scholar]
- Henson RN. Neuroimaging studies of priming. Progress in Neurobiology. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Henson RN, Goshen-Gottstein YG, Ganel T, Otten LJ, Quayle A, Rugg MD. Electrophysiological and haemodynamic correlates of face perception, recognition, and priming. Cerebral Cortex. 2003;13:793–805. doi: 10.1093/cercor/13.7.793. [DOI] [PubMed] [Google Scholar]
- Jeong B, Kubicki M. Reduced task-related suppression during semantic repetition priming in schizophrenia. Psychiatry Research: Neuroimaging. 2010;181:114–120. doi: 10.1016/j.pscychresns.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston PJ, Stojanov W, Devir H, Schall U. Functional MRI of facial emotion recognition deficits in schizophrenia and their electrophysiological correlates. European Journal of Neuroscience. 2005;22:1221–1232. doi: 10.1111/j.1460-9568.2005.04294.x. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophrenia Bulletin. 2010;36:1009–1019. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL. Perceptual specificity in visual object priming: functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia. 2001;39:184–199. doi: 10.1016/s0028-3932(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Lee J, Gosselin F, Wynn JK, Green MF. How do schizophrenia patients use visual information to decode facial emotion? Schizophrenia Bulletin. 2010;53:1–8. doi: 10.1093/schbul/sbq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal MK, Pandey R, Prasad AB. Facial expressions of emotions and schizophrenia. Schizophrenia Bulletin. 1998;24:399–412. doi: 10.1093/oxfordjournals.schbul.a033335. [DOI] [PubMed] [Google Scholar]
- Marie A, Gabrieli JDE, Vaidya C, Brown B, Pratto F, Zajonc RB, Shaw RJ. The mere exposure effect in patients with schizophrenia. Schizophrenia Bulletin. 2001;27:297–303. doi: 10.1093/oxfordjournals.schbul.a006875. [DOI] [PubMed] [Google Scholar]
- Marsolek CJ. Abstract-visual-form representations in the left cerebral hemisphere. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:375–386. doi: 10.1037//0096-1523.21.2.375. [DOI] [PubMed] [Google Scholar]
- Marsolek CJ, Kosslyn SM, Squire LR. Form-specific visual priming in the right cerebral hemisphere. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1992;18:492–508. doi: 10.1037//0278-7393.18.3.492. [DOI] [PubMed] [Google Scholar]
- Martin D, Cairns SA, Orme E, DeBruine LM, Jones BC, Macrae CN. Form-specific repetition priming for unfamiliar faces. Experimental Psychology. 2010;57:338–345. doi: 10.1027/1618-3169/a000040. [DOI] [PubMed] [Google Scholar]
- McBain R, Norton D, Chen Y. Differential roles of low and high spatial frequency content in abnormal facial emotion perception in schizophrenia. Schizophrenia Research. 2010;122:151–155. doi: 10.1016/j.schres.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton D, McBain R, Holt DJ, Ongur D, Chen Y. Association of impaired facial affect recognition with basic facial and visual processing deficits in schizophrenia. Biological Psychiatry. 2009;65:1094–1098. doi: 10.1016/j.biopsych.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Onitsuka T, Shenton ME, Kasai K, Nestor PG, Toner SK, Kikinis R, McCarley RW. Fusiform gyrus volume reduction and facial recognition in chronic schizophrenia. Archives of General Psychiatry. 2003;60:349–355. doi: 10.1001/archpsyc.60.4.349. [DOI] [PubMed] [Google Scholar]
- Onitsuka T, Niznikiewicz MA, Spencer KM, Frumin PM, Kuroki N, Lucia LC, Shenton ME, McCarley RW. Functional and structural deficits in brain regions subserving face perception in schizophrenia. American Journal of Psychiatry. 2006;163:455–462. doi: 10.1176/appi.ajp.163.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: an overview. Schizophrenia Bulletin. 2008;34:408–411. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Yovel G, Duchaine B. TMS evidence for the involvement of the right occipital face area in early face processing. Current Biology. 2007;17:1568–1573. doi: 10.1016/j.cub.2007.07.063. [DOI] [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Duchaine B. The role of the occipital face area in the cortical face perception network. Experimental Brain Research. 2011;209:481–493. doi: 10.1007/s00221-011-2579-1. [DOI] [PubMed] [Google Scholar]
- Quintana J, Wong T, Ortiz-Portillo E, Marder SR, Mazziotta JC. Right lateral fusiform gyrus dysfunction during facial information processing in schizophrenia. Biological Psychiatry. 2003;53:1099–1112. doi: 10.1016/s0006-3223(02)01784-5. [DOI] [PubMed] [Google Scholar]
- Rotshstein P, Henson RNA, Treves A, Driver J, Dolan RJ. Morphing Marilyn into Maggie dissociates physical and identity face representations in the brain. Nature Neuroscience. 2005;8:107–113. doi: 10.1038/nn1370. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Dobbins IG, Schnyer DM. Specificity of priming: a cognitive neuroscience perspective. Nature Reviews Neuroscience. 2004;5:853–862. doi: 10.1038/nrn1534. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL. Priming and the brain. Neuron. 1998;20:185–195. doi: 10.1016/s0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Dobbins IG, Nicholls L, Davis S, Verfaellie M, Schacter DL. Item to decision mapping in rapid response learning. Memory and Cognition. 2007;35:1472–1482. doi: 10.3758/bf03193617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz BL, Vaidya CJ, Howard JH, Deutsch SI. Attention to gaze and emotion in schizophrenia. Neuropsychology. 2010;24:711–720. doi: 10.1037/a0019562. [DOI] [PubMed] [Google Scholar]
- Schwartz BL, Rosse RB, Deutsch SI. Limits of the processing view in accounting for dissociations among memory measures in a clinical population. Memory and Cognition. 1993;21:63–72. doi: 10.3758/bf03211165. [DOI] [PubMed] [Google Scholar]
- Soler MJ, Ruiz JC, Vargas M, Dasi C, Fuentes I. Perceptual priming in schizophrenia evaluated by word fragment and word stem completion. Psychiatry Research. 2011;190:167–171. doi: 10.1016/j.psychres.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Goh J, Hebrank A, Sutton B, Jenkins L, Flicker BA, Park DC. Sustained happiness? Lack of repetition suppression in right-ventral visual cortex for happy faces. Social Cognitive and Affective Neuroscience. 2011;6:434–441. doi: 10.1093/scan/nsq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Schacter DL. Priming and human memory systems. Science. 1990;247:301–306. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Gabrieli JDE, Verfaellie M, Fleischman D, Askari N. Font-specific priming following global amnesia and occipital lobe damage. Neuropsychology. 1998;12:183–192. doi: 10.1037//0894-4105.12.2.183. [DOI] [PubMed] [Google Scholar]
- Wig GS, Buckner RL, Schacter DL. Repetition priming influences distinct brain systems: evidence from task-evoked data and resting-state correlations. Journal of Neurophysiology. 2009;101:2632–2648. doi: 10.1152/jn.91213.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Current Opinion in Neurobiology. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- Winston JS, Henson RN, Fine-Goulden MR, Dolan RJ. fMRI adaptation reveals dissociable neural representations of identity and expression in face perception. Journal of Neurophysiology. 2004;92:1830–1839. doi: 10.1152/jn.00155.2004. [DOI] [PubMed] [Google Scholar]
- Xu X, Biederman I. Loci of the release from fMRI adaptation for changes in facial expression, identity, and viewpoint. Journal of Vision. 2010;10:1–13. doi: 10.1167/10.14.36. [DOI] [PubMed] [Google Scholar]
- Yoon JH, D’Esposito M, Carter CS. Preserved function of the fusiform face area in schizophrenia as revealed by fMRI. Psychiatry Research. 2006;148:205–216. doi: 10.1016/j.pscychresns.2006.06.002. [DOI] [PubMed] [Google Scholar]