Abstract
Objective
We previously reported improved body composition and cardiovascular risk markers plus a small decrease in glucose tolerance with GH administration vs. placebo for 6 months to abdominally obese premenopausal women. The objective of this study was to determine whether the effects of GH treatment on cardiovascular risk markers, body composition, and glucose tolerance in obese women persist 6 months after GH withdrawal.
Design and patients
Fifty abdominally obese premenopausal women completed a trial of rhGH vs. placebo for 6 months; 39 women completed a subsequent 6-month withdrawal observation period.
Measurements
IGF-I, body composition by CT, 1H-MRS and DXA, serum cardiovascular risk markers, oral glucose tolerance test (OGTT)
Results
IGF-I standard deviation scores (SDS) within the GH group were −1.7±0.1 (pretreatment), −0.1±0.3 (after 6 months of GH) and −1.7±0.1 (6 months post-GH withdrawal). Six months after GH withdrawal, total abdominal and subcutaneous adipose tissue, total fat, trunk fat, trunk/extremity fat, hsCRP, apoB, LDL, and tPA were higher than at the 6-month (GH discontinuation) timepoint (p≤0.05). All body composition and cardiovascular risk markers that had improved with GH returned to baseline levels by 6 months after GH discontinuation, as did fasting and 2-hour OGTT glucose levels.
Conclusion
The effects of GH administration to abdominally obese premenopausal women have a short time-course. The beneficial effects on body composition and cardiovascular risk markers, and the side effect of altered glucose tolerance returned to pretreatment levels after GH withdrawal. There was no suppression of endogenous IGF-I levels, which returned to baseline after GH withdrawal.
Keywords: growth hormone, obesity, abdominal adiposity, body composition
Introduction
Abdominal adiposity is associated with impaired growth hormone (GH) secretion (1) and unfavorable cardiovascular risk marker and body composition profiles, including elevated high-sensitivity C-reactive protein (hsCRP), increased fat mass and decreased lean mass (2, 3). Several studies have demonstrated that GH treatment in individuals with adult-onset GH deficiency due to pituitary disease improves cardiovascular risk makers (4) and body composition (5–9) but can also cause abnormalities in glucose homeostasis (6). We recently reported results from a 6-month randomized, double-blind, placebo-controlled study, which demonstrated that GH treatment in abdominally obese premenopausal women improved body composition and cardiovascular risk markers, accompanied by a mild increase in both fasting and 2-hour glucose after oral tolerance test (OGTT) (10). These results are consistent with other studies in other obese populations, including obese men and post-menopausal women(11–14).
Important questions regarding GH treatment include: whether the beneficial effects of GH persist following GH withdrawal, whether GH-related effects reverse, and whether withdrawal of GH results in a rebound effect in which body composition overshoots pretreatment levels. In adults with adult-onset GH deficiency (GHD) due to pituitary disease, studies have shown that the effects of GH administration on cardiovascular risk markers and function do not persist following cessation of GH treatment (4, 15); however, results with regard to effects on body composition are conflicting. Both persistence and reversal of body fat reduction and increases in lean mass after GH withdrawal have been demonstrated (16, 17). In adults with HIV lipodystrophy treated with GH at treatment doses (18), and in healthy men with abdominal adiposity treated with supraphysiologic doses of GH (9), the beneficial effects of decreased visceral adiposity reversed after GH withdrawal, and in the HIV study visceral adiposity rebounded to higher levels than had been observed pretreatment. In addition, in one study, GH-induced insulin resistance rapidly reversed after GH withdrawal in adults with GHD (19).
We hypothesized that six months after withdrawal of GH therapy, the cardiovascular and body composition benefits and altered glucose homeostasis would reverse to pre-treatment levels with no rebound effect.
Subjects and Methods
Subjects
The inclusion and exclusion criteria have been previously stated (10). Briefly, premenopausal women ages 18–45 with BMI ≥ 25 kg/m2 waist circumference >88 cm, IGF-I level below the median for age-matched reference range, who were otherwise healthy were recruited through community advertisements. Seventy-nine women were randomized, fifty women completed the 6-month GH vs placebo trial, and thirty-nine women completed the 6-month withdrawal period.
Protocol
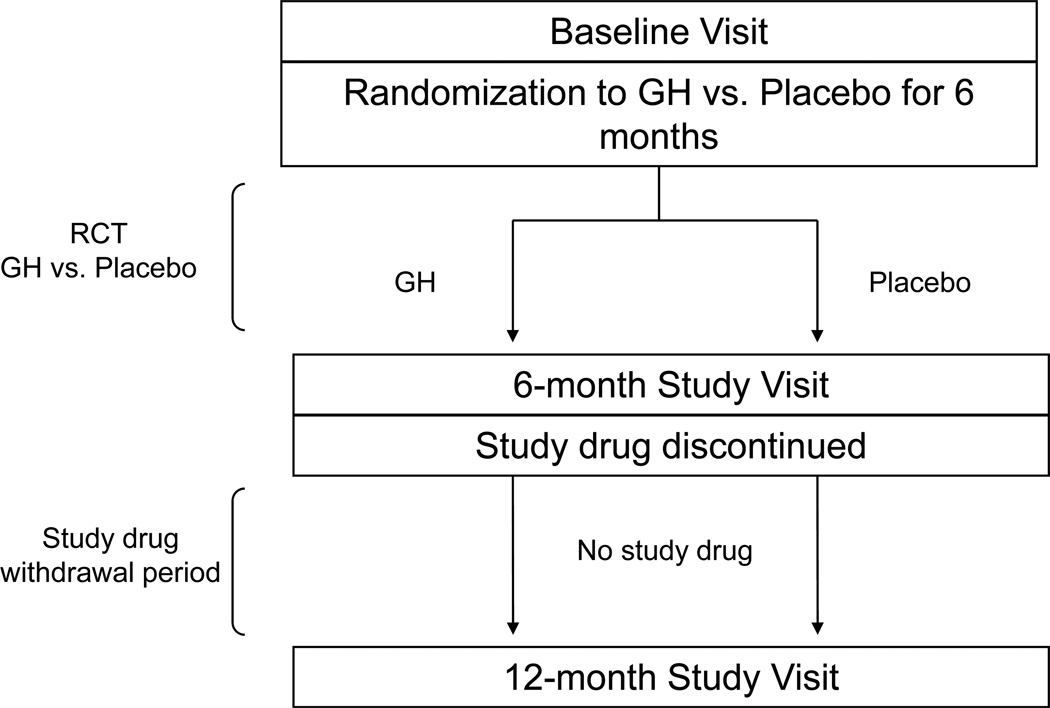
This was a single center, double-blind, randomized, placebo-controlled, parallel study performed on the Massachusetts General Hospital Clinical Research Center. The study was approved by the Partners Healthcare institutional review board. After full explanation of the purpose and nature of all procedures, written consent was obtained from each subject. The trial is registered at ClinicalTrials.gov, number NCT00131378. Subjects were randomized to recombinant human GH (Nutropin®, Genentech, Inc., South San Francisco, CA) or placebo for six months. Subsequently, subjects were followed for six months after study medication (GH or placebo) withdrawal (Figure 1). The starting dose of GH was 4 mcg/kg/d and was titrated by an independent unblinded investigator to target an IGF-I level in the upper half of the normal age-matched reference range. An independent data and safety monitoring board periodically reviewed the safety data, and drop criteria included a positive pregnancy test, fasting plasma glucose ≥ 7.0 mmol/L, or 2-hour oral glucose tolerance test (OGTT) glucose ≥ 11.1 mmol/L during the first six months. Study subjects were instructed not to modify exercise or eating habits during the duration of the study. Level of activity, including exercise, was assessed using the Paffenbarger questionnaire, a self-administered questionnaire that measures current levels of activity. Physical activity remained unchanged during the withdrawal period (p=0.8). The Massachusetts General Hospital research pharmacy dispensed active or placebo study drug, which were identical in appearance, to maintain double-blinding. The results of the 6-month GH vs placebo trial have been reported (10). This manuscript reports the results of the subsequent 6-month withdrawal period.
Figure 1.
Overview of study design. Obese premenopausal women were randomized to GH vs placebo for 6 months followed by a 6-month withdrawal period. This study reports the data from the 6-month withdrawal period.
Statistical Analysis
This study reports measurements performed at baseline, 6 months, and 12 months. A repeated measured analysis of variance was fit with terms for time and treatment×time, other than baseline. Two contrasts were estimated. The first tested for a treatment effect at 12 months and the second tested for a treatment effect on the difference between the 12-month and 6-month measurement. Probability value ≤ 0.05 was used to identify significance. Data are presented as mean±SEM.
Methods
IGF-I (solid-phase enzyme-labeled chemiluminescent immunometric assay, Immulite 2000 automated immunoanalyzer, Siemens Medical Systems, Erlangen, Germany; coefficient of variation (cv) < 5%), GH (immunoradiometric assay, Diagnostic Systems Laboratories Inc., Webster, TX; minimum detection limit 0.01 µg/L, cv < 6%), hsCRP (latex particle-enhanced immunoturbidmetric assay, Hitachi 911 analyzer, Roche Diagnostics, Indianapolis; cv < 6%), and other laboratory values were measured on blood samples drawn after an overnight fast. A 75-g, 2h OGTT was performed.
Body composition was assessed by computed tomography (CT), proton magnetic resonance spectroscopy (1H-MRS), and dual-energy x-ray absorptiometry (DXA.). CT at the level of the 4th lumbar vertebra was used to determine abdominal total adipose tissue (TAT), visceral adipose tissue (VAT), and subcutaneous adipose tissue (SAT). CT of the mid-thigh was used to determine thigh muscle cross-sectional area (cv < 1% for fat and muscle area) (20). 1H-MRS of calf muscle for intramyocellular lipids (IMCL) and liver for intrahepatic lipids (IHL) (3.0 Tesla MRI, Siemens Trio, Siemens Medical Systems, Erlangen, Germany; cv = 6% for IMCL and 8% for IHL) was performed as previously described (21, 22). DXA (Hologic QDR-4500, Hologic Inc, Waltham, MA; precision 1.7% for fat mass and 2.4% for fat-free mass) was used to measure fat and lean mass compartments.
Carotid intima-media thickness (IMT) (HDI 5000 ultrasound system, Philips Medical Systems, Bothwell, WA) was measured by a single, blinded, cardiologist with average cv 4.7%, as previously described (10). Mean IMT was determined by semiautomated edge detection software over the distal 1-cm segment of the right and left carotid arteries.
Results
Subjects
Fifty women completed the six-month randomized trial (n=28 in GH, n=22 in placebo), and thirty-nine women completed the subsequent six-month period after GH withdrawal (n=21 in GH, n=18 in placebo). At baseline, the mean age of subjects was 36±0.8 years, mean BMI was 34.9±0.6 kg/m2 (range 25.1–49.7 kg/m2), mean waist circumference was 109.6±1.4 cm, and mean IGF-I SDS was −1.7±0.05.
GH dosing and IGF-I levels
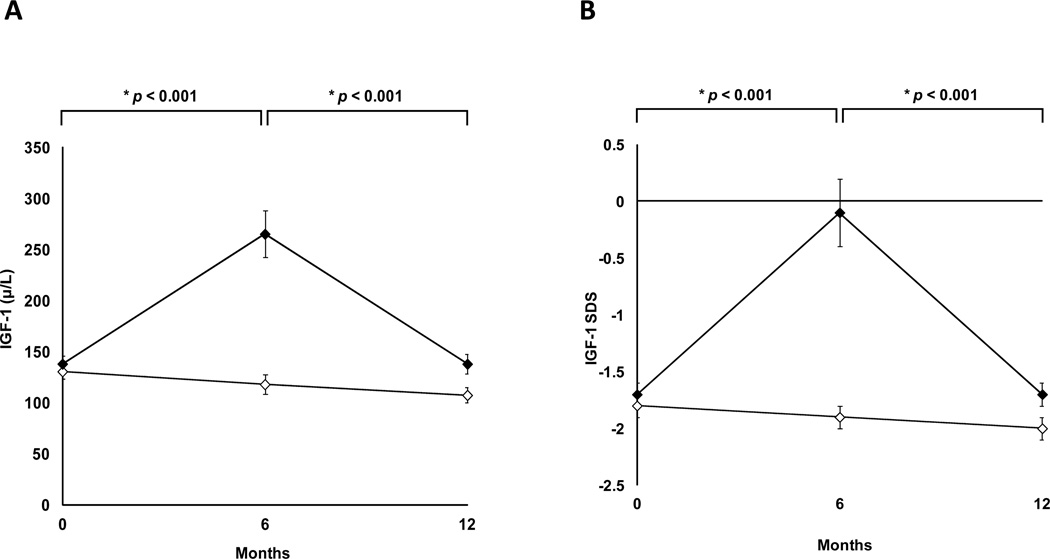
Mean IGF-I and SDS levels are shown in Figure 2. At baseline, IGF-I and SDS levels were comparable between the two groups. At 6 months, mean IGF-I was 265.1±23.0 µg/L (p<0.0001) and SDS was −0.13±0.27 (p<0.0001). At 12 months (six months after GH withdrawal), both absolute levels of IGF-I and IGF-I SDS were comparable to pretreatment levels, with no evidence of suppression of the GH axis. At 12 months, mean IGF-I was 137.8±9.8 µg/L in the GH group and 107.2±7.3 µg/L in the placebo group (p=0.11) with a mean SDS −1.67±0.10 in the GH group and −1.99±0.09 in the placebo group (p=0.11).
Figure 2.
Mean IGF-I (panel A) and IGF-I SDS (panel B) levels (± SEM) in women randomized to growth hormone (solid diamonds) or placebo (open diamonds) for the first six months, followed by 6-month withdrawal of study medication. p by repeated measures ANOVA between the two groups.
Body Composition
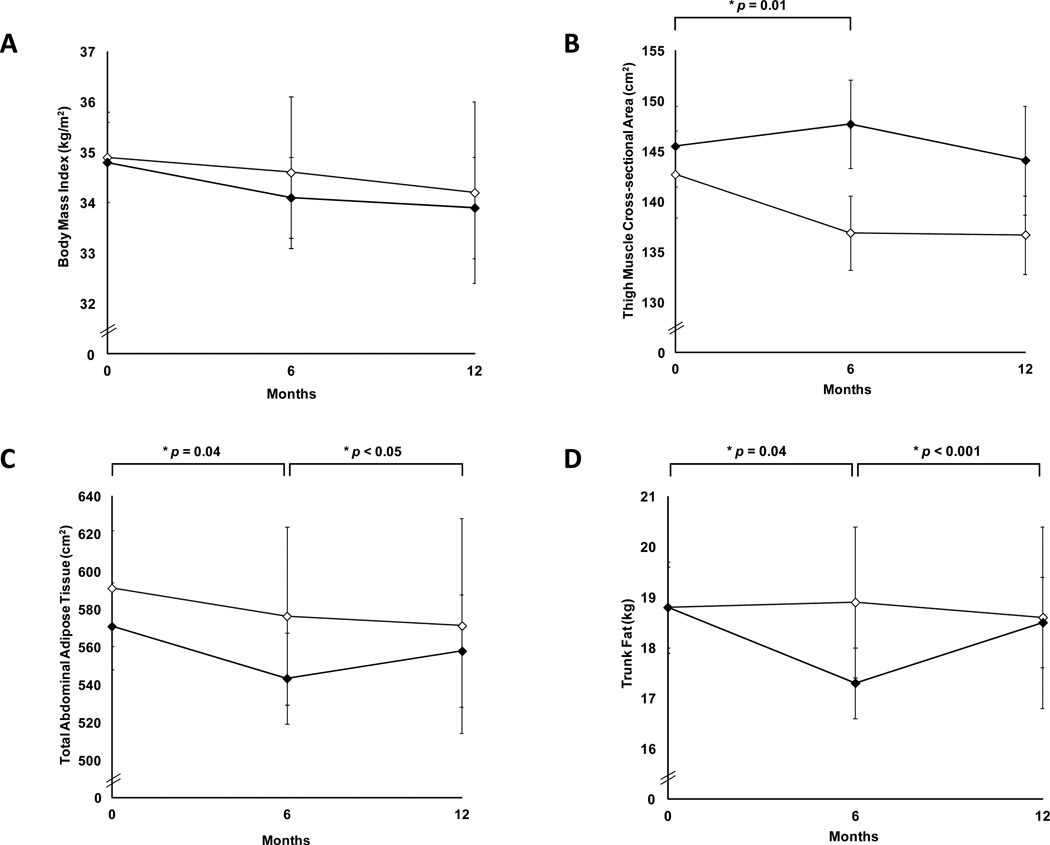
Following 6 months of GH withdrawal, body composition parameters that had improved with GH (decrease in TAT, SAT, trunk fat, and increase in thigh muscle and lean mass in GH vs placebo) returned to baseline levels at 12 months (p>0.05). TAT (p=0.05), SAT (p=0.01), total fat (p=0.001), trunk fat (p<0.001), and trunk/extremity fat (p=0.03) increased compared to the 6-month visit. There was no significant change in IMCL, IHL, VAT, and BMI during the 6-month treatment and 6-month withdrawal periods in the GH group vs. placebo (p=0.2 to 0.9). BMI, thigh muscle area, TAT, and trunk fat over the 12-month period (first 6 months receiving GH vs. placebo and subsequent 6 months receiving no study drug) are shown in Figure 3.
Figure 3.
Mean body mass index (panel A), thigh muscle cross-sectional area (panel B), total abdominal adipose tissue (panel C), and trunk fat (panel D) (± SEM) in women randomized to growth hormone (solid diamonds) or placebo (open diamonds) for the first six months, followed by 6-month withdrawal of study medication. p by repeated measures ANOVA between the two groups.
Cardiovascular Risk Markers
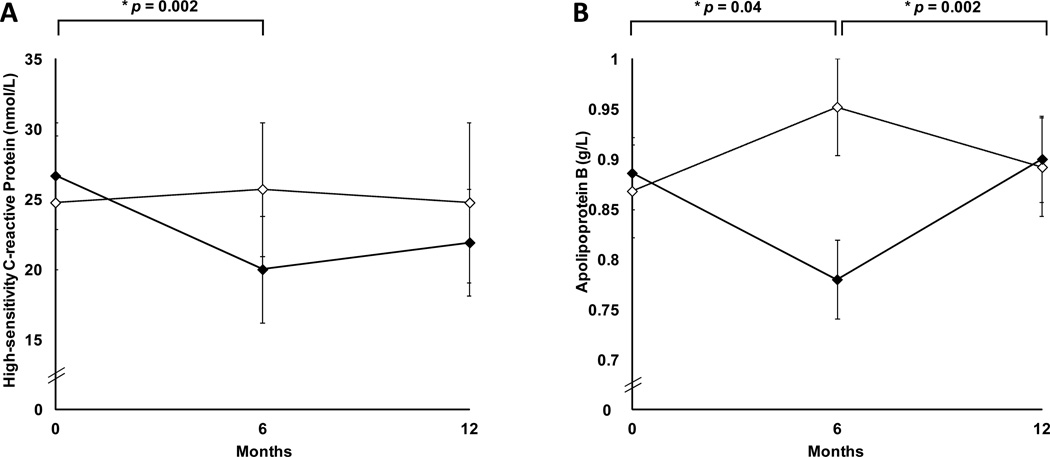
Following 6 months of GH withdrawal, cardiovascular risk markers which had decreased following 6 months of GH treatment compared to placebo, increased compared to the 6-month visit: tPA (p=0.01), apoB (p=0.002), LDL (p=0.05). All cardiovascular risk marker outcome measures that had improved with GH returned to pretreatment levels at 12 months by repeated-measures analysis of variance (p>0.05). hsCRP and apoB are shown in Figure 4. There was no significant change in serum cholesterol, triglycerides and carotid IMT, during the 6-month treatment and 6-month withdrawal periods in the GH group vs placebo (p=0.1 to 0.9).
Figure 4.
Mean high-sensitivity C-reactive protein (panel A) and apolipoprotein B (panel B) (± SEM) in women randomized to growth hormone (solid diamonds) or placebo (open diamonds) for the first six months, followed by 6-month withdrawal of study medication. p by repeated measures ANOVA between the two groups.
Glucose Parameters
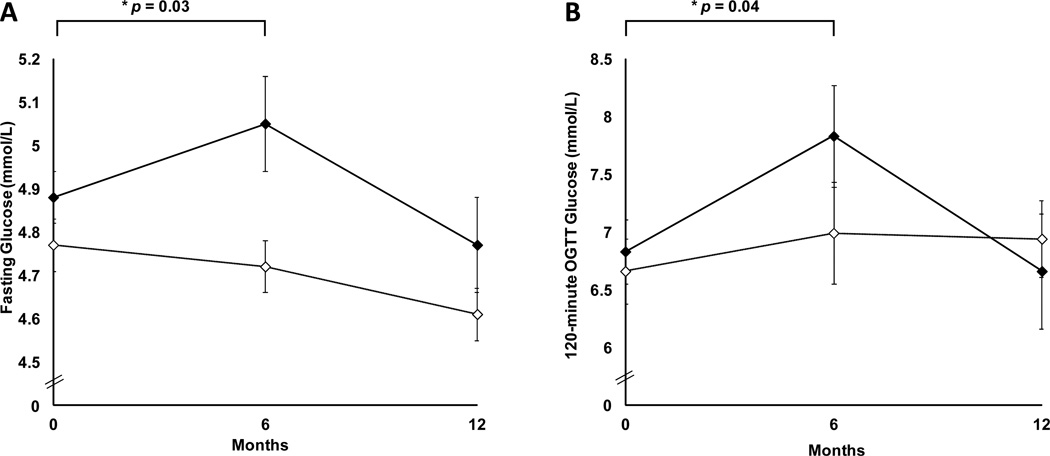
GH administration was associated with an increase in fasting and 2-hour OGTT glucose compared with placebo during 6 months of GH administration with four subjects having 2-hour OGTT glucose levels > 11.1 mmol/L. Three of these subjects underwent follow-up OGTTs at 9 months, which demonstrated normalization of glucose levels. No subjects had fasting glucose levels ≥ 7.0 mmol/L at any point during the study. All glucose parameters that had increased with GH returned to pre-treatment levels at 12 months by repeated-measures analysis of variance (p>0.05). Fasting glucose and 2-hour OGTT glucose are shown in Figure 5.
Figure 5.
Mean fasting glucose (panel A) and 120-minute oral glucose tolerance test glucose (panel B) levels (± SEM) in women randomized to growth hormone (solid diamonds) or placebo (open diamonds) for the first six months, followed by 6-month withdrawal of study medication. p by repeated measures ANOVA between the two groups.
Discussion
Our study demonstrates that in women with abdominal adiposity, 6 months of GH treatment does not exert long-term effects on the GH axis, cardiovascular risk markers, body composition, or glucose tolerance, following cessation of GH treatment. We previously reported results from a 6-month randomized, placebo-controlled study, which demonstrated that GH treatment in abdominally obese premenopausal women improved body composition and a number of cardiovascular risk markers. This included a decrease in total abdominal adipose tissue (TAT), subcutaneous abdominal adipose tissue (SAT), trunk fat, tissue plasminogen activator (tPA), apolipoprotein B (apoB), low-density lipoprotein (LDL), and high-sensitivity C-reactive protein (hsCRP), and an increase in thigh muscle and total lean mass(10). This was accompanied by a mild increase in both fasting and 2-hour glucose after oral tolerance test (OGTT). After 6 months of GH withdrawal, both IGF-I and IGF-I SDS returned to pre-treatment levels, with no evidence of GH axis suppression. All body composition and cardiovascular risk measures, which had improved with GH administration, reversed with GH withdrawal. In addition, GH-induced glucose intolerance also reversed after GH withdrawal.
Published data regarding whether GH exerts lasting effects after its discontinuation are conflicting. In a crossover study, 34 adults with GHD secondary to pituitary disease were randomized to receive GH or no GH treatment for 6 months and then crossed over to the other intervention. Improvements observed with GH administration reversed 6 months after GH withdrawal, with an increased total cholesterol/HDL ratio and increased hsCRP compared with the 6-month on-treatment levels (4). However, other studies have suggested that body composition effects associated with GH administration may last beyond the duration of the GH treatment. In a study of adults with GHD, GH treatment was administered for 18 months, followed by 6 months of GH withdrawal. In a crossover study, 40 males with GHD were randomized to GH or placebo for 18 months, followed by an 18-month crossover period. Although improvements to body fat percentage and lean mass observed during GH administration reversed during the 18-month placebo period, lean mass remained significantly higher compared to pre-treatment levels, suggesting a persistent effect of the prior GH treatment on this parameter (17). However, a study of GH withdrawal in a different population demonstrated very different results – that of a possible rebound effect on fat mass. In a crossover study of adults with HIV and lipodystrophy randomized to GH versus placebo for 18 months, visceral adipose tissue mass – which had decreased during the 18-month treatment period – increased to higher than pre-treatment levels 18 months after withdrawal (18). One might hypothesize that a higher GH dose might be more likely to result in prolonged effects. The mean dose administered in our study was higher than would have been expected to increase IGF-I levels within the normal range, based on data in hypopituitary patients(23). Nevertheless, the mean IGF-I SDS after 6 months of our study was −0.1, suggesting that we did not over-replace GH. The apparent discrepancy may reflect noncompliance in a portion of the patients, resulting in high apparent doses that were not self-administered reliably. In a study of adults with abdominal adiposity who received 6 months of supraphysiologic GH followed by 6 months of GH withdrawal, the beneficial effects of decreased visceral adiposity also reversed (9). From these studies, we cannot determine definitively whether dose affects length of GH effect. It should also be noted that we studied only women with IGF-I levels in the lower half of the normal range, and it is unknown whether our findings extend to obese women with higher endogenous IGF-I levels. Whether the apparently conflicting results of published studies are related to differences in populations studied (including degree of relative GH deficiency), doses of growth hormone and/or length of time of GH treatment and/or withdrawal remains unknown.
There are few published data regarding effects of GH withdrawal on glucose homeostasis. A recent study suggests that effects of GH are rapidly reversible. In a crossover study, 8 males with GHD on stable GH treatment were studied with hyperinsulinemic-euglycemic clamps after a 3-day period of GH withdrawal and were then randomized to no GH treatment or GH infusions. Although glucose infusion rates increased during GH administration, glucose infusion rates five hours after GH discontinuation were similar to those of controls, suggesting that GH-induced insulin resistance is rapidly reversible (19). These data are consistent with our data in which abnormalities in glucose homeostasis reversed after GH withdrawal.
In conclusion, our study suggests that the effects of GH administration to abdominally obese premenopausal women have a short time-course. The beneficial effects on body composition and cardiovascular risk outcome measures, and the side effect of altered glucose tolerance, reverse after GH withdrawal; all return to pretreatment levels. Importantly, there was no indication of suppression of endogenous GH levels below pre-treatment concentrations, as IGF-I levels returned to baseline after withdrawal of GH therapy.
Acknowledgements
Study medication and placebo only were supplied by Genentech, Inc.
Funding
This work was supported in part by National Institutes of Health Grants R01 HL-077674, UL1 RR-025758, K23 RR-23090, and T32 DK007028.
Footnotes
Declaration of Interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Vahl N, Jorgensen JO, Skjaerbaek C, Veldhuis JD, Orskov H, Christiansen JS. Abdominal adiposity rather than age and sex predicts mass and regularity of GH secretion in healthy adults. Am J Physiol. 1997;272:E1108–E1116. doi: 10.1152/ajpendo.1997.272.6.E1108. [DOI] [PubMed] [Google Scholar]
- 2.Cordido F, Garcia-Buela J, Sangiao-Alvarellos S, Martinez T, Vidal O. The decreased growth hormone response to growth hormone releasing hormone in obesity is associated to cardiometabolic risk factors. Mediators Inflamm. 2010;2010:434562. doi: 10.1155/2010/434562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller KK, Biller BM, Lipman JG, Bradwin G, Rifai N, Klibanski A. Truncal adiposity, relative growth hormone deficiency, and cardiovascular risk. J Clin Endocrinol Metab. 2005;90:768–774. doi: 10.1210/jc.2004-0894. [DOI] [PubMed] [Google Scholar]
- 4.Colao A, Di Somma C, Rota F, Pivonello R, Savanelli MC, Spiezia S, Lombardi G. Short-term effects of growth hormone (GH) treatment or deprivation on cardiovascular risk parameters and intima-media thickness at carotid arteries in patients with severe GH deficiency. J Clin Endocrinol Metab. 2005;90:2056–2062. doi: 10.1210/jc.2004-2247. [DOI] [PubMed] [Google Scholar]
- 5.Beauregard C, Utz AL, Schaub AE, Nachtigall L, Biller BM, Miller KK, Klibanski A. Growth hormone decreases visceral fat and improves cardiovascular risk markers in women with hypopituitarism: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2008;93:2063–2071. doi: 10.1210/jc.2007-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo J, You SM, Canavan B, Liebau J, Beltrani G, Koutkia P, Hemphill L, Lee H, Grinspoon S. Low-dose physiological growth hormone in patients with HIV and abdominal fat accumulation: a randomized controlled trial. JAMA. 2008;300:509–519. doi: 10.1001/jama.300.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meinhardt U, Nelson AE, Hansen JL, Birzniece V, Clifford D, Leung KC, Graham K, Ho KK. The effects of growth hormone on body composition and physical performance in recreational athletes: a randomized trial. Ann Intern Med. 2010;152:568–577. doi: 10.7326/0003-4819-152-9-201005040-00007. [DOI] [PubMed] [Google Scholar]
- 8.Sattler FR, Castaneda-Sceppa C, Binder EF, Schroeder ET, Wang Y, Bhasin S, Kawakubo M, Stewart Y, Yarasheski KE, Ulloor J, Colletti P, Roubenoff R, Azen SP. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab. 2009;94:1991–2001. doi: 10.1210/jc.2008-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasarica M, Zachwieja JJ, Dejonge L, Redman S, Smith SR. Effect of growth hormone on body composition and visceral adiposity in middle-aged men with visceral obesity. J Clin Endocrinol Metab. 2007;92:4265–4270. doi: 10.1210/jc.2007-0786. [DOI] [PubMed] [Google Scholar]
- 10.Bredella MA, Lin E, Brick DJ, Gerweck AV, Harrington LM, Torriani M, Thomas BJ, Schoenfeld DA, Breggia A, Rosen CJ, Hemphill LC, Wu Z, Rifai N, Utz AL, Miller K. Effects of growth hormone in women with abdominal adiposity: a 6-month randomized, double-blind, placebo-controlled trial. Eur J Endocrinol. 2012;166:601–611. doi: 10.1530/EJE-11-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franco C, Brandberg J, Lonn L, Andersson B, Bengtsson BA, Johannsson G. Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. J Clin Endocrinol Metab. 2005;90:1466–1474. doi: 10.1210/jc.2004-1657. [DOI] [PubMed] [Google Scholar]
- 12.Hong JW, Park JK, Lim CY, Kim SW, Chung YS, Kim SW, Lee EJ. A weekly administered sustained-release growth hormone reduces visceral fat and waist circumference in abdominal obesity. Horm Metab Res. 2011;43:956–961. doi: 10.1055/s-0031-1291246. [DOI] [PubMed] [Google Scholar]
- 13.Johannsson G, Marin P, Lonn L, Ottosson M, Stenlof K, Bjorntorp P, Sjostrom L, Bengtsson BA. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab. 1997;82:727–734. doi: 10.1210/jcem.82.3.3809. [DOI] [PubMed] [Google Scholar]
- 14.Mekala KC, Tritos NA. Effects of recombinant human growth hormone therapy in obesity in adults: a meta analysis. J Clin Endocrinol Metab. 2009;94:130–137. doi: 10.1210/jc.2008-1357. [DOI] [PubMed] [Google Scholar]
- 15.Cho GY, Jeong IK, Kim SH, Kim MK, Park WJ, Oh DJ, Yoo HJ. Effect of growth hormone on cardiac contractility in patients with adult onset growth hormone deficiency. Am J Cardiol. 2007;100:1035–1039. doi: 10.1016/j.amjcard.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 16.Gomez JM, Gomez N, Fiter J, Soler J. Effects of long-term treatment with GH in the bone mineral density of adults with hypopituitarism and GH deficiency and after discontinuation of GH replacement. Horm Metab Res. 2000;32:66–70. doi: 10.1055/s-2007-978591. [DOI] [PubMed] [Google Scholar]
- 17.Biller BM, Sesmilo G, Baum HB, Hayden D, Schoenfeld D, Klibanski A. Withdrawal of long-term physiological growth hormone (GH) administration: differential effects on bone density and body composition in men with adult-onset GH deficiency. J Clin Endocrinol Metab. 2000;85:970–976. doi: 10.1210/jcem.85.3.6474. [DOI] [PubMed] [Google Scholar]
- 18.Lo J, You SM, Liebau J, Lee H, Grinspoon S. Effects of low-dose growth hormone withdrawal in patients with HIV. Jama. 2010;304:272–274. doi: 10.1001/jama.2010.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krusenstjerna-Hafstrom T, Clasen BF, Moller N, Jessen N, Pedersen SB, Christiansen JS, Jorgensen JO. Growth hormone (GH)-induced insulin resistance is rapidly reversible: an experimental study in GH-deficient adults. J Clin Endocrinol Metab. 2011;96:2548–2557. doi: 10.1210/jc.2011-0273. [DOI] [PubMed] [Google Scholar]
- 20.Bredella MA, Ghomi RH, Thomas BJ, Torriani M, Brick DJ, Gerweck AV, Misra M, Klibanski A, Miller KK. Comparison of DXA and CT in the assessment of body composition in premenopausal women with obesity and anorexia nervosa. Obesity (Silver Spring) 2010;18:2227–2233. doi: 10.1038/oby.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bredella MA, Ghomi RH, Thomas BJ, Miller KK, Torriani M. Comparison of 3.0 T proton magnetic resonance spectroscopy short and long echo-time measures of intramyocellular lipids in obese and normal-weight women. J Magn Reson Imaging. 2010;32:388–393. doi: 10.1002/jmri.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bredella MA, Ghomi RH, Thomas BJ, Ouellette HA, Sahani DV, Miller KK, Torriani M. Breath-hold 1H-magnetic resonance spectroscopy for intrahepatic lipid quantification at 3 Tesla. J Comput Assist Tomogr. 2010;34:372–376. doi: 10.1097/RCT.0b013e3181cefb89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho KK. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol. 2007;157:695–700. doi: 10.1530/EJE-07-0631. [DOI] [PubMed] [Google Scholar]